Abstract
The survival rate for metastatic osteosarcoma has not improved for several decades, since the introduction and refinement of chemotherapy as a treatment in addition to surgery. Over two thirds of metastatic osteosarcoma patients, many of whom are children or adolescents, fail to exhibit durable responses and succumb to their disease. Concerted efforts have been made to increase survival rates through identification of candidate therapies via animal studies and early phase trials of novel treatments, but unfortunately, this work has produced negligible improvements to the survival rate for metastatic osteosarcoma patients. This review summarizes data from clinical trials of metastatic osteosarcoma therapies as well as pre-clinical studies that report efficacy of novel drugs against metastatic osteosarcoma in vivo. Considerations regarding the design of animal studies and clinical trials to improve survival outcomes for metastatic osteosarcoma patients are also discussed.
1. Introduction to Metastatic Osteosarcoma and Its Current Treatment Regimens
Osteosarcoma is the most common primary cancer of the bone. Its emergence is typically driven by mutations in the TP53 and RB1 genes of osteoblastic cells or their precursors in the extremities, such as the tibia or femur [1,2,3]. Osteosarcoma has an annual overall incidence rate of 3.1 per million [4]. It has a bimodal age distribution with a peak incidence of 4.2 per million in children and young adults, decreasing to 1.7 per million in adults aged 25–59, and a subsequent peak of 4.2 per million in older individuals [4].
The 5-year survival rate of osteosarcoma was as low as 20% when it was originally defined in the 1950s [5]. The introduction of chemotherapy markedly improved survival. An early randomized trial of adjuvant chemotherapy to treat osteosarcoma explored a combination of doxorubicin, cisplatin, cyclophosphamide, high-dose methotrexate, dactinomycin and bleomycin [6]. The authors reported a 2-year relapse-free survival rate of 66% versus 17% for the control (surveillance) group [6]. Another study later reported comparable survival rates to the multi-drug regimen using only doxorubicin, cisplatin and high-dose methotrexate [7]. A subsequent randomized trial compared the efficacy of doxorubicin and cisplatin versus doxorubicin, cisplatin, cyclophosphamide, high-dose methotrexate, dactinomycin, vincristine and bleomycin [8]. The trial achieved the same survival rate in both treatment groups with improved tolerability in the two-drug regimen group [8].
Most osteosarcoma patients currently receive doxorubicin, cisplatin and high-dose methotrexate (referred to as “MAP”) as first-line therapy, alongside limb-sparing surgery to remove the primary tumor. This regimen is associated with a 5-year survival rate of around 70% for localized disease. MAP is also administered as a first-line regimen to patients with metastases at diagnosis, although it is less effective for these patients: between 10% to 40% of them survive for 5 years or more after diagnosis.
Retrospective studies have underscored the utility of metastasectomies for patients with pulmonary metastases, including repeated operations to remove any subsequently detected lung lesions [9,10,11]. Evidence also supports intervention with the less invasive approach of radiofrequency ablation to destroy relatively small, uncalcified pulmonary osteosarcoma metastases [12].
A wide variety of systemic therapies have been explored for patients with relapsed or refractory osteosarcoma. Regorafenib (discussed below) is the only drug whose use as a second-line therapy was uniformly recommended by the National Comprehensive Cancer Network on the basis of high level (category 1) evidence, with high-dose ifosfamide plus etoposide and sorafenib receiving recommendations as second-line regimens based on less conclusive evidence [13].
Factors that portend particularly poor prognoses for patients with osteosarcoma metastases include: distant bone lesions (compared to the more common lung metastases) [14,15,16,17,18], bilateral (compared to unilateral) pulmonary metastases [16,19] and a greater number of lung nodules [14,20]. Metastases in relapsed patients were reportedly less responsive than metastases within previously untreated individuals, implying that prior chemotherapy exposure (even to different classes of agents) may select for chemo-resistant cells [21].
At diagnosis, around 20% of osteosarcoma patients have detectable metastases. The lungs make up 90% of metastatic sites, with other bones accounting for a further 8% to 10% [22,23]. Many patients who do not have detectable metastases at diagnosis are presumed to have micro-metastases that are undetectable using current imaging techniques [24,25]. One of the challenges in improving the outcome for those with macroscopic metastases is that some phase III clinical trials exclude patients with metastatic disease [23]. This can result in inconsistent treatment across institutes for patients with metastatic disease [23].
This review specifically summarizes pre-clinical and clinical trial data relating to novel therapies for metastatic osteosarcoma, to document progress towards improving outcomes for these patients.
2. Results of Published Trials
2.1. Chemotherapy
The standard neoadjuvant and adjuvant MAP chemotherapy regimen for patients with localized disease is also administered to patients with metastatic disease, before and after attempting to remove lesions by metastasectomy [23].
Various substitutions and additions have been explored, but unfortunately, none have yielded increased survival rates for patients with metastatic osteosarcoma.
Newer generations of anthracycline, such as pirarubicin, that reportedly have better tumor uptake and reduced toxicities have been examined as a substitute for doxorubicin [26]. A trial of 23 patients who were diagnosed with recurrent or refractory osteosarcoma were treated with pirarubicin and cisplatin had a median overall survival rate of 10 months [26]. Although efficacy was limited, the authors recommended follow-up trials with a greater cohort of patients given that all relapsed patients who did respond had metastatic disease [26].
Pemetrexed is an inhibitor of thymidylate synthase and has a broader range of action than methotrexate. A phase II trial was conducted to evaluate the efficacy of pemetrexed as a single agent in 32 metastatic osteosarcoma patients [27]. One patient in the cohort had a partial response and the median overall survival rate was only 5.5 months; the study did not meet its primary objective of five responses [27].
Cisplatin’s efficacy is hindered by dose-limiting nephrotoxicity. Trials have evaluated the efficacy of carboplatin, a less toxic platinating agent [28], as a replacement; however, several studies revealed that carboplatin was less effective than cisplatin at treating metastatic osteosarcoma [17,20,29]. Researchers have also attempted to minimize cisplatin-induced toxicity by administering the drug via inhalation to treat pulmonary cancers [30]. A phase I/II trial was established to evaluate the efficacy of inhaled lipid cisplatin in recurrent osteosarcoma patients with pulmonary metastases [31]. Of the 19 patients who received inhaled cisplatin, none exhibited common toxicities associated with cisplatin, such as nephrotoxicity and myelosuppression [31]. Three patients achieved a complete response, one a partial response and seven had stable disease [31]. The best responses were observed in patients with lesions less than 2 cm in size [31]. A subsequent phase II trial (NCT01650090) of inhaled cisplatin for metastatic osteosarcoma with a larger patient cohort was completed in 2018, but no results have been published to date.
One phase II trial evaluated the addition of ifosfamide to doxorubicin and cisplatin for metastatic disease but found no clinical benefit over standard treatment [32]. Although a total of 30 patients achieved a disease-free status, 21 relapsed with pulmonary metastases an average 15 months later resulting in an overall 5-year survival of only 18% and around 25% in similar study [33,34]. Interestingly, increasing the dose of ifosfamide to treat metastatic disease did not improve patient outcome [35].
The use of the topoisomerase II inhibitor etoposide as an additional agent, coupled with MAP plus ifosfamide, has been evaluated in two trials for metastatic osteosarcoma. A phase II/III trial evaluated the efficacy of this combination in 43 patients newly diagnosed with metastatic osteosarcoma [36]. The two-year survival rate was 52% and complete response rate 10% [36]. Significant toxicities were reported in the study, with two patients dying as a result of therapy, 83% of patients developing neutropenia and five suffering sepsis [36]. A later phase I/II trial studied the addition of etoposide to standard chemotherapy plus ifosfamide in 13 patients with metastatic osteosarcoma [37]. Only six patients completed the full regimen, with the remainder requiring dose reduction due to myelosuppression [37]. Median survival for patients with unresectable metastases was 13 months and 31 months for those who underwent successful metastasectomy [37]. Trial data to date suggest that the addition of etoposide into the chemotherapy regimen does not provide a substantial clinical benefit.
A phase II trial examined the efficacy of the water soluble camptothecin analogue topotecan, after promising data from in vivo testing on osteosarcoma xenografts [38,39]. Twenty-eight newly diagnosed patients with poor prognosis metastatic osteosarcoma were treated with standard chemotherapy plus the topoisomerase inhibitor topotecan, but only one partial response was observed [39]. Topotecan was well tolerated alongside chemotherapy but was not recommended for further study due to its disappointing efficacy [39].
A phase II trial examined the efficacy of high-dose thiotpeta (an alkylating agent) with autologous transplantation as an adjuvant to standard chemotherapy in 22 patients with relapsed metastatic osteosarcoma, but reported no significant clinical benefit compared to the 22 control patients who received chemotherapy alone [40]. Gemcitabine is an analogue of deoxycytidine and was trialed as a single agent for advanced sarcoma patients, including two with osteosarcoma, in a phase II trial based on promising activity in animal models of various non-osteosarcoma cancer types [41]. Of the 29 evaluable patients, one leiomyosarcoma patient experienced regression for five months and all other patients had progressive disease [42]. Another trial of gemcitabine documented two cases of progressive disease and four of stable disease in six osteosarcoma patients enrolled in a phase II study for pre-treated sarcoma patients [43].
L-alanosine is an inhibitor of de novo adenine synthesis. It was evaluated in 65 carcinoma and sarcoma patients, including seven osteosarcoma patients, three with metastases. Participants’ tumors featured methylthioadenosine phosphorylase deficiency [44], a determinant of sensitivity to L-alanosine [45]. No patients experienced objective responses. Two osteosarcoma patients had stable disease and the other five experienced progressive disease [44].
2.2. Bone-Modifying Agents
The bisphosphonate zoledronic acid inhibits bone resorption by suppressing osteoclast differentiation, and also exerts anti-cancer activities through incompletely defined pathways [46]. Zoledronic acid is approved to treat bone metastases from solid tumors. It was first trialed as an additive to chemotherapy to assess toxicity and feasibility in metastatic osteosarcoma patients in 2013 [47]. The authors found that it was safe to administer alongside chemotherapy, but any clinical benefits were difficult to define due to the small number of patients in the trial [47]. A subsequent trial conducted in a similar fashion with 318 patients, including 55 with metastases at diagnosis, found no clinical benefit for patients who received zoledronic acid and chemotherapy versus chemotherapy alone [48].
2.3. Stem Cell Rescue
Many osteosarcoma patients initially respond to chemotherapy, but a challenge in maintaining remission is balancing efficacy with the myelosuppressive activity of these treatments [49]. Autologous stem cell rescue combined with high-dose chemotherapy is an alternative treatment protocol for patients unlikely to respond to standard chemotherapy [49]. Several trials have been conducted over the past two decades using stem cell rescue to enable higher doses of treatment to be administered to patients with metastatic osteosarcoma. Unfortunately, survival rates remained unchanged compared to standard treatment protocols, and patients experienced more severe toxicities as a consequence of the increased chemotherapy doses [50,51,52,53].
2.4. Immunotherapy
The relatively high level of infiltrating lymphocytes in osteosarcomas compared to other sarcomas [40,54] have made them a promising candidate for immunotherapies [54,55]. One of the earliest trials of sole agent immunotherapy against metastatic osteosarcoma explored the efficacy of inhaled granulocyte macrophage colony stimulating factor (GM-CSF) against recurrent pulmonary metastases [56]. Although the treatment had low toxicity, the authors detected no immunostimulatory effects against pulmonary metastases and no improvement in patient outcome [56].
After encouraging results from treating non-metastatic osteosarcoma patients with the immune modulator liposomal muramyl tripeptide (mifamurtide) [57], addition of this agent to chemotherapy was explored in patients with metastatic disease [58,59]. Mifamurtide activates macrophages and monocytes to stimulate the production of cytokines, which may result in increased anti-tumor activity of infiltrating immune cells [59]. Metastatic osteosarcoma patients treated with chemotherapy plus mifamurtide took significantly longer to relapse than historical controls who just received chemotherapy [60]. However, in the context of a randomized controlled trial, mifamurtide unfortunately did not significantly boost the 5-year survival of metastatic osteosarcoma patients compared to those who received chemotherapy alone [58], although low participant numbers may have precluded detection of a subtle survival benefit [61]. No subsequent trials of mifamurtide in metastatic osteosarcoma have been conducted, but it has been approved by the European Medicines Agency to treat osteosarcoma patients aged between 2 and 30 [62].
The combination of recombinant interleukin 1α and etoposide, which was documented to provoke anti-tumor activity by lymphoid cells [63], was trialed in eight patients with relapsed metastatic osteosarcoma. Two had progressive disease and the rest partial or mixed responses [64]. Although the clinical response was modest, the authors interpreted these results as a good outcome considering the poor prognosis typically experienced by patients who relapse with metastatic disease [64]. Unfortunately, the trial was stopped early due to a halt in the production of recombinant interleukin 1α.
A high proportion of osteosarcomas, particularly pulmonary metastases, express programmed cell death protein-1 ligand (PD-L1). This suggests that metastases may be especially sensitive to PD-1 inhibitors such as pembrolizumab [55,65]. Several trials have evaluated the efficacy of pembrolizumab against metastatic and advanced osteosarcoma, but only one of 49 evaluable patients across three separate trials had a partial response to treatment [66,67,68]. Equally disappointingly, the majority of patients with metastatic disease who participated in trials of PD-1 inhibitors, such as nivolumab, camrelizumab and ipilimumab, experienced progressive disease [69,70,71].
Many osteosarcoma patients have human epidermal growth factor receptor 2 (HER2) positive tumors, which formed the basis of a trial evaluating HER2-specific chimeric antigen receptor modified T-cells (CAR T-cells) against HER2-positive sarcomas [72]. Unfortunately, CAR T-cell therapy was no more effective than PD-1 inhibition with 75% of osteosarcoma patients experiencing progressive disease and the remainder only stable disease [72]. Other immunotherapies that rely on the anti-cancer activity of cytotoxic lymphocytes, such as dendritic and T-cell receptor therapies, have failed to improve the outcome of patients with metastatic osteosarcoma [73,74].
Although the previously described immunotherapies failed to improve patient outcomes, a prospective study of metastatic osteosarcoma patients who received MAP in addition to IL-2 and lymphokine activated killer (LAK) cell reinfusion yielded more promising results [19]. Of 27 patients who received LAK cell reinfusion and IL-2, 11 remained alive at the time of publication with an overall survival rate of 45% at 130-month median follow up [19].
2.5. Drugs Targeting Tyrosine Kinases
Receptor tyrosine kinases represent a broad set of targets, some of which are often overexpressed in osteosarcomas, giving rise to a novel approach of treating metastatic osteosarcoma with tyrosine kinase inhibitors (TKI) [75]. Poor osteosarcoma patient outcome is associated with overexpression of HER2 [76], which prompted evaluation of the HER2 antibody trastuzumab with chemotherapy to treat HER2-positive metastatic osteosarcoma [77]. Patients who received trastuzumab plus chemotherapy had a 30-month overall survival rate of 59%, versus 50% for those who were only treated with chemotherapy [77]. Although trastuzumab was safe to administer to patients receiving chemotherapy, there was no significant difference in patient outcome [77].
OncoLar is a somatostatin analogue that inhibits IGF-1 production, which can suppress the growth of osteosarcoma in vitro [78] and metastasis in vivo [79]. Based on these data, OncoLar was evaluated in a phase I trial of metastatic or recurrent osteosarcoma in 19 patients. It was well tolerated but did not produce a clinical response in any patients [80].
Robatumumab is an IGF-1 receptor inhibitor that showed promising pre-clinical activity in subcutaneous patient-derived xenografts [81] and was evaluated in a phase II trial of sarcoma patients with resectable and un-resectable metastases [82]. Of 31 osteosarcoma patients with resectable metastases, one had a complete response and two had a partial response to treatment, whereas none of the 29 osteosarcoma patients with un-resectable metastases responded to treatment [82].
Many of the TKIs currently being evaluated have multiple targets, including receptor and intracellular tyrosine kinases that drive pro-tumorigenic pathways, such as angiogenesis and proliferation [83]. Data from clinical trials evaluating TKIs for sarcoma suggest that inhibition of multiple tyrosine kinases is more effective than targeting a single kinase [84].
Regorafenib is a multitarget TKI that has been tested for treating metastatic osteosarcoma across two randomized phase II clinical trials. The REGOBONE trial compared regorafenib versus placebo in patients with metastatic osteosarcoma who had failed to respond to chemotherapy. Regorafenib significantly delayed disease progression. Of 26 eligible treated patients, two had partial responses and 15 stable disease with the remainder showing disease progression [85]. SARC024 also trialed regorafenib in a similar fashion and documented a significant improvement in median progression-free survival vs. placebo (3.6 versus 1.7 months) [86]. Surprisingly, despite regorafenib markedly slowing progression in both trials, it unfortunately did not have a significant effect on overall survival [85,86]. As discussed below, imperfect correlations between surrogate endpoints and overall survival raise questions about the optimal primary endpoints for clinical trials. Insights into the basis of such discrepancies may unearth opportunities to build on the anti-progression activity of regorafenib (and perhaps other TKIs) to improve patient survival.
Sorafenib is another multitarget TKI that has been well studied in osteosarcoma patients. In a phase II trial of 35 patients with metastatic or advanced osteosarcoma, sorafenib treatment led to a reduction in tumor burden in 14% of patients, but only 29% had not progressed within 6 months of commencing treatment [87]. A subsequent phase II trial of sorafenib included everolimus in hope of blocking the mTOR pathway, which may confer resistance to TKI inhibition [88]. Therapy-related toxicity necessitated dose reductions in 66% of patients. Encouragingly, 45% of patients did not suffer disease progression within six months, although only 5% of patients survived for more than two years [88].
Cabozantinib is an inhibitor of VEGFR2 and MET and was studied in 43 osteosarcoma patients, of which 39 had pulmonary metastases, in a phase II clinical trial [89]. Similar results to the trials of sorafenib were observed, with a 6-month progression-free survival rate of 33% and only five patients experiencing partial responses [89]. Apatinib is an inhibitor of VEGFR2, which was studied in a phase II trial of 37 patients for advanced osteosarcoma and resulted in a four-month progression free survival rate of 57% [90]. Cediranib, which efficiently targets members of the VEGFR and PDGFR families [91], was assessed in a phase I trial that included four osteosarcoma patients. One experienced a 34% reduction in the size of their pulmonary metastases after two cycles of treatment, but the others did not respond [92]. Similarly, marginal efficacy was observed in the SARC009 phase II trial of dasatinib (a broad-spectrum kinase inhibitor [93]), which included 46 patients with advanced osteosarcoma. Tumors shrank in three of these patients and disease stabilized in another three, and 15% of the osteosarcoma patients survived for at least two years [94].
2.6. Other Drug Therapies
Other drug classes have been trialed for metastatic osteosarcoma based on promising pre-clinical data, but have yielded generally disappointing outcomes.
Glembatumumab vedotin is an antibody-drug conjugate that targets an anti-mitotic agent to cells expressing glycoprotein non-metastatic B protein, which is overexpressed in most osteosarcomas [95]. When tested in a phase II trial of 22 recurrent osteosarcoma patients, only one patient had a partial response and one patient’s death may have been attributed to glembatumumab vedotin therapy [96]. Radium 223 dichloride has a specificity for highly mineralized areas [97] and was evaluated in 18 high risk osteosarcoma patients, the majority of whom had bone metastases [98]. Minimal hematological toxicity was observed; however, the 12-month overall survival rate was only 9% [98]. Ecteinascidin 743, which had some activity against drug-resistant osteosarcoma cells in vitro [99], was evaluated in a phase II trial of 23 patients, with 88% bearing pulmonary metastases [100]. As a single agent, its efficacy was limited, with only three patients experiencing minor responses [100]. The mTOR inhibitor ridaforolimus was first evaluated in Ewing sarcoma patients in a phase I trial against advanced solid tumors, which reported 4 partial responses in 32 evaluable patients [101]. These results were the rationale for a phase II trial of ridaforolimus in 212 advanced sarcoma patients including 54 primary bone tumor patients, 51 of whom were diagnosed with pulmonary metastases [102]. Ridaforolimus was more effective in osteosarcoma patients than participants with soft tissue sarcomas. Three osteosarcoma patients achieved partial responses (two confirmed, one unconfirmed) and the bone cancer patients’ 6-month progression-free survival rate was 25% [102]. The authors assayed the levels of eight proteins within the mTOR pathway within tumors, but none predicted clinical responses [102].
Despite the numerous trials conducted to evaluate novel therapies for metastatic osteosarcoma, the long-term survival of patients remains unchanged (Table 1).

Table 1.
Summary of metastatic osteosarcoma clinical trials and their outcomes. Class of the investigational agent on which the study focused: C, chemotherapy; B, bone modifying agents; S, stem cell rescue; I, immunotherapy; T, tyrosine kinase inhibitors; O, other drug therapies. Responses: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease. OS, overall survival; PFS, progression free survival; EFS, event free survival.
3. Animal Studies
Many studies have been published evaluating the efficacy of candidate treatments for metastatic osteosarcoma using mouse models. These models are often generated by orthotopic or subcutaneous injection of human or murine osteosarcoma cells into mice, which can metastasize to the lungs over the course of an experiment (Table 2). The presence of a primary tumor in these models makes it difficult to ascertain whether a reduction in metastatic burden is a consequence of a smaller primary tumor in treated mice, seeding fewer cells to the lungs, and/or if the treatment acts directly on established metastases. A reduction in the size of metastases (in addition to their number) in treated mice may imply that the treatment targets osteosarcoma cells within the lungs. Further studies are necessary to confirm these results. Primary tumors are typically removed by surgery in clinical settings, yet this is rarely modeled in animal studies for logistical and ethical reasons.

Table 2.
Summary of studies reporting the efficacy of novel therapies (not yet evaluated in clinical trials) against metastatic osteosarcoma using pre-clinical models of mice bearing primary osteosarcomas. N, significantly reduced number of individual metastases compared to control mice; S, significantly reduced size of metastases compared to control mice; OT, orthotopic tumor; IM, intramuscular tumor; SC, subcutaneous tumor; I, isograft; X, xenograft.
It is sobering to reflect that the previously described clinical trials failed to recapitulate the exciting efficacy published using pre-clinical animal models. This mismatch may reflect inadequacies of these animal models, in addition to a publication bias favoring reporting of positive outcomes, which presumably would be more prominent in pre-clinical than clinical studies.
Directly seeding osteosarcoma cells to the lungs by intravenous injection can be used to establish a metastatic model without a primary tumor (modeling a context in which a patient’s tumor was surgically removed) [146,147,148,149,150]. These “experimental metastasis” preclinical models of osteosarcoma allow researchers to specifically evaluate whether a novel therapy can effectively target pulmonary metastases.
Although these models are a powerful tool, it can be difficult to determine if a drug is active against established metastases unless researchers commence treatment after metastases have formed. Authors evaluating therapies such as 3-hydroxyflavone, euxanthone, CXCR4, Timosaponin, TRAIL, Panobinostat, mycophenolic acid, zoledronic acid, rapamycin, parthenolide or quercetin began treatment prior to the injection of cells or shortly afterwards [145,147,148,151,152,153,154,155,156,157,158]. In those studies, efficacy may reflect action against tumor cells within the circulation and/or growing in the lungs. Destroying circulating tumor cells may be desirable, as could preventing cells from acquiring migratory phenotypes, intravasating, extravasating, forming micrometastases in distant sites or activating the proliferation of cells comprising dormant micrometastases. Pathways controlling these key steps of osteosarcoma metastases is an active and fascinating area of research, which has been recently reviewed [159,160]. However, the observation that surgical removal of primary tumors is usually insufficient to cure patients implies that most osteosarcoma patients harbor metastases (subdetectable or overt) when they are diagnosed. As discussed, the prognosis of patients with metastases that are large enough to be detected at diagnosis is much worse than those with ostensibly localized disease. Therapies that suppress early steps in the metastatic process seem unlikely to benefit osteosarcoma patients, particularly those with macro-metastatic disease (“locking the barn door after the horse has bolted”). It is conceivable that identification of molecular features of osteosarcoma cells possessing metastatic activity could facilitate development of targeted therapies capable of specifically eliminating those cells. Improving cure rates for patients with osteosarcoma metastases will probably require agents that can kill osteosarcoma cells comprising established metastases.
Studies in which authors waited at least one week post-cell injection prior to commencing treatment make a more compelling case for a drug’s efficacy against metastases, as the cells would have presumably arrived at the lungs and begun forming pulmonary tumors. The mTOR inhibitor rapamycin improved the survival rate of xenograft and isograft mice bearing pulmonary metastases compared to those treated with vehicle [161]. The oncolytic adenoviruses Delta24-RGD and VCN-01 both reduced the size and number of pulmonary metastases compared to control-treated mice in models of metastatic osteosarcoma using established cell lines and patient-derived cells [162]. Treatment of mice with the CXCR4 antagonist AMD3100 reduced the number of metastatic nodules in the lungs compared to vehicle-treated mice. In that study, the authors waited four weeks post-cell injection before starting treatment, so it is likely that metastases were established prior to treatment [163].
Ideally, researchers would confirm the presence of metastases in mice prior to starting treatment. Very few studies have been published in which the authors detected established metastases in the lungs of mice by microscopy (in pilot experiments) and/or in vivo imaging before beginning treatment. Nasarre et al. observed additive cooperation between PD-1 inhibitor and an antibody targeting Secreted Frizzled-Related Protein 2 (SFRP2) to reduce the number of metastases on the surface of mouse lungs by commencing treatment mice with the antibody in conjunction with a PD-1 inhibitor eight to twelve days after cells were intravenously injected [164]. The proteasome inhibitor ixazomib inhibited the growth of established pulmonary metastases after their presence was confirmed by bioluminescence imaging in two xenograft models, and moderately enhanced survival [165]. The kinase inhibitor sorafenib inhibited the growth of pulmonary metastases and reduced their size in a xenograft model of metastatic osteosarcoma, in addition to inhibiting the growth of subcutaneous osteosarcomas [166]. Sorefenib has since been evaluated to treat metastatic osteosarcoma in two clinical trials, as summarized above [87,88]. Another study found that the Smac mimetic LCL161 targeted established osteosarcoma metastases, inhibiting their growth or inducing their regression or elimination, significantly enhancing survival of mice compared to those treated with vehicle [167].
As an alternative to mouse xenografts and isografts, dogs present a useful model for evaluating new therapies for spontaneous metastatic osteosarcoma. Canine osteosarcoma shares many characteristics with human osteosarcoma, including immune infiltration, microenvironment, genetics and presentation of metastatic disease [168,169]. Although the incidence of osteosarcoma in humans is relatively low, it is 27 times higher dogs, which makes it easier to establish clinical trials with large patient numbers [168]. Some trials have been conducted using dogs as a model for metastatic osteosarcoma to evaluate new therapies, including Auranofin, which improved overall survival combined with standard care [170], and Palladia, which was ineffective as a single agent [171,172].
4. Current Clinical Trials
Of the clinical trials currently being conducted for metastatic osteosarcoma, almost half are evaluating immunotherapies such as mifamurtide, IL-2 or PD-1 inhibitors (Table 3, Figure 1). Tyrosine kinase inhibitors have also garnered a lot of interest, with multiple trials expanding on the published data of promising agents from this class, including regorafenib [85,86].

Table 3.
Summary of ongoing metastatic osteosarcoma clinical trials.
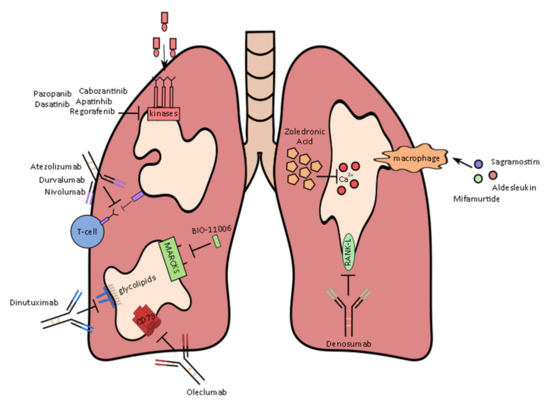
Figure 1.
Therapies and their mechanism of action currently being evaluated to treat metastatic osteosarcoma in clinical trials.
5. Future Directions
Despite intensive efforts to improve the outcome of patients diagnosed with osteosarcoma, including those with overt metastases, the survival rate for patients reported in clinical trials evaluating new therapies has only modestly increased at best. It seems the long-sought substantial improvements in patient survival will require additional basic biology research and development of drugs engaging relevant molecular targets, followed by well-designed animal experiments and phase II clinical trials, hopefully leading to the identification of promising candidate therapies for evaluation in phase III trials. Optimal clinical trial design will hasten progress towards this goal.
5.1. Endpoints and Comparators in Phase II Trials
Mineralization associated with osteosarcomas limits the utility of radiographic imaging for monitoring tumor responses to neo-adjuvant treatment [173,174]. Histological evaluation of resected primary tumors after pre-surgical chemotherapy has strong prognostic value, although the strength of this correlation diminished as treatment intensity increased [175]. Long-term survival after therapy hinges on the elimination of metastases (whether detectable or not), so the predictive power of primary tumor responses to chemotherapy implies that the cells that constitute a patient’s primary tumor and their metastases tend to exhibit similar chemo-sensitivity. It is, therefore, surprising that concordant histological responses between primary tumors and synchronous metastases were only recorded in half of the patients studied by Bacci et al., and heterogenous responses were observed among multiple metastases from some patients [34]. Some drugs appear to exert more toxicity towards subdetectable than overt metastases: replacing cisplatin with carboplatin did not alter the survival rate of patients with localized osteosarcoma, but severely diminished the proportion of patients with metastatic disease who survived for 5 years or more [20]. It may, therefore, be simplistic to assume that novel drugs that can provoke substantial necrosis in primary tumors will necessarily have the same destructive effect on metastases. Likewise, it is conceivable that some classes of drugs may target metastases better than primary tumors. The basis for differential sensitivity between primary osteosarcomas and metastases is unclear, but it could relate to distinct phenotypes of cancer cells that comprise primary versus metastatic tumors, differences in vascularization between primary tumors and metastases (which could influence intratumoral drug concentrations), or variation in the non-cancer cell composition of primary versus metastatic tumors. The strong prognostic influence of the metastatic site (e.g., bone or lymph node versus lung) highlights the differential drug sensitivities of osteosarcomas growing in different anatomical sites. These issues, coupled with the requirement for patients to have measurable tumors, reinforce the disadvantages of using objective response rates as primary endpoints in osteosarcoma trials [176,177].
The most meaningful endpoint for clinical trials of osteosarcoma treatments is overall survival (OS). In practice, however, progression-free survival (PFS) (or “event-free survival”; EFS) is more commonly used as a primary endpoint in phase II trials. Concerningly, while PFS and OS data correlated tightly in trials involving Ewing sarcoma patients [178], PFS was a less accurate predictor of OS in trials of localized osteosarcoma patients [179]. As mentioned above, delayed progression may not foretell prolonged survival, “progression” is more subjective than death, so bias must be avoided, and inconsistencies in the timing of progression monitoring between groups may lead to spurious apparent differences in outcomes [180,181]. In trials involving newly diagnosed patients with (seemingly) localized and resectable osteosarcomas, in many of whom “progression” would mean growth of micro-metastases to detectable size, a PFS endpoint may offer the advantage of speed while signifying a prognostically important event. If due caution is paid to its potential pitfalls, on balance PFS may be a reasonable primary endpoint in this context. However, the expected lifespans of patients with recurrent/relapsed disease and/or unresectable osteosarcomas are short. Hence, in trials involving such patients, overall survival may be a more suitable primary endpoint, as it would avoid the risks to data quality associated with PFS without substantially extending the trial’s duration.
Whether phase II trials should randomize patients to experimental therapies versus placebo is controversial. Some experts contend that single-arm trials with early endpoints can be informative and efficient, as historical benchmarks obviate the need to split relatively scarce patients between two treatment groups. The “New Agents for Osteosarcoma Task Force” devised a structured approach [177] to select agents for evaluation in phase III clinical trials, based on consideration of pre-clinical data and comparison of EFS measures from single-arm phase II trials with historical benchmarks [182]. Using this approach, the task force prioritized assessment of tyrosine kinase inhibitors [177]. However, others doubt the validity of using benchmarks from previous trials [176,183], arguing that participants in phase II trials should be randomized to receive the investigational agent or not, to allow a direct comparison of outcomes. Whether control patients are historical, or randomized participants within the trial, it is crucial to compare treatment efficacy between patients who share similar prognoses. Parameters including prior treatment, tumor resectability, the number of lung metastases and the presence/absence of extrapulmonary metastases could be used to stratify patients in each arm into groups sharing similar prognoses. Using PFS rather than OS as the primary endpoint is more fraught in single-arm than randomized trials. In single-arm trials, the definition of progression (including imaging sensitivity) and monitoring interval, in addition to patient prognoses, must match those used in the historical comparators for outcomes to be directly comparable. By careful selection of comparator datasets from previous trials, and ideally using OS as the primary endpoint, the logistical benefits of single-arm phase II trials may be achievable without unduly compromising the quality of the data and the strengths of conclusions that can be drawn from it.
5.2. Predicitive Biomarkers
In addition to providing insight into the potential utility of new therapies, clinical trials that incorporate predictive biomarker assays may reveal avenues for further research that may ultimately improve outcomes for patients. Understanding the physiological, cellular and/or biochemical factors that allow even a small proportion of patients to respond to a particular drug may facilitate personalized treatment approaches based on the features of patients’ primary tumors or metastases.
Biomarkers were explored to predict patient responses to ridaforolimus, but none of the eight markers assayed varied between patients who did or did not respond to treatment [102]. This may be a consequence of the authors’ use of archived tissue samples or could signify that the complexity of the mTOR pathway makes it difficult to identify a single component responsible for resistance/sensitivity to treatment [102].
A study comparing whole genome and exosome sequences between osteosarcoma patients’ primary tumors and their pulmonary metastases revealed that metastases had a much higher tumor mutational burden and genomic instability [184]. Metastases also had a higher level of infiltrating lymphocytes and increased expression of PD-L1, raising hope that they may be more sensitive to immune checkpoint inhibition [185]. In practice, the efficacy of immunotherapy for osteosarcoma has so far been tantalizing but limited. In the phase II trial of apatinib and the anti-PD-1 agent camrelizumab, the authors did not reach their prespecified threshold of 60% 6-month progression free survival [71]. They did, however, note that the two patients with overexpression of PD-L1 experienced durable disease control [71]. Expression of PD-L1 alone as a biomarker to predict patient response to PD-1 inhibitor immunotherapy has so far proved to be unreliable for most cancers [186]. An analysis of all FDA-approved checkpoint inhibitors found that PD-L1 expression only predicted improved responses in 30% of cases across numerous trials [186]. This figure is likely an overestimate as the analysis only examined successful trials that resulted in FDA approval [186]. Counterintuitively, the correlation between overexpression PD-L1 and efficacy of immune checkpoint inhibitors appears to differ between inhibitors that share molecular targets. For example, the PD-1 inhibitor atezolizumab was more effective in improving urothelial carcinoma patient outcomes for those with PD-L1-expressing tumors versus participants with PD-L1-negative tumors [187], whereas pembrolizumab improved patient outcome regardless of PD-L1 status [188]. In some cases, patients experienced durable responses to PD-1 inhibitor therapy, despite having PD-L1 negative tumors [189].
Several clinical trials of PD-1 inhibitors for carcinomas suggest that tumor mutational burden (TMB) may be useful for predicting patient response to immunotherapy [190,191,192,193]. High TMB predicted improved patient response to treatment independently of PD-L1 status in non-small-cell lung cancer patients [194]. The implications of high or low TMB in osteosarcoma patients is not well defined; however, osteosarcoma patients with a higher TMB were reported to be more likely to experience longer progression-free survival than patients with a lower TMB [195]. One case study of a patient with pulmonary osteosarcoma metastases and high TMB treated with PD-L1 inhibitor therapy reported a durable response to treatment, with the patient experiencing a remission for at least two years despite discontinuation of treatment due to therapy related toxicities [196]. A separate case study of an osteosarcoma patient with bone and lung metastases and a high TMB experienced a 33-month remission when treated with the PD-1 inhibitor pembrolizumab and controlled disease for 60 months [197]. The results from these two case studies, in addition to robust data from trials with larger cohorts of carcinoma patients, suggest that TMB may be a useful indicator of responsiveness to PD-1 inhibitors in osteosarcoma. Analysis of the TMB of historical samples from patients who were enrolled in PD-1 inhibitor clinical trials would help determine if there was a correlation between the level of TMB and response to treatment.
6. Conclusions
The paucity of better-than-expected survival outcomes in osteosarcoma clinical trials summarized above suggests that a dramatic improvement in outcomes for the majority of metastatic osteosarcoma patients will probably require targeting of a novel process or molecule, distinct from those engaged by agents used in clinical trials to date. Hopefully, ongoing pre-clinical research will uncover such game-changing novel targets. Until/unless those approaches bear fruit, extending lifespans for some osteosarcoma patients may hopefully be realized by assembling a panel of therapies that exhibit efficacy in subsets of patients, coupled with development of biomarker assays to enable tailoring of treatments to individual patients.
Author Contributions
Writing and preparation of original draft, M.A.H.; Review and editing of manuscript C.J.H. and M.A.H.; Funding acquisition, C.J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Kids’ Cancer Project, Cancer Council Victoria (grant number 1145995) and Tour De Cure. The APC was funded by La Trobe University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gianferante, D.M.; Mirabello, L.; Savage, S.A. Germline and somatic genetics of osteosarcoma—Connecting aetiology, biology and therapy. Nat. Rev. Endocrinol. 2017, 13, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Abarrategi, A.; Tornin, J.; Martinez-Cruzado, L.; Hamilton, A.; Martinez-Campos, E.; Rodrigo, J.P.; Gonzalez, M.V.; Baldini, N.; Garcia-Castro, J.; Rodriguez, R. Osteosarcoma: Cells-of-Origin, Cancer Stem Cells, and Targeted Therapies. Stem Cells Int. 2016, 2016, 3631764. [Google Scholar] [CrossRef] [PubMed]
- Walia, M.K.; Castillo-Tandazo, W.; Mutsaers, A.J.; Martin, T.J.; Walkley, C.R. Murine models of osteosarcoma: A piece of the translational puzzle. J. Cell Biochem. 2018, 119, 4241–4250. [Google Scholar] [CrossRef]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef]
- Coventry, M.B.; Dahlin, D.C. Osteogenic sarcoma; a critical analysis of 430 cases. J. Bone Joint. Surg. Am. 1957, 39, 741–758. [Google Scholar] [CrossRef]
- Link, M.P.; Goorin, A.M.; Miser, A.W.; Green, A.A.; Pratt, C.B.; Belasco, J.B.; Pritchard, J.; Malpas, J.S.; Baker, A.R.; Kirkpatrick, J.A.; et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N. Engl. J. Med. 1986, 314, 1600–1606. [Google Scholar] [CrossRef] [PubMed]
- Bramwell, V.H.; Burgers, M.; Sneath, R.; Souhami, R.; van Oosterom, A.T.; Voûte, P.A.; Rouesse, J.; Spooner, D.; Craft, A.W.; Somers, R.; et al. A comparison of two short intensive adjuvant chemotherapy regimens in operable osteosarcoma of limbs in children and young adults: The first study of the European Osteosarcoma Intergroup. J. Clin. Oncol. 1992, 10, 1579–1591. [Google Scholar] [CrossRef] [PubMed]
- Souhami, R.L.; Craft, A.W.; Van der Eijken, J.W.; Nooij, M.; Spooner, D.; Bramwell, V.H.; Wierzbicki, R.; Malcolm, A.J.; Kirkpatrick, A.; Uscinska, B.M.; et al. Randomised trial of two regimens of chemotherapy in operable osteosarcoma: A study of the European Osteosarcoma Intergroup. Lancet 1997, 350, 911–917. [Google Scholar] [CrossRef]
- Chen, F.; Miyahara, R.; Bando, T.; Okubo, K.; Watanabe, K.; Nakayama, T.; Toguchida, J.; Date, H. Repeat resection of pulmonary metastasis is beneficial for patients with osteosarcoma of the extremities. Interact. Cardiovasc. Thorac. Surg. 2009, 9, 649–653. [Google Scholar] [CrossRef]
- Briccoli, A.; Rocca, M.; Salone, M.; Bacci, G.; Ferrari, S.; Balladelli, A.; Mercuri, M. Resection of recurrent pulmonary metastases in patients with osteosarcoma. Cancer 2005, 104, 1721–1725. [Google Scholar] [CrossRef]
- Pastorino, U.; Palmerini, E.; Porcu, L.; Luksch, R.; Scanagatta, P.; Meazza, C.; Leuzzi, G.; Massimino, M.; Picci, P. Lung metastasectomy for osteosarcoma in children, adolescents, and young adults: Proof of permanent cure. Tumori J. 2021, 2021, 03008916211053048. [Google Scholar] [CrossRef] [PubMed]
- Saumet, L.; Deschamps, F.; Marec-Berard, P.; Gaspar, N.; Corradini, N.; Petit, P.; Sirvent, N.; Brugières, L. Radiofrequency ablation of metastases from osteosarcoma in patients under 25 years: The SCFE experience. Pediatr. Hematol. Oncol. 2015, 32, 41–49. [Google Scholar] [CrossRef] [PubMed]
- NCCN Guideline Bone Cancer 2022 v2 National Comprehensive Cancer Network Bone Cancer (ver. 2.2022). Available online: http://www.nccn.org/professionals/physician_gls/pdf/bone.pdf (accessed on 25 March 2022).
- Harris, M.B.; Gieser, P.; Goorin, A.M.; Ayala, A.; Shochat, S.J.; Ferguson, W.S.; Holbrook, T.; Link, M.P. Treatment of metastatic osteosarcoma at diagnosis: A Pediatric Oncology Group Study. J. Clin. Oncol. 1998, 16, 3641–3648. [Google Scholar] [CrossRef] [PubMed]
- Marina, N.M.; Pratt, C.B.; Rao, B.N.; Shema, S.J.; Meyer, W.H. Improved prognosis of children with osteosarcoma metastatic to the lung(s) at the time of diagnosis. Cancer 1992, 70, 2722–2727. [Google Scholar] [CrossRef]
- Kager, L.; Zoubek, A.; Pötschger, U.; Kastner, U.; Flege, S.; Kempf-Bielack, B.; Branscheid, D.; Kotz, R.; Salzer-Kuntschik, M.; Winkelmann, W.; et al. Primary metastatic osteosarcoma: Presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J. Clin. Oncol. 2003, 21, 2011–2018. [Google Scholar] [CrossRef]
- Ferguson, W.S.; Harris, M.B.; Goorin, A.M.; Gebhardt, M.C.; Link, M.P.; Shochat, S.J.; Siegal, G.P.; Devidas, M.; Grier, H.E. Presurgical window of carboplatin and surgery and multidrug chemotherapy for the treatment of newly diagnosed metastatic or unresectable osteosarcoma: Pediatric Oncology Group Trial. J. Pediatr. Hematol. Oncol. 2001, 23, 340–348. [Google Scholar] [CrossRef]
- Meyers, P.A.; Heller, G.; Healey, J.H.; Huvos, A.; Applewhite, A.; Sun, M.; LaQuaglia, M. Osteogenic sarcoma with clinically detectable metastasis at initial presentation. J. Clin. Oncol. 1993, 11, 449–453. [Google Scholar] [CrossRef]
- Meazza, C.; Cefalo, G.; Massimino, M.; Daolio, P.; Pastorino, U.; Scanagatta, P.; Morosi, C.; Podda, M.; Ferrari, A.; Terenziani, M.; et al. Primary metastatic osteosarcoma: Results of a prospective study in children given chemotherapy and interleukin-2. Med. Oncol. 2017, 34, 191. [Google Scholar] [CrossRef]
- Daw, N.C.; Billups, C.A.; Rodriguez-Galindo, C.; Carville, M.B.; Rao, B.N.; Cain, A.M.; Jenkins, J.J.; Neel, M.D.; Meyer, W.H. Metastatic osteosarcoma. Cancer 2006, 106, 403–412. [Google Scholar] [CrossRef]
- Harris, M.B.; Cantor, A.B.; Goorin, A.M.; Shochat, S.J.; Ayala, A.G.; Ferguson, W.S.; Holbrook, T.; Link, M.P. Treatment of osteosarcoma with ifosfamide: Comparison of response in pediatric patients with recurrent disease versus patients previously untreated: A Pediatric Oncology Group study. Med. Pediatr. Oncol. 1995, 24, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Marko, T.A.; Diessner, B.J.; Spector, L.G. Prevalence of Metastasis at Diagnosis of Osteosarcoma: An International Comparison. Pediatr. Blood Cancer 2016, 63, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Meazza, C.; Scanagatta, P. Metastatic osteosarcoma: A challenging multidisciplinary treatment. Exp. Rev. Anticancer. Ther. 2016, 16, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Geller, D.S.; Gorlick, R. Osteosarcoma: A review of diagnosis, management, and treatment strategies. Clin. Adv. Hematol. Oncol. HO 2010, 8, 705–718. [Google Scholar]
- Luetke, A.; Meyers, P.A.; Lewis, I.; Juergens, H. Osteosarcoma treatment—where do we stand? A state of the art review. Cancer Treat. Rev. 2014, 40, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.X.; He, A.N.; Tang, L.N.; Shen, Z.; Yao, Y. Evaluation of pirarubicin-cisplatin chemotherapy in the treatment for refractory and recurrent high-grade osteosarcoma: Experience of a single institute. Med. Oncol. 2012, 29, 2229–2233. [Google Scholar] [CrossRef]
- Duffaud, F.; Egerer, G.; Ferrari, S.; Rassam, H.; Boecker, U.; Bui-Nguyen, B. A phase II trial of second-line pemetrexed in adults with advanced/metastatic osteosarcoma. Eur. J. Cancer 2012, 48, 564–570. [Google Scholar] [CrossRef]
- McKeage, M.J. Comparative adverse effect profiles of platinum drugs. Drug Saf. 1995, 13, 228–244. [Google Scholar] [CrossRef]
- Petrilli, A.S.; Kechichian, R.; Broniscer, A.; Garcia, R.J.; Tanaka, C.; Francisco, J.; Lederman, H.; Odone Filho, V.; Camargo, O.P.; Bruniera, P.; et al. Activity of intraarterial carboplatin as a single agent in the treatment of newly diagnosed extremity osteosarcoma. Med. Pediatr. Oncol. 1999, 33, 71–75. [Google Scholar] [CrossRef]
- Wittgen, B.P.; Kunst, P.W.; van der Born, K.; van Wijk, A.W.; Perkins, W.; Pilkiewicz, F.G.; Perez-Soler, R.; Nicholson, S.; Peters, G.J.; Postmus, P.E. Phase I study of aerosolized SLIT cisplatin in the treatment of patients with carcinoma of the lung. Clin. Cancer Res. 2007, 13, 2414–2421. [Google Scholar] [CrossRef]
- Chou, A.J.; Gupta, R.; Bell, M.D.; Riewe, K.O.; Meyers, P.A.; Gorlick, R. Inhaled lipid cisplatin (ILC) in the treatment of patients with relapsed/progressive osteosarcoma metastatic to the lung. Pediatr. Blood Cancer 2013, 60, 580–586. [Google Scholar] [CrossRef]
- Voûte, P.A.; Souhami, R.L.; Nooij, M.; Somers, R.; Cortés-Funes, H.; van der Eijken, J.W.; Pringle, J.; Hogendoorn, P.C.; Kirkpatrick, A.; Uscinska, B.M.; et al. A phase II study of cisplatin, ifosfamide and doxorubicin in operable primary, axial skeletal and metastatic osteosarcoma. European Osteosarcoma Intergroup (EOI). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. ESMO 1999, 10, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Bacci, G.; Briccoli, A.; Ferrari, S.; Longhi, A.; Mercuri, M.; Capanna, R.; Donati, D.; Lari, S.; Forni, C.; DePaolis, M. Neoadjuvant chemotherapy for osteosarcoma of the extremity: Long-term results of the Rizzoli’s 4th protocol. Eur. J. Cancer 2001, 37, 2030–2039. [Google Scholar] [CrossRef]
- Bacci, G.; Briccoli, A.; Rocca, M.; Ferrari, S.; Donati, D.; Longhi, A.; Bertoni, F.; Bacchini, P.; Giacomini, S.; Forni, C.; et al. Neoadjuvant chemotherapy for osteosarcoma of the extremities with metastases at presentation: Recent experience at the Rizzoli Institute in 57 patients treated with cisplatin, doxorubicin, and a high dose of methotrexate and ifosfamide. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. ESMO 2003, 14, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Bacci, G.; Briccoli, A.; Ferrari, S.; Saeter, G.; Donati, D.; Longhi, A.; Manfrini, M.; Bertoni, F.; Rimondini, S.; Monti, C.; et al. Neoadjuvant chemotherapy for osteosarcoma of the extremities with synchronous lung metastases: Treatment with cisplatin, adriamycin and high dose of methotrexate and ifosfamide. Oncol. Rep. 2000, 7, 339–346. [Google Scholar] [CrossRef]
- Goorin, A.M.; Harris, M.B.; Bernstein, M.; Ferguson, W.; Devidas, M.; Siegal, G.P.; Gebhardt, M.C.; Schwartz, C.L.; Link, M.; Grier, H.E. Phase II/III trial of etoposide and high-dose ifosfamide in newly diagnosed metastatic osteosarcoma: A pediatric oncology group trial. J. Clin. Oncol. 2002, 20, 426–433. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A.; Meyer, T.; Michelagnoli, M.P.; Lewis, I.; Whelan, J.S. A phase I/II study of doxorubicin, ifosfamide, etoposide and interval methotrexate in patients with poor.r prognosis osteosarcoma. Pediatr. Blood Cancer 2006, 46, 345–350. [Google Scholar] [CrossRef]
- Houghton, P.J.; Cheshire, P.J.; Myers, L.; Stewart, C.F.; Synold, T.W.; Houghton, J.A. Evaluation of 9-dimethylaminomethyl-10-hydroxycamptothecin against xenografts derived from adult and childhood solid tumors. Cancer Chemother Pharm. 1992, 31, 229–239. [Google Scholar] [CrossRef]
- Seibel, N.L.; Krailo, M.; Chen, Z.; Healey, J.; Breitfeld, P.P.; Drachtman, R.; Greffe, B.; Nachman, J.; Nadel, H.; Sato, J.K.; et al. Upfront window trial of topotecan in previously untreated children and adolescents with poor prognosis metastatic osteosarcoma: Children’s Cancer Group (CCG) 7943. Cancer 2007, 109, 1646–1653. [Google Scholar] [CrossRef]
- Marec-Berard, P.; Dalban, C.; Gaspar, N.; Brugieres, L.; Gentet, J.C.; Lervat, C.; Corradini, N.; Castex, M.P.; Schmitt, C.; Pacquement, H.; et al. A multicentric randomized phase II clinical trial evaluating high-dose thiotepa as adjuvant treatment to standard chemotherapy in patients with resectable relapsed osteosarcoma. Eur. J. Cancer 2020, 125, 58–68. [Google Scholar] [CrossRef]
- Braakhuis, B.J.; Ruiz van Haperen, V.W.; Boven, E.; Veerman, G.; Peters, G.J. Schedule-dependent antitumor effect of gemcitabine in in vivo model system. Semin. Oncol. 1995, 22, 42–46. [Google Scholar]
- Okuno, S.; Edmonson, J.; Mahoney, M.; Buckner, J.C.; Frytak, S.; Galanis, E. Phase II trial of gemcitabine in advanced sarcomas. Cancer 2002, 94, 3225–3229. [Google Scholar] [CrossRef] [PubMed]
- Merimsky, O.; Meller, I.; Flusser, G.; Kollender, Y.; Issakov, J.; Weil-Ben-Arush, M.; Fenig, E.; Neuman, G.; Sapir, D.; Ariad, S.; et al. Gemcitabine in soft tissue or bone sarcoma resistant to standard chemotherapy: A phase II study. Cancer Chemother Pharm. 2000, 45, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Kindler, H.L.; Burris, H.A., 3rd; Sandler, A.B.; Oliff, I.A. A phase II multicenter study of L-alanosine, a potent inhibitor of adenine biosynthesis, in patients with MTAP-deficient cancer. Investig. New Drugs 2009, 27, 75–81. [Google Scholar] [CrossRef]
- Li, W.; Su, D.; Mizobuchi, H.; Martin, D.S.; Gu, B.; Gorlick, R.; Cole, P.; Bertino, J.R. Status of methylthioadenosine phosphorylase and its impact on cellular response to L-alanosine and methylmercaptopurine riboside in human soft tissue sarcoma cells. Oncol. Res. 2004, 14, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.; Li, H.; Zhai, Z.; Xu, J.; Dass, C.R.; Qin, A.; Dai, K. Zoledronic Acid: Pleiotropic Anti-Tumor Mechanism and Therapeutic Outlook for Osteosarcoma. Curr. Drug Targets 2018, 19, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Goldsby, R.E.; Fan, T.M.; Villaluna, D.; Wagner, L.M.; Isakoff, M.S.; Meyer, J.; Randall, R.L.; Lee, S.; Kim, G.; Bernstein, M.; et al. Feasibility and dose discovery analysis of zoledronic acid with concurrent chemotherapy in the treatment of newly diagnosed metastatic osteosarcoma: A report from the Children’s Oncology Group. Eur. J. Cancer 2013, 49, 2384–2391. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piperno-Neumann, S.; Le Deley, M.-C.; Rédini, F.; Pacquement, H.; Marec-Bérard, P.; Petit, P.; Brisse, H.; Lervat, C.; Gentet, J.-C.; Entz-Werlé, N.; et al. Zoledronate in combination with chemotherapy and surgery to treat osteosarcoma (OS2006): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016, 17, 1070–1080. [Google Scholar] [CrossRef]
- Meyers, P.A. High-dose therapy with autologous stem cell rescue for pediatric sarcomas. Curr. Opin. Oncol. 2004, 16, 120–125. [Google Scholar] [CrossRef]
- Fagioli, F.; Aglietta, M.; Tienghi, A.; Ferrari, S.; Brach del Prever, A.; Vassallo, E.; Palmero, A.; Biasin, E.; Bacci, G.; Picci, P.; et al. High-dose chemotherapy in the treatment of relapsed osteosarcoma: An Italian sarcoma group study. J. Clin. Oncol. 2002, 20, 2150–2156. [Google Scholar] [CrossRef]
- Boye, K.; Del Prever, A.B.; Eriksson, M.; Saeter, G.; Tienghi, A.; Lindholm, P.; Fagioli, F.; Skjeldal, S.; Ferrari, S.; Hall, K.S. High-dose chemotherapy with stem cell rescue in the primary treatment of metastatic and pelvic osteosarcoma: Final results of the ISG/SSG II study. Pediatr. Blood Cancer 2014, 61, 840–845. [Google Scholar] [CrossRef]
- Miniero, R.; Brach del Prever, A.; Vassallo, E.; Nesi, F.; Busca, A.; Fagioli, F.; Albiani, R.; Picci, P.; Bacci, G.; Madon, E. Feasibility of high-dose chemotherapy and autologous peripheral blood stem cell transplantation in children with high grade osteosarcoma. Bone Marrow Transplant. 1998, 22 (Suppl. S5), 37–40. [Google Scholar]
- Patel, S.R.; Papadopolous, N.; Raymond, A.K.; Donato, M.; Seong, C.M.; Yasko, A.W.; Lewis, V.O.; Lin, P.P.; Champlin, R.; Benjamin, R.S. A phase II study of cisplatin, doxorubicin, and ifosfamide with peripheral blood stem cell support in patients with skeletal osteosarcoma and variant bone tumors with a poor prognosis. Cancer 2004, 101, 156–163. [Google Scholar] [CrossRef]
- van Erp, A.E.M.; Versleijen-Jonkers, Y.M.H.; Hillebrandt-Roeffen, M.H.S.; van Houdt, L.; Gorris, M.A.J.; van Dam, L.S.; Mentzel, T.; Weidema, M.E.; Savci-Heijink, C.D.; Desar, I.M.E.; et al. Expression and clinical association of programmed cell death-1, programmed death-ligand-1 and CD8(+) lymphocytes in primary sarcomas is subtype dependent. Oncotarget 2017, 8, 71371–71384. [Google Scholar] [CrossRef] [PubMed]
- Koirala, P.; Roth, M.E.; Gill, J.; Piperdi, S.; Chinai, J.M.; Geller, D.S.; Hoang, B.H.; Park, A.; Fremed, M.A.; Zang, X.; et al. Immune infiltration and PD-L1 expression in the tumor microenvironment are prognostic in osteosarcoma. Sci. Rep. 2016, 6, 30093. [Google Scholar] [CrossRef] [PubMed]
- Arndt, C.A.; Koshkina, N.V.; Inwards, C.Y.; Hawkins, D.S.; Krailo, M.D.; Villaluna, D.; Anderson, P.M.; Goorin, A.M.; Blakely, M.L.; Bernstein, M.; et al. Inhaled granulocyte-macrophage colony stimulating factor for first pulmonary recurrence of osteosarcoma: Effects on disease-free survival and immunomodulation. a report from the Children’s Oncology Group. Clin. Cancer Res. 2010, 16, 4024–4030. [Google Scholar] [CrossRef] [PubMed]
- Meyers, P.A.; Schwartz, C.L.; Krailo, M.D.; Healey, J.H.; Bernstein, M.L.; Betcher, D.; Ferguson, W.S.; Gebhardt, M.C.; Goorin, A.M.; Harris, M.; et al. Osteosarcoma: The addition of muramyl tripeptide to chemotherapy improves overall survival--a report from the Children’s Oncology Group. J. Clin. Oncol. 2008, 26, 633–638. [Google Scholar] [CrossRef]
- Chou, A.J.; Kleinerman, E.S.; Krailo, M.D.; Chen, Z.; Betcher, D.L.; Healey, J.H.; Conrad, E.U., 3rd; Nieder, M.L.; Weiner, M.A.; Wells, R.J.; et al. Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic osteosarcoma: A report from the Children’s Oncology Group. Cancer 2009, 115, 5339–5348. [Google Scholar] [CrossRef]
- Kleinerman, E.S.; Jia, S.F.; Griffin, J.; Seibel, N.L.; Benjamin, R.S.; Jaffe, N. Phase II study of liposomal muramyl tripeptide in osteosarcoma: The cytokine cascade and monocyte activation following administration. J. Clin. Oncol. 1992, 10, 1310–1316. [Google Scholar] [CrossRef]
- Kleinerman, E.S.; Gano, J.B.; Johnston, D.A.; Benjamin, R.S.; Jaffe, N. Efficacy of liposomal muramyl tripeptide (CGP 19835A) in the treatment of relapsed osteosarcoma. Am. J. Clin. Oncol. 1995, 18, 93–99. [Google Scholar] [CrossRef]
- Jimmy, R.; Stern, C.; Lisy, K.; White, S. Effectiveness of mifamurtide in addition to standard chemotherapy for high-grade osteosarcoma: A systematic review. JBI Database Syst. Rev. Implement. Rep. 2017, 15, 2113–2152. [Google Scholar] [CrossRef]
- Meyers, P.A. Muramyl Tripeptide-Phosphatidyl Ethanolamine Encapsulated in Liposomes (L-MTP-PE) in the Treatment of Osteosarcoma. Adv. Exp. Med. Biol. 2020, 1257, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.F.; Zwelling, L.A.; McWatters, A.; An, T.; Kleinerman, E.S. Interleukin-1 alpha increases the cytotoxic activity of etoposide against human osteosarcoma cells. J. Exp. Ther. Oncol. 2002, 2, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Worth, L.L.; Jaffe, N.; Benjamin, R.S.; Papadopoulos, N.E.; Patel, S.; Raymond, A.K.; Jia, S.F.; Rodriguez, C.; Gano, J.; Gianan, M.A.; et al. Phase II study of recombinant interleukin 1alpha and etoposide in patients with relapsed osteosarcoma. Clin. Cancer Res. 1997, 3, 1721–1729. [Google Scholar]
- Wang, J.; Zhang, H.; Sun, X.; Wang, X.; Ren, T.; Huang, Y.; Zhang, R.; Zheng, B.; Guo, W. Exosomal PD-L1 and N-cadherin predict pulmonary metastasis progression for osteosarcoma patients. J. Nanobiotechnol. 2020, 18, 151. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- Le Cesne, A.; Marec-Berard, P.; Blay, J.Y.; Gaspar, N.; Bertucci, F.; Penel, N.; Bompas, E.; Cousin, S.; Toulmonde, M.; Bessede, A.; et al. Programmed cell death 1 (PD-1) targeting in patients with advanced osteosarcomas: Results from the PEMBROSARC study. Eur. J. Cancer 2019, 119, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Boye, K.; Longhi, A.; Guren, T.; Lorenz, S.; Naess, S.; Pierini, M.; Taksdal, I.; Lobmaier, I.; Cesari, M.; Paioli, A.; et al. Pembrolizumab in advanced osteosarcoma: Results of a single-arm, open-label, phase 2 trial. Cancer Immunol. Immunother. 2021, 70, 2617–2624. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Mahoney, M.R.; Van Tine, B.A.; Atkins, J.; Milhem, M.M.; Jahagirdar, B.N.; Antonescu, C.R.; Horvath, E.; Tap, W.D.; Schwartz, G.K.; et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018, 19, 416–426. [Google Scholar] [CrossRef]
- Merchant, M.S.; Wright, M.; Baird, K.; Wexler, L.H.; Rodriguez-Galindo, C.; Bernstein, D.; Delbrook, C.; Lodish, M.; Bishop, R.; Wolchok, J.D.; et al. Phase I Clinical Trial of Ipilimumab in Pediatric Patients with Advanced Solid Tumors. Clin. Cancer Res. 2016, 22, 1364–1370. [Google Scholar] [CrossRef]
- Xie, L.; Xu, J.; Sun, X.; Guo, W.; Gu, J.; Liu, K.; Zheng, B.; Ren, T.; Huang, Y.; Tang, X.; et al. Apatinib plus camrelizumab (anti-PD1 therapy, SHR-1210) for advanced osteosarcoma (APFAO) progressing after chemotherapy: A single-arm, open-label, phase 2 trial. J. Immunother. Cancer 2020, 8, e000798. [Google Scholar] [CrossRef]
- Ahmed, N.; Brawley, V.S.; Hegde, M.; Robertson, C.; Ghazi, A.; Gerken, C.; Liu, E.; Dakhova, O.; Ashoori, A.; Corder, A.; et al. Human Epidermal Growth Factor Receptor 2 (HER2) -Specific Chimeric Antigen Receptor-Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J. Clin. Oncol. 2015, 33, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Miwa, S.; Nishida, H.; Tanzawa, Y.; Takeuchi, A.; Hayashi, K.; Yamamoto, N.; Mizukoshi, E.; Nakamoto, Y.; Kaneko, S.; Tsuchiya, H. Phase 1/2 study of immunotherapy with dendritic cells pulsed with autologous tumor lysate in patients with refractory bone and soft tissue sarcoma. Cancer 2017, 123, 1576–1584. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Parker, L.L.; Lu, T.; Zheng, Z.; Toomey, M.A.; White, D.E.; Yao, X.; Li, Y.F.; Robbins, P.F.; Feldman, S.A.; et al. Treatment of Patients With Metastatic Cancer Using a Major Histocompatibility Complex Class II-Restricted T-Cell Receptor Targeting the Cancer Germline Antigen MAGE-A3. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 3322–3329. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Niu, X.; Yao, W. Receptor Tyrosine Kinases in Osteosarcoma Treatment: Which Is the Key Target? Front. Oncol. 2020, 10, 1642. [Google Scholar] [CrossRef] [PubMed]
- Scotlandi, K.; Manara, M.C.; Hattinger, C.M.; Benini, S.; Perdichizzi, S.; Pasello, M.; Bacci, G.; Zanella, L.; Bertoni, F.; Picci, P.; et al. Prognostic and therapeutic relevance of HER2 ex.xpr.ression in osteosarcoma and Ewing’s sarcoma. Eur. J. Cancer 2005, 41, 1349–1361. [Google Scholar] [CrossRef] [PubMed]
- Ebb, D.; Meyers, P.; Grier, H.; Bernstein, M.; Gorlick, R.; Lipshultz, S.E.; Krailo, M.; Devidas, M.; Barkauskas, D.A.; Siegal, G.P.; et al. Phase II trial of trastuzumab in combination with cytotoxic chemotherapy for treatment of metastatic osteosarcoma with human epidermal growth factor receptor 2 overexpression: A report from the children’s oncology group. J. Clin. Oncol. 2012, 30, 2545–2551. [Google Scholar] [CrossRef] [PubMed]
- Kappel, C.C.; Velez-Yanguas, M.C.; Hirschfeld, S.; Helman, L.J. Human osteosarcoma cell lines are dependent on insulin-like growth factor I for in vitro growth. Cancer Res. 1994, 54, 2803–2807. [Google Scholar]
- Pollak, M.; Sem, A.W.; Richard, M.; Tetenes, E.; Bell, R. Inhibition of Metastatic Behavior of Murine Osteosarcoma by Hypophysectomy. J. Natl. Cancer Inst. 1992, 84, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Mansky, P.J.; Liewehr, D.J.; Steinberg, S.M.; Chrousos, G.P.; Avila, N.A.; Long, L.; Bernstein, D.; Mackall, C.L.; Hawkins, D.S.; Helman, L.J. Treatment of Metastatic Osteosarcoma With the Somatostatin Analog OncoLar: Significant Reduction of Insulin-Like Growth Factor-1 Serum Levels. J. Pediatr. Hematol. Oncol. 2002, 24, 440–446. [Google Scholar] [CrossRef]
- Kolb, E.A.; Gorlick, R.; Houghton, P.J.; Morton, C.L.; Lock, R.; Carol, H.; Reynolds, C.P.; Maris, J.M.; Keir, S.T.; Billups, C.A.; et al. Initial testing (stage 1) of a monoclonal antibody (SCH 717454) against the IGF-1 receptor by the pediatric preclinical testing program. Pediatr. Blood Cancer 2008, 50, 1190–1197. [Google Scholar] [CrossRef]
- Anderson, P.M.; Bielack, S.S.; Gorlick, R.G.; Skubitz, K.; Daw, N.C.; Herzog, C.E.; Monge, O.R.; Lassaletta, A.; Boldrini, E.; Pápai, Z.; et al. A phase II study of clinical activity of SCH 717454 (robatumumab) in patients with relapsed osteosarcoma and Ewing sarcoma. Pediatr. Blood Cancer 2016, 63, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Jiang, S.; Shi, Y. Tyrosine kinase inhibitors for solid tumors in the past 20 years (2001–2020). J. Hematol. Oncol. 2020, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Just, M.A.; Van Mater, D.; Wagner, L.M. Receptor tyrosine kinase inhibitors for the treatment of osteosarcoma and Ewing sarcoma. Pediatr. Blood Cancer 2021, 68, e29084. [Google Scholar] [CrossRef]
- Duffaud, F.; Mir, O.; Boudou-Rouquette, P.; Piperno-Neumann, S.; Penel, N.; Bompas, E.; Delcambre, C.; Kalbacher, E.; Italiano, A.; Collard, O.; et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: A non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2019, 20, 120–133. [Google Scholar] [CrossRef]
- Davis, L.E.; Bolejack, V.; Ryan, C.W.; Ganjoo, K.N.; Loggers, E.T.; Chawla, S.; Agulnik, M.; Livingston, M.B.; Reed, D.; Keedy, V.; et al. Randomized Double-Blind Phase II Study of Regorafenib in Patients With Metastatic Osteosarcoma. J. Clin. Oncol. 2019, 37, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Grignani, G.; Palmerini, E.; Dileo, P.; Asaftei, S.D.; D’Ambrosio, L.; Pignochino, Y.; Mercuri, M.; Picci, P.; Fagioli, F.; Casali, P.G.; et al. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: An Italian Sarcoma Group study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Grignani, G.; Palmerini, E.; Ferraresi, V.; D’Ambrosio, L.; Bertulli, R.; Asaftei, S.D.; Tamburini, A.; Pignochino, Y.; Sangiolo, D.; Marchesi, E.; et al. Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: A non-randomised phase 2 clinical trial. Lancet Oncol. 2015, 16, 98–107. [Google Scholar] [CrossRef]
- Italiano, A.; Mir, O.; Mathoulin-Pelissier, S.; Penel, N.; Piperno-Neumann, S.; Bompas, E.; Chevreau, C.; Duffaud, F.; Entz-Werlé, N.; Saada, E.; et al. Cabozantinib in patients with advanced Ewing sarcoma or osteosarcoma (CABONE): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 446–455. [Google Scholar] [CrossRef]
- Xie, L.; Xu, J.; Sun, X.; Tang, X.; Yan, T.; Yang, R.; Guo, W. Apatinib for Advanced Osteosarcoma after Failure of Standard Multimodal Therapy: An Open Label Phase II Clinical Trial. Oncologist 2019, 24, e542–e550. [Google Scholar] [CrossRef]
- Wedge, S.R.; Kendrew, J.; Hennequin, L.F.; Valentine, P.J.; Barry, S.T.; Brave, S.R.; Smith, N.R.; James, N.H.; Dukes, M.; Curwen, J.O.; et al. AZD2171: A highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005, 65, 4389–4400. [Google Scholar] [CrossRef]
- Fox, E.; Aplenc, R.; Bagatell, R.; Chuk, M.K.; Dombi, E.; Goodspeed, W.; Goodwin, A.; Kromplewski, M.; Jayaprakash, N.; Marotti, M.; et al. A phase 1 trial and pharmacokinetic study of cediranib, an orally bioavailable pan-vascular endothelial growth factor receptor inhibitor, in children and adolescents with refractory solid tumors. J. Clin. Oncol. 2010, 28, 5174–5181. [Google Scholar] [CrossRef] [PubMed]
- Melnick, J.S.; Janes, J.; Kim, S.; Chang, J.Y.; Sipes, D.G.; Gunderson, D.; Jarnes, L.; Matzen, J.T.; Garcia, M.E.; Hood, T.L.; et al. An efficient rapid system for profiling the cellular activities of molecular libraries. Proc. Natl. Acad. Sci. USA 2006, 103, 3153–3158. [Google Scholar] [CrossRef] [PubMed]
- Schuetze, S.M.; Wathen, J.K.; Lucas, D.R.; Choy, E.; Samuels, B.L.; Staddon, A.P.; Ganjoo, K.N.; von Mehren, M.; Chow, W.A.; Loeb, D.M.; et al. SARC009: Phase 2 study of dasatinib in patients with previously treated, high-grade, advanced sarcoma. Cancer 2016, 122, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.; Barris, D.M.; Piperdi, S.; Kuo, V.; Everts, S.; Geller, D.; Houghton, P.; Kolb, E.A.; Hawthorne, T.; Gill, J.; et al. Targeting Glycoprotein NMB With Antibody-Drug Conjugate, Glembatumumab Vedotin, for the Treatment of Osteosarcoma. Pediatr. Blood Cancer 2016, 63, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Kopp, L.M.; Malempati, S.; Krailo, M.; Gao, Y.; Buxton, A.; Weigel, B.J.; Hawthorne, T.; Crowley, E.; Moscow, J.A.; Reid, J.M.; et al. Phase II trial of the glycoprotein non-metastatic B-targeted antibody-drug conjugate, glembatumumab vedotin (CDX-011), in recurrent osteosarcoma AOST1521: A report from the Children’s Oncology Group. Eur. J. Cancer 2019, 121, 177–183. [Google Scholar] [CrossRef]
- Deshayes, E.; Roumiguie, M.; Thibault, C.; Beuzeboc, P.; Cachin, F.; Hennequin, C.; Huglo, D.; Rozet, F.; Kassab-Chahmi, D.; Rebillard, X.; et al. Radium 223 dichloride for prostate cancer treatment. Drug Des. Devel. Ther. 2017, 11, 2643–2651. [Google Scholar] [CrossRef]
- Subbiah, V.; Anderson, P.M.; Kairemo, K.; Hess, K.; Huh, W.W.; Ravi, V.; Daw, N.C.; Somaiah, N.; Ludwig, J.A.; Benjamin, R.S.; et al. Alpha Particle Radium 223 Dichloride in High-risk Osteosarcoma: A Phase I Dose Escalation Trial. Clin. Cancer Res. 2019, 25, 3802–3810. [Google Scholar] [CrossRef]
- Scotlandi, K.; Perdichizzi, S.; Manara, M.C.; Serra, M.; Benini, S.; Cerisano, V.; Strammiello, R.; Mercuri, M.; Reverter-Branchat, G.; Faircloth, G.; et al. Effectiveness of Ecteinascidin-743 against drug-sensitive and -resistant bone tumor cells. Clin. Cancer Res. 2002, 8, 3893–3903. [Google Scholar]
- Laverdiere, C.; Kolb, E.A.; Supko, J.G.; Gorlick, R.; Meyers, P.A.; Maki, R.G.; Wexler, L.; Demetri, G.D.; Healey, J.H.; Huvos, A.G.; et al. Phase II study of ecteinascidin 743 in heavily pretreated patients with recurrent osteosarcoma. Cancer 2003, 98, 832–840. [Google Scholar] [CrossRef]
- Mita, M.M.; Mita, A.C.; Chu, Q.S.; Rowinsky, E.K.; Fetterly, G.J.; Goldston, M.; Patnaik, A.; Mathews, L.; Ricart, A.D.; Mays, T.; et al. Phase I trial of the novel mammalian target of rapamycin inhibitor deforolimus (AP23573; MK-8669) administered intravenously daily for 5 days every 2 weeks to patients with advanced malignancies. J. Clin. Oncol. 2008, 26, 361–367. [Google Scholar] [CrossRef]
- Chawla, S.P.; Staddon, A.P.; Baker, L.H.; Schuetze, S.M.; Tolcher, A.W.; D’Amato, G.Z.; Blay, J.Y.; Mita, M.M.; Sankhala, K.K.; Berk, L.; et al. Phase II study of the mammalian target of rapamycin inhibitor ridaforolimus in patients with advanced bone and soft tissue sarcomas. J. Clin. Oncol. 2012, 30, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Rainusso, N.; Lee, Y.C.; Dawson, B.; Coarfa, C.; Han, R.; Larson, J.L.; Shuck, R.; Kurenbekova, L.; Yustein, J.T. Tegavivint and the beta-Catenin/ALDH Axis in Chemotherapy-Resistant and Metastatic Osteosarcoma. J. Natl. Cancer Inst. 2019, 111, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Sun, M.; Jiang, Y.; Zhang, T.; Sun, W.; Wang, H.; Yin, F.; Wang, Z.; Sang, W.; Xu, J.; et al. Anlotinib, a novel small molecular tyrosine kinase inhibitor, suppresses growth and metastasis via dual blockade of VEGFR2 and MET in osteosarcoma. Int. J. Cancer 2019, 145, 979–993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, J.; Ren, T.; Huang, Y.; Liang, X.; Yu, Y.; Wang, W.; Niu, J.; Guo, W. Bone marrow mesenchymal stem cell-derived exosomal miR-206 inhibits osteosarcoma progression by targeting TRA2B. Cancer Lett. 2020, 490, 54–65. [Google Scholar] [CrossRef]
- Lamora, A.; Mullard, M.; Amiaud, J.; Brion, R.; Heymann, D.; Redini, F.; Verrecchia, F. Anticancer activity of halofuginone in a preclinical model of osteosarcoma: Inhibition of tumor growth and lung metastases. Oncotarget 2015, 6, 14413–14427. [Google Scholar] [CrossRef]
- Fang, S.; Tao, H.; Xia, K.; Guo, W. Proscillaridin A induces apoptosis and inhibits the metastasis of osteosarcoma in vitro and in vivo. Biochem. Biophys. Res. Commun. 2020, 521, 880–886. [Google Scholar] [CrossRef]
- Moukengue, B.; Brown, H.K.; Charrier, C.; Battaglia, S.; Baud’huin, M.; Quillard, T.; Pham, T.M.; Pateras, I.S.; Gorgoulis, V.G.; Helleday, T.; et al. TH1579, MTH1 inhibitor, delays tumour growth and inhibits metastases development in osteosarcoma model. EBioMedicine 2020, 53, 102704. [Google Scholar] [CrossRef]
- Kato, H.; Wakabayashi, H.; Naito, Y.; Kato, S.; Nakagawa, T.; Matsumine, A.; Sudo, A. Anti-tumor necrosis factor therapy inhibits lung metastasis in an osteosarcoma cell line. Oncology 2015, 88, 139–146. [Google Scholar] [CrossRef]
- Rodriguez-Nogales, C.; Moreno, H.; Zandueta, C.; Desmaele, D.; Lecanda, F.; Couvreur, P.; Blanco-Prieto, M.J. Combinatorial Nanomedicine Made of Squalenoyl-Gemcitabine and Edelfosine for the Treatment of Osteosarcoma. Cancers 2020, 12, 1895. [Google Scholar] [CrossRef]
- Shekhar, T.M.; Burvenich, I.J.G.; Harris, M.A.; Rigopoulos, A.; Zanker, D.; Spurling, A.; Parker, B.S.; Walkley, C.R.; Scott, A.M.; Hawkins, C.J. Smac mimetics LCL161 and GDC-0152 inhibit osteosarcoma growth and metastasis in mice. BMC Cancer 2019, 19, 924. [Google Scholar] [CrossRef]
- Maloney, C.; Kallis, M.P.; Edelman, M.; Tzanavaris, C.; Lesser, M.; Soffer, S.Z.; Symons, M.; Steinberg, B.M. Gefitinib Inhibits Invasion and Metastasis of Osteosarcoma via Inhibition of Macrophage Receptor Interacting Serine-Threonine Kinase 2. Mol. Cancer Ther. 2020, 19, 1340–1350. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Wei, Z.; Liu, J. MiRNA-1225 Inhibits Osteosarcoma Tumor Growth and Progression by Targeting YWHAZ. Onco. Targets Ther. 2021, 14, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, H.; Komizu, Y.; Ichihara, H.; Goto, K.; Ueoka, R. Hybrid liposomes inhibit tumor growth and lung metastasis of murine osteosarcoma cells. Cancer Med. 2013, 2, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Sueyoshi, T.; Jono, H.; Shinriki, S.; Ota, K.; Ota, T.; Tasaki, M.; Atsuyama, E.; Yakushiji, T.; Ueda, M.; Obayashi, K.; et al. Therapeutic approaches targeting midkine suppress tumor growth and lung metastasis in osteosarcoma. Cancer Lett. 2012, 316, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Gvozdenovic, A.; Boro, A.; Born, W.; Muff, R.; Fuchs, B. A bispecific antibody targeting IGF-IR and EGFR has tumor and metastasis suppressive activity in an orthotopic xenograft osteosarcoma mouse model. Am. J. Cancer Res. 2017, 7, 1435–1449. [Google Scholar] [PubMed]
- Zhu, H.; Chen, D.; Xie, X.; Li, Y.; Fan, T. Melittin inhibits lung metastasis of human osteosarcoma: Evidence of wnt/beta-catenin signaling pathway participation. Toxicon 2021, 198, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Lavaud, M.; Mullard, M.; Tesfaye, R.; Amiaud, J.; Legrand, M.; Danieau, G.; Brion, R.; Morice, S.; Regnier, L.; Dupuy, M.; et al. Overexpression of the Ubiquitin Specific Proteases USP43, USP41, USP27x and USP6 in Osteosarcoma Cell Lines: Inhibition of Osteosarcoma Tumor Growth and Lung Metastasis Development by the USP Antagonist PR619. Cells 2021, 10, 2268. [Google Scholar] [CrossRef]
- Topkas, E.; Cai, N.; Cumming, A.; Hazar-Rethinam, M.; Gannon, O.M.; Burgess, M.; Saunders, N.A.; Endo-Munoz, L. Auranofin is a potent suppressor of osteosarcoma metastasis. Oncotarget 2016, 7, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Zhang, Z.; Li, J.; Li, Q.; Hu, S.; Li, J.; Sun, M.; Cai, Z. Oleanolic acid derivative Dex-OA has potent anti-tumor and anti-metastatic activity on osteosarcoma cells in vitro and in vivo. Investig. New Drugs 2011, 29, 258–265. [Google Scholar] [CrossRef]
- Zhao, K.; Yang, S.Y.; Geng, J.; Gong, X.; Gong, W.; Shen, L.; Ning, B. Combination of anginex gene therapy and radiation decelerates the growth and pulmonary metastasis of human osteosarcoma xenografts. Cancer Med. 2018, 7, 2518–2529. [Google Scholar] [CrossRef] [PubMed]
- Broadhead, M.L.; Dass, C.R.; Choong, P.F. Systemically administered PEDF against primary and secondary tumours in a clinically relevant osteosarcoma model. Br. J. Cancer 2011, 105, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Lillo Osuna, M.A.; Garcia-Lopez, J.; El Ayachi, I.; Fatima, I.; Khalid, A.B.; Kumpati, J.; Slayden, A.V.; Seagroves, T.N.; Miranda-Carboni, G.A.; Krum, S.A. Activation of Estrogen Receptor Alpha by Decitabine Inhibits Osteosarcoma Growth and Metastasis. Cancer Res. 2019, 79, 1054–1068. [Google Scholar] [CrossRef]
- Xu, J.F.; Pan, X.H.; Zhang, S.J.; Zhao, C.; Qiu, B.S.; Gu, H.F.; Hong, J.F.; Cao, L.; Chen, Y.; Xia, B.; et al. CD47 blockade inhibits tumor progression human osteosarcoma in xenograft models. Oncotarget 2015, 6, 23662–23670. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Ichikawa, J.; Fujimaki, T.; Taniguchi, N.; Takayama, Y.; Haro, H. Gemcitabine and Rapamycin Exhibit Additive Effect against Osteosarcoma by Targeting Autophagy and Apoptosis. Cancers 2020, 12, 3097. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.H.; Zhang, P.; Yu, T.T.; Huang, H.K.; Zhang, L.L.; Yang, C.M.; Tan, T.; Yang, S.D.; Luo, X.J.; Luo, J.Y. Lycorine inhibits tumor growth of human osteosarcoma cells by blocking Wnt/β-catenin, ERK1/2/MAPK and PI3K/AKT signaling pathway. Am. J. Transl. Res. 2020, 12, 5381–5398. [Google Scholar]
- Lewis, V.O.; Devarajan, E.; Cardo-Vila, M.; Thomas, D.G.; Kleinerman, E.S.; Marchio, S.; Sidman, R.L.; Pasqualini, R.; Arap, W. BMTP-11 is active in preclinical models of human osteosarcoma and a candidate targeted drug for clinical translation. Proc. Natl. Acad. Sci. USA 2017, 114, 8065–8070. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Sumiyoshi, M. Antitumor and antimetastatic actions of dihydroxycoumarins (esculetin or fraxetin) through the inhibition of M2 macrophage differentiation in tumor-associated macrophages and/or G1 arrest in tumor cells. Eur. J. Pharmacol. 2015, 746, 115–125. [Google Scholar] [CrossRef]
- Shimizu, T.; Fuchimoto, Y.; Okita, H.; Fukuda, K.; Kitagawa, Y.; Ueno, S.; Kuroda, T. A curative treatment strategy using tumor debulking surgery combined with immune checkpoint inhibitors for advanced pediatric solid tumors: An in vivo study using a murine model of osteosarcoma. J. Pediatr. Surg. 2018, 53, 2460–2464. [Google Scholar] [CrossRef]
- Wang, F.; Pang, J.D.; Huang, L.L.; Wang, R.; Li, D.; Sun, K.; Wang, L.T.; Zhang, L.M. Nanoscale polysaccharide derivative as an AEG-1 siRNA carrier for effective osteosarcoma therapy. Int. J. Nanomed. 2018, 13, 857–875. [Google Scholar] [CrossRef]
- Biteau, K.; Guiho, R.; Chatelais, M.; Taurelle, J.; Chesneau, J.; Corradini, N.; Heymann, D.; Redini, F. L-MTP-PE and zoledronic acid combination in osteosarcoma: Preclinical evidence of positive therapeutic combination for clinical transfer. Am. J. Cancer Res. 2016, 6, 677–689. [Google Scholar]
- Watanabe, K.; Yui, Y.; Sasagawa, S.; Suzuki, K.; Kanamori, M.; Yasuda, T.; Kimura, T. Low-dose eribulin reduces lung metastasis of osteosarcoma in vitro and in vivo. Oncotarget 2019, 10, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Ren, T.; Huang, Y.; Guo, W. Apatinib inhibits migration and invasion as well as PD-L1 expression in osteosarcoma by targeting STAT3. Biochem. Biophys. Res. Commun. 2018, 495, 1695–1701. [Google Scholar] [CrossRef]
- Sumiyoshi, M.; Taniguchi, M.; Baba, K.; Kimura, Y. Antitumor and antimetastatic actions of xanthoangelol and 4-hydroxyderricin isolated from Angelica keiskei roots through the inhibited activation and differentiation of M2 macrophages. Phytomedicine 2015, 22, 759–767. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, Y.; Brown, H.K.; Patino-Garcia, A.; Heymann, D.; Blanco-Prieto, M.J. Oral administration of edelfosine encapsulated lipid nanoparticles causes regression of lung metastases in pre-clinical models of osteosarcoma. Cancer Lett. 2018, 430, 193–200. [Google Scholar] [CrossRef]
- Zhang, C.; Hong, C.S.; Hu, X.; Yang, C.; Wang, H.; Zhu, D.; Moon, S.; Dmitriev, P.; Lu, J.; Chiang, J.; et al. Inhibition of protein phosphatase 2A with the small molecule LB100 overcomes cell cycle arrest in osteosarcoma after cisplatin treatment. Cell Cycle 2015, 14, 2100–2108. [Google Scholar] [CrossRef]
- Crasto, J.A.; Fourman, M.S.; Morales-Restrepo, A.; Mahjoub, A.; Mandell, J.B.; Ramnath, K.; Tebbets, J.C.; Watters, R.J.; Weiss, K.R. Disulfiram reduces metastatic osteosarcoma tumor burden in an immunocompetent Balb/c or-thotopic mouse model. Oncotarget 2018, 9, 30163–30172. [Google Scholar] [CrossRef]
- Zheng, B.; Ren, T.; Huang, Y.; Sun, K.; Wang, S.; Bao, X.; Liu, K.; Guo, W. PD-1 axis expression in musculoskeletal tumors and antitumor effect of nivolumab in osteosarcoma model of humanized mouse. J. Hematol. Oncol. 2018, 11, 16. [Google Scholar] [CrossRef]
- Koto, K.; Horie, N.; Kimura, S.; Murata, H.; Sakabe, T.; Matsui, T.; Watanabe, M.; Adachi, S.; Maekawa, T.; Fushiki, S.; et al. Clinically relevant dose of zoledronic acid inhibits spontaneous lung metastasis in a murine osteosarcoma model. Cancer Lett. 2009, 274, 271–278. [Google Scholar] [CrossRef]
- Brounais, B.; Chipoy, C.; Mori, K.; Charrier, C.; Battaglia, S.; Pilet, P.; Richards, C.D.; Heymann, D.; Rédini, F.; Blanchard, F. Oncostatin M induces bone loss and sensitizes rat osteosarcoma to the antitumor effect of Midostaurin in vivo. Clin. Cancer Res. 2008, 14, 5400–5409. [Google Scholar] [CrossRef]
- Naruse, T.; Nishida, Y.; Hosono, K.; Ishiguro, N. Meloxicam inhibits osteosarcoma growth, invasiveness and metastasis by COX-2-dependent and independent routes. Carcinogenesis 2006, 27, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Ek, E.T.; Dass, C.R.; Contreras, K.G.; Choong, P.F. Inhibition of orthotopic osteosarcoma growth and metastasis by multitargeted antitumor activities of pigment epithelium-derived factor. Clin. Exp. Metastasis 2007, 24, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Ek, E.T.; Dass, C.R.; Contreras, K.G.; Choong, P.F. PEDF-derived synthetic peptides exhibit antitumor activity in an orthotopic model of human osteosarcoma. J. Orthop Res. 2007, 25, 1671–1680. [Google Scholar] [CrossRef]
- Gao, Y.S.; Mei, J.; Tong, T.L.; Hu, M.; Xue, H.M.; Cai, X.S. Inhibitory effects of VEGF-siRNA mediated by adenovirus on osteosarcoma-bearing nude mice. Cancer Biother. Radiopharm. 2009, 24, 243–247. [Google Scholar] [CrossRef]
- Kishida, Y.; Yoshikawa, H.; Myoui, A. Parthenolide, a natural inhibitor of Nuclear Factor-kappaB, inhibits lung colonization of murine osteosarcoma cells. Clin. Cancer Res. 2007, 13, 59–67. [Google Scholar] [CrossRef]
- Harris, M.A.; Hons, B.; Shekhar, T.M.; Coupland, L.A.; Miles, M.A.; Hawkins, C.J. Transient NK Cell Depletion Facilitates Pulmonary Osteosarcoma Metastases After Intravenous Inoculation in Athymic Mice. J. Adolesc. Young Adult Oncol. 2020, 9, 667–671. [Google Scholar] [CrossRef]
- Klangjorhor, J.; Chaiyawat, P.; Teeyakasem, P.; Sirikaew, N.; Phanphaisarn, A.; Settakorn, J.; Lirdprapamongkol, K.; Yama, S.; Svasti, J.; Pruksakorn, D. Mycophenolic acid is a drug with the potential to be repurposed for suppressing tumor growth and metastasis in osteosarcoma treatment. Int. J. Cancer 2020, 146, 3397–3409. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.-H.; Chen, P.-N.; Hsieh, Y.-H.; Lin, C.-Y.; Cheng, F.-Y.; Chiu, P.-C.; Chu, S.-C.; Hsieh, Y.-S. 3-Hydroxyflavone inhibits human osteosarcoma U2OS and 143B cells metastasis by affecting EMT and repressing u-PA/MMP-2 via FAK-Src to MEK/ERK and RhoA/MLC2 pathways and reduces 143B tumor growth in vivo. Food Chem. Toxicol. 2016, 97, 177–186. [Google Scholar] [CrossRef]
- Tome, Y.; Kimura, H.; Maehara, H.; Sugimoto, N.; Bouvet, M.; Tsuchiya, H.; Kanaya, F.; Hoffman, R.M. High lung-metastatic variant of human osteosarcoma cells, selected by passage of lung metastasis in nude mice, is associated with increased expression of alpha(v)beta(3) integrin. Anticancer. Res. 2013, 33, 3623–3627. [Google Scholar] [PubMed]
- Igarashi, K.; Kawaguchi, K.; Kiyuna, T.; Murakami, T.; Yamamoto, N.; Hayashi, K.; Kimura, H.; Miwa, S.; Tsuchiya, H.; Hoffman, R.M. Antimetastatic Efficacy of the Combination of Caffeine and Valproic Acid on an Orthotopic Human Osteosarcoma Cell Line Model in Nude Mice. Anticancer. Res. 2017, 37, 1005–1011. [Google Scholar] [CrossRef]
- Chen, X.; Deng, M.; Zhou, X.; Wang, X.; Ye, Y.; Zhu, J.; Jiang, H.; Chen, X.; Zha, W. Euxanthone Impairs the Metastatic Potential of Osteosarcoma by Reducing COX-2 Expression. Anat. Rec. 2019, 302, 1399–1408. [Google Scholar] [CrossRef]
- Portella, L.; Vitale, R.; De Luca, S.; D’Alterio, C.; Ierano, C.; Napolitano, M.; Riccio, A.; Polimeno, M.N.; Monfregola, L.; Barbieri, A.; et al. Preclinical development of a novel class of CXCR4 antagonist impairing solid tumors growth and metastases. PLoS ONE 2013, 8, e74548. [Google Scholar] [CrossRef]
- Hsieh, Y.H.; Hsu, W.H.; Yang, S.F.; Liu, C.J.; Lu, K.H.; Wang, P.H.; Lin, R.C. Potential Antimetastatic Effect of Timosaponin AIII against Human Osteosarcoma Cells through Regulating the Integrin/FAK/Cofilin Axis. Pharmaceuticals 2021, 14, 260. [Google Scholar] [CrossRef]
- Picarda, G.; Lamoureux, F.; Geffroy, L.; Delepine, P.; Montier, T.; Laud, K.; Tirode, F.; Delattre, O.; Heymann, D.; Rédini, F. Preclinical evidence that use of TRAIL in Ewing’s sarcoma and osteosarcoma therapy inhibits tumor growth, prevents osteolysis, and increases animal survival. Clin. Cancer Res. 2010, 16, 2363–2374. [Google Scholar] [CrossRef]
- McGuire, J.J.; Nerlakanti, N.; Lo, C.H.; Tauro, M.; Utset-Ward, T.J.; Reed, D.R.; Lynch, C.C. Histone deacetylase inhibition prevents the growth of primary and metastatic osteosarcoma. Int. J. Cancer 2020, 147, 2811–2823. [Google Scholar] [CrossRef]
- Lan, H.; Hong, W.; Fan, P.; Qian, D.; Zhu, J.; Bai, B. Quercetin Inhibits Cell Migration and Invasion in Human Osteosarcoma Cells. Cell Physiol. Biochem. 2017, 43, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Ory, B.; Heymann, M.F.; Kamijo, A.; Gouin, F.; Heymann, D.; Redini, F. Zoledronic acid suppresses lung metastases and prolongs overall survival of osteosarcoma-bearing mice. Cancer 2005, 104, 2522–2529. [Google Scholar] [CrossRef]
- Wan, X.; Mendoza, A.; Khanna, C.; Helman, L.J. Rapamycin inhibits ezrin-mediated metastatic behavior in a murine model of osteosarcoma. Cancer Res. 2005, 65, 2406–2411. [Google Scholar] [CrossRef]
- Fan, T.M.; Roberts, R.D.; Lizardo, M.M. Understanding and Modeling Metastasis Biology to Improve Therapeutic Strategies for Combating Osteosarcoma Progression. Front. Oncol. 2020, 10, 13. [Google Scholar] [CrossRef]
- Sheng, G.; Gao, Y.; Yang, Y.; Wu, H. Osteosarcoma and Metastasis. Front. Oncol. 2021, 11, 780264. [Google Scholar] [CrossRef]
- Morrow, J.J.; Mendoza, A.; Koyen, A.; Lizardo, M.M.; Ren, L.; Waybright, T.J.; Hansen, R.J.; Gustafson, D.L.; Zhou, M.; Fan, T.M.; et al. mTOR Inhibition Mitigates Enhanced mRNA Translation Associated with the Metastatic Phenotype of Osteosarcoma Cells In Vivo. Clin. Cancer Res. 2016, 22, 6129–6141. [Google Scholar] [CrossRef]
- Martinez-Velez, N.; Xipell, E.; Vera, B.; Acanda de la Rocha, A.; Zalacain, M.; Marrodan, L.; Gonzalez-Huarriz, M.; Toledo, G.; Cascallo, M.; Alemany, R.; et al. The Oncolytic Adenovirus VCN-01 as Therapeutic Approach Against Pediatric Osteosarcoma. Clin. Cancer Res. 2016, 22, 2217–2225. [Google Scholar] [CrossRef]
- Jiang, C.; Fang, X.; Zhang, H.; Wang, X.; Li, M.; Jiang, W.; Tian, F.; Zhu, L.; Bian, Z. AMD3100 combined with triptolide inhibit proliferation, invasion and metastasis and induce apoptosis of human U2OS osteosarcoma cells. Biomed. Pharmacother. 2017, 86, 677–685. [Google Scholar] [CrossRef]
- Nasarre, P.; Garcia, D.I.; Siegel, J.B.; Bonilla, I.V.; Mukherjee, R.; Hilliard, E.; Chakraborty, P.; Nasarre, C.; Yustein, J.T.; Lang, M.; et al. Overcoming PD-1 Inhibitor Resistance with a Monoclonal Antibody to Secreted Frizzled-Related Protein 2 in Metastatic Osteosarcoma. Cancers 2021, 13, 2696. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.A.; Miles, M.A.; Shekhar, T.M.; Cerra, C.; Georgy, S.R.; Ryan, S.D.; Cannon, C.M.; Hawkins, C.J. The proteasome inhibitor ixazomib inhibits the formation and growth of pulmonary and abdominal osteosarcoma metastases in mice. Cancers 2020, 12, 1207. [Google Scholar] [CrossRef]
- Pignochino, Y.; Grignani, G.; Cavalloni, G.; Motta, M.; Tapparo, M.; Bruno, S.; Bottos, A.; Gammaitoni, L.; Migliardi, G.; Camussi, G.; et al. Sorafenib blocks tumour growth, angiogenesis and metastatic potential in preclinical models of osteosarcoma through a mechanism potentially involving the inhibition of ERK1/2, MCL-1 and ezrin pathways. Mol. Cancer 2009, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.A.; Shekhar, T.M.; Miles, M.A.; Cerra, C.; Hawkins, C.J. The smac mimetic LCL161 targets established pulmonary osteosarcoma metastases in mice. Clin. Exp. Metastasis 2021, 38, 441–449. [Google Scholar] [CrossRef]
- Simpson, S.; Dunning, M.D.; de Brot, S.; Grau-Roma, L.; Mongan, N.P.; Rutland, C.S. Comparative review of human and canine osteosarcoma: Morphology, epidemiology, prognosis, treatment and genetics. Acta Vet. Scand. 2017, 59, 71. [Google Scholar] [CrossRef]
- Mueller, F.; Fuchs, B.; Kaser-Hotz, B. Comparative biology of human and canine osteosarcoma. Anticancer Res. 2007, 27, 155–164. [Google Scholar] [PubMed]
- Endo-Munoz, L.; Bennett, T.C.; Topkas, E.; Wu, S.Y.; Thamm, D.H.; Brockley, L.; Cooper, M.; Sommerville, S.; Thomson, M.; O’Connell, K.; et al. Auranofin improves overall survival when combined with standard of care in a pilot study involving dogs with osteosarcoma. Vet. Comp. Oncol. 2020, 18, 206–213. [Google Scholar] [CrossRef]
- Laver, T.; London, C.A.; Vail, D.M.; Biller, B.J.; Coy, J.; Thamm, D.H. Prospective evaluation of toceranib phosphate in metastatic canine osteosarcoma. Vet. Comp. Oncol. 2018, 16, E23–E29. [Google Scholar] [CrossRef]
- London, C.; Mathie, T.; Stingle, N.; Clifford, C.; Haney, S.; Klein, M.K.; Beaver, L.; Vickery, K.; Vail, D.M.; Hershey, B.; et al. Preliminary evidence for biologic activity of toceranib phosphate (Palladia(®)) in solid tumours. Vet. Comp. Oncol. 2012, 10, 194–205. [Google Scholar] [CrossRef]
- Padhani, A.R.; Ollivier, L. The RECIST (Response Evaluation Criteria in Solid Tumors) criteria: Implications for diagnostic radiologists. Br. J. Radiol. 2001, 74, 983–986. [Google Scholar] [CrossRef] [PubMed]
- McHugh, K.; Kao, S. Response evaluation criteria in solid tumours (RECIST): Problems and need for modifications in paediatric oncology? Br. J. Radiol. 2003, 76, 433–436. [Google Scholar] [CrossRef]
- Bishop, M.W.; Chang, Y.C.; Krailo, M.D.; Meyers, P.A.; Provisor, A.J.; Schwartz, C.L.; Marina, N.M.; Teot, L.A.; Gebhardt, M.C.; Gorlick, R.; et al. Assessing the Prognostic Significance of Histologic Response in Osteosarcoma: A Comparison of Outcomes on CCG-782 and INT0133-A Report From the Children’s Oncology Group Bone Tumor Committee. Pediatr. Blood Cancer 2016, 63, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Thebault, E.; Piperno-Neumann, S.; Tran, D.; Pacquement, H.; Marec-Berard, P.; Lervat, C.; Castex, M.P.; Cleirec, M.; Bompas, E.; Vannier, J.P.; et al. Successive Osteosarcoma Relapses after the First Line O2006/Sarcome-09 Trial: What Can We Learn for Further Phase-II Trials? Cancers 2021, 13, 1683. [Google Scholar] [CrossRef]
- Whittle, S.B.; Offer, K.; Roberts, R.D.; LeBlanc, A.; London, C.; Majzner, R.G.; Huang, A.Y.; Houghton, P.; Alejandro Sweet Cordero, E.; Grohar, P.J.; et al. Charting a path for prioritization of novel agents for clinical trials in osteosarcoma: A report from the Children’s Oncology Group New Agents for Osteosarcoma Task Force. Pediatr. Blood Cancer 2021, 68, e29188. [Google Scholar] [CrossRef]
- Tanaka, K.; Kawano, M.; Iwasaki, T.; Itonaga, I.; Tsumura, H. A meta-analytic evaluation of the correlation between event-free survival and overall survival in randomized controlled trials of newly diagnosed Ewing sarcoma. BMC Cancer 2020, 20, 379. [Google Scholar] [CrossRef]
- Tanaka, K.; Kawano, M.; Iwasaki, T.; Matsuda, S.; Itonaga, I.; Tsumura, H. Surrogate endpoints for overall survival in randomised controlled trials of localised osteosarcoma: A meta-analytic evaluation. Sci. Rep. 2020, 10, 8573. [Google Scholar] [CrossRef] [PubMed]
- Samaille, T.; Moreau Bachelard, C.; Coquan, E.; du Rusquec, P.; Paoletti, X.; Le Tourneau, C. Impact of the timing of tumor assessments on median progression-free survival in clinical trials in advanced cancer patients. ESMO Open 2021, 7, 100366. [Google Scholar] [CrossRef] [PubMed]
- Dancey, J.E.; Dodd, L.E.; Ford, R.; Kaplan, R.; Mooney, M.; Rubinstein, L.; Schwartz, L.H.; Shankar, L.; Therasse, P. Recommendations for the assessment of progression in randomised cancer treatment trials. Eur. J. Cancer 2009, 45, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Lagmay, J.P.; Krailo, M.D.; Dang, H.; Kim, A.; Hawkins, D.S.; Beaty, O., 3rd; Widemann, B.C.; Zwerdling, T.; Bomgaars, L.; Langevin, A.M.; et al. Outcome of Patients With Recurrent Osteosarcoma Enrolled in Seven Phase II Trials Through Children’s Cancer Group, Pediatric Oncology Group, and Children’s Oncology Group: Learning From the Past to Move Forward. J. Clin. Oncol. 2016, 34, 3031–3038. [Google Scholar] [CrossRef]
- Omer, N.; Le Deley, M.C.; Piperno-Neumann, S.; Marec-Berard, P.; Italiano, A.; Corradini, N.; Bellera, C.; Brugières, L.; Gaspar, N. Phase-II trials in osteosarcoma recurrences: A systematic review of past experience. Eur. J. Cancer 2017, 75, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Niu, X.; Wang, Z.; Song, C.L.; Huang, Z.; Chen, K.N.; Duan, J.; Bai, H.; Xu, J.; Zhao, J.; et al. Multiregion Sequencing Reveals the Genetic Heterogeneity and Evolutionary History of Osteosarcoma and Matched Pulmonary Metastases. Cancer Res. 2019, 79, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Li, B.; Wang, S.; Chen, T.; Ye, Z. Innate Immune Cells: A Potential and Promising Cell Population for Treating Osteosarcoma. Front. Immunol. 2019, 10, 1114. [Google Scholar] [CrossRef]
- Davis, A.A.; Patel, V.G. The role of PD-L1 expression as a predictive biomarker: An analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Stenehjem, D.D.; Tran, D.; Nkrumah, M.A.; Gupta, S. PD1/PDL1 inhibitors for the treatment of advanced urothelial bladder cancer. Onco. Targets Ther. 2018, 11, 5973–5989. [Google Scholar] [CrossRef] [PubMed]
- Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Vuky, J.; Powles, T.; Plimack, E.R.; Hahn, N.M.; de Wit, R.; Pang, L.; et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 1483–1492. [Google Scholar] [CrossRef]
- Carbone, D.P.; Reck, M.; Paz-Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.M.; Ciuleanu, T.E.; Badin, F.; et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.-O.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322. [Google Scholar] [CrossRef]
- Chida, K.; Kawazoe, A.; Kawazu, M.; Suzuki, T.; Nakamura, Y.; Nakatsura, T.; Kuwata, T.; Ueno, T.; Kuboki, Y.; Kotani, D.; et al. A Low Tumor Mutational Burden and PTEN Mutations Are Predictors of a Negative Response to PD-1 Blockade in MSI-H/dMMR Gastrointestinal Tumors. Clin. Cancer Res. 2021, 27, 3714–3724. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Nathanson, T.; Rizvi, H.; Creelan, B.C.; Sanchez-Vega, F.; Ahuja, A.; Ni, A.; Novik, J.B.; Mangarin, L.M.B.; Abu-Akeel, M.; et al. Genomic Features of Response to Combination Immunotherapy in Patients with Advanced Non-Small-Cell Lung Cancer. Cancer Cell 2018, 33, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Yang, Y.; Guo, W.; Che, D.; Xu, J.; Sun, X.; Liu, K.; Ren, T.; Liu, X.; Yang, Y.; et al. The Clinical Implications of Tumor Mutational Burden in Osteosarcoma. Front. Oncol. 2020, 10, 595527. [Google Scholar] [CrossRef] [PubMed]
- Sterz, U.; Grube, M.; Herr, W.; Menhart, K.; Wendl, C.; Vogelhuber, M. Case Report: Dual Checkpoint Inhibition in Advanced Metastatic Osteosarcoma Results in Remission of All Tumor Manifestations-A Report of a Stunning Success in a 37-Year-Old Patient. Front. Oncol. 2021, 11, 684733. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wang, F.; Huang, J.; Zhou, Y.; Yang, Q.; Qian, G.; Zhou, C.; Min, D.; Song, L.; Shen, Z. Case Report: Sequential Chemotherapy and Immunotherapy Produce Sustained Response in Osteosarcoma With High Tumor Mutational Burden. Front. Endocrinol. 2021, 12, 625226. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).