A Focus on Enterochromaffin Cells among the Enteroendocrine Cells: Localization, Morphology, and Role
Abstract
1. Introduction
2. Enteroendocrine Cells
2.1. Role of Enteroendocrine Cells in the Brain-Gut Axis
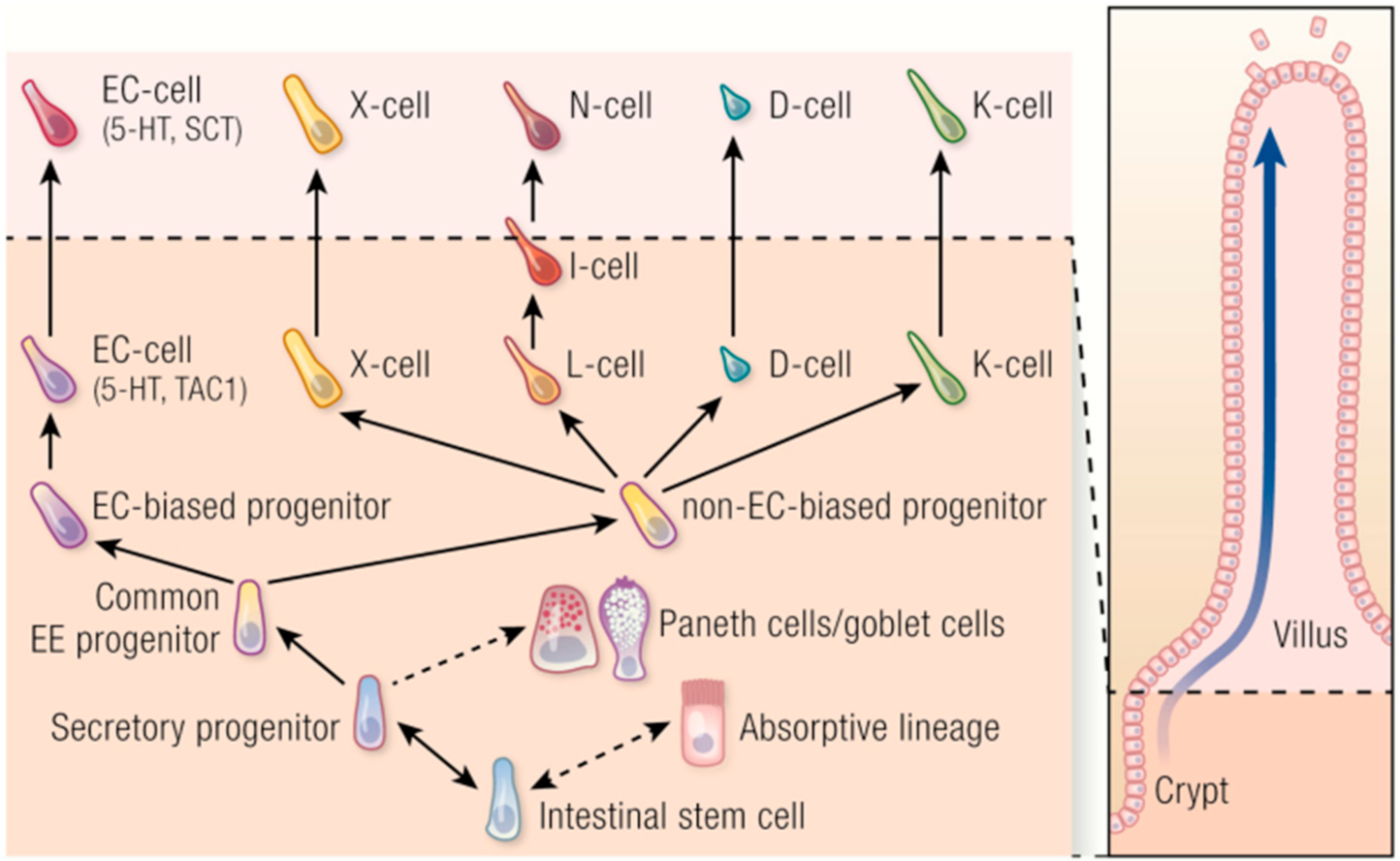
2.2. Enteroendocrine Cells and Plasticity
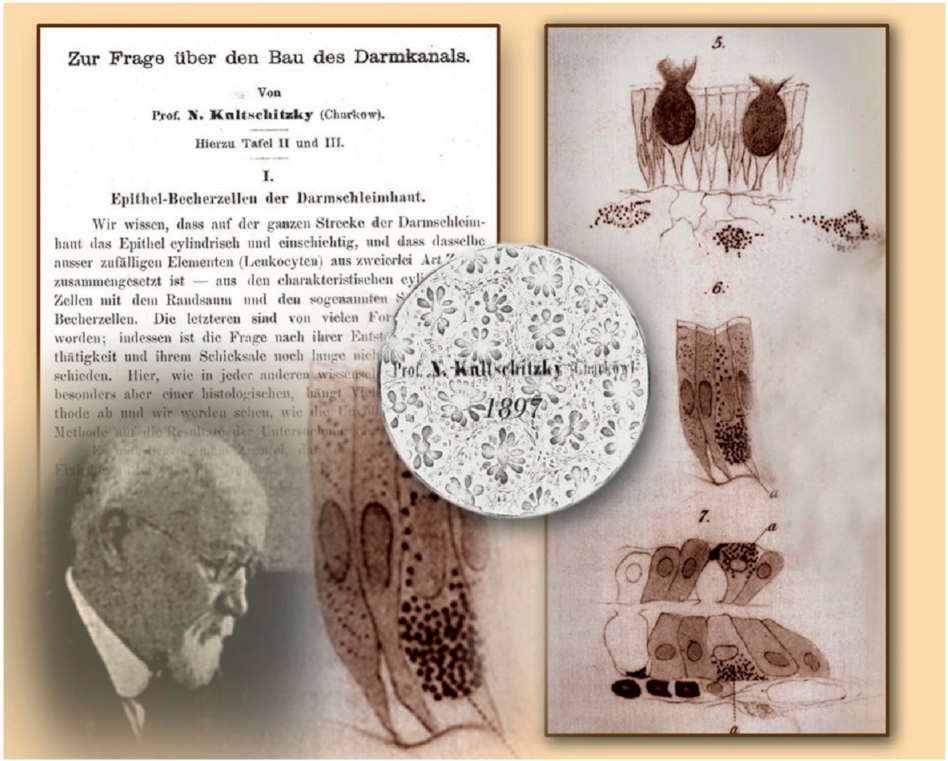
3. History of Enterochromaffin Cells
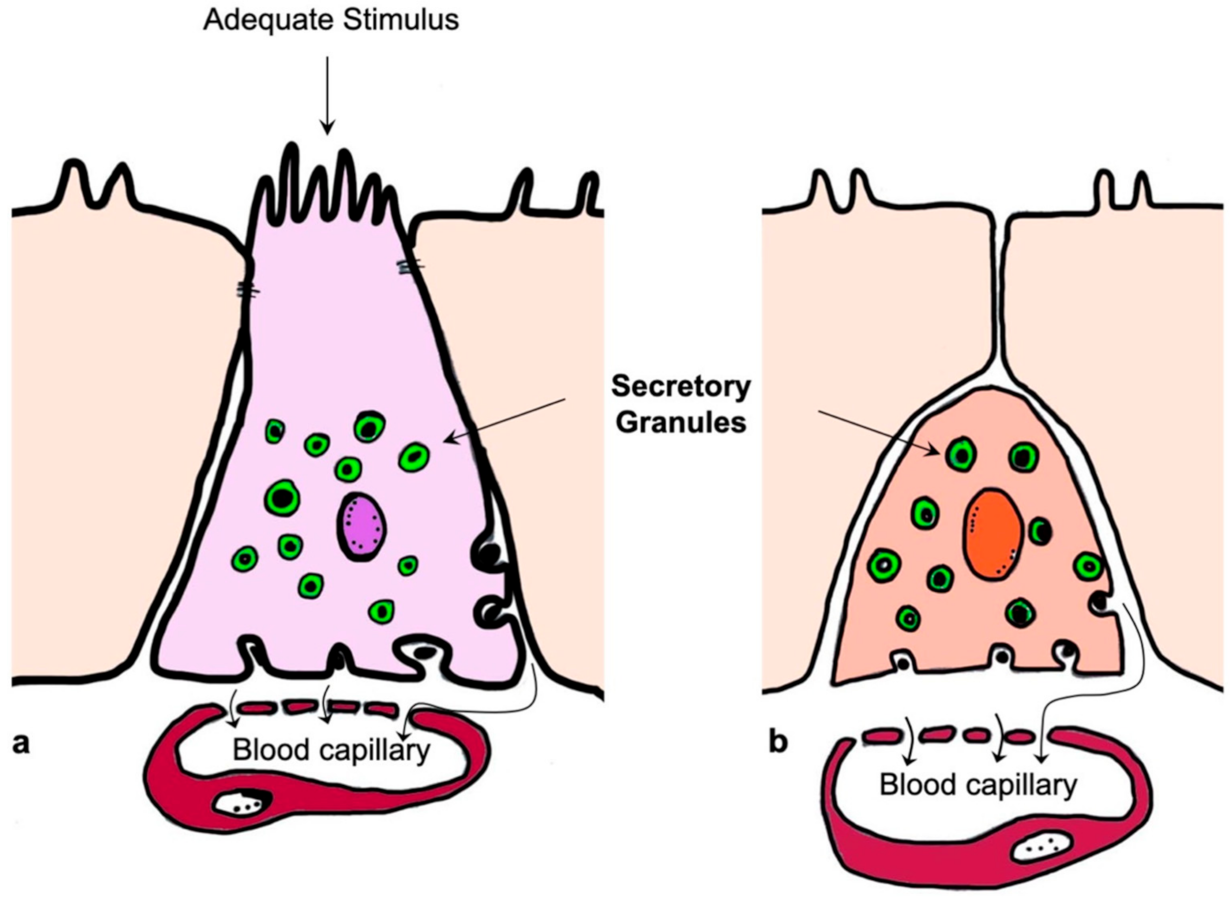
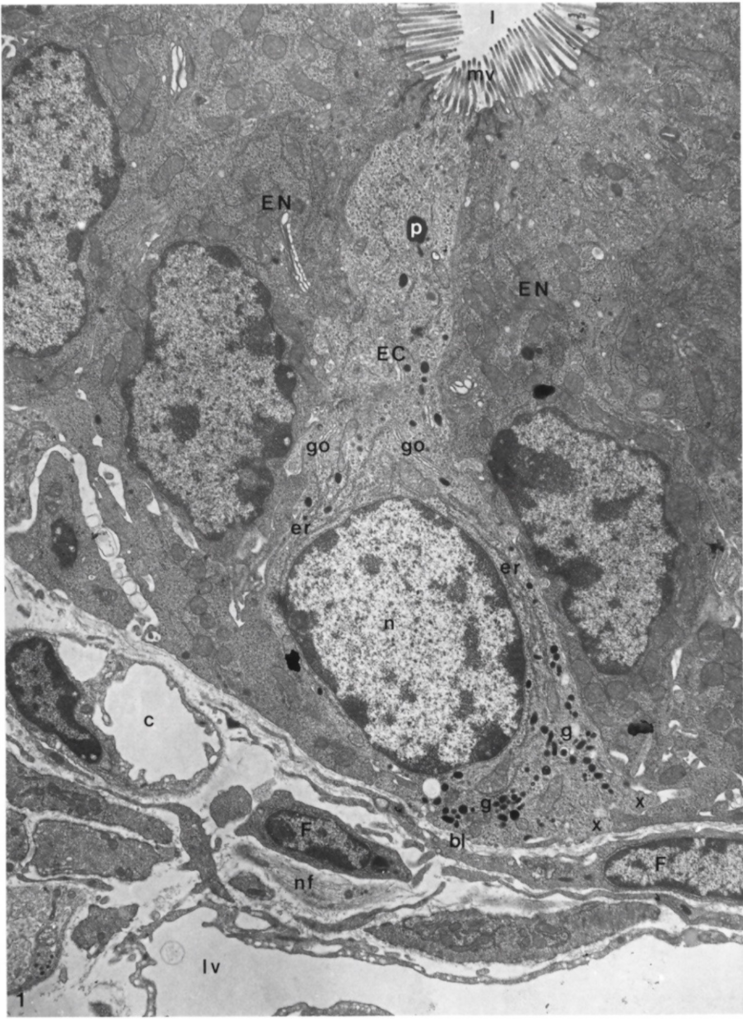
4. Ultrastructural Features of Enterochromaffin Cells and Their Bipolarity
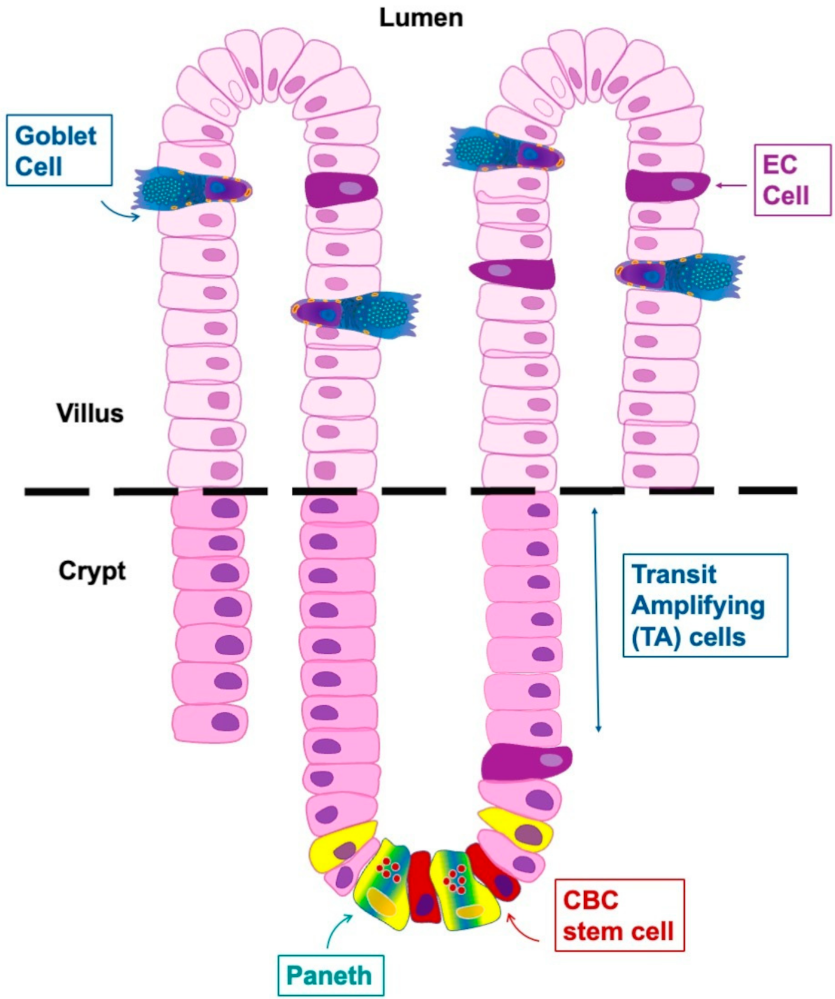
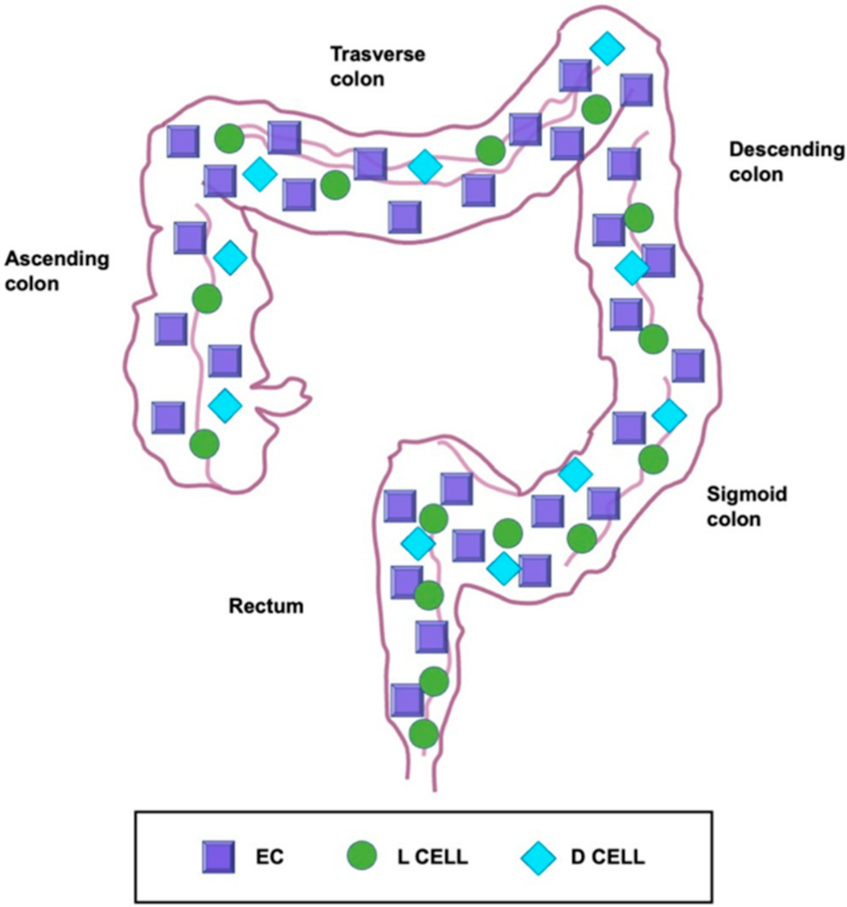
5. Localization of Enterochromaffin Cells
6. Heterogeneity of Enterochromaffin Cells
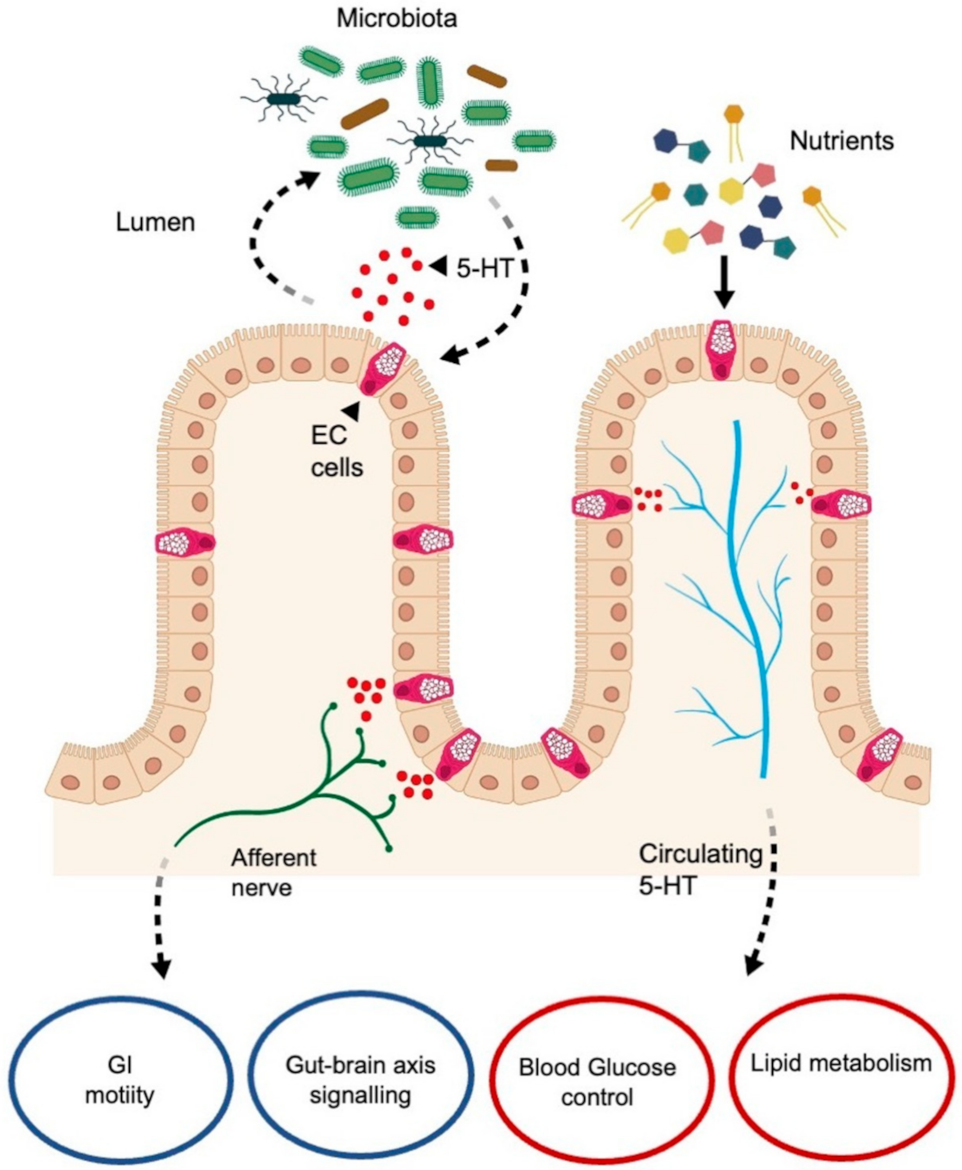
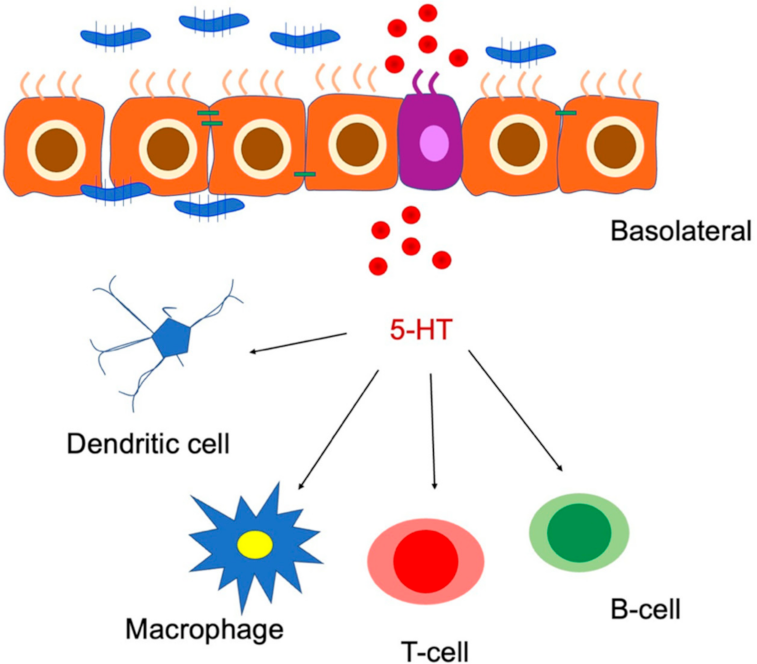
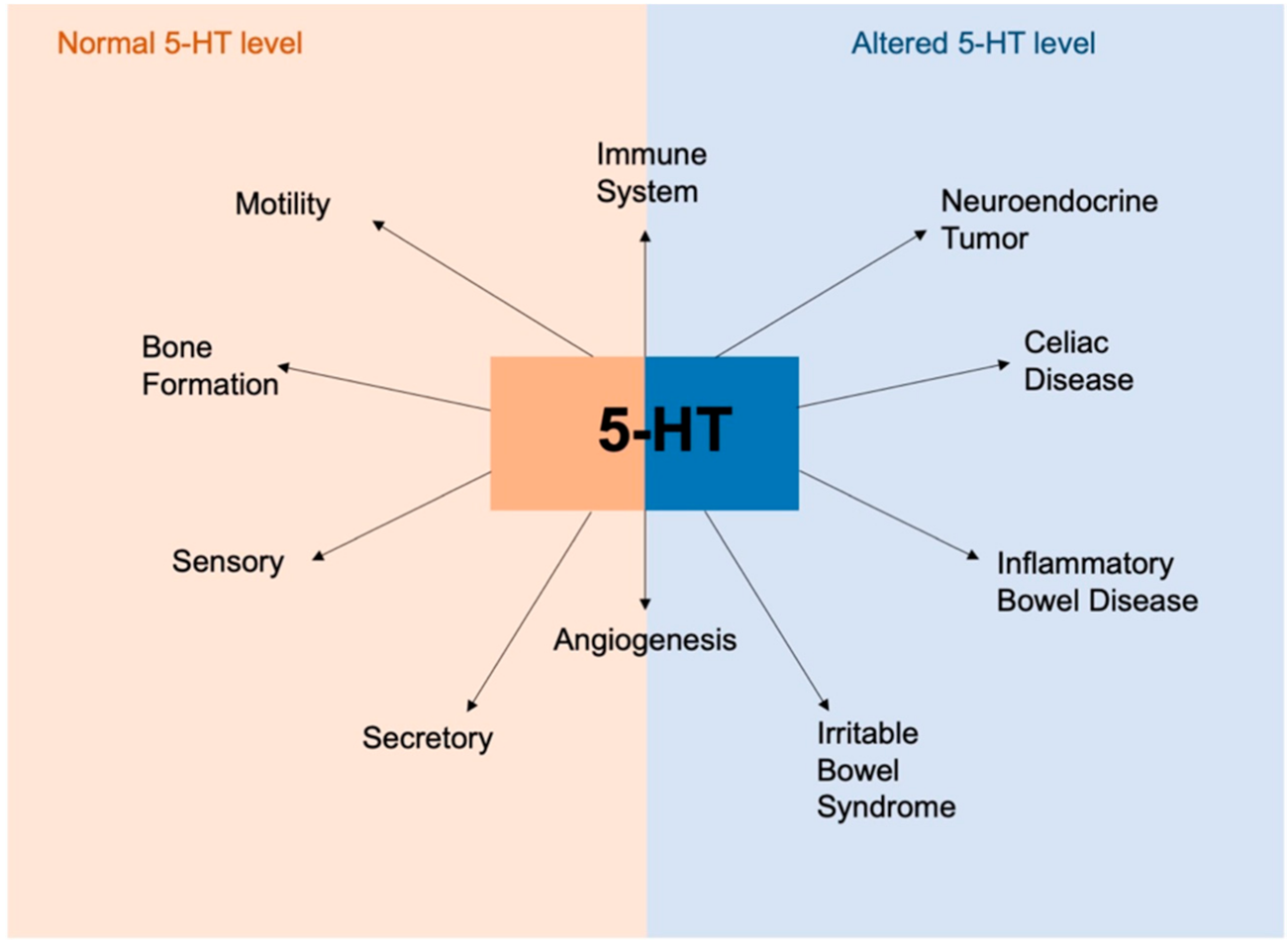
6.1. Serotonin in Enterochromaffin Cells
Serotonin–Gut Microbiota Interaction
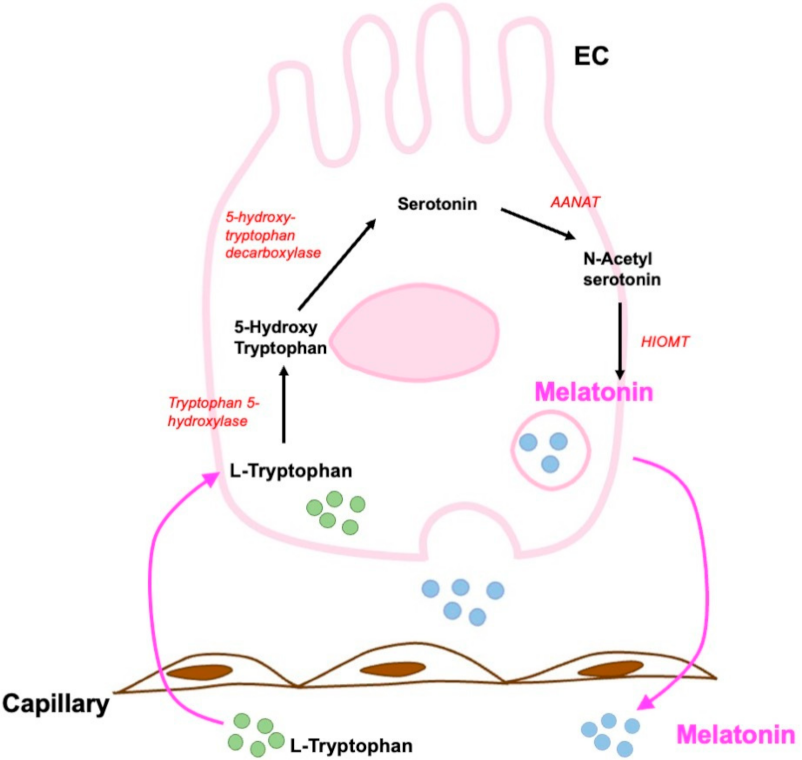
6.2. Melatonin and Enterochromaffin Cells
Role of Melatonin in Gastrointestinal Tract
6.3. Substance P and Enterochromaffin Cells
7. Enterochromaffin Cells and Diet in Pathology
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Öhman, L.; Törnblom, H.; Simrén, M. Crosstalk at the mucosal border: Importance of the gut microenvironment in IBS. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 36–49. [Google Scholar] [CrossRef]
- Bellono, N.W.; Bayrer, J.R.; Leitch, D.B.; Castro, J.; Zhang, C.; O’Donnell, T.; Brierley, S.M.; Ingraham, H.A.; Julius, D. Enterochromaffin Cells are Gut Chemosensors that Couple to Sensory Neural Pathway. Cell 2017, 170, 185.e16–198.e16. [Google Scholar] [CrossRef] [PubMed]
- Beumer, J.; Gehart, H.; Clevers, H. Enteroendocrine Dynamics- New Tools Reveal Hormonal Plasticity in the Gut. Endocr. Rev. 2020, 41, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Brierley, S.M.; Linden, D.R. Neuroplasticity and disfunction after gastrointestinal inflammation. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Gribble, F.M.; Reimann, F. Enteroendocrine Cells: Chemosensors in the Intestinal Epithelium. Annu. Rev. Physiol. 2016, 78, 277–299. [Google Scholar] [CrossRef] [PubMed]
- Mazzawi, T.; El-Salhy, M. Effect of diet and individual dietary guidance on gastrointestinal endocrine cells in patients with irritable bowel syndome (Review). Int. J. Mol. Med. 2017, 40, 943–952. [Google Scholar] [CrossRef]
- Rehfeld, J.F. A centenary of gastrointestinal endocrinology. Horm. Metab. Res. 2004, 36, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Latorre, R.; Sternini, C.; De Giorgio, R.; Greenwood-Van Meerveld, B. Enteroendocrine cells: A review of their role in brain-gut communication. Neurogastroenterol. Motil. 2016, 28, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Pearse, A.G.; Polak, J.M. Neural crest origin of the endocrine polypeptide (APUD) cells of the gastrointestinal tract and pancreas. Gut 1971, 12, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Ray, S.K.; Singh, N.K.; Johnston, B.; Leiter, A.B. Basic helix-loop-helix transcription factors and enteroendocrine cell differentiation. Diabetes Obes. Metab. 2011, 13 (Suppl. S1), 5–12. [Google Scholar] [CrossRef] [PubMed]
- Engelstoft, M.S.; Egerod, K.L.; Lund, M.L.; Schwartz, T.W. Enteroendocrine cell types revisited. Curr. Opin. Pharmacol. 2013, 13, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.A.; Sun, E.W.; Martin, A.M.; Keating, D.J. The ever-changing roles of serotonin. Int. J. Biochem. Cell Biol. 2020, 125, 105776. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.M.; Namkung, J.; Go, Y.; Shong, K.E.; Kim, K.; Kim, H.; Park, B.Y.; Lee, H.W.; Jeon, Y.H.; Song, J.; et al. Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nat. Commun. 2015, 6, 6794. [Google Scholar] [CrossRef] [PubMed]
- Sundler, F.; Bouttcher, G.; Ekblad, E.; Haukanson, R. The Neuroendocrine System of the Gut. Acta Oncol. 1989, 28, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Beyak, M.J.; Bulmer, D.C.E.; Jiang, W.; Keating, C.; Rong, W.; Grundy, D. Extrinsic sensory afferent nerve innervating the gastrointestinal tract. In Physiology of the Gastrointestinal Tract, 4th ed.; Johnson, L.R., Barrett, K.E., Ghishan, F.K., Merchant, J.L., Said, H.M., Wood, J.D., Eds.; Elsevier: San Diego, CA, USA, 2006; pp. 685–726. [Google Scholar]
- Raybould, H.E. Gut Chemosensing: Interaction between gut endocrine cells and visceral afferents. Auton. Neurosci. 2010, 153, 41–46. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M. Possible role of intestinal stem cells in the pathophysiology of irritable bowel syndrome. World J. Gastroenterol. 2020, 26, 1427–1438. [Google Scholar] [CrossRef] [PubMed]
- Gehart, H.; van Es, J.H.; Hamer, K.; Beumer, J.; Kretzschmar, K.; Dekkers, J.F.; Rios, A.; Clevers, H. Identification of Enteroendocrine Regulators by Real-time Single-Cell Differentiation Mapping. Cell 2019, 176, 1158.e16–1173.e16. [Google Scholar] [CrossRef]
- Yan, K.S.; Gevaert, O.; Zheng, G.X.Y.; Anchang, B.; Probert, C.S.; Larkin, K.A.; Davies, P.S.; Cheng, Z.F.; Kaddis, J.S.; Han, A.; et al. Intestinal Enteroendocrine Lineage Cells Possess Homeostatic and Injury-inducible Stem Cell Activity. Cell Stem Cell 2017, 21, 78.e6–90.e6. [Google Scholar] [CrossRef]
- Modlin, I.M.; Kidd, M.; Pfragner, R.; Eick, G.N.; Champaneria, M.C. The functional characterization of normal and neoplastic human enterochromaffin cells. J. Clin. Endocrinol. Metab. 2006, 91, 2340–2348. [Google Scholar] [CrossRef] [PubMed]
- Haber, A.L.; Biton, M.; Rogel, N.; Herbst, R.H.; Shekhar, K.; Smillie, C.; Burgin, G.; Delorey, T.M.; Howitt, M.R.; Katz, Y.; et al. A single-cell survey of the small intestinal epithelium. Nature 2017, 551, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.Y.; Xavier Wong, H.L.; Zang, K.H.; Li, X.; Bian, Z.X. Enterochromaffin cell hyperplasia in the gut: Factors, mechanism and therapeutic clues. Life Sci. 2019, 239, 116886. [Google Scholar] [CrossRef] [PubMed]
- Gunawardene, A.R.; Corfe, B.M.; Staton, C.A. Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int. J. Exp. Pathol. 2011, 92, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Drozdov, I.; Modlin, I.M.; Kidd, M.; Goloubinov, V.V. From Leningrad to London: The saga of Kulchitsky and the legacy of the enterochromaffin cell. Neuroendocrinology 2009, 89, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Ahlman, H.; Nilsson, O. The gut as the largest endocrine organ in the body. Ann. Oncol. 2001, 12 (Suppl. S2), S63–S68. [Google Scholar] [CrossRef]
- Koo, A.; Fothergill, L.J.; Kuramoto, H.; Furness, J.B. 5-HT containing enteroendocrine cells characterised by morphologies, patterns of hormone co-expression, and relationships with nerve fibres in the mouse gastrointestinal tract. Histochem. Cell Biol. 2021, 155, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Kulchitsky, N. Zur Frage über den Bau des Darmkanals. Arch. Mikr. Anat. 1897, 49, 7–35. [Google Scholar] [CrossRef]
- Heidenhain, R.P. Untersuchungen uber den Bau der Labdrusen. Arch. Mikr. Anat. 1970, 6, 368. [Google Scholar] [CrossRef]
- Simard, L.C.; Van Campenhout, E. The Embryonic Development of Argentaffin Cells in the Chick Intestine. Anat. Rec. 1932, 53, 141–159. [Google Scholar] [CrossRef]
- Ciaccio, M. Sur une nouvelle espece cellulaire dans les glandes de Lieberkuhn. C. R. Seances Soc. Biol. Fil. 1906, 60, 76–77. [Google Scholar]
- Solcia, E.; Capella, R.; Buffa, R.; Fiocca, R.; Frigerio, B.; Usellini, L. Identification, ultrastructure and classification of gut endocrine cells and related growths. Investig. Cell Pathol. 1980, 3, 37–49. [Google Scholar]
- Solcia, E.; Creutzfeldt, W.; Falkmer, S.; Fujita, T.; Greider, M.; Grossman, M.; Grube, D.; Hakanson, R.; Larsson, L.-I.; Lechago, J.; et al. Human gastroenteropancreatic endocrine-paracrine cells: Santa Monica 1980 classification. Cellular basis of chemical messengers in the digestive system. UCLA Forum Med. Sci. 1981, 23, 159–165. [Google Scholar]
- Wade, P.R.; Westfall, J.A. Ultrastructure of enterochromaffin cells and associated neural and vascular elements in the mouse duodenum. Cell Tissue Rev. 1985, 241, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Kuramoto, H.; Kadowaki, M.; Sakamoto, H.; Yuasa, K.; Todo, A.; Shirai, R. Distinct morphology of serotonin-containing enterochromaffin (EC) cells in the rat distal colon. Arch. Histol. Cytol. 2007, 70, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Forssmann, W.G.; Orci, L.; Pictet, R.; Renold, A.E.; Rouiller, C. The endocrine cells in the epithelium of the gastrointestinal mucosa of the rat. J. Cell Biol. 1969, 40, 692–715. [Google Scholar] [CrossRef]
- Ferreira, M.N. Argentaffin and other “endocrine” cells of the small intestine in the adult mouse. I. Ultrastructure and classification. Am. J. Anat. 1971, 131, 315–329. [Google Scholar] [CrossRef]
- Håkanson, R.; Chen, D.; Andersson, K.; Monstein, H.J.; Zhao, C.M.; Ryberg, B.; Sundler, F.; Mattsson, H. The biology and physiology of the ECL cell. Yale J. Biol. Med. 1994, 67, 123–134. [Google Scholar]
- Nilsson, O.; Ahlman, H.; Gefferd, M.; Dahlström, A.; Ericson, L.E. Bipolarity of duodenal enterochromaffin cells in the rat. Cell Tissue Res. 1987, 248, 49–54. [Google Scholar] [CrossRef]
- Gustafsson, B.I.; Bakke, I.; Tømmerȧs, K.; Waldum, H.L. A new method for visualization of gut mucosal cells, describing the enterochromaffin cell in the rat gastrointestinal tract. Scand. J. Gastroenterol. 2006, 41, 390–395. [Google Scholar] [CrossRef]
- O’Hara, J.R.; Ho, W.; Linden, D.R.; Mawe, G.M.; Sharkey, K.A. Enteroendocrine cells and 5-HT availability are altered in mucosa of guinea pigs with TNBS ileitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G998–G1007. [Google Scholar] [CrossRef]
- Spiller, R. Serotonin and GI clinical disorders. Neuropharmacology 2008, 55, 1072–1080. [Google Scholar] [CrossRef]
- Vincent, K.M.; Sharp, J.W.; Raybould, H.E. Intestinal glucose-induced calcium-calmodulin kinase signaling in the gut-brain axis in awake rats. Neurogastroenterol. Motil. 2011, 23, e282–e293. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, R.; Zhan, G.; Wang, D.; Tan, X.; Xu, H. Enterochromaffin Cells: Sentinels to Gut Microbiota in Hyperalgesia? Front. Cell. Infect. Microbiol. 2021, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Luo, J.; Li, J.; Kim, G.; Stewart, A.; Urban, J.F., Jr.; Huang, Y.; Chen, S.; Wu, L.G.; Chesler, A.; et al. Interleukin-33 Promotes Serotonin Release from Enterochromaffin Cells for Intestinal Homeostasis. Immunity 2021, 54, 151.e6–163.e6. [Google Scholar] [CrossRef] [PubMed]
- Sjölund, K.; Sandén, G.; Hȧkanson, R.; Sundler, F. Endocrine cells in human intestine: An immunocytochemical Study. Gastroenterology 1983, 85, 1120–1130. [Google Scholar] [CrossRef]
- Portela-Gomes, G.M.; Grimelius, L.; Petersson, R.; Bergström, R. Enterochromaffin Cells in the rat gastrointestinal tract. Aspects of factors influencing quantification. Upsala J. Med. Sci. 1984, 89, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Trier, J.S. Morphology of the epithelium of the distal esophagus in patients with midesophageal peptic strictures. Gastroenterology 1970, 58, 444–461. [Google Scholar] [CrossRef]
- Bordi, C.; D’adda, T.; Azzoni, C.; Ferraro, G. Classification of gastric endocrine cells at the light and electron microscopical levels. Microsc. Res. Tech. 2000, 48, 258–271. [Google Scholar] [CrossRef]
- Hunne, B.; Stebbing, M.J.; McQuade, R.M.; Furness, J.B. Distributions and relationships of chemically defined enteroendocrine cells in the rat gastric mucosa. Cell Tissue Res. 2019, 378, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Cristina, M.L.; Lehy, T.; Zeitoun, P.; Dufougeray, F. Fine structural classification and comparative distribution of endocrine cells in normal human large Intestine. Gastroenterology 1978, 75, 20–28. [Google Scholar] [CrossRef]
- Cetin, Y. Secretin-cells of the mammalian intestine contain serotonin. Histochemistry 1990, 93, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Diwakarla, S.; Fothergill, L.J.; Fakhry, J.; Callaghan, B.; Furness, J.B. Heterogeneity of enterochromaffin cells within the gastrointestinal tract. Neurogastroenterol. Motil. 2017, 29, e13101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Duan, J.Z.; Lei, N.; Xing, H.; Shao, Y.; Yang, G.B. Cellular bases for interactions between immunocytes and enteroendocrine cells in the intestinal mucosal barrier of rhesus macaques. Cell Tissue Res. 2012, 350, 135–141. [Google Scholar] [CrossRef]
- Polak, J.M.; Heitz, P.; Pearse, A.G. Differential localisation of substance P and motilin. Scand. J. Gastroenterol. Suppl. 1976, 39, 39–42. [Google Scholar] [PubMed]
- Spohn, S.N.; Mawe, G.M. Non-conventional features of peripheral serotonin signalling—The gut and beyond. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Bearcroft, C.P.; Perrett, D.; Farthing, M.J. 5-hydroxytryptamine release into human jejunum by cholera toxin. Gut 1996, 39, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Grondahl, M.L.; Thorbøll, J.E.; Hansen, M.B.; Skadhauge, E. Regional differences in the effect of cholera toxin and enterotoxigenic Escherichia coli infection on electrolyte and fluid transport in the porcine small intestine. Zent. Vet. A 1998, 45, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.B.; Witte, A.B. The Role of serotonin in intestinal luminal sensing and secretion. Acta Physiol. 2008, 193, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Matthes, S.; Bader, M. Peripheral Serotonin Synthesis as a New Drug Target. Trends Pharmacol. Sci. 2018, 39, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Gershon, M.D. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Trevisani, M.; Siemens, J.; Materazzi, S.; Bautista, D.M.; Nassini, R.; Campi, B.; Imamachi, N.; Andrè, E.; Patacchini, R.; Cottrell, G.S.; et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. USA 2007, 104, 13519–13524. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Lumsden, A.L.; Young, R.L.; Jessup, C.F.; Spencer, N.J.; Keating, D.J. The nutrient-sensing repertoires of mouse enterochromaffin cells differ between duodenum and colon. Neurogastroenterol. Motil. 2017, 29, e13046. [Google Scholar] [CrossRef] [PubMed]
- Balen, D.; Ljubojevic, M.; Breljak, D.; Brzica, H.; Zlender, V.; Koepsell, H.; Sabolic, I. Revised immunolocalization of the Na+-D-glucose cotransporter SGLT1 in rat organs with an improved antibody. Am. J. Physiol. Cell Physiol. 2008, 295, C475–C489. [Google Scholar] [CrossRef] [PubMed]
- Sotak, M.; Marks, J.; Unwin, R.J. Putative tissue location and function of the SLC5 family member SGLT3. Exp. Physiol. 2017, 102, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Kidd, M.; Modlin, I.M.; Gustafsson, B.I.; Drozdov, I.; Hauso, O.; Pfragner, R. Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants, and ofactants. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G260–G272. [Google Scholar] [CrossRef] [PubMed]
- Everest, P. Stress and bacteria: Microbial endocrinology. Gut 2007, 56, 1037–1038. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Camilleri, M.; Carlson, P.J.; Cremonini, F.; Feber, I.; Stephens, D.; McKinzie, S.; Zinsmeister, A.R.; Urrutia, R. Association of distinct alpha(2) adrenoceptor and serotonin transporter polymorphisms with constipation and somatic symptoms in functional gastrointestinal disords. Gut 2004, 53, 829–837. [Google Scholar] [CrossRef]
- Westlund, K.N.; Zhang, L.P.; Ma, F.; Nesemeier, R.; Ruiz, J.C.; Ostertag, E.M.; Crawford, J.S.; Babinski, K.; Marcinkiewicz, M.M. A rat knockout model implicates TRPC4 in visceral pain sensation. Neuroscience 2014, 262, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Knutson, K.; Alcaino, C.; Linden, D.R.; Gibbons, S.J.; Kashyap, P.; Grover, M.; Oeckler, R.; Gottlieb, P.A.; Li, H.J.; et al. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J. Physiol. 2017, 595, 79–91. [Google Scholar] [CrossRef]
- Williams, E.K.; Chang, R.B.; Strochlic, D.E.; Umans, B.D.; Lowell, B.B.; Liberles, S.D. Sensory Neurons that Detect Stretch and Nutrients in the Digestive System. Cell 2016, 166, 209–221. [Google Scholar] [CrossRef]
- Crane, J.D.; Palanivel, R.; Mottillo, E.P.; Bujak, A.L.; Wang, H.; Ford, R.J.; Collins, A.; Blümer, R.M.; Fullerton, M.D.; Yabut, J.M.; et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat. Med. 2015, 21, 166–172. [Google Scholar] [CrossRef]
- Young, R.L.; Lumsden, A.L.; Keating, D.J. Gut Serotonin is a Regulator of Obesity and Metabolism. Gastroenterology 2015, 149, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Akiba, Y.; Maruta, K.; Narimatsu, K.; Said, H.; Kaji, I.; Kuri, A.; Iwamoto, K.I.; Kuwahara, A.; Kaunitz, J.D. FFA2 activation combined with ulcerogenic COX inhibition induces duodenal mucosal injury via the 5-HT pathway in rats. Am. J. Physiol. Gastrointest. Liver. Physiol. 2017, 313, G117–G128. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Zhang, S.; Meng, Y.; Feng, Y.; Wang, Y.; Wang, E.; Pan, X.; Zhu, R.; Fan, H.; Pang, S.; et al. Female serotonin transporter-knockout rat: A potential model of irritable bowel syndrome. FASEB J. 2021, 35, e21701. [Google Scholar] [CrossRef]
- Ahern, G.P. 5-HT and the immune system. Curr. Opin. Pharmacol. 2011, 11, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Banskota, S.; Ghia, J.E.; Khan, W.I. Serotonin in the gut: Blessing or a curse. Biochimie 2019, 161, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Raikhlin, N.T.; Kvetnoy, I.M.; Tolkachev, V.N. Melatonin may be synthesised in enterochromaffin cells. Nature 1975, 255, 344–345. [Google Scholar] [CrossRef]
- Pal, P.K.; Sarkar, S.; Chattopadhyay, A.; Tan, D.X.; Bandyopadhyay, D. Enterochromaffin cells as the souce of melatonin: Key findings and functional relevance in mammals. Melatonin Res. 2019, 2, 61–81. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-X.; Mayo, J.C.; Sainz, R.M.; Leon, J.; Bandyopadhyay, D. Neurally-mediated and neurally-indipendent benefical actions of melatonin in the gastrointestinal tract. J. Physiol. Pharmacol. 2003, 54 (Suppl. 4), 113–125. [Google Scholar]
- Voisin, P.; Namboodiri, M.A.; Klein, D.C. Arylamine N-acetyltransferase and arylalkylamine N-acetyltransferase in the mammalian pineal gland. J. Biol. Chem. 1984, 259, 10913–10918. [Google Scholar] [CrossRef]
- Axelrod, J.; Weissbach, H. Enzymatic O-methylation of N-acetylserotonin to melatonin. Science 1960, 131, 1312. [Google Scholar] [CrossRef]
- Motilva, V.; Cabeza, J.; Alarcon de la Lastra, C. New issues about melatonin and its effects on the digestive system. Curr. Pharm. Des. 2001, 7, 909–931. [Google Scholar] [CrossRef] [PubMed]
- Chow, P.H.; Lee, P.N.; Poon, A.M.S.; Shiu, S.Y.W.; Pang, S.F. The gastrointestinal system: A site of melatonin paracrine action. Melatonin: A universal photoperiodic signal with diverse actions. Front. Horm. Res. 1996, 21, 123–132. [Google Scholar] [CrossRef]
- Menendez-Pelaez, A.; Buzzell, G.R. Harderian gland indoles. In Harderian Glands: Porphyrin Metabolism, Behavioral and Endocrine Effects; Webb, S.M., Hoffman, R.A., Puig-Domingo, M.L., Reiter, R.J., Eds.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 219–234. [Google Scholar]
- Moretti, E.; Favero, G.; Rodella, L.F.; Rezzani, R. Melatonin’s Antineoplastic Potential Against Glioblastoma. Cells 2020, 9, 599. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, A.; Brzozowska, I.M.; Hoa, N.; Jones, M.K.; Tarnawski, A.S. Melatonin signaling in mitochondria in mitochondria extends beyond neurons and neuroprotection: Implications for angiogenesis and cardio/gastroprotection. Proc. Natl. Acad. Sci. USA 2018, 115, 1942–1943. [Google Scholar] [CrossRef] [PubMed]
- Bubenik, G.A. Thirty four years since the discovery of gastrointestinal melatonin. J. Physiol. Pharmacol. 2008, 59, 33–51. [Google Scholar]
- Thor, P.J.; Krolczyk, G.; Gil, K.; Zurowski, D.; Nowak, L. Melatonin and serotonin effects on gastrointestinal motility. J. Physiol. Pharmacol. 2007, 58 (Suppl. S6), 97–103. [Google Scholar]
- Huether, G. The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia 1993, 49, 665–670. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Han, H.; Chen, S.; Gao, J.; Liu, G.; Wu, X.; Deng, J.; Yu, Q.; Huang, X.; et al. Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high-fat diet-fed mice. J. Pineal Res. 2018, 65, e12524. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, e00013. [Google Scholar] [CrossRef]
- Von Euler, U.S.; Gaddum, J.H. An unidentified depressor substance in certain tissue extracts. J. Physiol. 1931, 72, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Pearse, A.G.; Polak, J.M. Immunocytochemical localization of substance P in mammalian intestine. Histochemistry 1975, 41, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Sokolski, K.N.; Lechago, J. Human colonic substance P-producing cells are a separate population from the serotonin-producing enterochromaffin cells. J. Histochem. Cytochem. 1984, 32, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Heitz, P.; Polak, J.M.; Timson, D.M.; Pearse, A.G. Enterochromaffin cells as the endocrine source of gastrointestinal substance P. Histochemistry 1976, 49, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Pernow, B. Studies on substance P: Purification, occurrence and biological actions. Acta Physiol. Scand. Suppl. 1953, 29, 1–89. [Google Scholar]
- Bryant, M.G.; Bloom, S.R.; Polak, J.M.; Albuquerque, R.H.; Modlin, I.; Pearse, A.G.E. Possible dual role for vasoactive intestinal peptide as gastrointestinal hormone and neurotransmitter substance. Lancet 1976, 1, 991–993. [Google Scholar] [CrossRef]
- Alumets, J.; Håkanson, R.; Ingemansson, S.; Sundler, F. Substance P and 5-HT in granules isolated from an intestinal argentaffin carcinoid. Histochemistry 1977, 52, 217–222. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, T.M.; O’Connell, J.; O’Brien, D.I.; Goode, T.; Bredin, C.B.; Shanahan, F. The role of substance P in inflammatory disease. J. Cell. Physiol. 2004, 201, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Koon, H.W.; Pothoulakis, C. Immunomodulatory propertiers of substance P: The gastrointestinal system as a model. Ann. N. Y. Acad. Sci. 2006, 1088, 23–40. [Google Scholar] [CrossRef]
- Taylor, C.T.; Keely, S.J. The autonomic nervous system and inflammatory bowel disease. Auton. Neurosci. 2007, 133, 104–114. [Google Scholar] [CrossRef]
- Lakhan, S.E.; Kirchgessner, A. Neuroinflammation in inflammatory bowel disease. J. Neuroinflamm. 2010, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Machida, T.; Takano, Y.; Iizuka, K.; Machida, M.; Hirafuji, M. Methotrexate causes acute hyperplasia of enterochromaffin cells containing substance P in the intestinal mucosa of rats. J. Pharmacol. Sci. 2017, 133, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Obara, Y.; Machida, T.; Takano, Y.; Shiga, S.; Suzuki, A.; Hamaue, N.; Iizuka, K.; Hirafuji, M. Cisplatin increases the number of enterochromaffin cells containing substance P in rat Intestine. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 847–858. [Google Scholar] [CrossRef]
- Bruen, C.M.; O’Halloran, F.; Cashman, K.D.; Giblin, L. The effects of food components on hormonal signalling in gastrointestinal enteroendocrine cells. Food Funct. 2012, 3, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Stasi, C.; Bellini, M.; Bassotti, G.; Blandizzi, C.; Milani, S. Serotonin receptors and their role in the pathophysiology and therapy of irritable bowel syndrome. Tech. Coloproctol. 2014, 18, 613–621. [Google Scholar] [CrossRef]
- Duca, F.A.; Lam, T.K. Gut microbiota, nutrient sensing and energy balance. Diabetes Obes. Metab. 2014, 16 (Suppl. S1), 68–76. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.C. Irritable bowel syndrome and diet. Curr. Opin. Endocrinol. Diabetes Obes. 2022, 29, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Szklany, K.; Engen, P.A.; Naqib, A.; Green, S.J.; Keshavarzian, A.; Lopez Rincon, A.; Siebrand, C.J.; Diks, M.A.P.; van de Kaa, M.; Garssen, J.; et al. Dietary Supplementation throughout Life with Non-Digestible Oligosaccharides and/or n-3 Poly-Unsaturated Fatty Acids in Healthy Mice Modulates the Gut-Immune System-Brain Axis. Nutrients 2021, 14, 173. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Zacharko-Siembida, A.; Valverde Piedra, J.L.V.; Strzałka, B.; Arciszewski, M.B. Red kidney bean (Phaseolus vulgaris) lectin stimulation increases the number of enterochromaffin cells in the small intestine of suckling piglets. J. Vet. Res. 2014, 58, 289–294. [Google Scholar] [CrossRef][Green Version]
- Zacharko-Siembida, A.; Arciszewski, M.; Valverde Piedra, J.; Tomaszewska, E.; Szymanczyk, S.; Muszyński, S.; Dobrowolski, P.; Mozel, S.; Schwarz, T. Expression of serotonin, somatostatin, and glucagon-like peptide 1 (GLP1) in the intestinal neuroendocrine cells of pigs fed with population rye type and hybrid rye type grains. Med. Weter 2019, 75, 6251-2019. [Google Scholar] [CrossRef]
- Martin, A.M.; Jones, L.A.; Jessup, C.F.; Sun, E.W.; Keating, D.J. Diet differentially regulates enterochromaffin cell serotonin content, density and nutrient sensitivity in the mouse small and large intestine. Neurogastroenterol. Motil. 2020, 32, e13869. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, A.L.; Martin, A.M.; Sun, E.W.; Schober, G.; Isaacs, N.J.; Pezos, N.; Wattchow, D.A.; de Fontgalland, D.; Rabbitt, P.; Hollington, P.; et al. Sugar Responses of Human Enterochromaffin Cells Depend on Gut Region, Sex, and Body Mass. Nutrients 2019, 11, 234. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed]
- Le Beyec, J.; Pelletier, A.L.; Arapis, K.; Hourseau, M.; Cluzeaud, F.; Descatoire, V.; Ducroc, R.; Aparicio, T.; Joly, F.; Couvelard, A.; et al. Overexpression of gastric leptin precedes adipocyte leptin during high-fat diet and is linked to 5HT-containing enterochromaffin cells. Int. J. Obes. 2014, 38, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yang, W.; Li, Y.; Cong, Y. Enteroendocrine Cells: Sensing Gut Microbiota and Regulating Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2020, 26, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Mazzawi, T.; El-Salhy, M. Changes in duodenal enteroendocrine cells in patients with irritable bowel syndrome following dietary guidance. Exp. Biol. Med. 2017, 242, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Moran-Ramos, S.; Tovar, A.R.; Torres, N. Diet: Friend or foe of enteroendocrine cells—How it interacts with enteroendocrine cells. Adv. Nutr. 2012, 3, 8–20. [Google Scholar] [CrossRef] [PubMed]
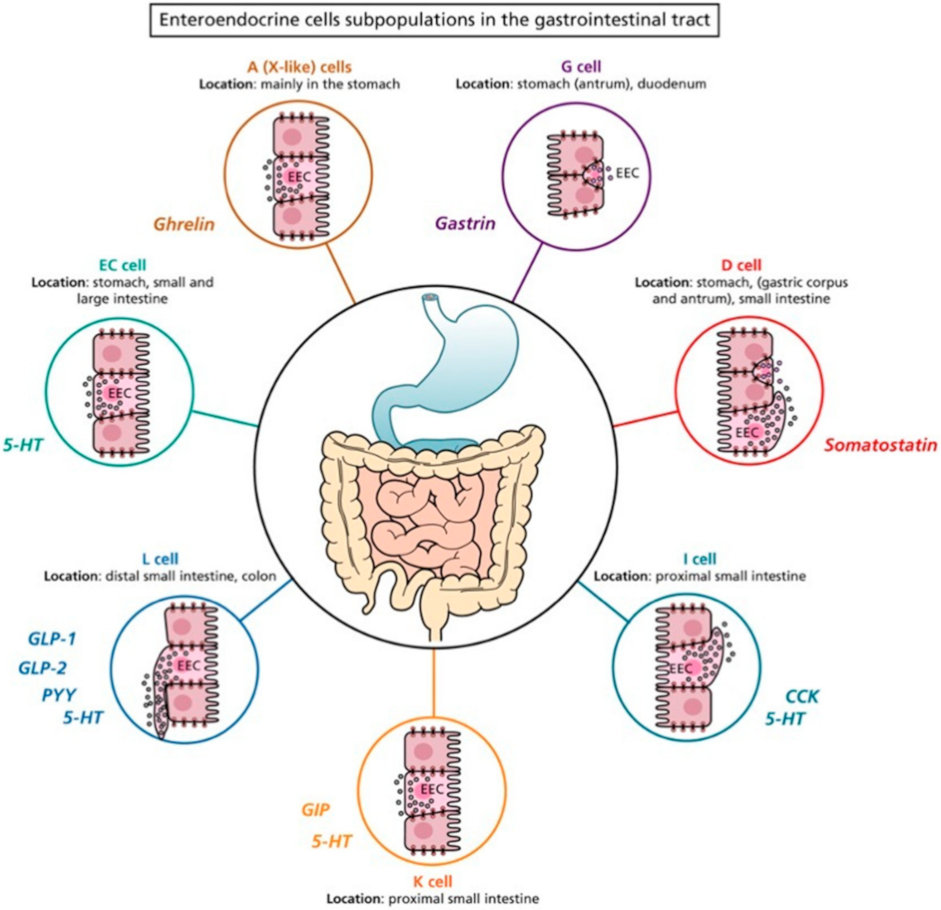


| EECs | Location | Hormones |
|---|---|---|
| A (X-like) cells | Stomach (corpus) | Ghrelin |
| D cells | Stomach (pylorum) Proximal segment of the small intestine | Somatostatin |
| EC cells | Stomach (pylorum), small and large intestine | 5-HT |
| G cells | Stomach (antrum), duodenum | Gastrin |
| I cells | Small intestine Distal portion small intestine | CCK 5-HT |
| L cells | Distal portion of small intestine Colon (mainly in the proximal portion) Large intestine | GLP-1, GLP-2, PYY 5-HT |
| K cells | Small intestine | GIP 5-HT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezzani, R.; Franco, C.; Franceschetti, L.; Gianò, M.; Favero, G. A Focus on Enterochromaffin Cells among the Enteroendocrine Cells: Localization, Morphology, and Role. Int. J. Mol. Sci. 2022, 23, 3758. https://doi.org/10.3390/ijms23073758
Rezzani R, Franco C, Franceschetti L, Gianò M, Favero G. A Focus on Enterochromaffin Cells among the Enteroendocrine Cells: Localization, Morphology, and Role. International Journal of Molecular Sciences. 2022; 23(7):3758. https://doi.org/10.3390/ijms23073758
Chicago/Turabian StyleRezzani, Rita, Caterina Franco, Lorenzo Franceschetti, Marzia Gianò, and Gaia Favero. 2022. "A Focus on Enterochromaffin Cells among the Enteroendocrine Cells: Localization, Morphology, and Role" International Journal of Molecular Sciences 23, no. 7: 3758. https://doi.org/10.3390/ijms23073758
APA StyleRezzani, R., Franco, C., Franceschetti, L., Gianò, M., & Favero, G. (2022). A Focus on Enterochromaffin Cells among the Enteroendocrine Cells: Localization, Morphology, and Role. International Journal of Molecular Sciences, 23(7), 3758. https://doi.org/10.3390/ijms23073758








