PACAP-38 and PAC1 Receptor Alterations in Plasma and Cardiac Tissue Samples of Heart Failure Patients
Abstract
:1. Introduction
2. Results
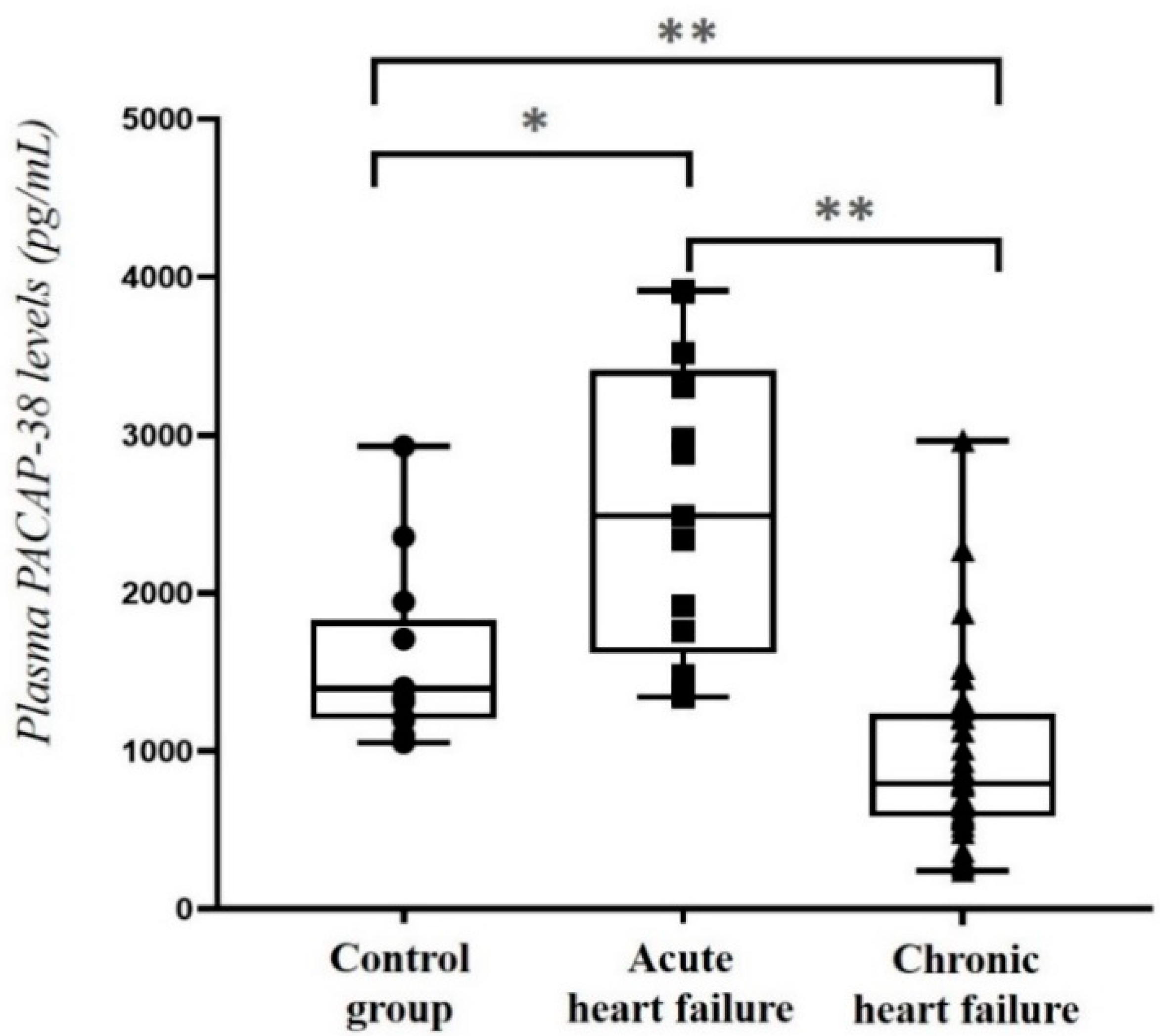
2.1. Comparison of Plasma PACAP-38 Levels in HF Patients and Healthy Control Individuals
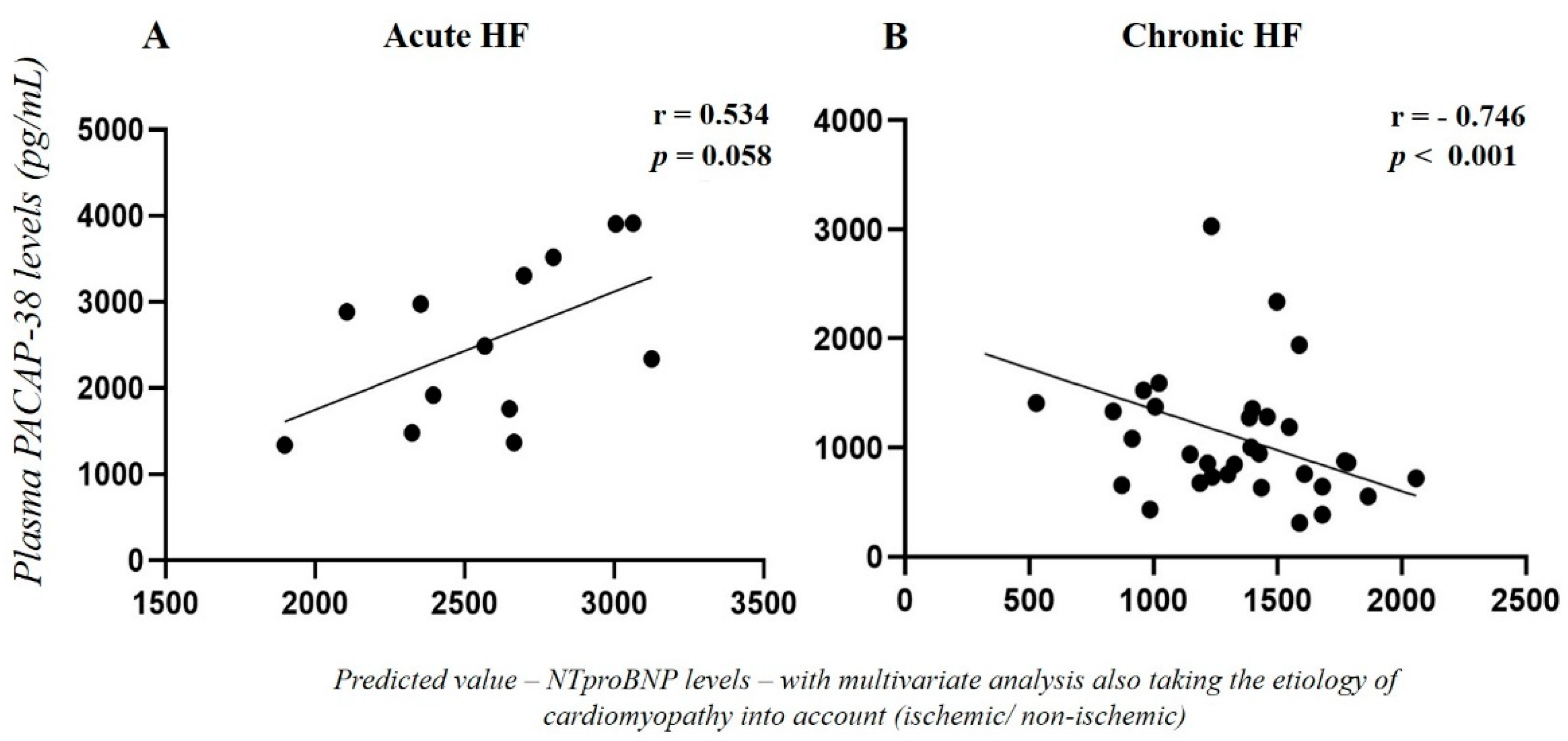
2.2. Correlation of Plasma PACAP-38 Levels with NT-proBNP Levels in HF Patients
2.3. Correlation of Plasma PACAP-38 Levels with Different Clinical and Laboratory Parameters
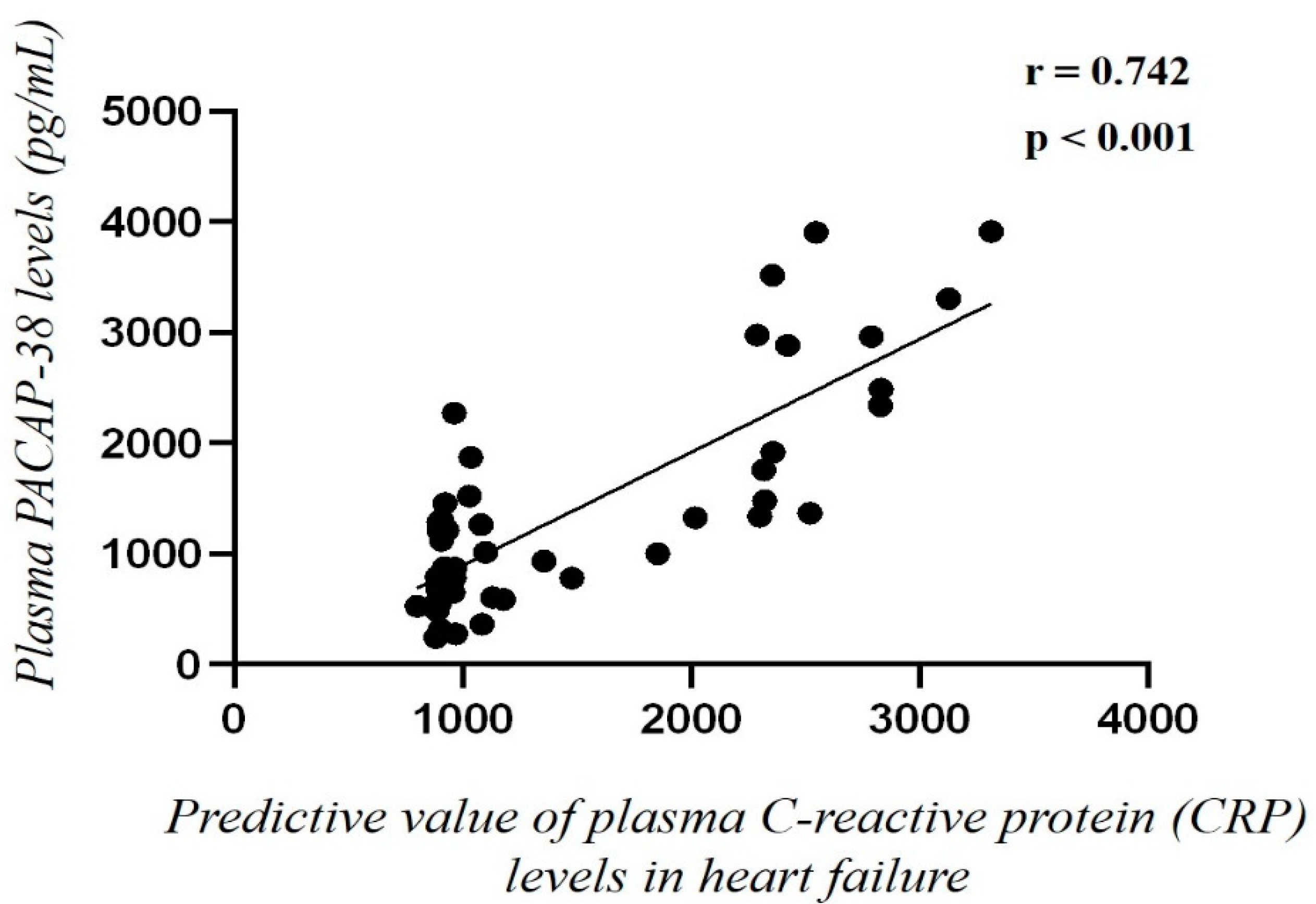
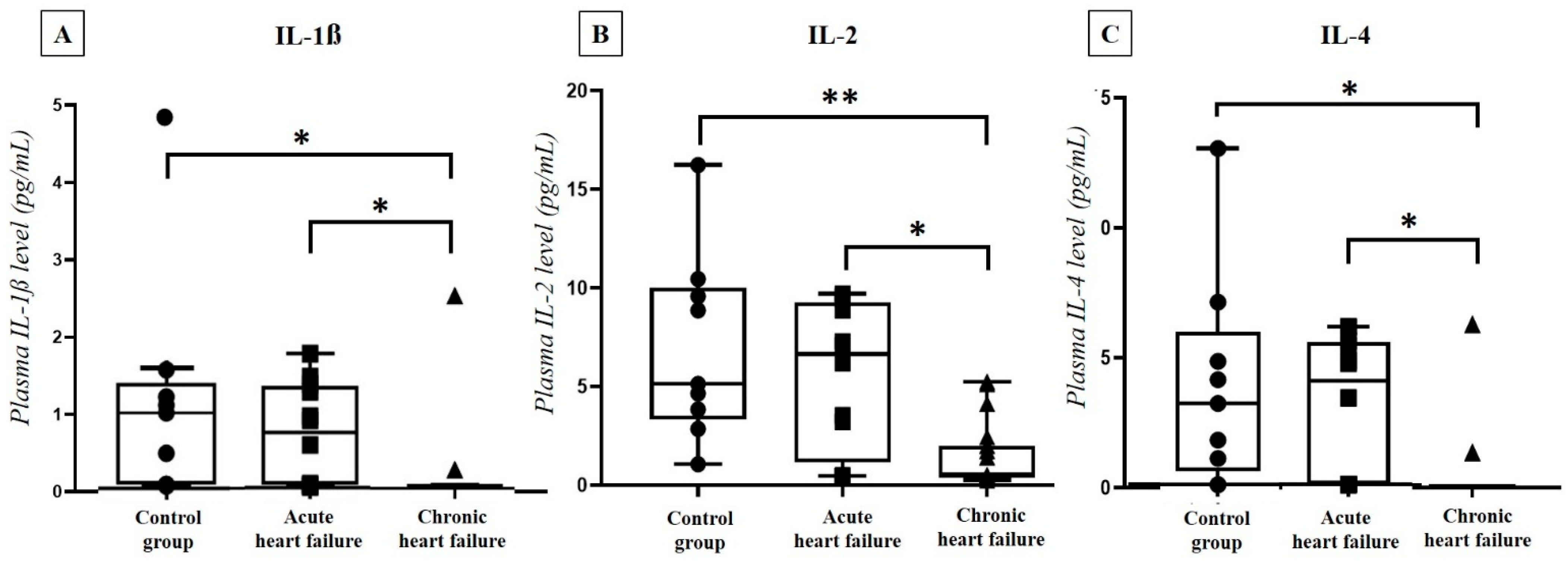
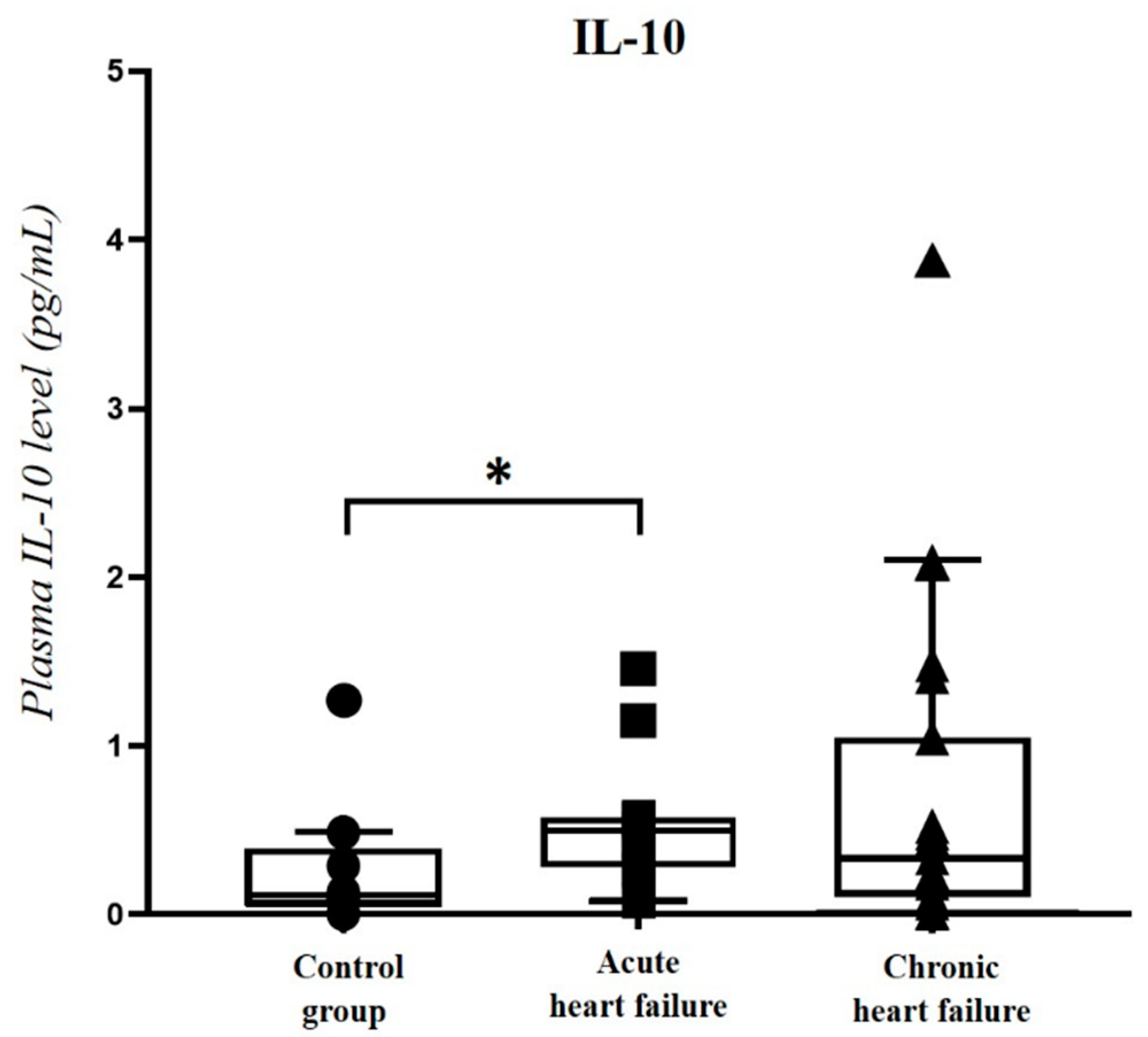
2.4. Correlation of Plasma PACAP-38 Levels with Pro- and Anti-Inflammatory Cytokines
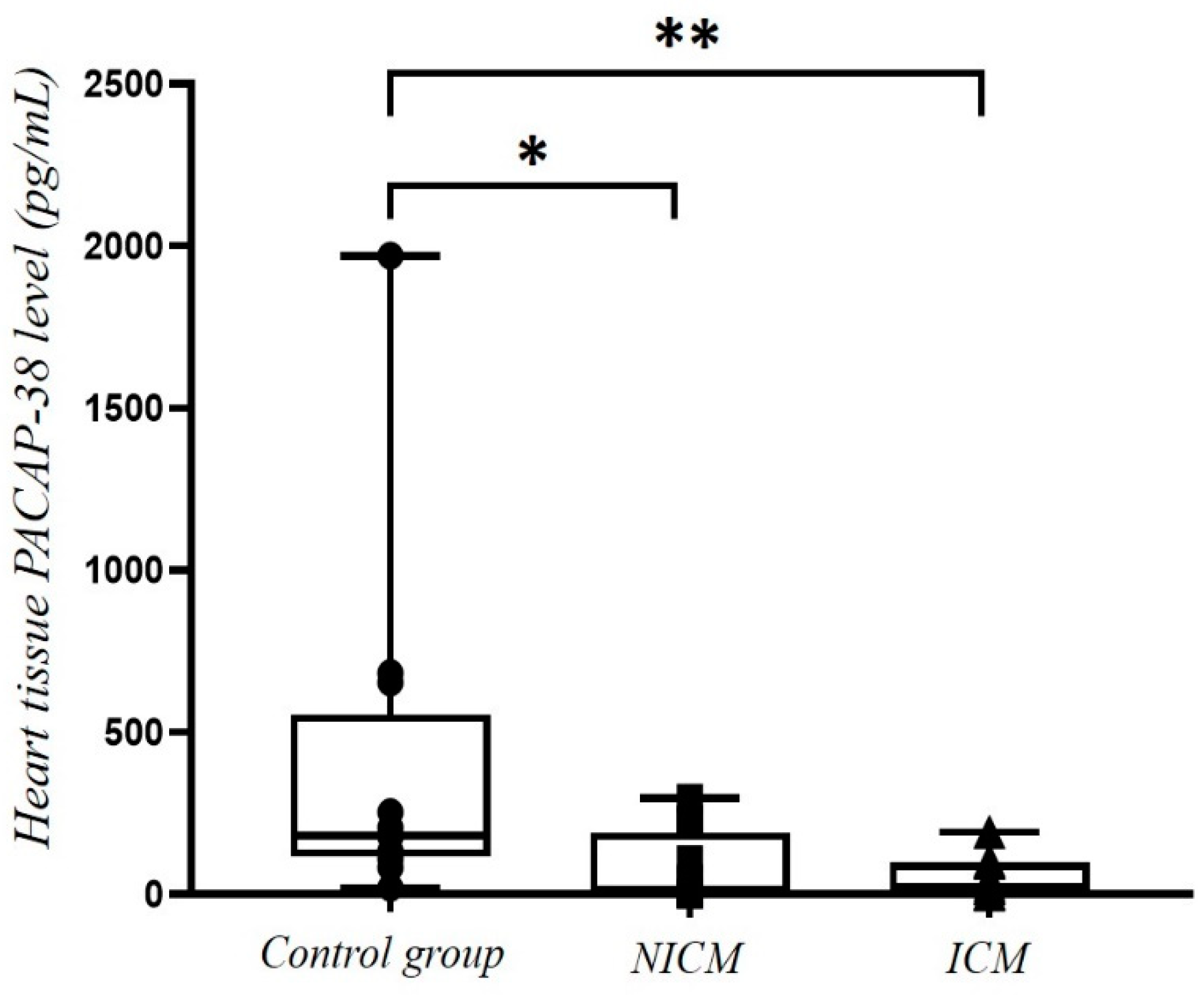
2.5. Comparison of PACAP-38 Levels in Heart Tissue Lysate of Non-Ischemic Cardiomyopathy (NICM) and Ischemic Cardiomyopathy (ICM) Patients and Healthy Controls
2.6. Correlation of Tissue PACAP-38 Levels with Different Clinical and Laboratory Parameters
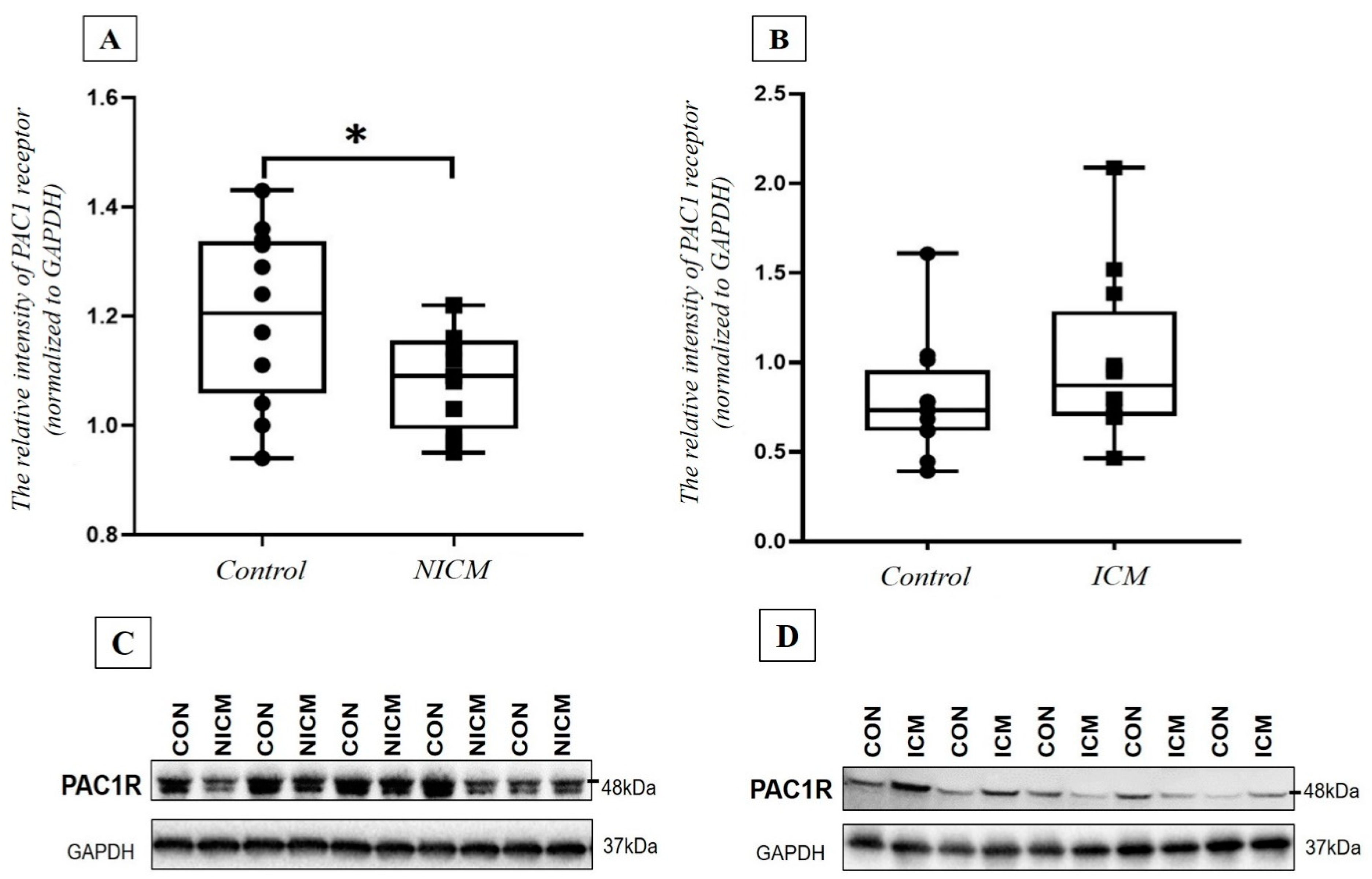
2.7. Comparison of PAC1 Receptor Level in the Heart Tissue Lysate of NICM and ICM Patients and Healthy Controls
3. Discussion
4. Materials and Methods
4.1. Plasma and Serum Samples of HF Patients
4.2. Cardiac Tissue Samples of HF Patients
4.3. Measurement of PACAP-38 Like Immunoreactivity (LI) by ELISA
4.4. Measurement of Pro- and Anti-Inflammatory Cytokine Levels with LUMINEX Array
4.5. Measurement of PAC1 Receptor Level by Western Blot Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.T.; Tseng, Y.T.; Chu, T.W.; Chen, J.; Lai, M.Y.; Tang, W.R.; Shiao, C.C. N-terminal pro b-type natriuretic peptide (NT-pro-BNP)-based score can predict in-hospital mortality in patients with heart failure. Sci. Rep. 2016, 6, 29590. [Google Scholar] [CrossRef]
- Taylor, K.S.; Verbakel, J.Y.; Feakins, B.G.; Price, C.P.; Perera, R.; Bankhead, C.; Pluddemann, A. Diagnostic accuracy of point-of-care natriuretic peptide testing for chronic heart failure in ambulatory care: Systematic review and meta-analysis. BMJ 2018, 361, k1450. [Google Scholar] [CrossRef] [Green Version]
- Pan, W.; Yang, D.; Yu, P.; Yu, H. Comparison of predictive value of NT-proBNP, sST2 and MMPs in heart failure patients with different ejection fractions. BMC Cardiovasc. Disord. 2020, 20, 208. [Google Scholar] [CrossRef]
- Hedayat, M.; Mahmoudi, M.J.; Rose, N.R.; Rezaei, N. Proinflammatory cytokines in heart failure: Double-edged swords. Heart Fail. Rev. 2010, 15, 543–562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bauersachs, J.; Langer, H.F. Immune mechanisms in heart failure. Eur. J. Heart Fail. 2017, 19, 1379–1389. [Google Scholar] [CrossRef] [Green Version]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.; Hashimoto, H.; Galas, L.; et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef] [PubMed]
- Bian, N.; Du, G.; Ip, M.F.; Ding, J.; Chang, Q.; Li, Z. Pituitary adenylate cyclase-activating polypeptide attenuates tumor necrosis factor-α-induced apoptosis in endothelial colony-forming cells. Biomed. Rep. 2017, 7, 11–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racz, B.; Gasz, B.; Borsiczky, B.; Gallyas, F., Jr.; Tamas, A.; Jozsa, R.; Lubics, A.; Kiss, P.; Roth, E.; Ferencz, A.; et al. Protective effects of pituitary adenylate cyclase activating polypeptide in endothelial cells against oxidative stress-induced apoptosis. Gen. Comp. Endocrinol. 2007, 153, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Racz, B.; Gasz, B.; Gallyas, F., Jr.; Kiss, P.; Tamas, A.; Szanto, Z.; Lubics, A.; Lengvari, I.; Toth, G.; Hegyi, O.; et al. PKA-Bad-14-3-3 and Akt-Bad-14-3-3 signaling pathways are involved in the protective effects of PACAP against ischemia/reperfusion-induced cardiomyocyte apoptosis. Regul. Pept. 2008, 145, 105–115. [Google Scholar] [CrossRef]
- Reglodi, D.; Tamas, A. Pituitary Adenylate Cyclase Activating Polypeptide–PACAP, 1st ed.; Springer: Cham, Switzerland, 2016; ISBN 978-3-319-35135-3. [Google Scholar]
- Roth, E.; Weber, G.; Kiss, P.; Horvath, G.; Toth, G.; Gasz, B.; Ferencz, A.; Gallyas, F., Jr.; Reglodi, D.; Racz, B. Effects of PACAP and preconditioning against ischemia/reperfusion-induced cardiomyocyte apoptosis in vitro. Ann. N. Y. Acad. Sci. 2009, 1163, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Seaborn, T.; Masmoudi-Kouli, O.; Fournier, A.; Vaudry, H.; Vaudry, D. Protective effects of pituitary adenylate cyclase-activating polypeptide (PACAP) against apoptosis. Curr. Pharm. Des. 2011, 17, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Sano, H.; Miyata, A.; Horio, T.; Nishikimi, T.; Matsuo, H.; Kangawa, K. The effect of pituitary adenylate cyclase activating polypeptide on cultured rat cardiocytes as a cardioprotective factor. Regul. Pept. 2002, 109, 107–113. [Google Scholar] [CrossRef]
- Gasz, B.; Racz, B.; Roth, E.; Borsiczky, B.; Tamas, A.; Boronkai, A.; Gallyas, F., Jr.; Toth, G.; Reglodi, D. PACAP inhibits oxidative stress-induced activation of MAP kinase-dependent apoptotic pathway in cultured cardiomyocytes. Ann. N. Y. Acad. Sci. 2006, 1070, 293–297. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Szobi, A.; Gonçalvesova, E.; Varga, Z.V.; Leszek, P.; Kusmierczyk, M.; Hulman, M.; Kyselovič, J.; Ferdinandy, P.; Adameova, A. Analysis of necroptotic proteins in failing human hearts. J. Transl. Med. 2017, 15, 86. [Google Scholar] [CrossRef] [Green Version]
- Songbo, M.; Lang, H.; Xinyong, C.; Bin, X.; Ping, Z.; Liang, S. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol. Lett. 2019, 307, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Nakamachi, T.; Ohtaki, H.; Yofu, S.; Sato, A.; Endo, K.; Iso, Y.; Suzuki, H.; Takeyama, Y.; Shintani, N.; et al. Cardioprotective effect of endogenous pituitary adenylate cyclase activating polypeptide on doxorubicin-induced cardiomyopathy in mice. Circ. J. 2010, 74, 1183–1190. [Google Scholar] [CrossRef] [Green Version]
- Subramaniam, V.; Chuang, G.; Xia, H.; Burn, B.; Bradley, J.; Maderdrut, J.L.; Coy, D.H.; Varner, K.J. Pituitary adenylate cyclase-activating polypeptide (PACAP) protects against mitoxantrone-induced cardiac injury in mice. Peptides 2017, 95, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.; Hein, L.; Brede, M.; Jahns, R.; Engelhardt, S.; Grone, H.J.; Schutz, G. Pulmonary hypertension and right heart failure in pituitary adenylate cyclase-activating polypeptide type I receptor-deficient mice. Circulation 2004, 110, 3245–3251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabo, D.; Sarszegi, Z.; Polgar, B.; Saghy, E.; Nemeth, A.; Reglodi, D.; Makkos, A.; Gorbe, A.; Helyes, Z.; Ferdinandy, P.; et al. PACAP-38 in acute ST-segment elevation myocardial infarction in humans and pigs: A translational study. Int. J. Mol. Sci. 2021, 22, 2883. [Google Scholar] [CrossRef]
- Perna, E.R.; Macin, S.M.; Canella, J.P.; Augier, N.; Stival, J.L.; Cialzeta, J.R.; Pitzus, A.E.; Garcia, E.H.; Obregon, R.; Brizuela, M.; et al. Ongoing myocardial injury in stable severe heart failure: Value of cardiac troponin T monitoring for high-risk patient identification. Circulation 2004, 110, 2376–2382. [Google Scholar] [CrossRef]
- Tamas, A.; Toth, D.; Pham, D.; Loibl, C.; Rendeki, S.; Csontos, C.; Rozanovic, M.; Bogar, L.; Polgar, B.; Nemeth, J.; et al. Changes of pituitary adenylate cyclase activating polypeptide (PACAP-38) level in polytrauma patients in the early post-traumatic period. Peptides 2021, 146, 170645. [Google Scholar] [CrossRef]
- Sarszegi, Z.; Szabo, D.; Gaszner, B.; Konyi, A.; Reglodi, D.; Nemeth, J.; Lelesz, B.; Polgar, B.; Jungling, A.; Tamas, A. Examination of pituitary adenylate cyclase-activating polypeptide (PACAP-38) as a potential biomarker in heart failure patients. J. Mol. Neurosci. 2019, 68, 368–376. [Google Scholar] [CrossRef]
- Mueller, C.; McDonald, K.; de Boer, R.A.; Maisel, A.; Cleland, J.; Kozhuharov, N.; Coats, A.; Metra, M.; Mebazaa, A.; Ruschitzka, F.; et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur. J. Heart Fail. 2019, 21, 715–731. [Google Scholar] [CrossRef] [Green Version]
- Gage, J.R.; Fonarow, G.; Hamilton, M.; Widawski, M.; Martínez-Maza, O.; Vredevoe, D.L. Beta blocker and angiotensin-converting enzyme inhibitor therapy is associated with decreased Th1/Th2 cytokine ratios and inflammatory cytokine production in patients with chronic heart failure. Neuroimmunomodulation 2004, 11, 173–180. [Google Scholar] [CrossRef]
- Lappegård, K.T.; Bjørnstad, H.; Mollnes, T.E.; Hovland, A. Effect of cardiac resynchronization therapy on inflammation in congestive heart failure: A review. Scand. J. Immunol. 2015, 82, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Bartekova, M.; Radosinska, J.; Jelemensky, M.; Dhalla, N.S. Role of cytokines and inflammation in heart function during health and disease. Heart Fail. Rev. 2018, 23, 733–758. [Google Scholar] [CrossRef] [PubMed]
- Hanna, A.; Frangogiannis, N.G. Inflammatory cytokines and chemokines as therapeutic targets in heart failure. Cardiovasc. Drugs Ther. 2020, 34, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Ge, J.; Li, X. Association of IL-1β polymorphisms and plasma levels with chronic heart failure: A case-control study in Chinese patients. Eur. J. Infl. 2018, 16, 2058739218818686. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Cen, Z.; Wei, B.; Wu, W.; Zhou, Q. Increased circulating interleukin 10-secreting B cells in patients with dilated cardiomyopathy. Int. J. Clin. Exp. Pathol. 2015, 8, 8107–8114. [Google Scholar]
- Levick, S.P.; Goldspink, P.H. Could interferon-gamma be a therapeutic target for treating heart failure? Heart Fail. Rev. 2014, 19, 227–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, E.S.; Packer, M.; Lo, K.H.; Fasanmade, A.A.; Willerson, J.T. Anti-TNF therapy against congestive heart failure investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 2003, 107, 3133–3140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumura, T.; Tsushima, K.; Ohtaki, E.; Misu, K.; Tohbaru, T.; Asano, R.; Nagayama, M.; Kitahara, K.; Umemura, J.; Sumiyoshi, T.; et al. Effects of carvedilol on plasma levels of interleukin-6 and tumor necrosis factor-alpha in nine patients with dilated cardiomyopathy. J. Cardiol. 2002, 39, 253–257. [Google Scholar]
- Szanto, Z.; Sarszegi, Z.; Reglodi, D.; Nemeth, J.; Szabadfi, K.; Kiss, P.; Varga, A.; Banki, E.; Csanaky, K.; Gaszner, B.; et al. PACAP-38 immunoreactivity in human malignant tumor samples and cardiac diseases. J. Mol. Neurosci. 2012, 48, 667–673. [Google Scholar] [CrossRef]
- Alston, E.N.; Parrish, D.C.; Hasan, W.; Tharp, K.; Pahlmeyer, L.; Habecker, B.A. Cardiac ischemia-reperfusion regulates sympathetic neuropeptide expression through gp130-dependent and independent mechanisms. Neuropeptides 2011, 45, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Miller, W.L.; Hartman, A.K.; Burritt, M.F.; Burnett, J.C., Jr.; Jaffe, A.S. Mortality in end stage heart failure is associated with paradoxically low NT-pro BNP and BNP levels: “natriuretic peptide exhaustion”? J. Card. Fail. 2004, 10, s45. [Google Scholar] [CrossRef]
- Dadson, K.; Hauck, L.; Billia, F. Molecular mechanisms in cardiomyopathy. Clin. Sci. 2017, 131, 1375–1392. [Google Scholar] [CrossRef] [PubMed]
- Tomai, F.; Crea, F.; Chiariello, L.; Gioffrè, P.A. Ischemic preconditioning in humans: Models, mediators, and clinical relevance. Circulation 1999, 100, 559–563. [Google Scholar] [CrossRef]
- Varga, Z.V.; Pipicz, M.; Baan, J.A.; Baranyai, T.; Koncsos, G.; Leszek, P.; Kuśmierczyk, M.; Sánchez-Cabo, F.; García-Pavía, P.; Brenner, G.J.; et al. Alternative splicing of NOX4 in the failing human heart. Front. Physiol. 2017, 8, 935. [Google Scholar] [CrossRef] [Green Version]
- Baranyai, T.; Herczeg, K.; Onodi, Z.; Voszka, I.; Modos, K.; Marton, N.; Nagy, G.; Mager, I.; Wood, M.J.; El Andaloussi, S.; et al. Isolation of exosomes from blood plasma: Qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS ONE 2015, 10, e0145686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Acute HF (n = 13) | Chronic HF (n = 33) | Control Group (n = 13) | |
|---|---|---|---|
| Mean age (year) | 66.5 ± 3.7 | 65.9 ± 3.8 | 65.8 ± 4.0 |
| Gender | 33% women 67% men | 34.3% women 65.7% men | 31% women 69% men |
| Mean ejection fraction (%) | 33.1% | 30.3% | 38.5% |
| NYHA stage | III–IV. st. | II. st. | I. st. |
| Cardiovascular status | decompensated | compensated | no heart failure |
| Comorbidities | |||
| Hypertension | 69.2% | 87.9% | 46.1% |
| Diabetes mellitus | 46.2% | 42.4% | 15.4% |
| Atrial fibrillation | 53.8% | 36.4% | 7.7% |
| Medical therapy | |||
| ACEI/ARB | 100% | 100% | 38.5% |
| β-blocker | 100% | 100% | 38.5% |
| MRA | 76.9% | 81.8% | 0% |
| Diuretics | 92.3% | 84.8% | 15.4% |
| Ivabradine | 23.1% | 9.0% | 0% |
| Digoxin | 15.4% | 15.2% | 0% |
| Correlation Coefficient (r) | Significance (p) | |
|---|---|---|
| Comorbidities | ||
| Hypertension | r = −0.095 | p = 0.532 |
| Diabetes mellitus | r = 0.003 | p = 0.983 |
| Atrial fibrillation | r = 0.064 | p = 0.671 |
| Therapy | ||
| ACEI/ARB | - | - |
| β-blocker | - | - |
| MRA | r = 0.031 | p = 0.178 |
| Diuretics | r = 0.081 | p = 0.708 |
| Ivabradin | r = 0.206 | p = 0.326 |
| Digoxin | r = 0.048 | p = 0.822 |
| CRT | r = 0.005 | p = 0.973 |
| ICD | r = 0.067 | p = 0.659 |
| Echocardiographic parameters | ||
| EF (%) | r = 0.113 | p = 0.456 |
| LV-EDD (mm) | r = 0.063 | p = 0.689 |
| RV-EDD (mm) | r = −0.012 | p = 0.938 |
| Mitral regurgitation | r = 0.045 | p = 0.776 |
| Tricuspid regurgitation | r = 0.034 | p = 0.827 |
| IVC (mm) | r = 0.067 | p = 0.671 |
| Laboratory parameters | ||
| Cholesterol | r = 0.043 | p = 0.736 |
| LDL cholesterol | r = 0.183 | p = 0.474 |
| HDL cholesterol | r = 0.041 | p = 0.826 |
| Triglycerides | r = 0.033 | p = 0.354 |
| Blood urea nitrogen | r = 0.010 | p = 0.946 |
| Serum creatinine | r = 0.100 | p = 0.514 |
| Cytokines | Correlation Test | Multivariate Analysis | ||
|---|---|---|---|---|
| Correlation Coefficient (r) | Significance (p) | Correlation Coefficient (r) | Significance (p) | |
| IL-1 β | r = 0.539 * | p = 0.002 | r = 0.780 ** | p < 0.001 |
| IL-2 | r = 0.494 * | p = 0.005 | r = 0.812 ** | p < 0.001 |
| IL-4 | r = 0.481 * | p = 0.006 | r = 0.800 ** | p < 0.001 |
| IL-10 | r = 0.367 * | p = 0.042 | r = 0.799 ** | p < 0.001 |
| NICM (n = 11) | ICM (n = 12) | |
|---|---|---|
| Mean age (years) | 39.18 ± 3.4 | 59.42 ± 3.8 |
| Gender | 90.9% men 9.1% women | 91.7% men 8.3% women |
| Echocardiographic parameters | ||
| End-diastolic diameter (mm) | 75.45 ± 3.1 | 72.92 ± 1.9 |
| End-systolic diameter (mm) | 67.34 ± 2.9 | 65.75 ± 1.8 |
| Ejection fraction (%) | 17.09 ± 1.4 | 21.92 ± 2.3 |
| Hemodynamic parameters | ||
| Cardiac output (L/min) | 4.03 ± 0.22 | 4.28 ± 0.56 |
| Mean heart rate (bpm) | 104.5 ± 3.1 | 74.33 ± 4.9 |
| Mean systolic blood pressure (mmHg) | 99.56 ± 3.4 | 107.17 ± 4.9 |
| Mean diastolic blood pressure (mmHg) | 62.89 ± 4.1 | 56.58 ± 4.6 |
| Correlation Coefficient (r) | Significance (p) | |
|---|---|---|
| NT-proBNP (pg/mL) | ||
| All patients | r = −0.167 | p = 0.435 |
| NICM | r = −0.041 | p = 0.899 |
| ICM | r = −0.254 | p = 0.425 |
| with multivariate analysis | r = −0.187 | p = 0.688 |
| Echocardiographic parameters | ||
| EF (%) | r = 0.146 | p = 0.494 |
| LV-EDD (mm) | r = 0.167 | p = 0.369 |
| RV-EDD (mm) | r = −0.177 | p = 0.407 |
| Posterior wall thickness (mm) | r = 0.240 | p = 0.451 |
| septal wall thickness (mm) | r = 0.197 | p = 0.540 |
| Routine laboratory tests | ||
| Cholesterol | r = 0.068 | p = 0.751 |
| LDL cholesterol | r = 0.089 | p = 0.693 |
| HDL cholesterol | r = 0.057 | p = 0.766 |
| Triglycerides | r = 0.129 | p = 0.567 |
| Blood urea nitrogen | r = −0.031 | p = 0.887 |
| Creatinine | r = −0.122 | p = 0.578 |
| Sodium | r = −0.280 | p = 0.196 |
| Potassium | r = −0.307 | p = 0.154 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, D.; Sárszegi, Z.; Polgár, B.; Sághy, É.; Reglődi, D.; Tóth, T.; Onódi, Z.; Leszek, P.; Varga, Z.V.; Helyes, Z.; et al. PACAP-38 and PAC1 Receptor Alterations in Plasma and Cardiac Tissue Samples of Heart Failure Patients. Int. J. Mol. Sci. 2022, 23, 3715. https://doi.org/10.3390/ijms23073715
Szabó D, Sárszegi Z, Polgár B, Sághy É, Reglődi D, Tóth T, Onódi Z, Leszek P, Varga ZV, Helyes Z, et al. PACAP-38 and PAC1 Receptor Alterations in Plasma and Cardiac Tissue Samples of Heart Failure Patients. International Journal of Molecular Sciences. 2022; 23(7):3715. https://doi.org/10.3390/ijms23073715
Chicago/Turabian StyleSzabó, Dóra, Zsolt Sárszegi, Beáta Polgár, Éva Sághy, Dóra Reglődi, Tünde Tóth, Zsófia Onódi, Przemyslaw Leszek, Zoltán V. Varga, Zsuzsanna Helyes, and et al. 2022. "PACAP-38 and PAC1 Receptor Alterations in Plasma and Cardiac Tissue Samples of Heart Failure Patients" International Journal of Molecular Sciences 23, no. 7: 3715. https://doi.org/10.3390/ijms23073715
APA StyleSzabó, D., Sárszegi, Z., Polgár, B., Sághy, É., Reglődi, D., Tóth, T., Onódi, Z., Leszek, P., Varga, Z. V., Helyes, Z., Kemény, Á., Ferdinandy, P., & Tamás, A. (2022). PACAP-38 and PAC1 Receptor Alterations in Plasma and Cardiac Tissue Samples of Heart Failure Patients. International Journal of Molecular Sciences, 23(7), 3715. https://doi.org/10.3390/ijms23073715










