Decoding the Bell-Shaped Calcium Spikes in Phosphorylation Cycles of Flagella
Abstract
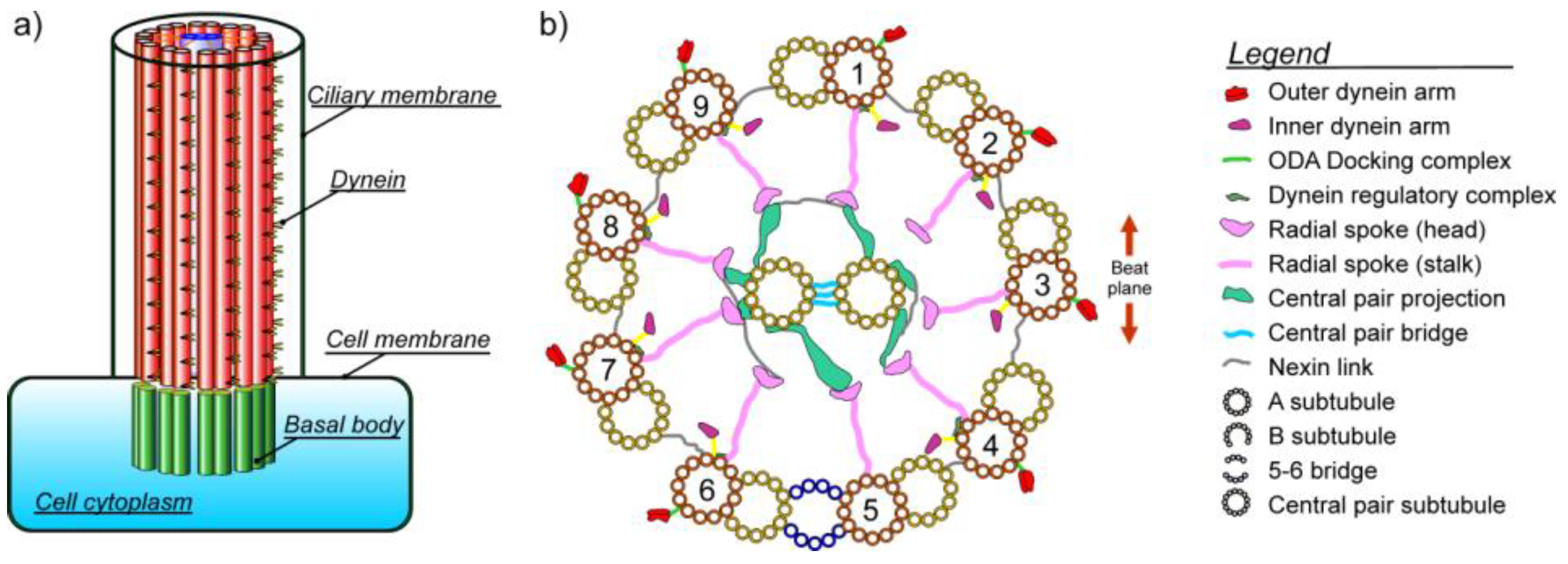
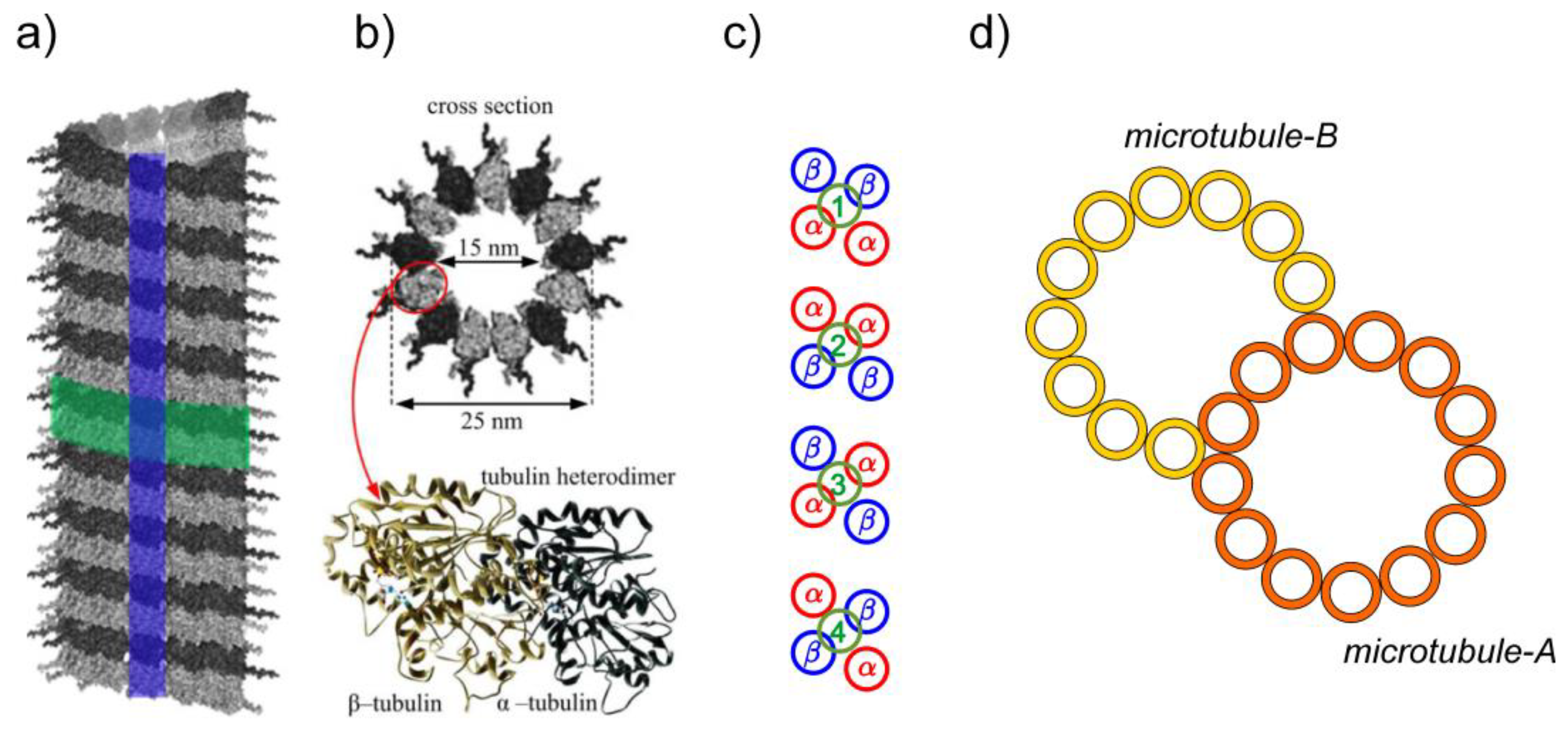
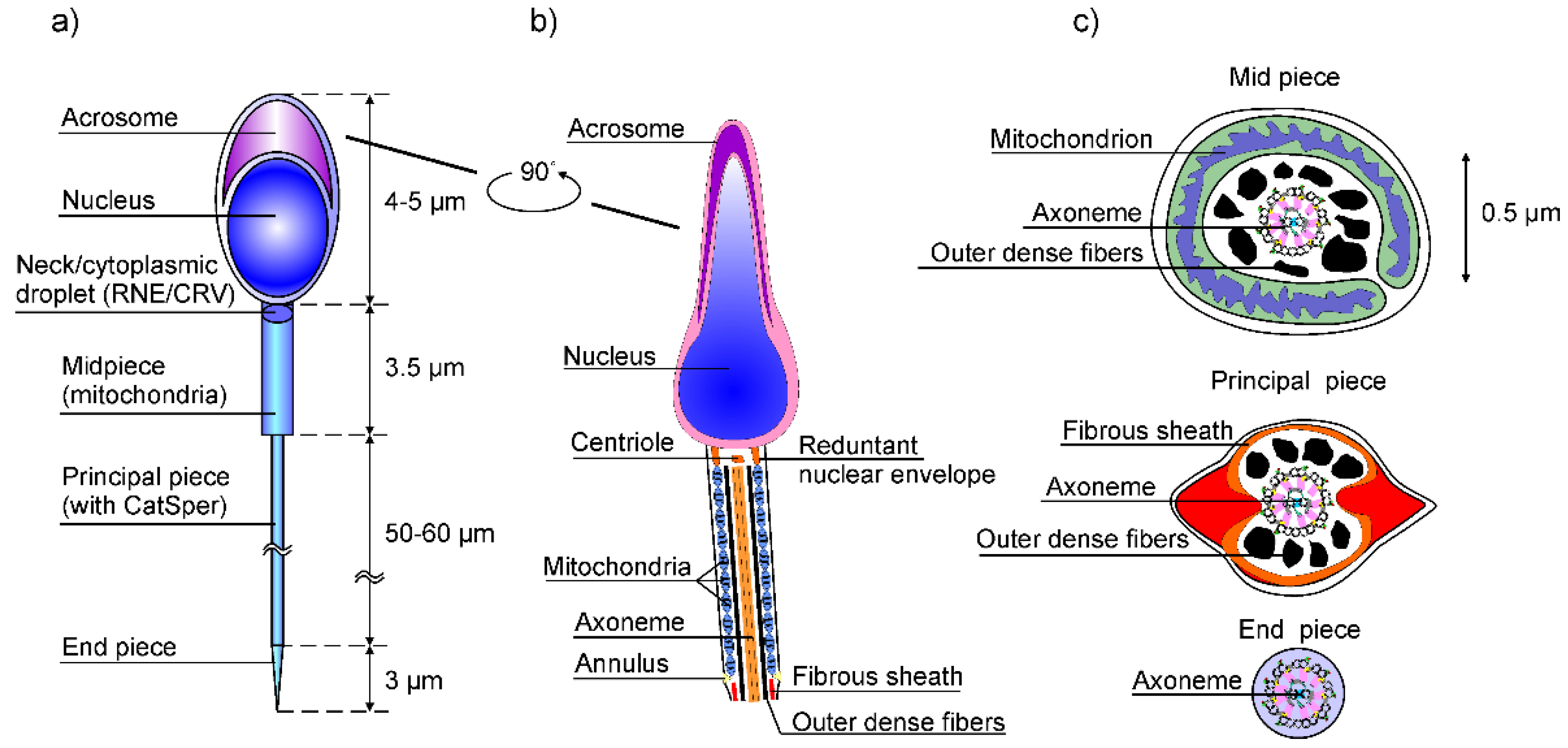
1. Introduction
1.1. Microtubules
1.2. Dyneins
1.3. Radial Spokes
1.4. Bending of Axoneme
2. Principles of Calcium Signaling
3. Calcium Signaling in Flagella
- In the case of the human sperm, the signaling is triggered by a stimulus (progesterone or nitrogen monoxide -NO) that generates Ca2+ signal through Cat-sper channels;
- It activates the ON mechanism that feeds Ca2+ into flagellum and sperm head. Since flagella are thin cylinders with a very large surface-to-volume ratio [16], these Ca2+ fluxes are efficiently injected into the cytosol.
- The pulses of Ca2+ ions function as messengers, which stimulate several axonemal proteins, primarily CaM, calaxin and IC138 of IDA-I1, to perform the control of dyneins and to modulate flagellary beats [17].
- Eventually the OFF mechanism, composed of pumps and ionic exchangers, removes Ca2+ from the cytoplasm to internal Ca2+ stores and buffers, as well as out of flagella, in order to restore the resting state [18].
4. The Principal Sensors for Ca2+ Signals in Flagella
5. Mechanism for Propagation of Intracellular Calcium Waves—The Particular Aspect of Flagellar Waves
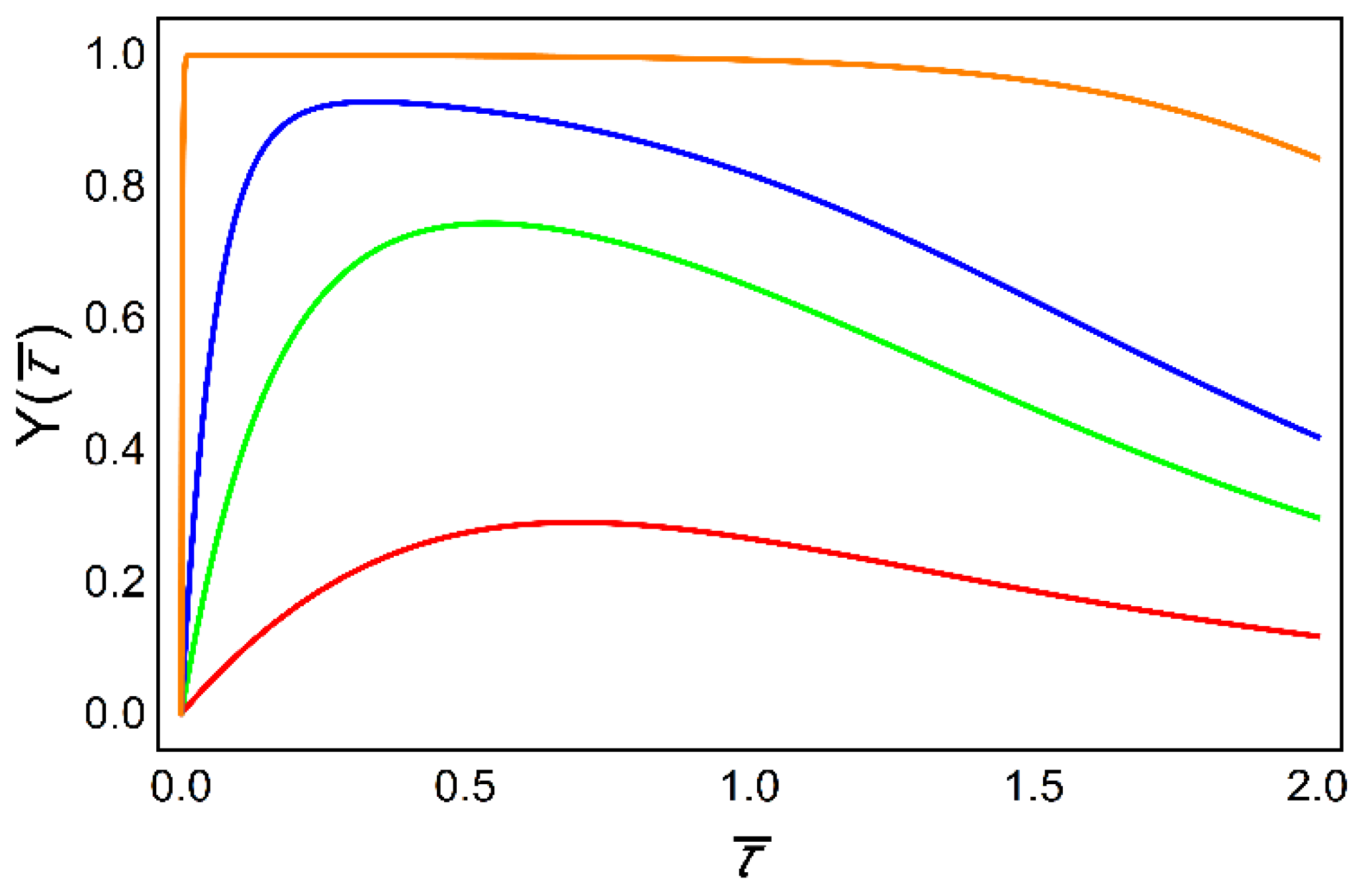
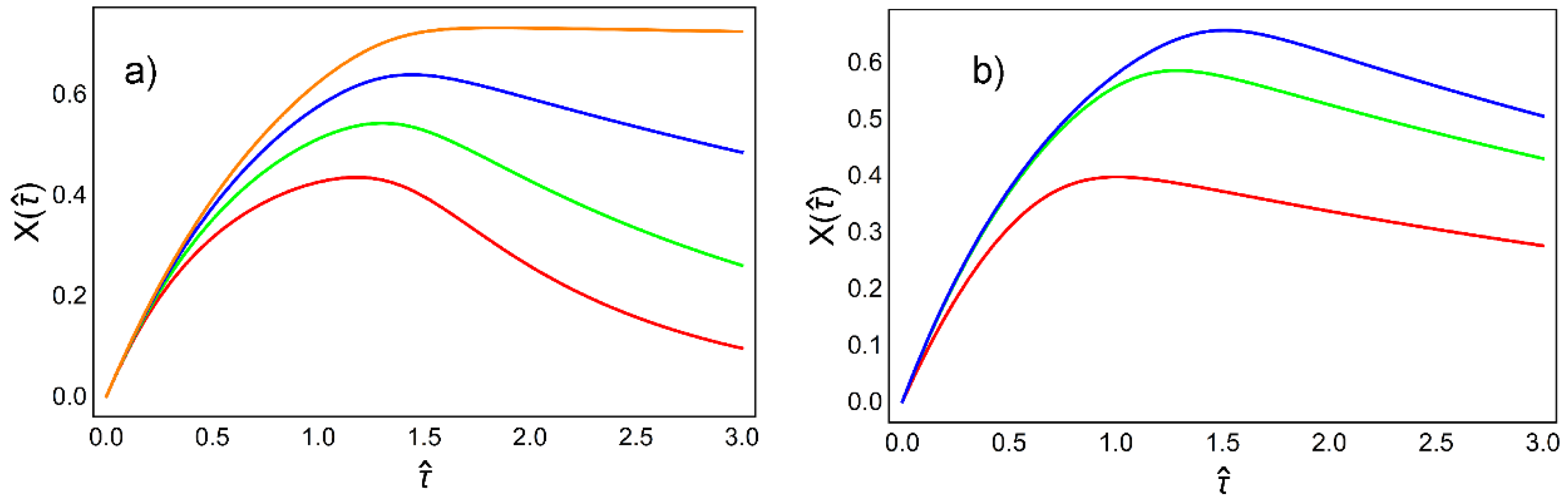
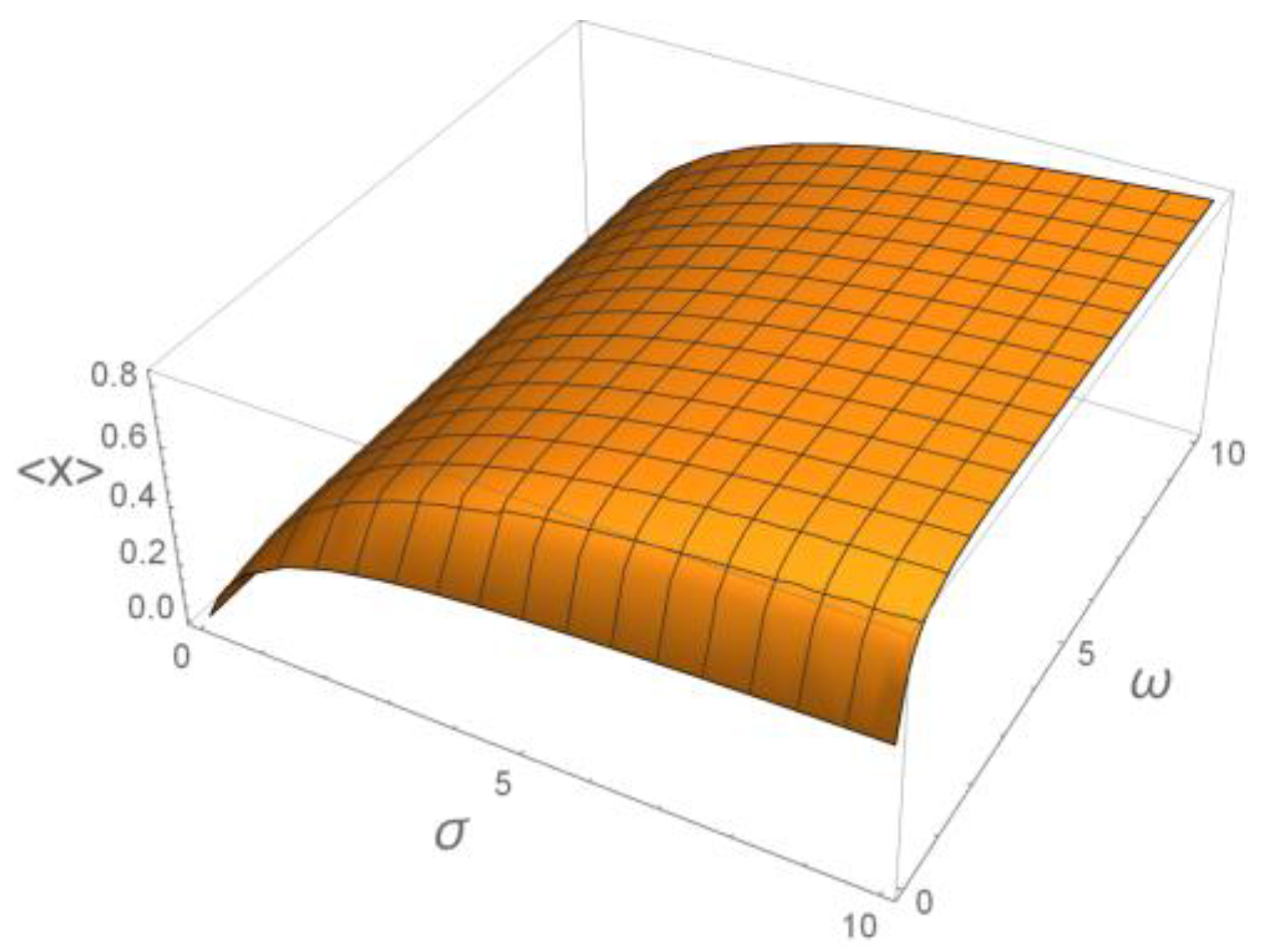
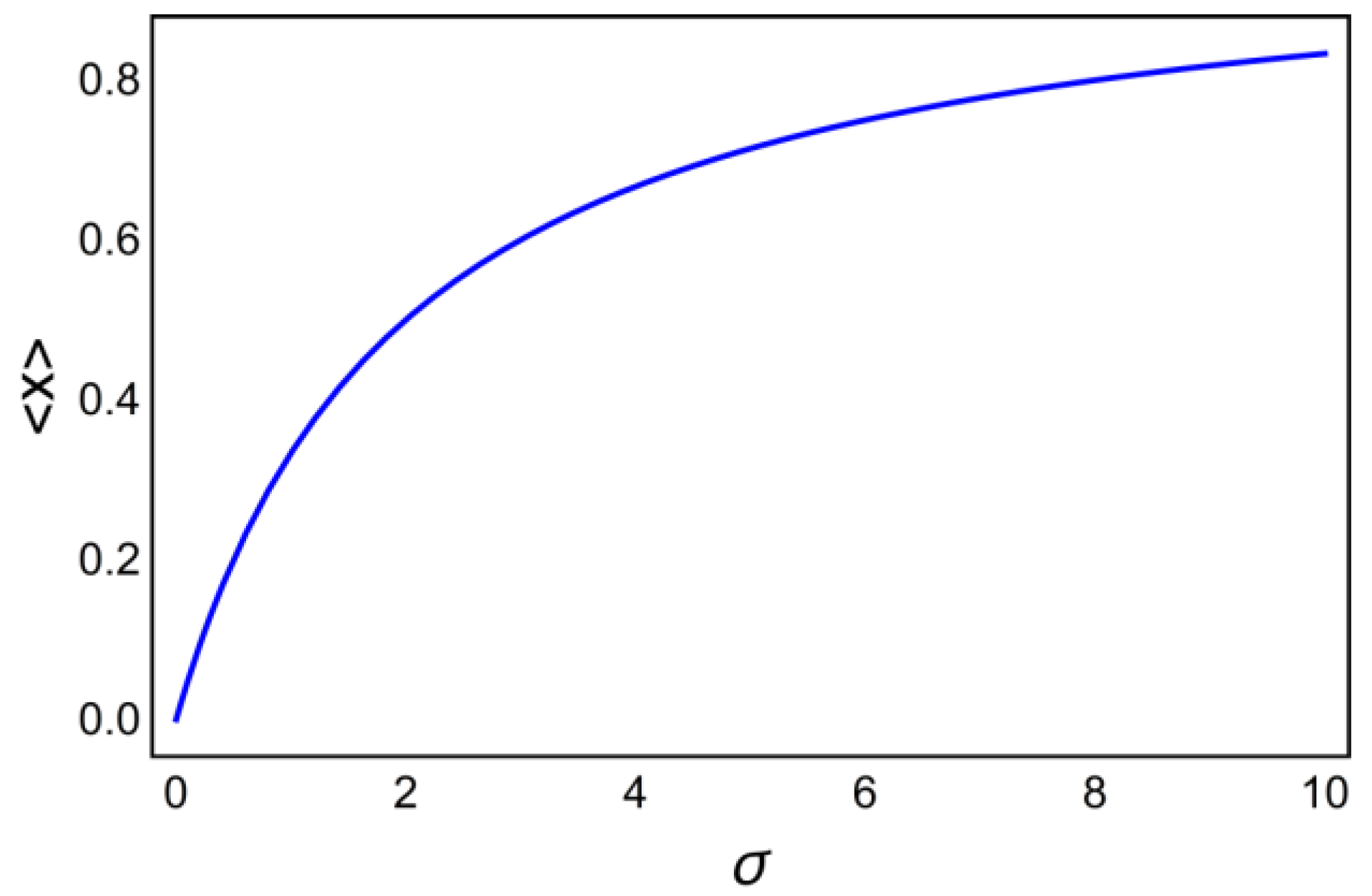
6. Decoding of Axonemal “Ca2+ Clouds” by Phosphorylation Cycles
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MT | microtubule |
| MTD | microtubule doublet |
| CP | central pair |
| RS | radial spoke |
| ATP | adenosine triphosphate |
| IDA | inner dynein arm |
| ODA | outer dynein arm |
| DHC | dynein heavy chain |
| LC | light chain |
| IC | intermediate chain |
| CaM | calmodulin |
| MIA | modifier of IDA |
| CSC | spoke-associated complex |
| N-DRC | nexin-dynein regulatory complex |
| ER | endoplasmic reticulum |
| SR | sarcoplasmic reticulum |
| CICR | calcium induced calcium release |
| PKC | protein kinase C |
| IP3 | inositol triphosphate |
| IP3R | inositol triphosphate receptor |
| CICI | calcium induced calcium influx |
| CTT | carboxyl terminal tail |
References
- Satir, P.; Christiansen, S.T. Overview of structure and function of mammalian cilia. Annu. Rev. Physiol. 2007, 69, 377–400. [Google Scholar] [CrossRef] [PubMed]
- Satarić, M.V.; Zdravković, S.; Nemeš, T.; Satarić, B.M. Calcium signaling modulates the dynaimcs of cilia and flagella. Eur. Biophys. J. 2020, 49, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T. Axoneme structure from motile cilia. Cold Spring Harb. Perspect. Biol. 2017, 9, a028076. [Google Scholar] [CrossRef] [PubMed]
- Stepanek, L.; Pigino, G. Microtubule doublets are double-track railways for intraflagellar transport trains. Science 2016, 352, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, R.; Yagi, T. Functional diversity of axonemal dyneins as assessed by in vitro and in vivo motility assays of Chlamydomonas mutants. Zool. Sci. 2014, 31, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Viswanadha, R.; Sale, W.S.; Porter, M.E. Ciliar Motility: Regulation of axonemal dynein motors. Cold Spring Harb. Perspect. Biol. 2017, 9, a018325. [Google Scholar] [CrossRef]
- Lindemann, C.B.; Leich, K.A. Flagellar and ciliary beating: The proven and the possible. J. Cell Sci. 2010, 123, 519–528. [Google Scholar] [CrossRef]
- King, S.M.; Sale, W.S. Fifty years of microtubule sliding in cilia. Mol. Biol. Cell 2018, 29, 698–701. [Google Scholar] [CrossRef]
- Bootman, M.D.; Collins, T.J.; Peppiatt, C.M.; Prothero, L.S.; MacKenzie, L.; De Smet, P.; Travers, M.; Tovey, S.C.; Seo, J.T.; Berridge, M.J.; et al. Calcium signaling—An overview. Semin. Cell. Dev. Biol. 2001, 12, 3–10. [Google Scholar] [CrossRef]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versality and universality of calcium signalling. Net. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef]
- Burgoyne, R.D. Neuronal calcium sensor proteins: Generating diversity in neuronal Ca2+ signaling. Nat. Rev. Neurosci. 2007, 8, 182–193. [Google Scholar]
- Srivastava, N.; Pande, M. Mitochondrion: Features, functions and comparative analysis of specific probes in detecting sperm cell damages. Asian. Pac. J. Rep. 2016, 5, 445–452. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Z.; Ping, P.; Wang, G.; Yuan, X.; Sun, F. Outer dense fibers stabilize the axoneme to maintain sperm motility. J. Cell Mol. Med. 2018, 22, 1755–1768. [Google Scholar] [CrossRef]
- Jouaville, L.S.; Ichas, F.; Holmuhamedov, E.L.; Camacho, P.; Lechleiter, J.D. Synchronization of calcium waves by mitochondrial substrates in Xenopus laevis oocytes. Nature 1995, 377, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Inaba, K. Calcium sensors of ciliary outer arm dynein; functions and phylogenic considerations for eukaryotic evolution. Cilia 2015, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Satarić, M.V.; Nemeš, T.; Sekulić, D.; Tuszynski, J.A. How signals of calcium ions initiate the beats of cilia and flagella. BioSystems 2019, 182, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Shiba, K.; Okai, M.; Takahashi, Y.; Shitaka, Y.; Oiwa, K.; Tanokura, M.; Inaba, K. Calaxin drives sperm chemotaxis by Ca2+ medicated direct modulation of a dynein motor. Proc. Natl. Acad. Sci. USA 2012, 109, 20497–20502. [Google Scholar] [CrossRef] [PubMed]
- Lishko, P.V.; Botchkina, I.L.; Kirichok, Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature 2011, 471, 387–391. [Google Scholar] [CrossRef]
- Publicover, S.; Harper, C.V.; Barratt, C. Ca2+ signaling in sperm--making the most of what you’ve got. Nat. Cell Biol. 2007, 9, 235–242. [Google Scholar] [CrossRef]
- Darszon, A.; Nishikagi, T.; Beltran, C.; Treviño, C.L. Calcium channels in the development, maturation and function of spermatozoa. Physiol. Rev. 2011, 91, 1305–1355. [Google Scholar]
- Satarić, M.V.; Sekulić, D.; Živanov, M. Solitonic ionic currents along microtubules. J. Comput. Theor. Nanosci. 2010, 7, 1–10. [Google Scholar] [CrossRef]
- White, D.; de Lamirande, E.; Gagnon, C. Protein kinase C is an important signaling mediator associated with motility of intact sea urchin spermatozoa. J. Exp. Biol. 2007, 210, 4053–4064. [Google Scholar] [CrossRef] [PubMed]
- Wargo, M.J.; Dymek, E.E.; Smith, E.F. Calmodulin and PF6 are components of a complex that localizes to the C1 microtubule of the flagellar central apparatus. J. Cell Sci. 2005, 118, 4655–4665. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Igushi, A.; Takemura, A. Roles of calmodulin and calcium/calmodulin-dependent protein kinase in flagellar motility regulation in the coral Acropora digitifera. Mar. Biotechnol. 2009, 11, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Shiba, K.; Baba, S.A.; Fujiwara, E.; Inaba, K. Calaxin is essential for the transmission of Ca2+ dependent asymmetric waves in sperm flagella. bioRxiv 2020, 109, 50. [Google Scholar] [CrossRef]
- Gilkey, J.C.; Jaffe, L.F.; Ridgway, E.B.; Reynolds, G.T. A free calcium wave traverses the activating egg of the medaka, Oryzias latipes. J. Cell Biol. 1978, 76, 448–466. [Google Scholar] [CrossRef]
- Jaffe, L.F. Fast calcium waves. Cell Calcium 2010, 48, 102–113. [Google Scholar] [CrossRef]
- Atri, A.; Amundson, J.; Clapham, D.; Sneyd, J. A single-pool model for intracellular calcium oscillations and waves in the Xenopus laevis oocyte. Biophys. J. 1993, 65, 1727–1739. [Google Scholar] [CrossRef]
- Keller, M.; Kao, J.P.Y.; Egger, M.; Niggli, E. Calcium waves driven by “sensitization” wave-fronts. Cardiovasc. Res. 2007, 74, 39–45. [Google Scholar] [CrossRef]
- Jaffe, L.F. Stretch-activated calcium channels relay fast calcium waves propagated by calcium-induced calcium influx. Biol. Cell 2007, 99, 175–184. [Google Scholar] [CrossRef]
- Huang, J.B.; Kindzelskii, A.L.; Clark, A.J.; Petty, H.R. Identification of calcium channels promoting calcium spikes and waves in HT1080 tumor cells: Their apparent roles in cell motility and invasion. Cancer Res. 2004, 64, 2482–2489. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, S.T.; Schëväert, D.; Swan, M.A.; Mortimer, D. Quantitative observations of flagellar motility of capacitating human spermatozoa. Hum. Reprod. 1997, 12, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Ishijima, S.; Baba, S.A.; Mohri, H.; Suarez, S.S. Quantitative analysis of flagellar movement in hyperactivated and acrosome-reacted golden hamster spermatozoa. Mol. Reprod. Dev. 2002, 61, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Satarić, M.V.; Ilić, D.I.; Ralević, N.; Tuszynski, J.A. A nonlinear model of ionic wave propagation along microtubules. Eur. Biophys. J. 2009, 38, 637–647. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Wood, C.D.; Darszon, A.; Whitaker, M. Speract induces calcium oscillations in the sperm tail. J. Cell Biol. 2003, 161, 89–101. [Google Scholar] [CrossRef]
- Dolmetsch, R.E.; Lewis, R.S.; Goodnow, C.C.; Healy, J.I. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 1997, 386, 855–858. [Google Scholar] [CrossRef]
- Reither, G.; Schaefer, M.; Lipp, P. PKCα: A versatile key for decoding the cellular calcium toolkit. J. Cell Biol. 2006, 174, 521–533. [Google Scholar] [CrossRef]
- Dupont, G.; Houart, G.; De Konick, P. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations: A simple model. Cell Calcium 2003, 34, 485–497. [Google Scholar] [CrossRef]
- Schingmann, K.; Michaut, M.A.; McElwee, J.L.; Wolf, C.; Travis, A.J.; Turner, R.M. Calmodulin and CaMKII in the sperm Principal Piece: Evidence for a Motility-Related Calcium/Calmodulin Pathway. J. Androl. 2007, 28, 706–716. [Google Scholar] [CrossRef]
- Hunley, C.; Marucho, M. Electrical propagation of condensed and diffuse ions along actin filaments. bioRxiv 2021, 50, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Zahran, E.H.M. Exact travelling wave solutions of nano-ionic solitons and nano-ionic current of microtubules using the -expansion method. Adv. Nanoparticles 2015, 4, 25–36. [Google Scholar] [CrossRef][Green Version]
- Sirisubtawee, S.; Koonprasert, S. Exact traveling wave solutions of certain nonlinear partial differential equations using the -expansion method, Hindawi. Adv. Theor. Math. Phys. 2018, 2018. [Google Scholar] [CrossRef]
- Kayum, M.A.; Seadawy, A.R.; Akbar, A.M.; Sugati, T.G. Stable solutions to the nonlinear RLC transmission line equation and the Sinh-Poisson equation arising in Mathematical Physics. Open Phys. 2020, 18, 710–725. [Google Scholar] [CrossRef]
- Koldenkova, V.P.; Nagai, T. Genetically encoded Ca2+ indicators; Properties and evaluation. Biochem. Biophys. Acta 2013, 1833, 1787–1797. [Google Scholar] [CrossRef]
- Salazar, C.; Politi, A.Z.; Höfer, T. Decoding of calcium oscillations by phosphorylation cycles: Analytic results. Biophys. J. 2008, 94, 1203–1215. [Google Scholar] [CrossRef]
- Böhmer, M.; Van, Q.; Weyand, I.; Hagen, V.; Beyermann, M.; Matsumoto, M.; Hoshi, M.; Hildebrand, E.; Kaupp, U.B. Ca2+ spikes in the flagellum control chemotactic behavior of sperm. EMBO J. 2005, 24, 2741–2752. [Google Scholar] [CrossRef]
- Priego-Espinosa, D.A.; Darszon, A.; Guerrero, A.; González-Cota, A.L.; Nishikagi, T.; Martinez-Mekler, G.; Carneiro, J. Modular analysis of the control of flagellar Ca2+-spike trains produced by CatSper and CaV channels in sea urchin sperm. PLoS Comput. Biol. 2020. [Google Scholar] [CrossRef]



| Our Model (Satarić et al.) | Estimated Speeds [μm/s] | Experimental Evidence | Experimental Speed [μm/s] |
|---|---|---|---|
| 2009 [34] | 6000-overestimated | / | / |
| 2010 [21] | 160–240 | Huang et al. [31] | 100 |
| 2019 [16] | 530 | Mortimer et al. [32] | 500 |
| 2020 [2] | 620 | Ishijima et al. [33] | 700 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satarić, M.; Nemeš, T.; Tuszynski, J. Decoding the Bell-Shaped Calcium Spikes in Phosphorylation Cycles of Flagella. Int. J. Mol. Sci. 2022, 23, 3760. https://doi.org/10.3390/ijms23073760
Satarić M, Nemeš T, Tuszynski J. Decoding the Bell-Shaped Calcium Spikes in Phosphorylation Cycles of Flagella. International Journal of Molecular Sciences. 2022; 23(7):3760. https://doi.org/10.3390/ijms23073760
Chicago/Turabian StyleSatarić, Miljko, Tomas Nemeš, and Jack Tuszynski. 2022. "Decoding the Bell-Shaped Calcium Spikes in Phosphorylation Cycles of Flagella" International Journal of Molecular Sciences 23, no. 7: 3760. https://doi.org/10.3390/ijms23073760
APA StyleSatarić, M., Nemeš, T., & Tuszynski, J. (2022). Decoding the Bell-Shaped Calcium Spikes in Phosphorylation Cycles of Flagella. International Journal of Molecular Sciences, 23(7), 3760. https://doi.org/10.3390/ijms23073760







