GABAA Receptor Autoantibodies Decrease GABAergic Synaptic Transmission in the Hippocampal CA3 Network
Abstract
:1. Introduction
2. Results
2.1. GABAA Receptor Autoantibodies (rAb-IP2) Reduce Inhibitory Postsynaptic Signaling in Hippocampal CA1 Pyramidal Cells
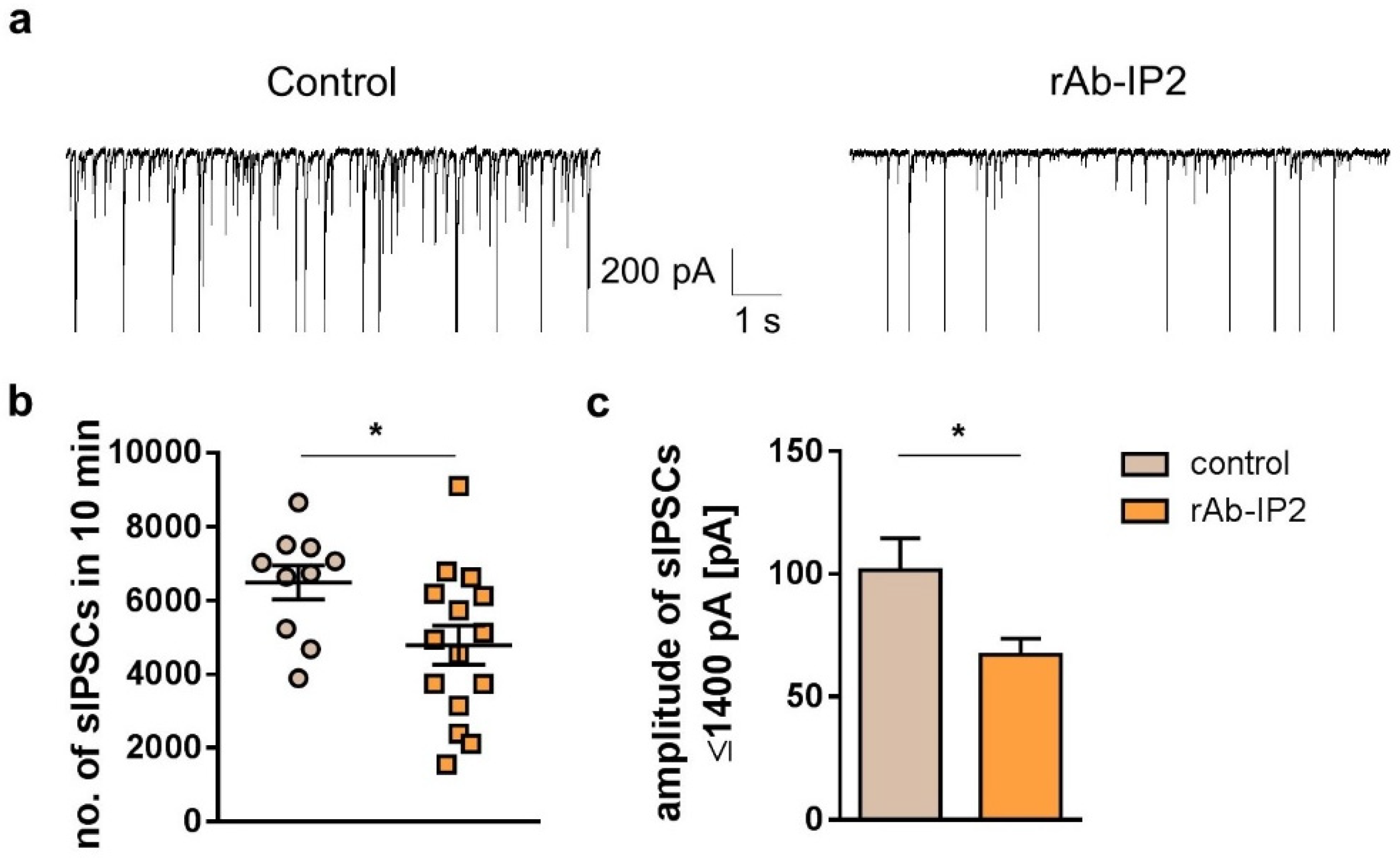
2.2. GABAA Receptor Autoantibodies Decrease GABAergic Synaptic Transmission in Hippocampal CA3 Network
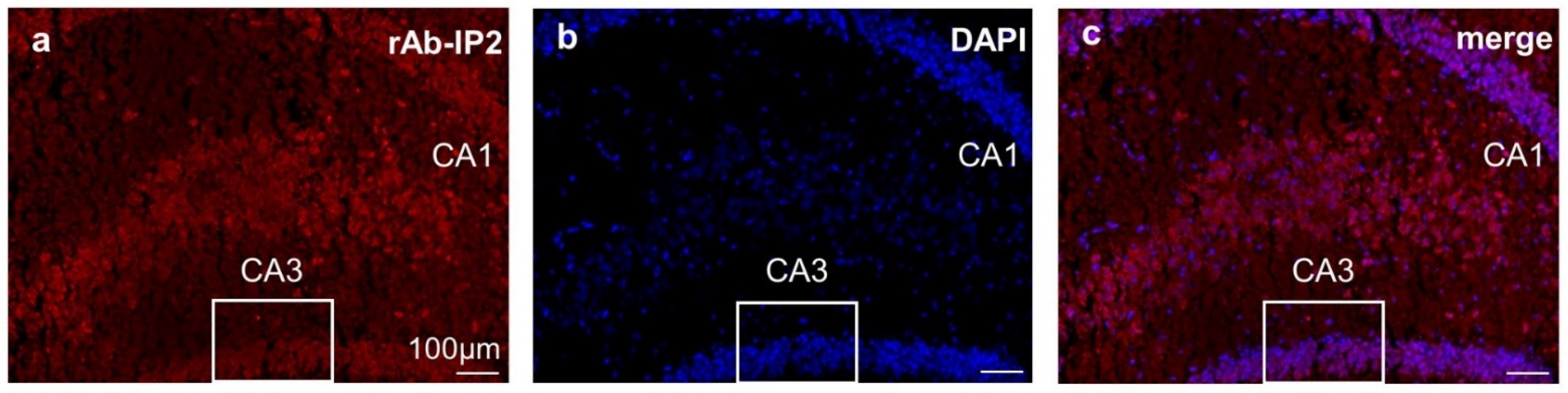
2.3. Immunohistochemical Staining Confirmed GABAA Receptor Autoantibody Binding to Hippocampal Tissue
3. Discussion
4. Materials and Methods
4.1. Clinical Samples
4.2. Animals
4.3. Preparation of Acute Murine Brain Slices
4.4. Incubation of Acute Murine Brain Slices with the GABAAR Autoantibody rAb-IP2
4.5. Electrophysiological Recordings by Patch-Clamp Technique
Voltage-Clamp Analysis
4.6. Immunohistochemistry
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jacob, T.C.; Moss, S.J.; Jurd, R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat. Rev. Neurosci. 2008, 9, 331–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, R.W.; Sieghart, W. GABA A receptors: Subtypes provide diversity of function and pharmacology. Neuropharmacology 2009, 56, 141–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrant, M.; Nusser, Z. Variations on an inhibitory theme: Phasic and tonic activation of GABA(A) receptors. Nat. Rev. Neurosci. 2005, 6, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, U.; Knoflach, F. Beyond classical benzodiazepines: Novel therapeutic potential of GABAA receptor subtypes. Nat. Rev. Drug Discov. 2011, 10, 685–697. [Google Scholar] [CrossRef] [Green Version]
- Petit-Pedrol, M.; Armangue, T.; Peng, X.; Bataller, L.; Cellucci, T.; Davis, R.; McCracken, L.; Martinez-Hernandez, E.; Mason, W.P.; Kruer, M.C.; et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: A case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol. 2014, 13, 276–286. [Google Scholar] [CrossRef] [Green Version]
- Spatola, M.; Petit-Pedrol, M.; Simabukuro, M.M.; Armangue, T.; Castro, F.J.; Barcelo Artigues, M.I.; Julià Benique, M.R.; Benson, L.; Gorman, M.; Felipe, A.; et al. Investigations in GABAA receptor antibody-associated encephalitis. Neurology 2017, 88, 1012–1020. [Google Scholar] [CrossRef] [Green Version]
- Pettingill, P.; Kramer, H.B.; Coebergh, J.A.; Pettingill, R.; Maxwell, S.; Nibber, A.; Malaspina, A.; Jacob, A.; Irani, S.R.; Buckley, C.; et al. Antibodies to GABAA receptor α1 and γ2 subunits: Clinical and serologic characterization. Neurology 2015, 84, 1233–1241. [Google Scholar] [CrossRef] [Green Version]
- Ohkawa, T.; Satake, S.; Yokoi, N.; Miyazaki, Y.; Ohshita, T.; Sobue, G.; Takashima, H.; Watanabe, O.; Fukata, Y.; Fukata, M. Identification and characterization of GABA(A) receptor autoantibodies in autoimmune encephalitis. J. Neurosci. 2014, 34, 8151–8163. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Huang, Z.; Ding, L.; Deel, M.E.; Arain, F.M.; Murray, C.R.; Patel, R.S.; Flanagan, C.D.; Gallagher, M.J. Altered cortical GABAA receptor composition, physiology, and endocytosis in a mouse model of a human genetic absence epilepsy syndrome. J. Biol. Chem. 2013, 288, 21458–21472. [Google Scholar] [CrossRef] [Green Version]
- Dalmau, J.; Graus, F. Antibody-Mediated Encephalitis. N. Engl. J. Med. 2018, 378, 840–851. [Google Scholar] [CrossRef] [Green Version]
- Bracher, A.; Alcalá, C.; Ferrer, J.; Melzer, N.; Hohlfeld, R.; Casanova, B.; Beltrán, E.; Dornmair, K. An expanded parenchymal CD8+ T cell clone in GABAA receptor encephalitis. Ann. Clin. Transl. Neurol. 2020, 7, 239–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreye, J.; Wright, S.K.; van Casteren, A.; Stöffler, L.; Machule, M.L.; Reincke, S.M.; Nikolaus, M.; van Hoof, S.; Sanchez-Sendin, E.; Homeyer, M.A.; et al. Encephalitis patient-derived monoclonal GABAA receptor antibodies cause epileptic seizures. J. Exp. Med. 2021, 218, e20210012. [Google Scholar] [CrossRef] [PubMed]
- Brändle, S.M.; Cerina, M.; Weber, S.; Held, K.; Menke, A.F.; Alcalá, C.; Gebert, D.; Herrmann, A.M.; Pellkofer, H.; Gerdes, L.A.; et al. Cross-reactivity of a pathogenic autoantibody to a tumor antigen in GABAA receptor encephalitis. Proc. Natl. Acad. Sci. USA 2021, 118, e1916337118. [Google Scholar] [CrossRef] [PubMed]
- Spruston, N. Pyramidal neurons: Dendritic structure and synaptic integration. Nat. Rev. Neurosci. 2008, 9, 206–221. [Google Scholar] [CrossRef]
- Lopes da Silva, F.H.; Witter, M.P.; Boeijinga, P.H.; Lohman, A.H. Anatomic organization and physiology of the limbic cortex. Physiol. Rev. 1990, 70, 453–511. [Google Scholar] [CrossRef]
- Le Duigou, C.; Simonnet, J.; Teleñczuk, M.T.; Fricker, D.; Miles, R. Recurrent synapses and circuits in the CA3 region of the hippocampus: An associative network. Front. Cell Neurosci. 2014, 7, 262. [Google Scholar] [CrossRef]
- Jefferys, J.G. Basic mechanisms of focal epilepsies. Exp. Physiol. 1990, 75, 127–162. [Google Scholar] [CrossRef] [Green Version]
- Wittner, L.; Miles, R. Factors defining a pacemaker region for synchrony in the hippocampus. J. Physiol. 2007, 584, 867–883. [Google Scholar] [CrossRef]
- Stoop, R.; Pralong, E. Functional connections and epileptic spread between hippocampus, entorhinal cortex and amygdala in a modified horizontal slice preparation of the rat brain. Eur. J. Neurosci. 2000, 12, 3651–3663. [Google Scholar] [CrossRef]
- van Groen, T.; Wyss, J.M. Extrinsic projections from area CA1 of the rat hippocampus: Olfactory, cortical, subcortical, and bilateral hippocampal formation projections. J. Comp. Neurol. 1990, 302, 515–528. [Google Scholar] [CrossRef]
- Heldt, S.A.; Ressler, K.J. Forebrain and midbrain distribution of major benzodiazepine-sensitive GABAA receptor subunits in the adult C57 mouse as assessed with in situ hybridization. Neuroscience 2007, 150, 370–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hörtnagl, H.; Tasan, R.O.; Wieselthaler, A.; Kirchmair, E.; Sieghart, W.; Sperk, G. Patterns of mRNA and protein expression for 12 GABAA receptor subunits in the mouse brain. Neuroscience 2013, 236, 345–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisden, W.; Seeburg, P.H. GABAA receptor channels: From subunits to functional entities. Curr. Opin. Neurobiol. 1992, 2, 263–269. [Google Scholar] [CrossRef]
- Gao, Y.; Heldt, S.A. Enrichment of GABAA Receptor α-Subunits on the Axonal Initial Segment Shows Regional Differences. Front. Cell Neurosci. 2016, 10, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minier, F.; Sigel, E. Positioning of the alpha-subunit isoforms confers a functional signature to gamma-aminobutyric acid type A receptors. Proc. Natl. Acad. Sci. USA 2004, 101, 7769–7774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tia, S.; Wang, J.F.; Kotchabhakdi, N.; Vicini, S. Distinct deactivation and desensitization kinetics of recombinant GABAA receptors. Neuropharmacology 1996, 35, 1375–1382. [Google Scholar] [CrossRef]
- Brooks-Kayal, A.R.; Shumate, M.D.; Jin, H.; Rikhter, T.Y.; Coulter, D.A. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat. Med. 1998, 4, 1166–1172, Erratum in Nat. Med. 1999, 5, 590. [Google Scholar] [CrossRef]
- Maljevic, S.; Krampfl, K.; Cobilanschi, J.; Tilgen, N.; Beyer, S.; Weber, Y.G.; Schlesinger, F.; Ursu, D.; Melzer, W.; Cossette, P.; et al. A mutation in the GABA(A) receptor alpha(1)-subunit is associated with absence epilepsy. Ann. Neurol. 2006, 59, 983–987. [Google Scholar] [CrossRef]
- Macdonald, R.L.; Gallagher, M.J.; Feng, H.J.; Kang, J. GABA(A) receptor epilepsy mutations. Biochem. Pharmacol. 2004, 68, 1497–1506. [Google Scholar] [CrossRef]
- Planagumà, J.; Leypoldt, F.; Mannara, F.; Gutiérrez-Cuesta, J.; Martín-García, E.; Aguilar, E.; Titulaer, M.J.; Petit-Pedrol, M.; Jain, A.; Balice-Gordon, R.; et al. Human N-methyl D-aspartate receptor antibodies alter memory and behaviour in mice. Brain 2015, 138, 94–109. [Google Scholar] [CrossRef]
- Symmonds, M.; Moran, C.H.; Leite, M.I.; Buckley, C.; Irani, S.R.; Stephan, K.E.; Friston, K.J.; Moran, R.J. Ion channels in EEG: Isolating channel dysfunction in NMDA receptor antibody encephalitis. Brain 2018, 141, 1691–1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puttachary, S.; Sharma, S.; Tse, K.; Beamer, E.; Sexton, A.; Crutison, J.; Thippeswamy, T. Immediate Epileptogenesis after Kainate-Induced Status Epilepticus in C57BL/6J Mice: Evidence from Long Term Continuous Video-EEG Telemetry. PLoS ONE 2015, 10, e0131705. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.K.; Rosch, R.E.; Wilson, M.A.; Upadhya, M.A.; Dhangar, D.R.; Clarke-Bland, C.; Wahid, T.T.; Barman, S.; Goebels, N.; Kreye, J.; et al. Multimodal electrophysiological analyses reveal that reduced synaptic excitatory neurotransmission underlies seizures in a model of NMDAR antibody-mediated encephalitis. Commun. Biol. 2021, 4, 1106. [Google Scholar] [CrossRef] [PubMed]
- Palpagama, T.H.; Sagniez, M.; Kim, S.; Waldvogel, H.J.; Faull, R.L.; Kwakowsky, A. GABAA Receptors Are Well Preserved in the Hippocampus of Aged Mice. eNeuro 2019, 6, ENEURO.0496-18.2019. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menke, A.F.; Ismail, F.S.; Dornmair, K.; Cerina, M.; Meuth, S.G.; Melzer, N. GABAA Receptor Autoantibodies Decrease GABAergic Synaptic Transmission in the Hippocampal CA3 Network. Int. J. Mol. Sci. 2022, 23, 3707. https://doi.org/10.3390/ijms23073707
Menke AF, Ismail FS, Dornmair K, Cerina M, Meuth SG, Melzer N. GABAA Receptor Autoantibodies Decrease GABAergic Synaptic Transmission in the Hippocampal CA3 Network. International Journal of Molecular Sciences. 2022; 23(7):3707. https://doi.org/10.3390/ijms23073707
Chicago/Turabian StyleMenke, Amélie F., Fatme Seval Ismail, Klaus Dornmair, Manuela Cerina, Sven G. Meuth, and Nico Melzer. 2022. "GABAA Receptor Autoantibodies Decrease GABAergic Synaptic Transmission in the Hippocampal CA3 Network" International Journal of Molecular Sciences 23, no. 7: 3707. https://doi.org/10.3390/ijms23073707
APA StyleMenke, A. F., Ismail, F. S., Dornmair, K., Cerina, M., Meuth, S. G., & Melzer, N. (2022). GABAA Receptor Autoantibodies Decrease GABAergic Synaptic Transmission in the Hippocampal CA3 Network. International Journal of Molecular Sciences, 23(7), 3707. https://doi.org/10.3390/ijms23073707





