Cation Permeability of Voltage-Gated Hair Cell Ca2+ Channels of the Vertebrate Labyrinth
Abstract
1. Introduction
2. Results
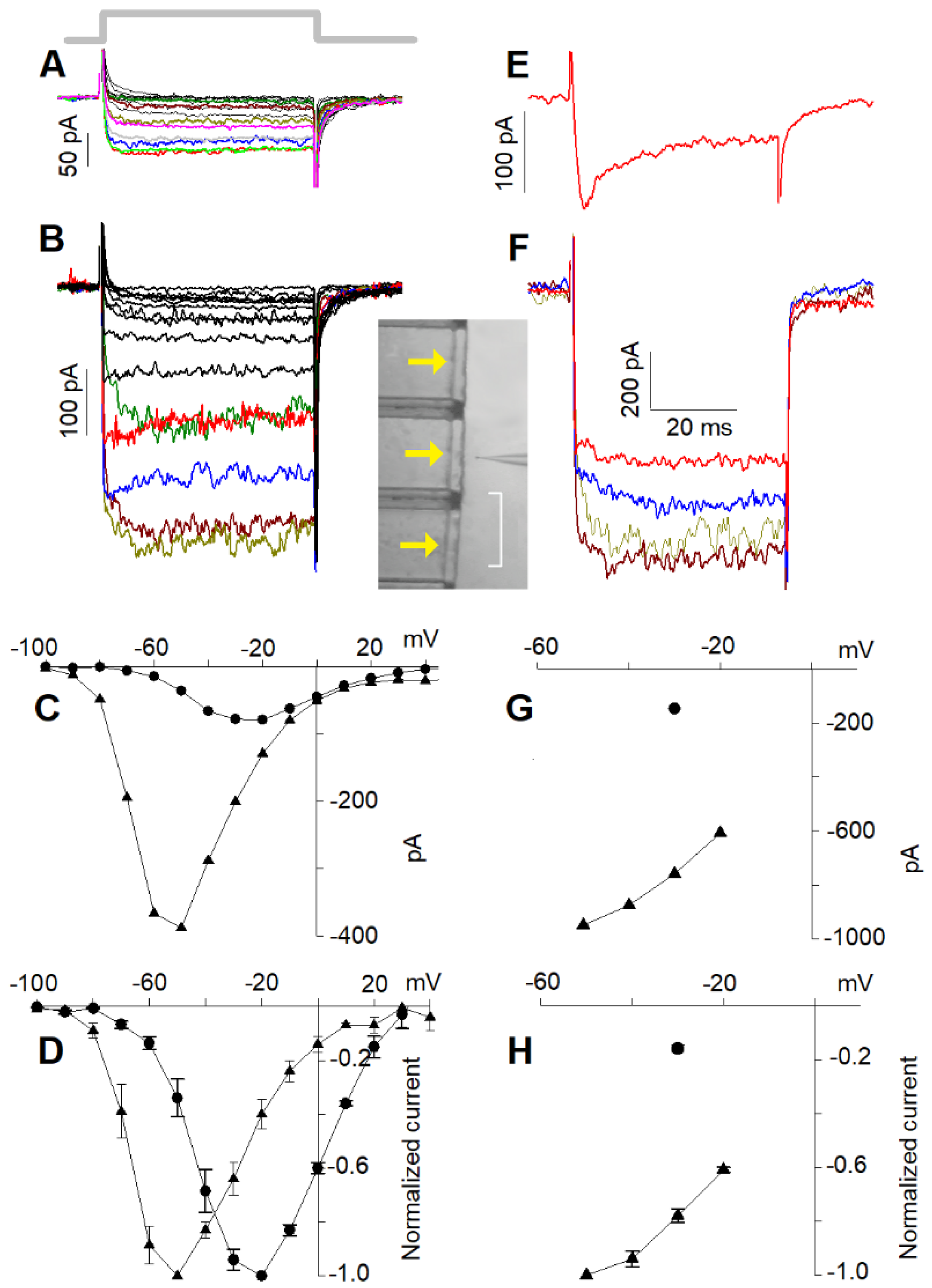
2.1. Isolation of L- and Putative R-Type Components
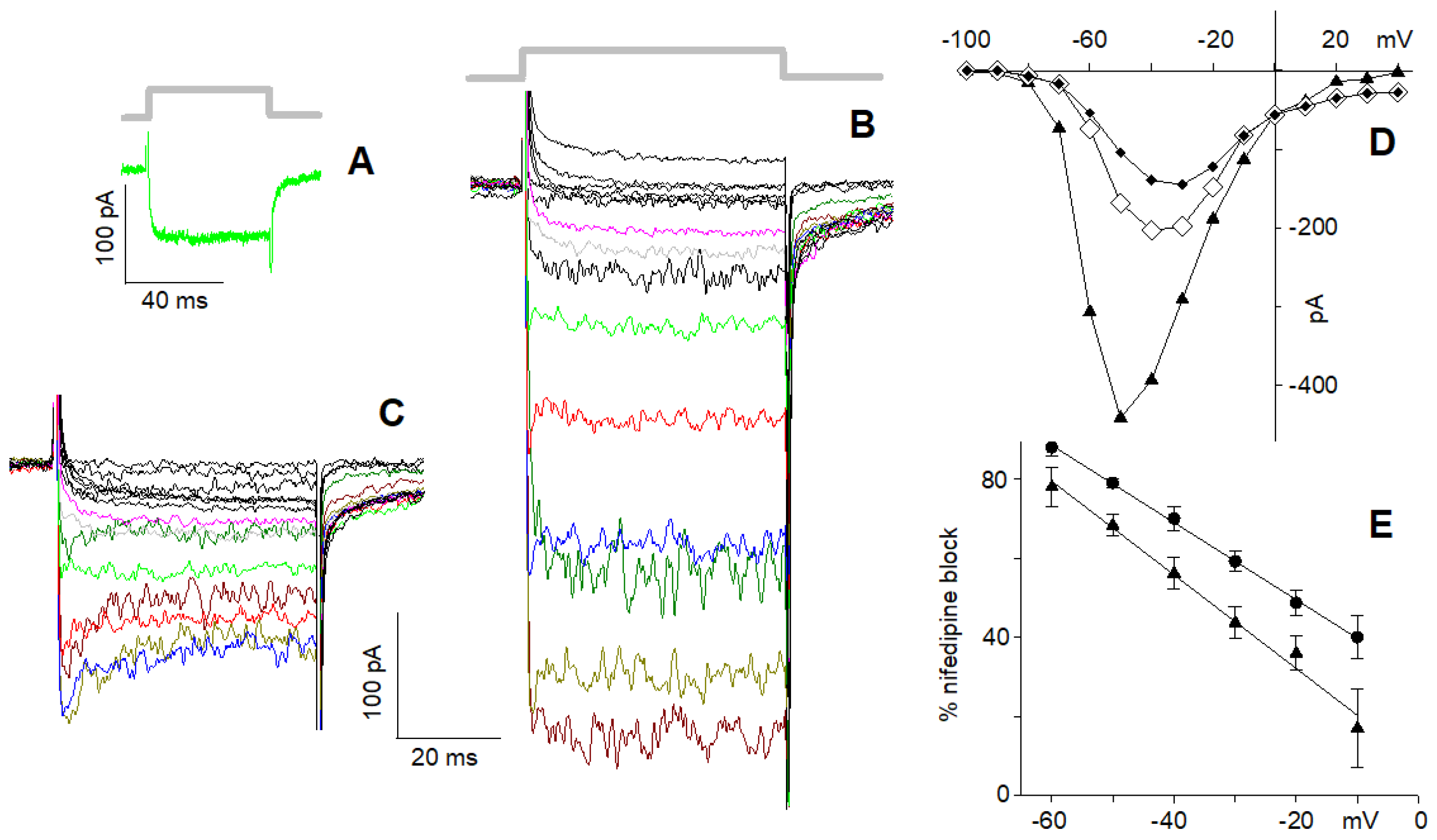
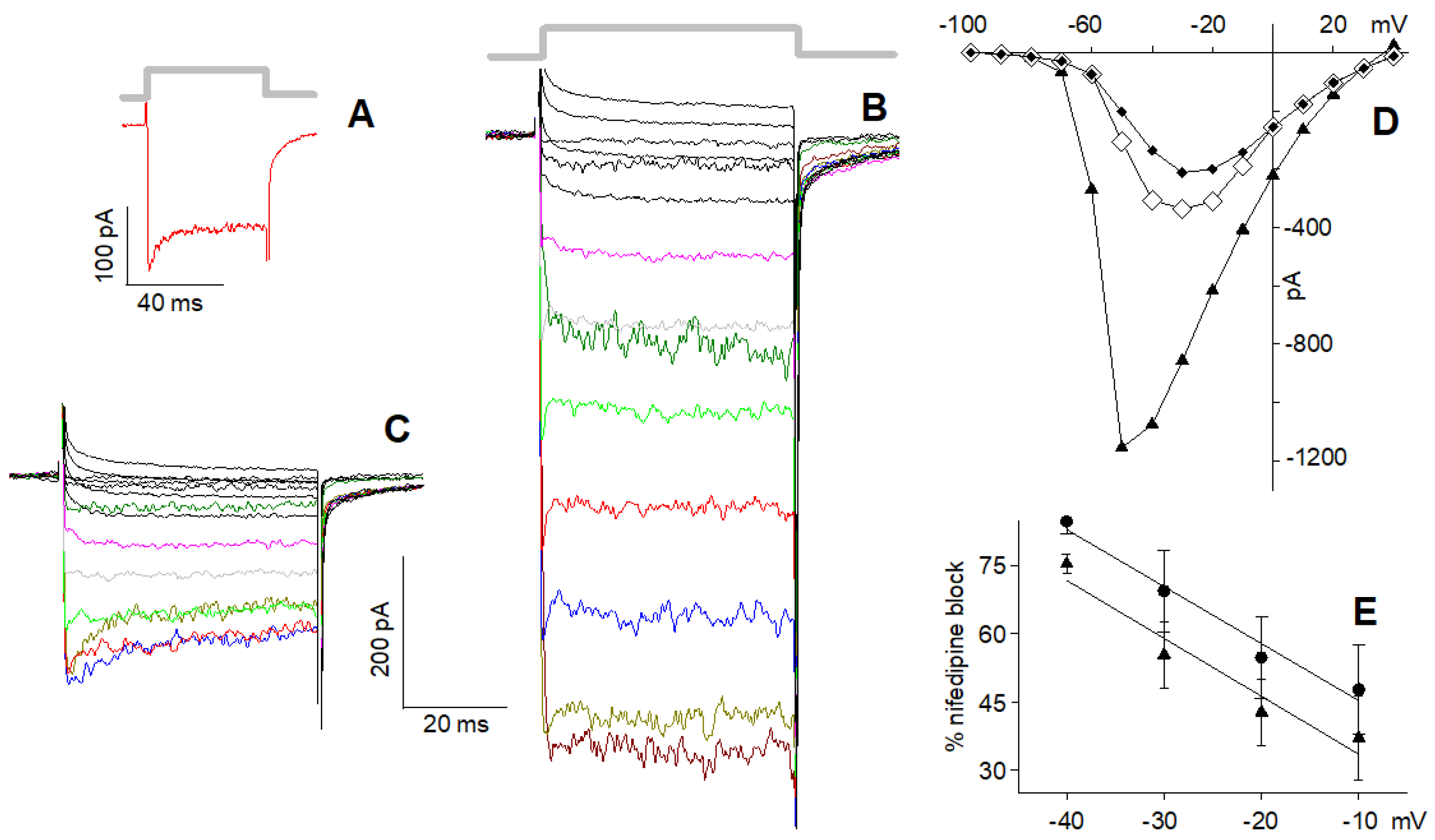
2.2. Current Elicited by Depolarisation in 10 nM Ca2+
2.3. L-Type Channel Blockers
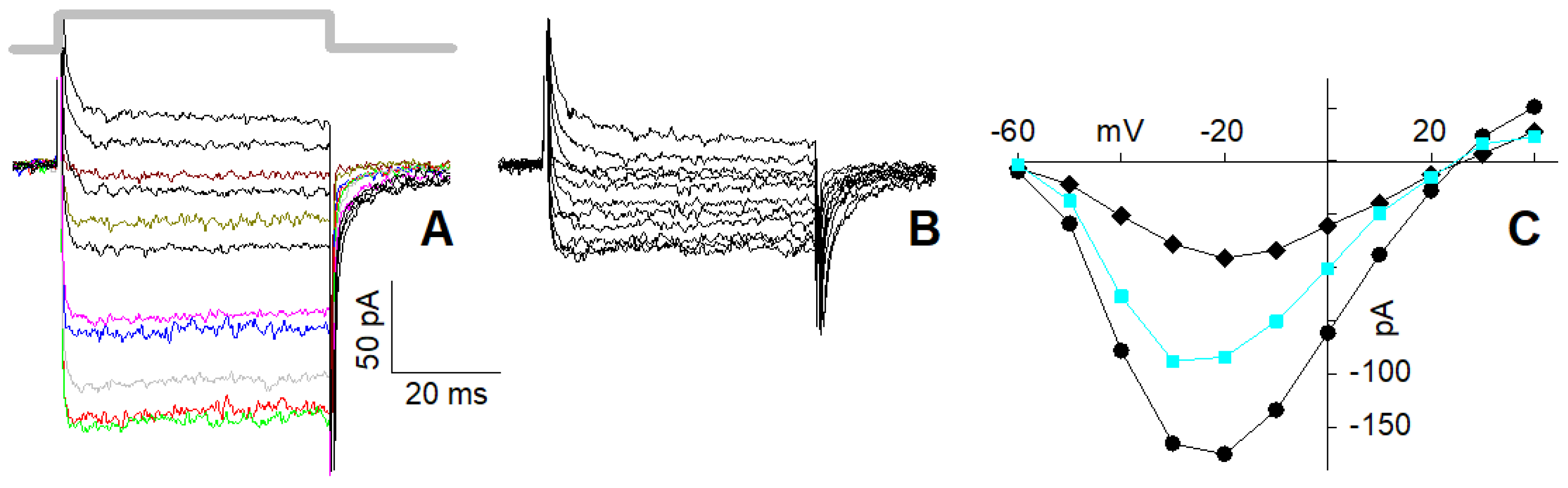
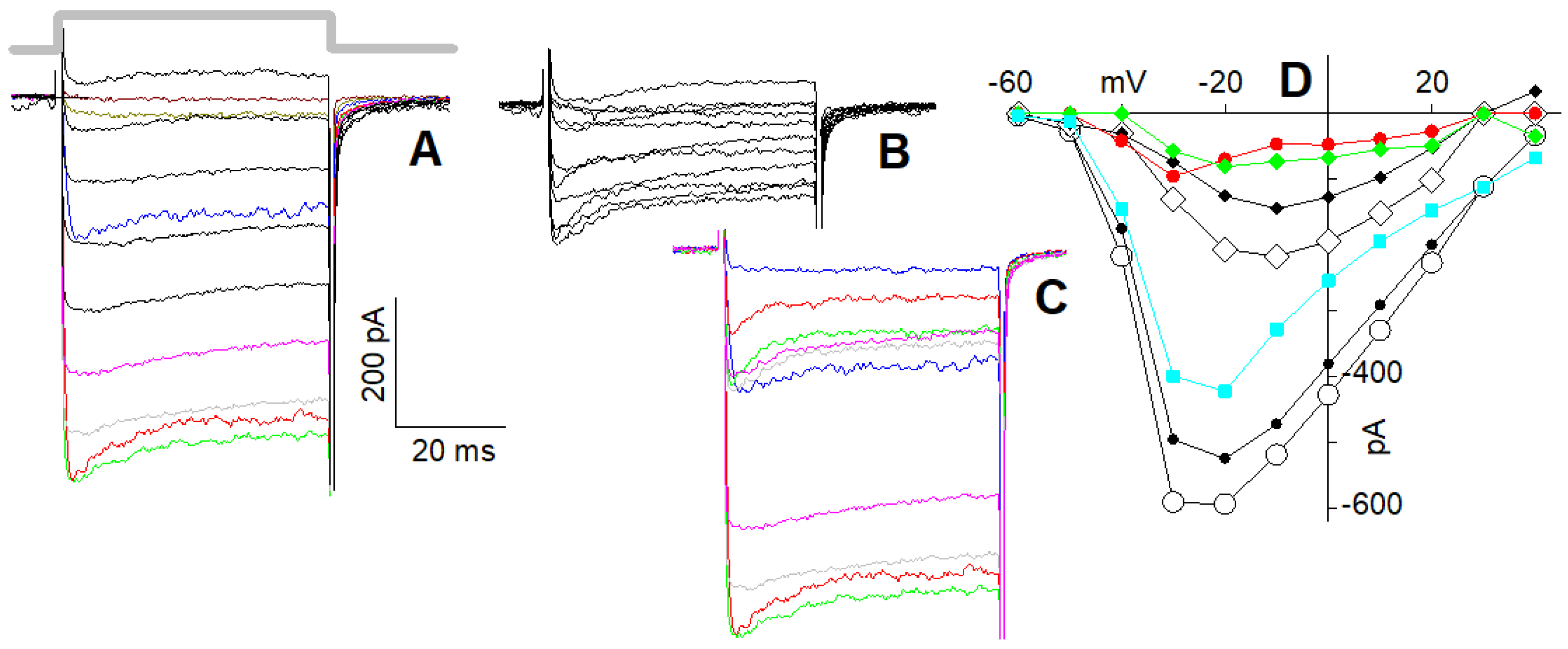
2.4. I–Vs of L + R2 and of L + R1 + R2 Currents in 4 mM Ca2+
2.5. I–Vs of L + R2 and of L + R1 + R2 Currents in 10 nM Ca2+
3. Discussion
4. Materials and Methods
4.1. Animal and Solutions
4.2. Patch Clamp Recording and Fast Solution Application
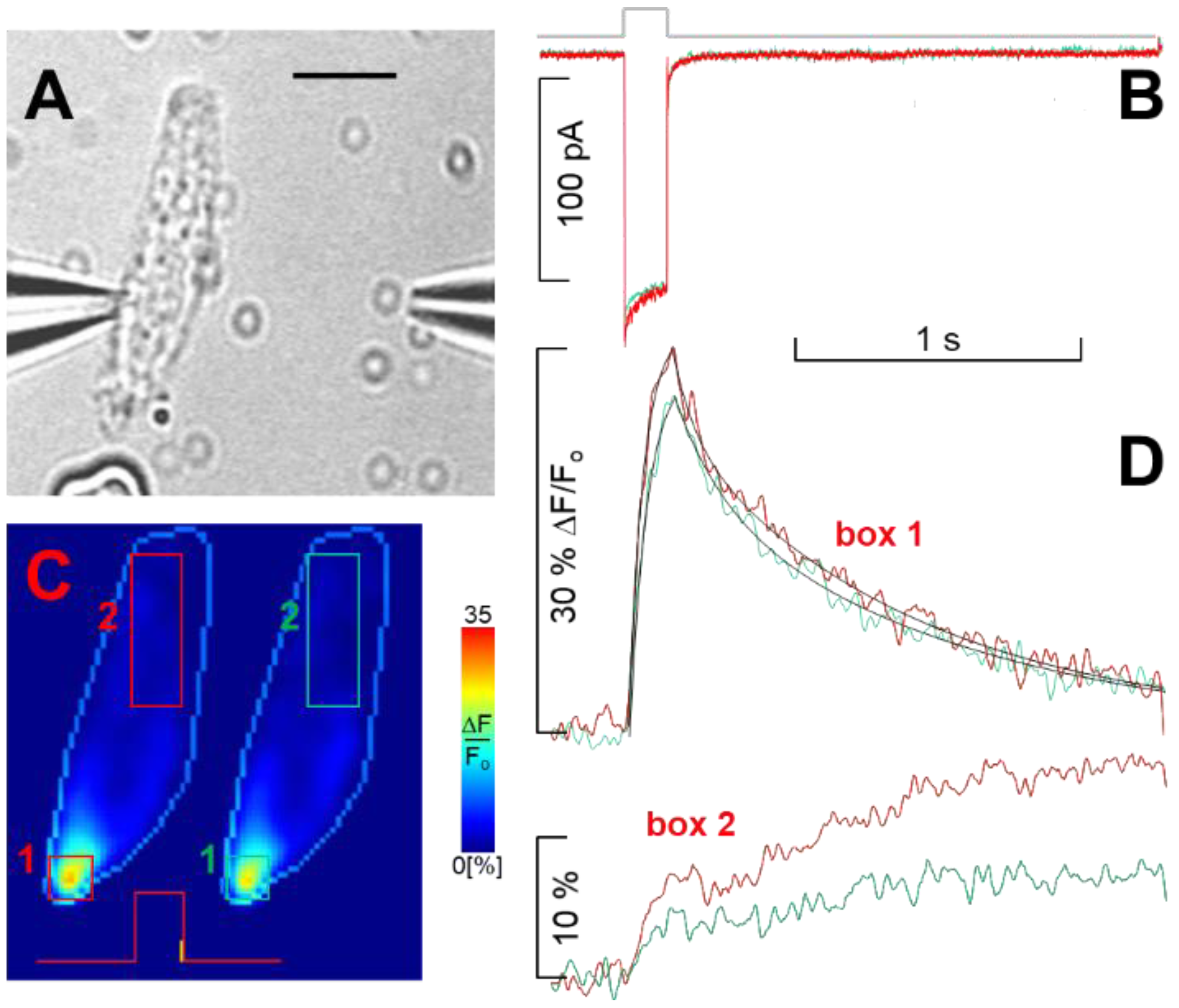
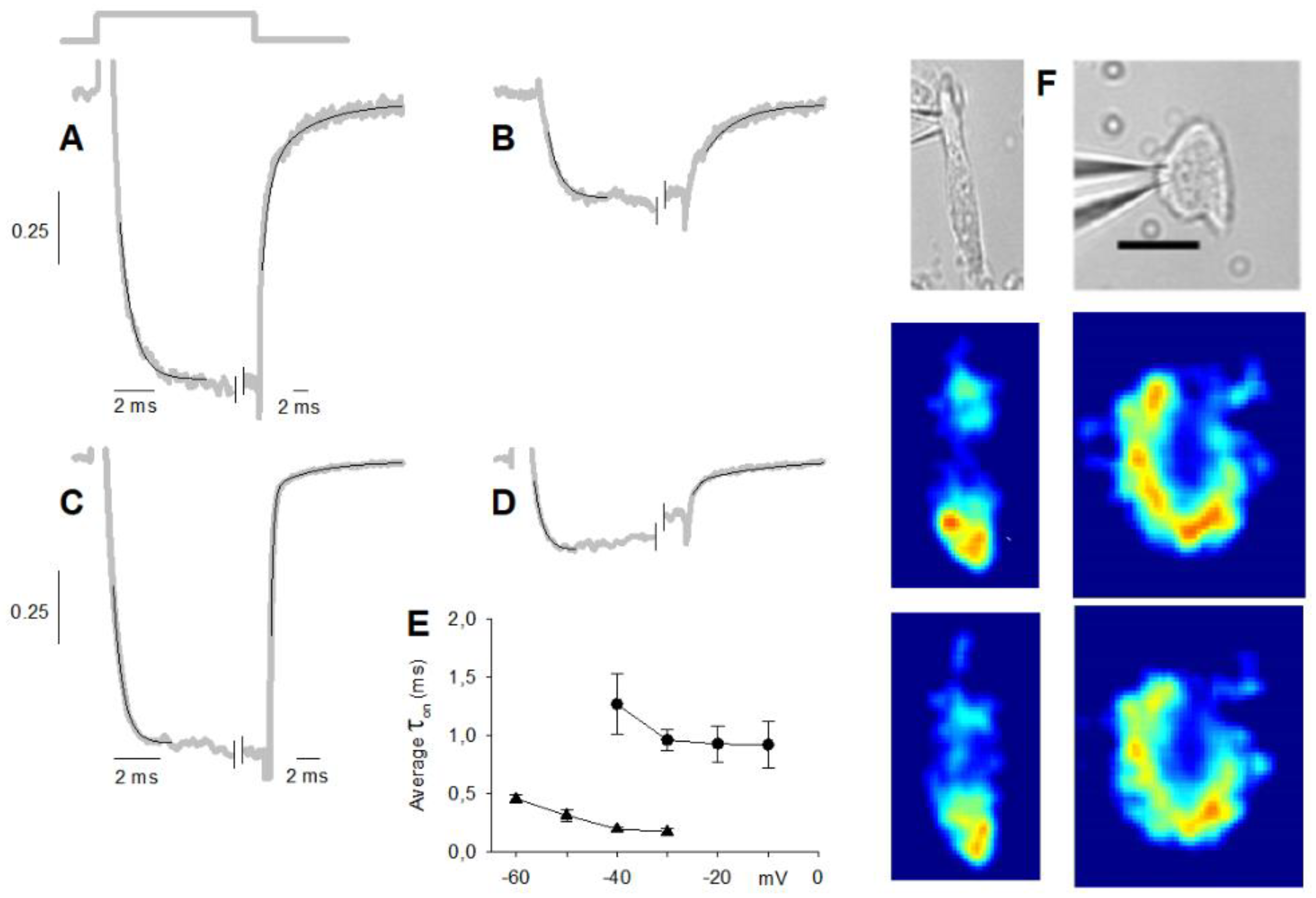
4.3. Fast Fluorescence Imaging and Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rossi, M.L.; Martini, M.; Pelucchi, B.; Fesce, R. The quantal nature of transmitter release at the cytoneural junction of the frog labyrinth. J. Physiol. 1994, 478, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.; Rossi, M.L.; Rubbini, G.; Rispoli, G. Calcium currents in hair cells isolated from semicircular canals of the frog. Biophys. J. 2000, 78, 1240–1254. [Google Scholar] [CrossRef][Green Version]
- Fuchs, P. The synaptic physiology of cochlear hair cells. Audiol. Neurootol. 2002, 7, 40–44. [Google Scholar] [CrossRef]
- Pangrsic, T.; Singer, J.H.; Koschak, A. Voltage-Gated Calcium Channels: Key Players in Sensory Coding in the Retina and the Inner Ear. Physiol. Rev. 2018, 98, 2063–2096. [Google Scholar] [CrossRef] [PubMed]
- Striessnig, J.; Pinggera, A.; Kaur, G.; Bock, G.; Tuluc, P. L-type Ca2+ channels in heart and brain. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2014, 3, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Mei, C.; Dong, H.; Nisenbaum, E.; Thielhelm, T.; Nourbakhsh, A.; Yan, D.; Smeal, M.; Lundberg, Y.; Hoffer, M.E.; Angeli, S.; et al. Genetics and the Individualized Therapy of Vestibular Disorders. Front. Neurol. 2021, 12, 633207. [Google Scholar] [CrossRef]
- Arab, S.F.; Düwel, P.; Jüngling, E.; Westhofen, M.; Lückhoff, A. Inhibition of voltage-gated calcium currents in type II vestibular hair cells by cinnarizine. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004, 369, 570–575. [Google Scholar] [CrossRef]
- Volkov, A.G.; Paula, S.; Deamer, D.W. Two mechanisms of permeation of small neutral molecules and hydrated ions across phospholipid bilayers. Bioelectrochem. Bioenerg. 1997, 42, 153–160. [Google Scholar] [CrossRef]
- Almers, W.; McCleskey, E.W.; Palade, P.T. A non-selective cation conductance in frog muscle membrane blocked by micromolar external calcium ions. J. Physiol. 1984, 353, 565–583. [Google Scholar] [CrossRef]
- Almers, W.; McCleskey, E.W. Non-selective conductance in calcium channels of frog muscle: Calcium selectivity in a single-file pore. J. Physiol. 1984, 353, 585–608. [Google Scholar] [CrossRef]
- Art, J.J.; Fettiplace, R.; Wu, Y.C. The effects of low calcium on the voltage-dependent conductances involved in tuning of turtle hair cells. J. Physiol. 1993, 470, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Schnee, M.E.; Ricci, A.J. Biophysical and pharmacological characterization of voltage-gated calcium currents in turtle auditory hair cells. J. Physiol. 2003, 549, 697–717. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; DeMaria, C.D.; Erickson, M.G.; Mori, M.X.; Alseikhan, B.A.; Yue, D.T. Unified mechanisms of Ca2+ regulation across the Ca2+ channel family. Neuron 2003, 39, 951–960. [Google Scholar] [CrossRef]
- Rodriguez-Contreras, A.; Yamoah, E.N. Direct measurements of single-channel Ca2+ currents in bullfrog hair cells reveals two distinct channel subtypes. J. Physiol. 2001, 534, 669–689. [Google Scholar] [CrossRef]
- Su, Z.L.; Jiang, S.C.; Gu, R.; Yang, W.P. Two types of calcium channels in bullfrog saccular hair cells. Hear. Res. 1995, 87, 62–68. [Google Scholar] [CrossRef]
- Rodriguez-Contreras, A.; Nonner, W.; Yamoah, E.N. Ca2+ transport properties and determinants of anomalous mole fraction effects of single voltage-gated Ca2+ channels in hair cells from bullfrog saccule. J. Physiol. 2002, 538, 729–745. [Google Scholar] [CrossRef]
- Kollmar, R.; Montgomery, L.G.; Fak, J.; Henry, L.J.; Hudspeth, A.J. Predominance of the 1D subunit in L-type voltage-gated Ca2+ channels of hair cells in the chicken’s cochlea. Proc. Natl. Acad. Sci. USA 1997, 94, 14883–14888. [Google Scholar] [CrossRef]
- Bao, H.; Wong, W.H.; Goldberg, J.M.; Eatock, R.A. Voltage-gated calcium channel currents in type I and type II hair cells isolated from the rat crista. J. Neurophysiol. 2003, 90, 155–164. [Google Scholar] [CrossRef]
- Ertel, E.A.; Campbell, K.P.; Harpold, M.M.; Hofmann, F.; Mori, Y.; Perez-Reyes, E.; Schwartz, A.; Snutch, T.P.; Tanabe, T.; Birnbaumer, L.; et al. Nomenclature of voltage-gated calcium channels. Neuron 2000, 25, 533–535. [Google Scholar] [CrossRef]
- Hering, S. β-Subunits: Fine tuning of Ca2+ channel block. Trends Pharmacol. Sci. 2002, 23, 509–513. [Google Scholar] [CrossRef]
- Tamse, C.T.; Xu, Y.; Song, H.; Nie, L.; Yamoah, E.N. Protein kinase A mediates voltage-dependent facilitation of Ca2+ current in presynaptic hair cells in Hermissenda crassicornis. J. Neurophysiol. 2003, 89, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Koschak, A.; Reimer, D.; Huber, I.; Grabner, M.; Glossmann, H.; Engel, J.; Striessnig, J. Alpha 1D (Cav1.3) subunits can form L-type Ca2+ channels activating at negative voltages. J. Biol. Chem. 2001, 276, 22100–22106. [Google Scholar] [CrossRef]
- Xu, W.; Lipscombe, D. Neuronal Ca(V)1.3alpha(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J. Neurosci. 2001, 21, 5944–5951. [Google Scholar] [CrossRef] [PubMed]
- Lopez, I.; Ishiyama, G.; Acuna, D.; Ishiyama, A.; Baloh, R.W. Immunolocalization of voltage-gated calcium channel alpha1 subunits in the chinchilla cochlea. Cell Tissue Res. 2003, 313, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Waka, N.; Knipper, M.; Engel, J. Localization of the calcium channel subunits Cav1.2 (alpha1C) and Cav2.3 (alpha1E) in the mouse organ of Corti. Histol. Histopathol. 2003, 18, 1115–1123. [Google Scholar] [CrossRef]
- Platzer, J.; Engel, J.; Schrott-Fischer, A.; Stephan, K.; Bova, S.; Chen, H.; Zheng, H.; Striessnig, J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell 2000, 102, 89–97. [Google Scholar] [CrossRef]
- Lundt, A.; Soós, J.; Seidel, R.; Henseler, C.; Müller, R.; Raj Ginde, V.; Imran Arshaad, M.; Ehninger, D.; Hescheler, J.; Sachinidis, A.; et al. Functional implications of Cav 2.3 R-type voltage-gated calcium channels in the murine auditory system-novel vistas from brainstem-evoked response audiometry. Eur. J. Neurosci. 2020, 51, 1583–1604. [Google Scholar] [CrossRef]
- Randall, A.D.; Tsien, R.W. Contrasting biophysical and pharmacological properties of T-type and R-type calcium channels. Neuropharmacology 1997, 36, 879–893. [Google Scholar] [CrossRef]
- Weiss, N.; Zamponi, G.W. T-type calcium channels: From molecule to therapeutic opportunities. Int. J. Biochem. Cell Biol. 2019, 108, 34–39. [Google Scholar] [CrossRef]
- Smith, C.L.; Abdallah, S.; Wong, Y.Y.; Le, P.; Harracksingh, A.N.; Artinian, L.; Tamvacakis, A.N.; Rehder, V.; Reese, T.S.; Senatore, A. Evolutionary insights into T-type Ca2+ channel structure, function, and ion selectivity from the Trichoplax adhaerens homologue. J. Gen. Physiol. 2017, 149, 483–510. [Google Scholar] [CrossRef]
- Quinn, R.K.; Drury, H.R.; Cresswell, E.T.; Tadros, M.A.; Nayagam, B.A.; Callister, R.J.; Brichta, A.M.; Lim, R. Expression and Physiology of Voltage-Gated Sodium Channels in Developing Human Inner Ear. Front. Neurosci. 2021, 15, 733291. [Google Scholar] [CrossRef] [PubMed]
- Masetto, S.; Bosica, M.; Correia, M.J.; Ottersen, O.P.; Zucca, G.; Perin, P.; Valli, P. Na+ currents in vestibular type I and type II hair cells of the embryo and adult chicken. J. Neurophysiol. 2003, 90, 1266–1278. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rispoli, G.; Martini, M.; Rossi, M.L.; Mammano, F. Dynamics of intracellular calcium in hair cells isolated from the semicircular canal of the frog. Cell Calcium 2001, 30, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Laryushkin, D.P.; Maiorov, S.A.; Zinchenko, V.P.; Gaidin, S.G.; Kosenkov, A.M. Role of L-Type Voltage-Gated Calcium Channels in Epileptiform Activity of Neurons. Int. J. Mol. Sci. 2021, 22, 10342. [Google Scholar] [CrossRef] [PubMed]
- García, M.C.; Hernández-Gallegos, Z.; Escamilla, J.; Sánchez, J.A. Calciseptine, a Ca2+ channel blocker, has agonist actions on L-type Ca2+ currents of frog and mammalian skeletal muscle. J. Membr. Biol. 2001, 184, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Du, L.; Peterson, B.Z. Calcicludine Binding to the Outer Pore of L-type Calcium Channels is Allosterically Coupled to Dihydropyridine Binding. Biochemistry 2007, 46, 7590–7598. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Green, G.E.M.; Khan, K.M.; Beisel, D.W.; Drescher, M.J.; Hatfield, J.S.; Drescher, D.G. Calcium channel subunits in the mouse cochlea. J. Neurochem. 1996, 67, 37–45. [Google Scholar] [CrossRef]
- Tang, L.; Gamal El-Din, T.M.; Payandeh, J.; Martinez, G.Q.; Heard, T.M.; Scheuer, T.; Zheng, N.; Catterall, W.A. Structural basis for Ca2+ selectivity of a voltage-gated calcium channel. Nature 2014, 505, 56–61. [Google Scholar] [CrossRef]
- Tang, L.; Gamal El-Din, T.M.; Swanson, T.M.; Pryde, D.C.; Scheuer, T.; Zheng, N.; Catterall, W.A. Structural basis for inhibition of a voltage-gated Ca2+ channel by Ca2+ antagonist drugs. Nature 2016, 537, 117–121. [Google Scholar] [CrossRef]
- Sakipov, S.; Sobolevsky, A.I.; Kurnikova, M.G. Ion Permeation Mechanism in Epithelial Calcium Channel TRVP6. Sci. Rep. 2018, 8, 5715. [Google Scholar] [CrossRef]
- Doyle, D.A.; Morais Cabral, J.H.; Pfuetzner, R.A.; Kuo, A.; Gulbis, J.M.; Cohen, S.L.; Chait, B.T.M.; MacKinnon, R. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 1998, 280, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.; Gheri, A.; Pusch, M.; Moran, O. Use dependence of tetrodotoxin block of sodium channels: A revival of the trapped-ion mechanism. Biophys. J. 1996, 71, 1295–1312. [Google Scholar] [CrossRef]
- Martini, M.; Rossi, M.L.; Farinelli, F.; Moriondo, A.; Mammano, F.; Rispoli, G. No evidence for calcium electrogenic exchanger in frog semicircular canal hair cells. Eur. J. Neurosci. 2002, 16, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.purdue.edu/agsoftware/winmax/ (accessed on 28 February 2022).

| 4 mM Ca2+ | 10 nM Ca2+ | 4 mM Ca2+ | 10 nM Ca2+ | |||||
|---|---|---|---|---|---|---|---|---|
| L + R2 (n = 21) | R2 * (n = 11) | L + R2 (n = 21) | R2 * (n = 8) | L + R1 + R2 (n = 9) | R1 + R2 * (n = 6) | L + R1 + R2 (n = 9) | R1 + R2 * (n = 6) | |
| τon (ms) | 0.70 ± 0.06 | 0.75 ± 0.11 | 0.36 ± 0.02 | 0.38 ± 0.03 | 0.39 ± 0.12 | 0.40 ± 0.04 | 0.37 ± 0.04 | 0.28 ± 0.04 |
| τoff1 (ms) | 1.18 ± 0.16 | 0.36 ± 0.02 | 0.89 ± 0.19 | 0.29 ± 0.02 | 0.32 ± 0.08 | |||
| τoff2 (ms) | 9.1 ± 1.1 | 5.8 ± 0.5 | 12.0 ± 1.8 | 6.3 ± 1.0 | 10.6 ± 1.0 | 6.6 ± 1.6 | 11.6 ± 1.9 | 5.8 ± 1.7 |
| Peak (pA) | 156 ± 14 | 421 ± 80 | 144 ± 31 | 317 ± 71 | ||||
| Plateau (pA) | 95.8 ± 7.8 a | 39.4 ± 4.1 b | 457 ± 36 c | 100 ± 8.0 d | 359 ± 66 e | 95 ± 22 f | 1250 ± 140 | 207 ± 62 g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martini, M.; Rispoli, G. Cation Permeability of Voltage-Gated Hair Cell Ca2+ Channels of the Vertebrate Labyrinth. Int. J. Mol. Sci. 2022, 23, 3786. https://doi.org/10.3390/ijms23073786
Martini M, Rispoli G. Cation Permeability of Voltage-Gated Hair Cell Ca2+ Channels of the Vertebrate Labyrinth. International Journal of Molecular Sciences. 2022; 23(7):3786. https://doi.org/10.3390/ijms23073786
Chicago/Turabian StyleMartini, Marta, and Giorgio Rispoli. 2022. "Cation Permeability of Voltage-Gated Hair Cell Ca2+ Channels of the Vertebrate Labyrinth" International Journal of Molecular Sciences 23, no. 7: 3786. https://doi.org/10.3390/ijms23073786
APA StyleMartini, M., & Rispoli, G. (2022). Cation Permeability of Voltage-Gated Hair Cell Ca2+ Channels of the Vertebrate Labyrinth. International Journal of Molecular Sciences, 23(7), 3786. https://doi.org/10.3390/ijms23073786





