Enteroendocrine System and Gut Barrier in Metabolic Disorders
Abstract
1. Introduction
2. The Enteroendocrine System
2.1. Enteroendocrine Cell Types
2.2. Focus on GLP-1 and GIP Incretin Enterohormones
2.3. Mechanisms of Enterohormone Secretion
3. Models to Study Enteroendocrine Cells
3.1. In Vitro Cell-Based Models
3.2. Enteroids
3.3. Exploring Enteroendocrine Cells in Enteroids
4. Enteroendocrine System in Metabolic Disorders
4.1. Diets and Enteroendocrine System
4.2. Impact of Bariatric Surgery on Enteroendocrine System
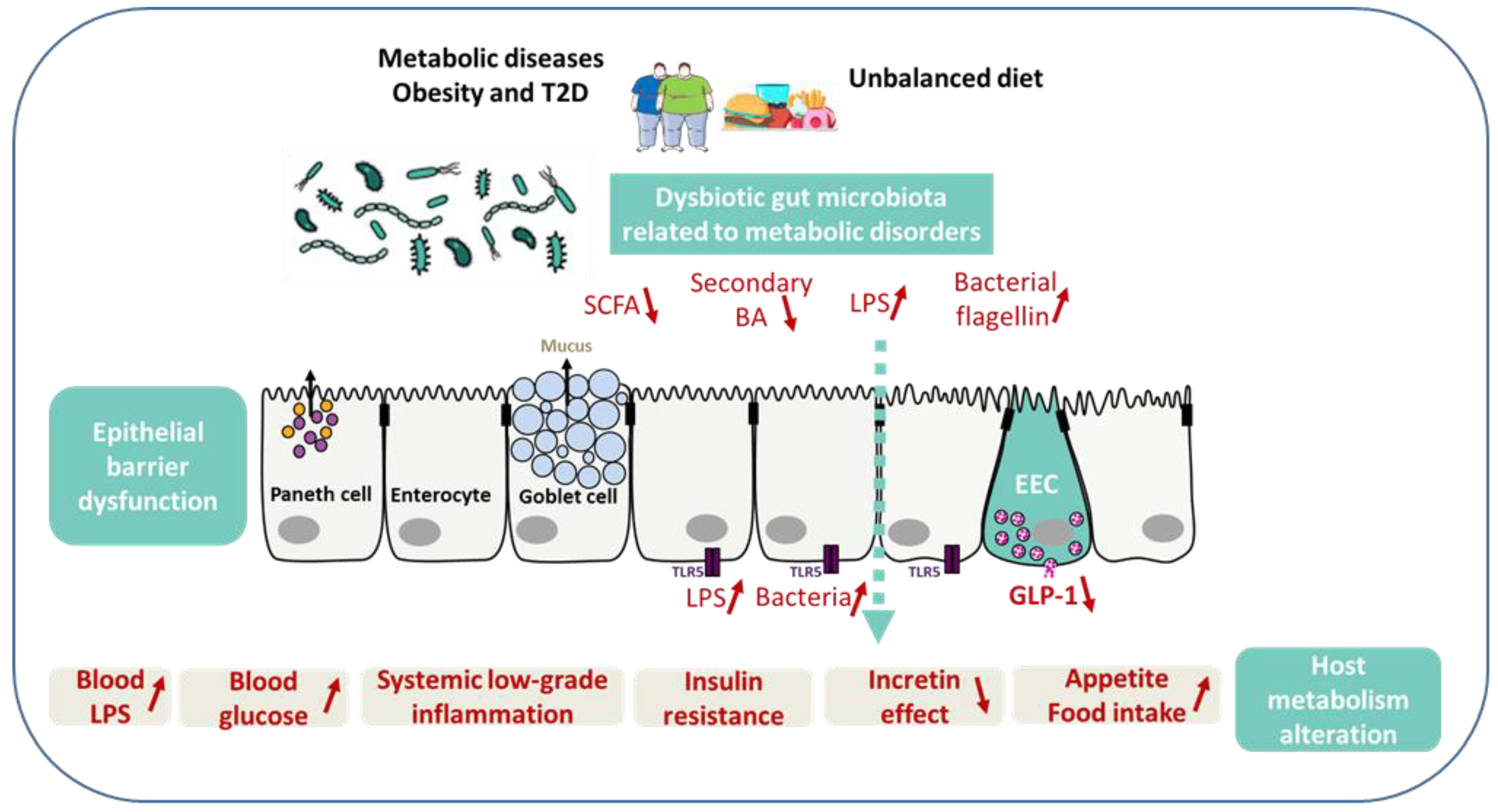
5. Microbiota, Enteroendocrine System, and Gut Barrier in Metabolic Disorders
5.1. SCFA, Secondary Bile Acids, and GLP-2
5.2. LPS, Flagellin, and Hyperglycemia
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Darwich, A.S.; Aslam, U.; Ashcroft, D.M.; Rostami-Hodjegan, A. Meta-Analysis of the Turnover of Intestinal Epithelia in Preclinical Animal Species and Humans. Drug Metab. Dispos. 2014, 42, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. The Intestinal Crypt, a Prototype Stem Cell Compartment. Cell 2013, 154, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Beumer, J.; Clevers, H. Regulation and Plasticity of Intestinal Stem Cells during Homeostasis and Regeneration. Development 2016, 143, 3639–3649. [Google Scholar] [CrossRef] [PubMed]
- Gerbe, F.; Legraverend, C.; Jay, P. The Intestinal Epithelium Tuft Cells: Specification and Function. Cell Mol. Life Sci. 2012, 69, 2907–2917. [Google Scholar] [CrossRef]
- Farin, H.F.; Van Es, J.H.; Clevers, H. Redundant Sources of Wnt Regulate Intestinal Stem Cells and Promote Formation of Paneth Cells. Gastroenterology 2012, 143, 1518–1529.e7. [Google Scholar] [CrossRef]
- Gerbe, F.; Sidot, E.; Smyth, D.J.; Ohmoto, M.; Matsumoto, I.; Dardalhon, V.; Cesses, P.; Garnier, L.; Pouzolles, M.; Brulin, B.; et al. Intestinal Epithelial Tuft Cells Initiate Type 2 Mucosal Immunity to Helminth Parasites. Nature 2016, 529, 226–230. [Google Scholar] [CrossRef]
- Xu, Y.-M.; Gao, Q.; Zhang, J.-Z.; Lu, Y.-T.; Xing, D.-M.; Qin, Y.-Q.; Fang, J. Prolyl Hydroxylase 3 Controls the Intestine Goblet Cell Generation through Stabilizing ATOH1. Cell Death Differ. 2020, 27, 2131–2142. [Google Scholar] [CrossRef]
- Latorre, R.; Sternini, C.; De Giorgio, R.; Greenwood-Van Meerveld, B. Enteroendocrine Cells: A Review of Their Role In Brain-Gut Communication. Neurogastroenterol. Motil. 2016, 28, 620–630. [Google Scholar] [CrossRef]
- Habib, A.M.; Richards, P.; Cairns, L.S.; Rogers, G.J.; Bannon, C.A.M.; Parker, H.E.; Morley, T.C.E.; Yeo, G.S.H.; Reimann, F.; Gribble, F.M. Overlap of Endocrine Hormone Expression in the Mouse Intestine Revealed by Transcriptional Profiling and Flow Cytometry. Endocrinology 2012, 153, 3054–3065. [Google Scholar] [CrossRef]
- Habib, A.M.; Richards, P.; Rogers, G.J.; Reimann, F.; Gribble, F.M. Co-Localisation and Secretion of Glucagon-like Peptide 1 and Peptide YY from Primary Cultured Human L Cells. Diabetologia 2013, 56, 1413–1416. [Google Scholar] [CrossRef]
- Grunddal, K.V.; Ratner, C.F.; Svendsen, B.; Sommer, F.; Engelstoft, M.S.; Madsen, A.N.; Pedersen, J.; Nøhr, M.K.; Egerod, K.L.; Nawrocki, A.R.; et al. Neurotensin Is Coexpressed, Coreleased, and Acts Together With GLP-1 and PYY in Enteroendocrine Control of Metabolism. Endocrinology 2016, 157, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-J.; Robinson, E.S.; Rivera, L.R.; McMillan, P.J.; Testro, A.; Nikfarjam, M.; Bravo, D.M.; Furness, J.B. Glucagon-like Peptide 1 and Peptide YY Are in Separate Storage Organelles in Enteroendocrine Cells. Cell Tissue Res. 2014, 357, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Haber, A.L.; Biton, M.; Rogel, N.; Herbst, R.H.; Shekhar, K.; Smillie, C.; Burgin, G.; Delorey, T.M.; Howitt, M.R.; Katz, Y.; et al. A Single-Cell Survey of the Small Intestinal Epithelium. Nature 2017, 551, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Gehart, H.; van Es, J.H.; Hamer, K.; Beumer, J.; Kretzschmar, K.; Dekkers, J.F.; Rios, A.; Clevers, H. Identification of Enteroendocrine Regulators by Real-Time Single-Cell Differentiation Mapping. Cell 2019, 176, 1158–1173.e16. [Google Scholar] [CrossRef] [PubMed]
- Beumer, J.; Artegiani, B.; Post, Y.; Reimann, F.; Gribble, F.; Nguyen, T.N.; Zeng, H.; Van den Born, M.; Van Es, J.H.; Clevers, H. Enteroendocrine Cells Switch Hormone Expression along the Crypt-to-Villus BMP Signalling Gradient. Nat. Cell Biol. 2018, 20, 909–916. [Google Scholar] [CrossRef]
- Paternoster, S.; Falasca, M. Dissecting the Physiology and Pathophysiology of Glucagon-Like Peptide-1. Front. Endocrinol. (Lausanne) 2018, 9, 584. [Google Scholar] [CrossRef]
- Jenny, M.; Uhl, C.; Roche, C.; Duluc, I.; Guillermin, V.; Guillemot, F.; Jensen, J.; Kedinger, M.; Gradwohl, G. Neurogenin3 Is Differentially Required for Endocrine Cell Fate Specification in the Intestinal and Gastric Epithelium. EMBO J. 2002, 21, 6338–6347. [Google Scholar] [CrossRef]
- Ye, D.Z.; Kaestner, K.H. Foxa1 and Foxa2 Control the Differentiation of Goblet and Enteroendocrine L- and D-Cells in Mice. Gastroenterology 2009, 137, 2052–2062. [Google Scholar] [CrossRef]
- Beucher, A.; Gjernes, E.; Collin, C.; Courtney, M.; Meunier, A.; Collombat, P.; Gradwohl, G. The Homeodomain-Containing Transcription Factors Arx and Pax4 Control Enteroendocrine Subtype Specification in Mice. PLoS ONE 2012, 7, e36449. [Google Scholar] [CrossRef]
- Desai, S.; Loomis, Z.; Pugh-Bernard, A.; Schrunk, J.; Doyle, M.J.; Minic, A.; McCoy, E.; Sussel, L. Nkx2.2 Regulates Cell Fate Choice in the Enteroendocrine Cell Lineages of the Intestine. Dev. Biol. 2008, 313, 58–66. [Google Scholar] [CrossRef]
- Hill, M.E.; Asa, S.L.; Drucker, D.J. Essential Requirement for Pax6 in Control of Enteroendocrine Proglucagon Gene Transcription. Mol. Endocrinol. 1999, 13, 1474–1486. [Google Scholar] [CrossRef] [PubMed]
- Terry, N.A.; Walp, E.R.; Lee, R.A.; Kaestner, K.H.; May, C.L. Impaired Enteroendocrine Development in Intestinal-Specific Islet1 Mouse Mutants Causes Impaired Glucose Homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G979–G991. [Google Scholar] [CrossRef] [PubMed]
- Piccand, J.; Vagne, C.; Blot, F.; Meunier, A.; Beucher, A.; Strasser, P.; Lund, M.L.; Ghimire, S.; Nivlet, L.; Lapp, C.; et al. Rfx6 Promotes the Differentiation of Peptide-Secreting Enteroendocrine Cells While Repressing Genetic Programs Controlling Serotonin Production. Mol. Metab. 2019, 29, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Gribble, F.M.; Reimann, F. Function and Mechanisms of Enteroendocrine Cells and Gut Hormones in Metabolism. Nat. Rev. Endocrinol. 2019, 15, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Raja, A. Physiology, Gastric Inhibitory Peptide. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Miyawaki, K.; Yamada, Y.; Ban, N.; Ihara, Y.; Tsukiyama, K.; Zhou, H.; Fujimoto, S.; Oku, A.; Tsuda, K.; Toyokuni, S.; et al. Inhibition of Gastric Inhibitory Polypeptide Signaling Prevents Obesity. Nat. Med. 2002, 8, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Hamasaki, A.; Yamane, S.; Muraoka, A.; Joo, E.; Fujita, K.; Inagaki, N. Plasma Gastric Inhibitory Polypeptide and Glucagon-like Peptide-1 Levels after Glucose Loading Are Associated with Different Factors in Japanese Subjects. J. Diabetes Investig. 2011, 2, 193–199. [Google Scholar] [CrossRef]
- Sandoval, D.A.; D’Alessio, D.A. Physiology of Proglucagon Peptides: Role of Glucagon and GLP-1 in Health and Disease. Physiol. Rev. 2015, 95, 513–548. [Google Scholar] [CrossRef]
- Mentlein, R.; Gallwitz, B.; Schmidt, W.E. Dipeptidyl-Peptidase IV Hydrolyses Gastric Inhibitory Polypeptide, Glucagon-like Peptide-1(7-36)Amide, Peptide Histidine Methionine and Is Responsible for Their Degradation in Human Serum. Eur. J. Biochem. 1993, 214, 829–835. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Biology of Incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Holst, J.J. The Physiology of Glucagon-like Peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like Peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Reimann, F.; Gribble, F.M. Glucose-Sensing in Glucagon-like Peptide-1-Secreting Cells. Diabetes 2002, 51, 2757–2763. [Google Scholar] [CrossRef] [PubMed]
- Reimann, F.; Habib, A.M.; Tolhurst, G.; Parker, H.E.; Rogers, G.J.; Gribble, F.M. Glucose Sensing in L Cells: A Primary Cell Study. Cell Metab. 2008, 8, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Moss, C.E.; Glass, L.L.; Diakogiannaki, E.; Pais, R.; Lenaghan, C.; Smith, D.M.; Wedin, M.; Bohlooly-Y, M.; Gribble, F.M.; Reimann, F. Lipid Derivatives Activate GPR119 and Trigger GLP-1 Secretion in Primary Murine L-Cells. Peptides 2016, 77, 16–20. [Google Scholar] [CrossRef]
- Wheeler, S.E.; Stacey, H.M.; Nahaei, Y.; Hale, S.J.; Hardy, A.B.; Reimann, F.; Gribble, F.M.; Larraufie, P.; Gaisano, H.Y.; Brubaker, P.L. The SNARE Protein Syntaxin-1a Plays an Essential Role in Biphasic Exocytosis of the Incretin Hormone Glucagon-Like Peptide 1. Diabetes 2017, 66, 2327–2338. [Google Scholar] [CrossRef] [PubMed]
- Ohara-Imaizumi, M.; Aoyagi, K.; Akimoto, Y.; Nakamichi, Y.; Nishiwaki, C.; Kawakami, H.; Nagamatsu, S. Imaging Exocytosis of Single Glucagon-like Peptide-1 Containing Granules in a Murine Enteroendocrine Cell Line with Total Internal Reflection Fluorescent Microscopy. Biochem. Biophys. Res. Commun. 2009, 390, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Grant, S.G.; Yiangou, Y.; Ghatei, M.A.; Bloom, S.R.; Bautch, V.L.; Solcia, E.; Polak, J.M. Development of Neuroendocrine Tumors in the Gastrointestinal Tract of Transgenic Mice. Heterogeneity of Hormone Expression. Am. J. Pathol. 1990, 136, 1349–1363. [Google Scholar]
- Drucker, D.J.; Jin, T.; Asa, S.L.; Young, T.A.; Brubaker, P.L. Activation of Proglucagon Gene Transcription by Protein Kinase-A in a Novel Mouse Enteroendocrine Cell Line. Mol. Endocrinol. 1994, 8, 1646–1655. [Google Scholar] [CrossRef]
- Kuhre, R.E.; Wewer Albrechtsen, N.J.; Deacon, C.F.; Balk-Møller, E.; Rehfeld, J.F.; Reimann, F.; Gribble, F.M.; Holst, J.J. Peptide Production and Secretion in GLUTag, NCI-H716, and STC-1 Cells: A Comparison to Native L-Cells. J. Mol. Endocrinol. 2016, 56, 201–211. [Google Scholar] [CrossRef]
- Psichas, A.; Tolhurst, G.; Brighton, C.A.; Gribble, F.M.; Reimann, F. Mixed Primary Cultures of Murine Small Intestine Intended for the Study of Gut Hormone Secretion and Live Cell Imaging of Enteroendocrine Cells. J. Vis. Exp. 2017. [Google Scholar] [CrossRef]
- Goldspink, D.A.; Reimann, F.; Gribble, F.M. Models and Tools for Studying Enteroendocrine Cells. Endocrinology 2018, 159, 3874–3884. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.P.; Larraufie, P.; Richards, P.; Kay, R.G.; Galvin, S.G.; Miedzybrodzka, E.L.; Leiter, A.; Li, H.J.; Glass, L.L.; Ma, M.K.L.; et al. Comparison of Human and Murine Enteroendocrine Cells by Transcriptomic and Peptidomic Profiling. Diabetes 2019, 68, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Nagatake, T.; Fujita, H.; Minato, N.; Hamazaki, Y. Enteroendocrine Cells Are Specifically Marked by Cell Surface Expression of Claudin-4 in Mouse Small Intestine. PLoS ONE 2014, 9, e90638. [Google Scholar] [CrossRef]
- Glass, L.L.; Calero-Nieto, F.J.; Jawaid, W.; Larraufie, P.; Kay, R.G.; Göttgens, B.; Reimann, F.; Gribble, F.M. Single-Cell RNA-Sequencing Reveals a Distinct Population of Proglucagon-Expressing Cells Specific to the Mouse Upper Small Intestine. Mol. Metab. 2017, 6, 1296–1303. [Google Scholar] [CrossRef]
- Richards, P.; Pais, R.; Habib, A.M.; Brighton, C.A.; Yeo, G.S.H.; Reimann, F.; Gribble, F.M. High Fat Diet Impairs the Function of Glucagon-like Peptide-1 Producing L-Cells. Peptides 2016, 77, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Osinski, C.; Le Gléau, L.; Poitou, C.; de Toro-Martin, J.; Genser, L.; Fradet, M.; Soula, H.A.; Leturque, A.; Blugeon, C.; Jourdren, L.; et al. Type 2 Diabetes Is Associated with Impaired Jejunal Enteroendocrine GLP-1 Cell Lineage in Human Obesity. Int. J. Obes. (Lond.) 2021, 45, 170–183. [Google Scholar] [CrossRef]
- Le Gléau, L.; Rouault, C.; Osinski, C.; Prifti, E.; Soula, H.A.; Debédat, J.; Busieau, P.; Amouyal, C.; Clément, K.; Andreelli, F.; et al. Intestinal Alteration of α-Gustducin and Sweet Taste Signaling Pathway in Metabolic Diseases Is Partly Rescued after Weight Loss and Diabetes Remission. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E417–E432. [Google Scholar] [CrossRef]
- Diakogiannaki, E.; Pais, R.; Tolhurst, G.; Parker, H.E.; Horscroft, J.; Rauscher, B.; Zietek, T.; Daniel, H.; Gribble, F.M.; Reimann, F. Oligopeptides Stimulate Glucagon-like Peptide-1 Secretion in Mice through Proton-Coupled Uptake and the Calcium-Sensing Receptor. Diabetologia 2013, 56, 2688–2696. [Google Scholar] [CrossRef]
- Dusaulcy, R.; Handgraaf, S.; Skarupelova, S.; Visentin, F.; Vesin, C.; Heddad-Masson, M.; Reimann, F.; Gribble, F.; Philippe, J.; Gosmain, Y. Functional and Molecular Adaptations of Enteroendocrine L-Cells in Male Obese Mice Are Associated With Preservation of Pancreatic α-Cell Function and Prevention of Hyperglycemia. Endocrinology 2016, 157, 3832–3843. [Google Scholar] [CrossRef]
- Basak, O.; Beumer, J.; Wiebrands, K.; Seno, H.; van Oudenaarden, A.; Clevers, H. Induced Quiescence of Lgr5+ Stem Cells in Intestinal Organoids Enables Differentiation of Hormone-Producing Enteroendocrine Cells. Cell Stem Cell 2017, 20, 177–190.e4. [Google Scholar] [CrossRef]
- Rahmani, S.; Breyner, N.M.; Su, H.-M.; Verdu, E.F.; Didar, T.F. Intestinal Organoids: A New Paradigm for Engineering Intestinal Epithelium in Vitro. Biomaterials 2019, 194, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 Stem Cells Build Crypt-Villus Structures in Vitro without a Mesenchymal Niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.J.; Van Es, J.H.; Van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-Term Expansion of Epithelial Organoids from Human Colon, Adenoma, Adenocarcinoma, and Barrett’s Epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef]
- Middendorp, S.; Schneeberger, K.; Wiegerinck, C.L.; Mokry, M.; Akkerman, R.D.L.; van Wijngaarden, S.; Clevers, H.; Nieuwenhuis, E.E.S. Adult Stem Cells in the Small Intestine Are Intrinsically Programmed with Their Location-Specific Function. Stem Cells 2014, 32, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Zachos, N.C.; Kovbasnjuk, O.; Foulke-Abel, J.; In, J.; Blutt, S.E.; de Jonge, H.R.; Estes, M.K.; Donowitz, M. Human Enteroids/Colonoids and Intestinal Organoids Functionally Recapitulate Normal Intestinal Physiology and Pathophysiology. J. Biol. Chem. 2016, 291, 3759–3766. [Google Scholar] [CrossRef]
- Beumer, J.; Puschhof, J.; Bauzá-Martinez, J.; Martínez-Silgado, A.; Elmentaite, R.; James, K.R.; Ross, A.; Hendriks, D.; Artegiani, B.; Busslinger, G.A.; et al. High-Resolution MRNA and Secretome Atlas of Human Enteroendocrine Cells. Cell 2020, 181, 1291–1306.e19. [Google Scholar] [CrossRef]
- Ohki, J.; Sakashita, A.; Aihara, E.; Inaba, A.; Uchiyama, H.; Matsumoto, M.; Ninomiya, Y.; Yamane, T.; Oishi, Y.; Iwatsuki, K. Comparative Analysis of Enteroendocrine Cells and Their Hormones between Mouse Intestinal Organoids and Native Tissues. Biosci. Biotechnol. Biochem. 2020, 84, 936–942. [Google Scholar] [CrossRef]
- Stroulios, G.; Stahl, M.; Elstone, F.; Chang, W.; Louis, S.; Eaves, A.; Simmini, S.; Conder, R.K. Culture Methods to Study Apical-Specific Interactions Using Intestinal Organoid Models. J. Vis. Exp. 2021. [Google Scholar] [CrossRef]
- Co, J.Y.; Margalef-Català, M.; Li, X.; Mah, A.T.; Kuo, C.J.; Monack, D.M.; Amieva, M.R. Controlling Epithelial Polarity: A Human Enteroid Model for Host-Pathogen Interactions. Cell Rep. 2019, 26, 2509–2520.e4. [Google Scholar] [CrossRef]
- VanDussen, K.L.; Marinshaw, J.M.; Shaikh, N.; Miyoshi, H.; Moon, C.; Tarr, P.I.; Ciorba, M.A.; Stappenbeck, T.S. Development of an Enhanced Human Gastrointestinal Epithelial Culture System to Facilitate Patient-Based Assays. Gut 2015, 64, 911–920. [Google Scholar] [CrossRef]
- Petersen, N.; Reimann, F.; van Es, J.H.; van den Berg, B.M.; Kroone, C.; Pais, R.; Jansen, E.; Clevers, H.; Gribble, F.M.; de Koning, E.J.P. Targeting Development of Incretin-Producing Cells Increases Insulin Secretion. J. Clin. Investig. 2015, 125, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Petersen, N.; Frimurer, T.M.; Terndrup Pedersen, M.; Egerod, K.L.; Wewer Albrechtsen, N.J.; Holst, J.J.; Grapin-Botton, A.; Jensen, K.B.; Schwartz, T.W. Inhibiting RHOA Signaling in Mice Increases Glucose Tolerance and Numbers of Enteroendocrine and Other Secretory Cells in the Intestine. Gastroenterology 2018, 155, 1164–1176.e2. [Google Scholar] [CrossRef] [PubMed]
- Chang-Graham, A.L.; Danhof, H.A.; Engevik, M.A.; Tomaro-Duchesneau, C.; Karandikar, U.C.; Estes, M.K.; Versalovic, J.; Britton, R.A.; Hyser, J.M. Human Intestinal Enteroids With Inducible Neurogenin-3 Expression as a Novel Model of Gut Hormone Secretion. Cell Mol. Gastroenterol. Hepatol. 2019, 8, 209–229. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.C.; Weber, G.J.; van Sambeek, D.M.; Soares, J.W.; Racicot, K.; Breault, D.T. Intestinal Enteroids Recapitulate the Effects of Short-Chain Fatty Acids on the Intestinal Epithelium. PLoS ONE 2020, 15, e0230231. [Google Scholar] [CrossRef]
- Petersen, N.; Reimann, F.; Bartfeld, S.; Farin, H.F.; Ringnalda, F.C.; Vries, R.G.J.; van den Brink, S.; Clevers, H.; Gribble, F.M.; de Koning, E.J.P. Generation of L-Cells in Mouse and Human Small Intestine Organoids. Diabetes 2014, 63, 410–420. [Google Scholar] [CrossRef]
- Zietek, T.; Rath, E.; Haller, D.; Daniel, H. Intestinal Organoids for Assessing Nutrient Transport, Sensing and Incretin Secretion. Sci. Rep. 2015, 5, 16831. [Google Scholar] [CrossRef]
- Goldspink, D.A.; Lu, V.B.; Miedzybrodzka, E.L.; Smith, C.A.; Foreman, R.E.; Billing, L.J.; Kay, R.G.; Reimann, F.; Gribble, F.M. Labeling and Characterization of Human GLP-1-Secreting L-Cells in Primary Ileal Organoid Culture. Cell Rep. 2020, 31, 107833. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Trends in Adult Body-Mass Index in 200 Countries from 1975 to 2014: A Pooled Analysis of 1698 Population-Based Measurement Studies with 19·2 Million Participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide Trends in Body-Mass Index, Underweight, Overweight, and Obesity from 1975 to 2016: A Pooled Analysis of 2416 Population-Based Measurement Studies in 128·9 Million Children, Adolescents, and Adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Batterham, R.L.; Heffron, H.; Kapoor, S.; Chivers, J.E.; Chandarana, K.; Herzog, H.; Le Roux, C.W.; Thomas, E.L.; Bell, J.D.; Withers, D.J. Critical Role for Peptide YY in Protein-Mediated Satiation and Body-Weight Regulation. Cell Metab. 2006, 4, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Verdich, C.; Toubro, S.; Buemann, B.; Lysgård Madsen, J.; Juul Holst, J.; Astrup, A. The Role of Postprandial Releases of Insulin and Incretin Hormones in Meal-Induced Satiety--Effect of Obesity and Weight Reduction. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Carr, R.D.; Larsen, M.O.; Jelic, K.; Lindgren, O.; Vikman, J.; Holst, J.J.; Deacon, C.F.; Ahrén, B. Secretion and Dipeptidyl Peptidase-4-Mediated Metabolism of Incretin Hormones after a Mixed Meal or Glucose Ingestion in Obese Compared to Lean, Nondiabetic Men. J. Clin. Endocrinol. Metab. 2010, 95, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Gerspach, A.C.; Wölnerhanssen, B.; Beglinger, B.; Nessenius, F.; Napitupulu, M.; Schulte, F.H.; Steinert, R.E.; Beglinger, C. Gastric and Intestinal Satiation in Obese and Normal Weight Healthy People. Physiol. Behav. 2014, 129, 265–271. [Google Scholar] [CrossRef]
- Færch, K.; Torekov, S.S.; Vistisen, D.; Johansen, N.B.; Witte, D.R.; Jonsson, A.; Pedersen, O.; Hansen, T.; Lauritzen, T.; Sandbæk, A.; et al. GLP-1 Response to Oral Glucose Is Reduced in Prediabetes, Screen-Detected Type 2 Diabetes, and Obesity and Influenced by Sex: The ADDITION-PRO Study. Diabetes 2015, 64, 2513–2525. [Google Scholar] [CrossRef]
- Nauck, M.; Stöckmann, F.; Ebert, R.; Creutzfeldt, W. Reduced Incretin Effect in Type 2 (Non-Insulin-Dependent) Diabetes. Diabetologia 1986, 29, 46–52. [Google Scholar] [CrossRef]
- Meier, J.J.; Nauck, M.A. Incretins and the Development of Type 2 Diabetes. Curr. Diab. Rep. 2006, 6, 194–201. [Google Scholar] [CrossRef]
- Vilsbøll, T.; Krarup, T.; Sonne, J.; Madsbad, S.; Vølund, A.; Juul, A.G.; Holst, J.J. Incretin Secretion in Relation to Meal Size and Body Weight in Healthy Subjects and People with Type 1 and Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2003, 88, 2706–2713. [Google Scholar] [CrossRef]
- Skrha, J.; Hilgertová, J.; Jarolímková, M.; Kunešová, M.; Hill, M. Meal Test for Glucose-Dependent Insulinotropic Peptide (GIP) in Obese and Type 2 Diabetic Patients. Physiol. Res. 2010, 59, 749–755. [Google Scholar] [CrossRef]
- Ahrén, B. Incretin Dysfunction in Type 2 Diabetes: Clinical Impact and Future Perspectives. Diabetes Metab. 2013, 39, 195–201. [Google Scholar] [CrossRef]
- Aulinger, B.A.; Vahl, T.P.; Prigeon, R.L.; D’Alessio, D.A.; Elder, D.A. The Incretin Effect in Obese Adolescents with and without Type 2 Diabetes: Impaired or Intact? Am. J. Physiol. Endocrinol. Metab. 2016, 310, E774–E781. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, K.; Holst, J.J.; Baller, B.; Ellrichmann, M.; Nauck, M.A.; Schmidt, W.E.; Meier, J.J. Predictors of Incretin Concentrations in Subjects with Normal, Impaired, and Diabetic Glucose Tolerance. Diabetes 2008, 57, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.F.; Vrang, N.; Sangild, P.T.; Jelsing, J. Novel Insight into the Distribution of L-Cells in the Rat Intestinal Tract. Am. J. Transl. Res. 2013, 5, 347–358. [Google Scholar]
- Theodorakis, M.J.; Carlson, O.; Michopoulos, S.; Doyle, M.E.; Juhaszova, M.; Petraki, K.; Egan, J.M. Human Duodenal Enteroendocrine Cells: Source of Both Incretin Peptides, GLP-1 and GIP. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E550–E559. [Google Scholar] [CrossRef]
- Kampmann, K.; Ueberberg, S.; Menge, B.A.; Breuer, T.G.K.; Uhl, W.; Tannapfel, A.; Meier, J.J. Abundance and Turnover of GLP-1 Producing L-Cells in Ileal Mucosa Are Not Different in Patients with and without Type 2 Diabetes. Metabolism 2016, 65, 84–91. [Google Scholar] [CrossRef]
- Wölnerhanssen, B.K.; Moran, A.W.; Burdyga, G.; Meyer-Gerspach, A.C.; Peterli, R.; Manz, M.; Thumshirn, M.; Daly, K.; Beglinger, C.; Shirazi-Beechey, S.P. Deregulation of Transcription Factors Controlling Intestinal Epithelial Cell Differentiation; a Predisposing Factor for Reduced Enteroendocrine Cell Number in Morbidly Obese Individuals. Sci. Rep. 2017, 7, 8174. [Google Scholar] [CrossRef]
- Wang, B.; Bobe, G.; LaPres, J.J.; Bourquin, L.D. High Sucrose Diets Promote Intestinal Epithelial Cell Proliferation and Tumorigenesis in APC(Min) Mice by Increasing Insulin and IGF-I Levels. Nutr. Cancer 2009, 61, 81–93. [Google Scholar] [CrossRef]
- Beyaz, S.; Mana, M.D.; Roper, J.; Kedrin, D.; Saadatpour, A.; Hong, S.-J.; Bauer-Rowe, K.E.; Xifaras, M.E.; Akkad, A.; Arias, E.; et al. High-Fat Diet Enhances Stemness and Tumorigenicity of Intestinal Progenitors. Nature 2016, 531, 53–58. [Google Scholar] [CrossRef]
- Aranias, T.; Grosfeld, A.; Poitou, C.; Omar, A.A.; Le Gall, M.; Miquel, S.; Garbin, K.; Ribeiro, A.; Bouillot, J.-L.; Bado, A.; et al. Lipid-Rich Diet Enhances L-Cell Density in Obese Subjects and in Mice through Improved L-Cell Differentiation. J. Nutr. Sci. 2015, 4, e22. [Google Scholar] [CrossRef]
- Otten, J.; Andersson, J.; Ståhl, J.; Stomby, A.; Saleh, A.; Waling, M.; Ryberg, M.; Hauksson, J.; Svensson, M.; Johansson, B.; et al. Exercise Training Adds Cardiometabolic Benefits of a Paleolithic Diet in Type 2 Diabetes Mellitus. J. Am. Heart Assoc. 2019, 8, e010634. [Google Scholar] [CrossRef]
- Jang, H.-J.; Kokrashvili, Z.; Theodorakis, M.J.; Carlson, O.D.; Kim, B.-J.; Zhou, J.; Kim, H.H.; Xu, X.; Chan, S.L.; Juhaszova, M.; et al. Gut-Expressed Gustducin and Taste Receptors Regulate Secretion of Glucagon-like Peptide-1. Proc. Natl. Acad. Sci. USA 2007, 104, 15069–15074. [Google Scholar] [CrossRef]
- Nuzzo, A.; Czernichow, S.; Hertig, A.; Ledoux, S.; Poghosyan, T.; Quilliot, D.; Le Gall, M.; Bado, A.; Joly, F. Prevention and Treatment of Nutritional Complications after Bariatric Surgery. Lancet Gastroenterol. Hepatol. 2021, 6, 238–251. [Google Scholar] [CrossRef]
- Korner, J.; Bessler, M.; Cirilo, L.J.; Conwell, I.M.; Daud, A.; Restuccia, N.L.; Wardlaw, S.L. Effects of Roux-En-Y Gastric Bypass Surgery on Fasting and Postprandial Concentrations of Plasma Ghrelin, Peptide YY, and Insulin. J. Clin. Endocrinol. Metab. 2005, 90, 359–365. [Google Scholar] [CrossRef] [PubMed]
- le Roux, C.W.; Welbourn, R.; Werling, M.; Osborne, A.; Kokkinos, A.; Laurenius, A.; Lönroth, H.; Fändriks, L.; Ghatei, M.A.; Bloom, S.R.; et al. Gut Hormones as Mediators of Appetite and Weight Loss after Roux-En-Y Gastric Bypass. Ann. Surg. 2007, 246, 780–785. [Google Scholar] [CrossRef]
- Jørgensen, N.B.; Jacobsen, S.H.; Dirksen, C.; Bojsen-Møller, K.N.; Naver, L.; Hvolris, L.; Clausen, T.R.; Wulff, B.S.; Worm, D.; Lindqvist Hansen, D.; et al. Acute and Long-Term Effects of Roux-En-Y Gastric Bypass on Glucose Metabolism in Subjects with Type 2 Diabetes and Normal Glucose Tolerance. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E122–E131. [Google Scholar] [CrossRef] [PubMed]
- Yousseif, A.; Emmanuel, J.; Karra, E.; Millet, Q.; Elkalaawy, M.; Jenkinson, A.D.; Hashemi, M.; Adamo, M.; Finer, N.; Fiennes, A.G.; et al. Differential Effects of Laparoscopic Sleeve Gastrectomy and Laparoscopic Gastric Bypass on Appetite, Circulating Acyl-Ghrelin, Peptide YY3-36 and Active GLP-1 Levels in Non-Diabetic Humans. Obes. Surg. 2014, 24, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Svane, M.S.; Bojsen-Møller, K.N.; Martinussen, C.; Dirksen, C.; Madsen, J.L.; Reitelseder, S.; Holm, L.; Rehfeld, J.F.; Kristiansen, V.B.; van Hall, G.; et al. Postprandial Nutrient Handling and Gastrointestinal Hormone Secretion After Roux-En-Y Gastric Bypass vs Sleeve Gastrectomy. Gastroenterology 2019, 156, 1627–1641.e1. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Singh, R.P.; Pothier, C.E.; Nissen, S.E.; et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes—5-Year Outcomes. N. Engl. J. Med. 2017, 376, 641–651. [Google Scholar] [CrossRef]
- Han, Y.; Jia, Y.; Wang, H.; Cao, L.; Zhao, Y. Comparative Analysis of Weight Loss and Resolution of Comorbidities between Laparoscopic Sleeve Gastrectomy and Roux-En-Y Gastric Bypass: A Systematic Review and Meta-Analysis Based on 18 Studies. Int. J. Surg. 2020, 76, 101–110. [Google Scholar] [CrossRef]
- Larraufie, P.; Roberts, G.P.; McGavigan, A.K.; Kay, R.G.; Li, J.; Leiter, A.; Melvin, A.; Biggs, E.K.; Ravn, P.; Davy, K.; et al. Important Role of the GLP-1 Axis for Glucose Homeostasis after Bariatric Surgery. Cell Rep. 2019, 26, 1399–1408.e6. [Google Scholar] [CrossRef]
- Martinussen, C.; Bojsen-Møller, K.N.; Dirksen, C.; Svane, M.S.; Kristiansen, V.B.; Hartmann, B.; Holst, J.J.; Madsbad, S. Augmented GLP-1 Secretion as Seen After Gastric Bypass May Be Obtained by Delaying Carbohydrate Digestion. J. Clin. Endocrinol. Metab. 2019, 104, 3233–3244. [Google Scholar] [CrossRef] [PubMed]
- Wallenius, V.; Dirinck, E.; Fändriks, L.; Maleckas, A.; le Roux, C.W.; Thorell, A. Glycemic Control after Sleeve Gastrectomy and Roux-En-Y Gastric Bypass in Obese Subjects with Type 2 Diabetes Mellitus. Obes. Surg. 2018, 28, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Nergård, B.J.; Lindqvist, A.; Gislason, H.G.; Groop, L.; Ekelund, M.; Wierup, N.; Hedenbro, J.L. Mucosal Glucagon-like Peptide-1 and Glucose-Dependent Insulinotropic Polypeptide Cell Numbers in the Super-Obese Human Foregut after Gastric Bypass. Surg. Obes. Relat. Dis. 2015, 11, 1237–1246. [Google Scholar] [CrossRef]
- Rhee, N.A.; Wahlgren, C.D.; Pedersen, J.; Mortensen, B.; Langholz, E.; Wandall, E.P.; Friis, S.U.; Vilmann, P.; Paulsen, S.J.; Kristiansen, V.B.; et al. Effect of Roux-En-Y Gastric Bypass on the Distribution and Hormone Expression of Small-Intestinal Enteroendocrine Cells in Obese Patients with Type 2 Diabetes. Diabetologia 2015, 58, 2254–2258. [Google Scholar] [CrossRef]
- Cavin, J.-B.; Couvelard, A.; Lebtahi, R.; Ducroc, R.; Arapis, K.; Voitellier, E.; Cluzeaud, F.; Gillard, L.; Hourseau, M.; Mikail, N.; et al. Differences in Alimentary Glucose Absorption and Intestinal Disposal of Blood Glucose After Roux-En-Y Gastric Bypass vs. Sleeve Gastrectomy. Gastroenterology 2016, 150, 454–464.e9. [Google Scholar] [CrossRef]
- Mumphrey, M.B.; Patterson, L.M.; Zheng, H.; Berthoud, H.-R. Roux-En-Y Gastric Bypass Surgery Increases Number but Not Density of CCK-, GLP-1-, 5-HT-, and Neurotensin-Expressing Enteroendocrine Cells in Rats. Neurogastroenterol. Motil. 2013, 25, e70–e79. [Google Scholar] [CrossRef]
- Mumphrey, M.B.; Hao, Z.; Townsend, R.L.; Patterson, L.M.; Berthoud, H.-R. Sleeve Gastrectomy Does Not Cause Hypertrophy and Reprogramming of Intestinal Glucose Metabolism in Rats. Obes. Surg. 2015, 25, 1468–1473. [Google Scholar] [CrossRef][Green Version]
- Arapis, K.; Cavin, J.B.; Gillard, L.; Cluzeaud, F.; Lettéron, P.; Ducroc, R.; Le Beyec, J.; Hourseau, M.; Couvelard, A.; Marmuse, J.-P.; et al. Remodeling of the Residual Gastric Mucosa after Roux-En-y Gastric Bypass or Vertical Sleeve Gastrectomy in Diet-Induced Obese Rats. PLoS ONE 2015, 10, e0121414. [Google Scholar] [CrossRef]
- Ribeiro-Parenti, L.; Jarry, A.-C.; Cavin, J.-B.; Willemetz, A.; Le Beyec, J.; Sannier, A.; Benadda, S.; Pelletier, A.-L.; Hourseau, M.; Léger, T.; et al. Bariatric Surgery Induces a New Gastric Mucosa Phenotype with Increased Functional Glucagon-like Peptide-1 Expressing Cells. Nat. Commun. 2021, 12, 110. [Google Scholar] [CrossRef]
- Peiris, M.; Aktar, R.; Raynel, S.; Hao, Z.; Mumphrey, M.B.; Berthoud, H.-R.; Blackshaw, L.A. Effects of Obesity and Gastric Bypass Surgery on Nutrient Sensors, Endocrine Cells, and Mucosal Innervation of the Mouse Colon. Nutrients 2018, 10, 1529. [Google Scholar] [CrossRef]
- Agus, A.; Clément, K.; Sokol, H. Gut Microbiota-Derived Metabolites as Central Regulators in Metabolic Disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Goodarzi, M.O. Metabolites Linking the Gut Microbiome with Risk for Type 2 Diabetes. Curr. Nutr. Rep. 2020, 9, 83–93. [Google Scholar] [CrossRef]
- Khan, M.T.; Nieuwdorp, M.; Bäckhed, F. Microbial Modulation of Insulin Sensitivity. Cell Metab. 2014, 20, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Freeland, K.R.; Wilson, C.; Wolever, T.M.S. Adaptation of Colonic Fermentation and Glucagon-like Peptide-1 Secretion with Increased Wheat Fibre Intake for 1 Year in Hyperinsulinaemic Human Subjects. Br. J. Nutr. 2010, 103, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Hoste, S.; Guiot, Y.; Delzenne, N.M. Dietary Non-Digestible Carbohydrates Promote L-Cell Differentiation in the Proximal Colon of Rats. Br. J. Nutr. 2007, 98, 32–37. [Google Scholar] [CrossRef]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The Short Chain Fatty Acid Propionate Stimulates GLP-1 and PYY Secretion via Free Fatty Acid Receptor 2 in Rodents. Int. J. Obes. (Lond.) 2015, 39, 424–429. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-Chain Fatty Acids Stimulate Glucagon-like Peptide-1 Secretion via the G-Protein-Coupled Receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef]
- Yadav, H.; Lee, J.-H.; Lloyd, J.; Walter, P.; Rane, S.G. Beneficial Metabolic Effects of a Probiotic via Butyrate-Induced GLP-1 Hormone Secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef]
- Wichmann, A.; Allahyar, A.; Greiner, T.U.; Plovier, H.; Lundén, G.Ö.; Larsson, T.; Drucker, D.J.; Delzenne, N.M.; Cani, P.D.; Bäckhed, F. Microbial Modulation of Energy Availability in the Colon Regulates Intestinal Transit. Cell Host. Microbe 2013, 14, 582–590. [Google Scholar] [CrossRef]
- Zhao, M.; Ren, K.; Xiong, X.; Cheng, M.; Zhang, Z.; Huang, Z.; Han, X.; Yang, X.; Alejandro, E.U.; Ruan, H.-B. Protein O-GlcNAc Modification Links Dietary and Gut Microbial Cues to the Differentiation of Enteroendocrine L Cells. Cell Rep. 2020, 32, 108013. [Google Scholar] [CrossRef]
- Arora, T.; Akrami, R.; Pais, R.; Bergqvist, L.; Johansson, B.R.; Schwartz, T.W.; Reimann, F.; Gribble, F.M.; Bäckhed, F. Microbial Regulation of the L Cell Transcriptome. Sci. Rep. 2018, 8, 1207. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-Mediated Bile Acid Sensing Controls Glucose Homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Kanazawa, A.; Watada, H. Type 2 Diabetes and Bacteremia. ANM 2017, 71, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Reijnders, D.; Goossens, G.H.; Hermes, G.D.A.; Neis, E.P.J.G.; van der Beek, C.M.; Most, J.; Holst, J.J.; Lenaerts, K.; Kootte, R.S.; Nieuwdorp, M.; et al. Effects of Gut Microbiota Manipulation by Antibiotics on Host Metabolism in Obese Humans: A Randomized Double-Blind Placebo-Controlled Trial. Cell Metab. 2016, 24, 63–74. [Google Scholar] [CrossRef]
- Vrieze, A.; Out, C.; Fuentes, S.; Jonker, L.; Reuling, I.; Kootte, R.S.; van Nood, E.; Holleman, F.; Knaapen, M.; Romijn, J.A.; et al. Impact of Oral Vancomycin on Gut Microbiota, Bile Acid Metabolism, and Insulin Sensitivity. J. Hepatol. 2014, 60, 824–831. [Google Scholar] [CrossRef]
- Dubé, P.E.; Brubaker, P.L. Frontiers in Glucagon-like Peptide-2: Multiple Actions, Multiple Mediators. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E460–E465. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in Gut Microbiota Control Inflammation in Obese Mice through a Mechanism Involving GLP-2-Driven Improvement of Gut Permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Chen, J.; Yi, K.; Peng, L.; Xie, J.; Gou, X.; Peng, T.; Tang, L. Phlorizin Ameliorates Obesity-Associated Endotoxemia and Insulin Resistance in High-Fat Diet-Fed Mice by Targeting the Gut Microbiota and Intestinal Barrier Integrity. Gut Microbes 2020, 12, 1–18. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.G.; Muccioli, G.M.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; François, P.; et al. Responses of Gut Microbiota and Glucose and Lipid Metabolism to Prebiotics in Genetic Obese and Diet-Induced Leptin-Resistant Mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Warmbrunn, M.V.; Nieuwdorp, M.; Clément, K. Metabolism and Metabolic Disorders and the Microbiome: The Intestinal Microbiota Associated With Obesity, Lipid Metabolism, and Metabolic Health-Pathophysiology and Therapeutic Strategies. Gastroenterology 2021, 160, 573–599. [Google Scholar] [CrossRef]
- Ghezzal, S.; Postal, B.G.; Quevrain, E.; Brot, L.; Seksik, P.; Leturque, A.; Thenet, S.; Carrière, V. Palmitic Acid Damages Gut Epithelium Integrity and Initiates Inflammatory Cytokine Production. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158530. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, F.; Smith, K.D.; Ozinsky, A.; Hawn, T.R.; Yi, E.C.; Goodlett, D.R.; Eng, J.K.; Akira, S.; Underhill, D.M.; Aderem, A. The Innate Immune Response to Bacterial Flagellin Is Mediated by Toll-like Receptor 5. Nature 2001, 410, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Ley, R.E.; Gewirtz, A.T. Intestinal Epithelial Cell Toll-like Receptor 5 Regulates the Intestinal Microbiota to Prevent Low-Grade Inflammation and Metabolic Syndrome in Mice. Gastroenterology 2014, 147, 1363–1377.e17. [Google Scholar] [CrossRef]
- Yang, J.; Yan, H. TLR5: Beyond the Recognition of Flagellin. Cell Mol. Immunol. 2017, 14, 1017–1019. [Google Scholar] [CrossRef]
- Vijay-Kumar, M.; Aitken, J.D.; Carvalho, F.A.; Cullender, T.C.; Mwangi, S.; Srinivasan, S.; Sitaraman, S.V.; Knight, R.; Ley, R.E.; Gewirtz, A.T. Metabolic Syndrome and Altered Gut Microbiota in Mice Lacking Toll-like Receptor 5. Science 2010, 328, 228–231. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M.; et al. Hyperglycemia Drives Intestinal Barrier Dysfunction and Risk for Enteric Infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef]
- Genser, L.; Aguanno, D.; Soula, H.A.; Dong, L.; Trystram, L.; Assmann, K.; Salem, J.-E.; Vaillant, J.-C.; Oppert, J.-M.; Laugerette, F.; et al. Increased Jejunal Permeability in Human Obesity Is Revealed by a Lipid Challenge and Is Linked to Inflammation and Type 2 Diabetes. J. Pathol. 2018, 246, 217–230. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osinski, C.; Moret, D.; Clément, K.; Serradas, P.; Ribeiro, A. Enteroendocrine System and Gut Barrier in Metabolic Disorders. Int. J. Mol. Sci. 2022, 23, 3732. https://doi.org/10.3390/ijms23073732
Osinski C, Moret D, Clément K, Serradas P, Ribeiro A. Enteroendocrine System and Gut Barrier in Metabolic Disorders. International Journal of Molecular Sciences. 2022; 23(7):3732. https://doi.org/10.3390/ijms23073732
Chicago/Turabian StyleOsinski, Céline, Dounia Moret, Karine Clément, Patricia Serradas, and Agnès Ribeiro. 2022. "Enteroendocrine System and Gut Barrier in Metabolic Disorders" International Journal of Molecular Sciences 23, no. 7: 3732. https://doi.org/10.3390/ijms23073732
APA StyleOsinski, C., Moret, D., Clément, K., Serradas, P., & Ribeiro, A. (2022). Enteroendocrine System and Gut Barrier in Metabolic Disorders. International Journal of Molecular Sciences, 23(7), 3732. https://doi.org/10.3390/ijms23073732





