Factors Affecting the Stability of Platinum(II) Complexes with 1,2,4-Triazolo[1,5-a]pyrimidine Derivatives and Tetrahydrothiophene-1-Oxide or Diphenyl Sulfoxide
Abstract
:1. Introduction
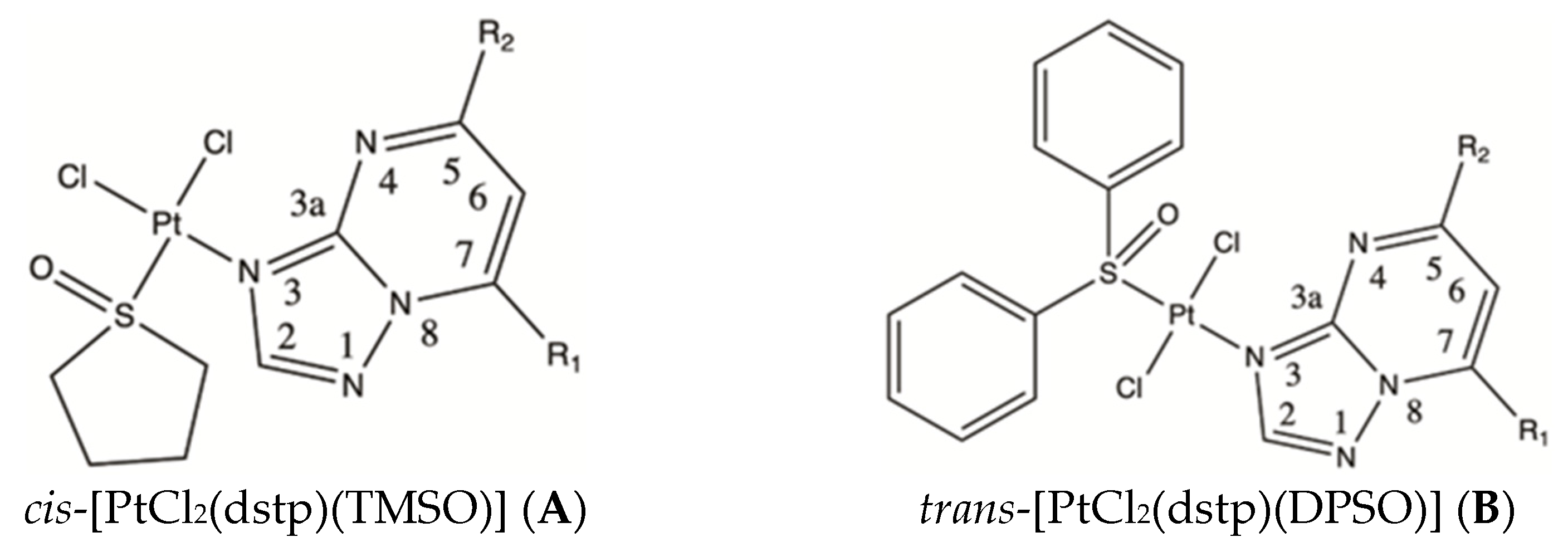
2. Results and Discussion
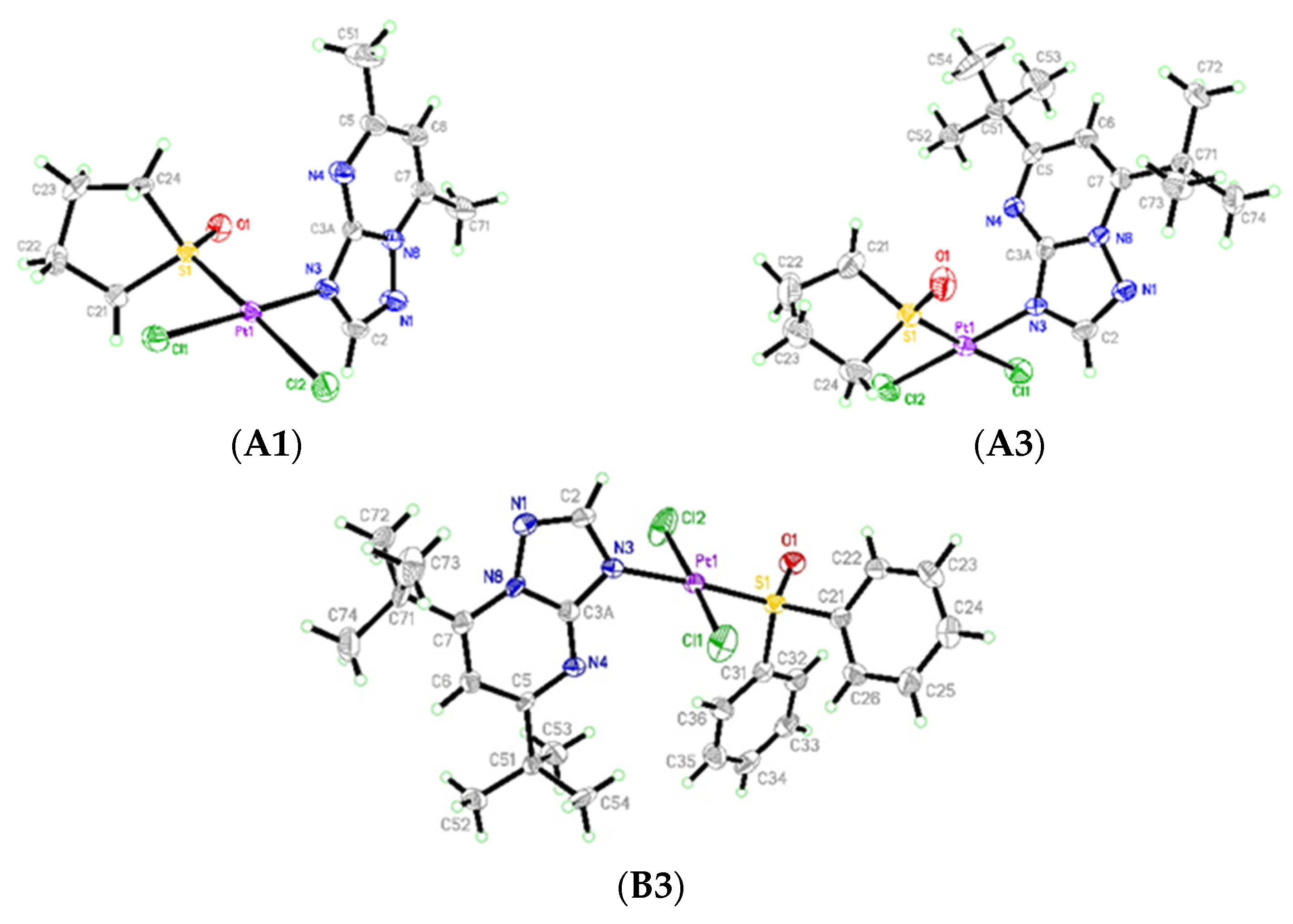
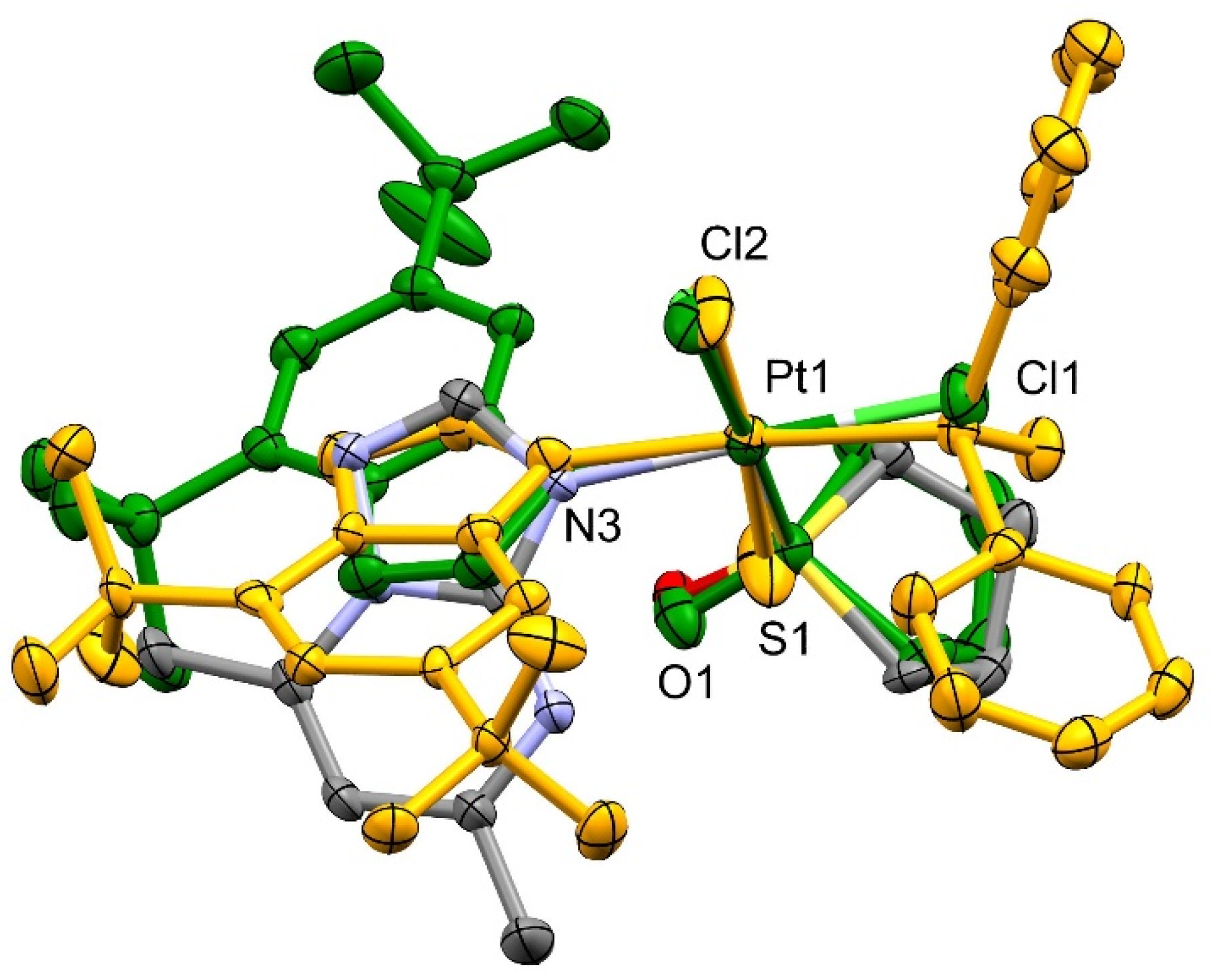
2.1. Crystal Structures of cis-[PtCl2(dmtp)(TMSO)] (A1), cis-[PtCl2(dbtp)(TMSO)] (A3), and trans-[PtCl2(dbtp)(DPSO)] (B3)
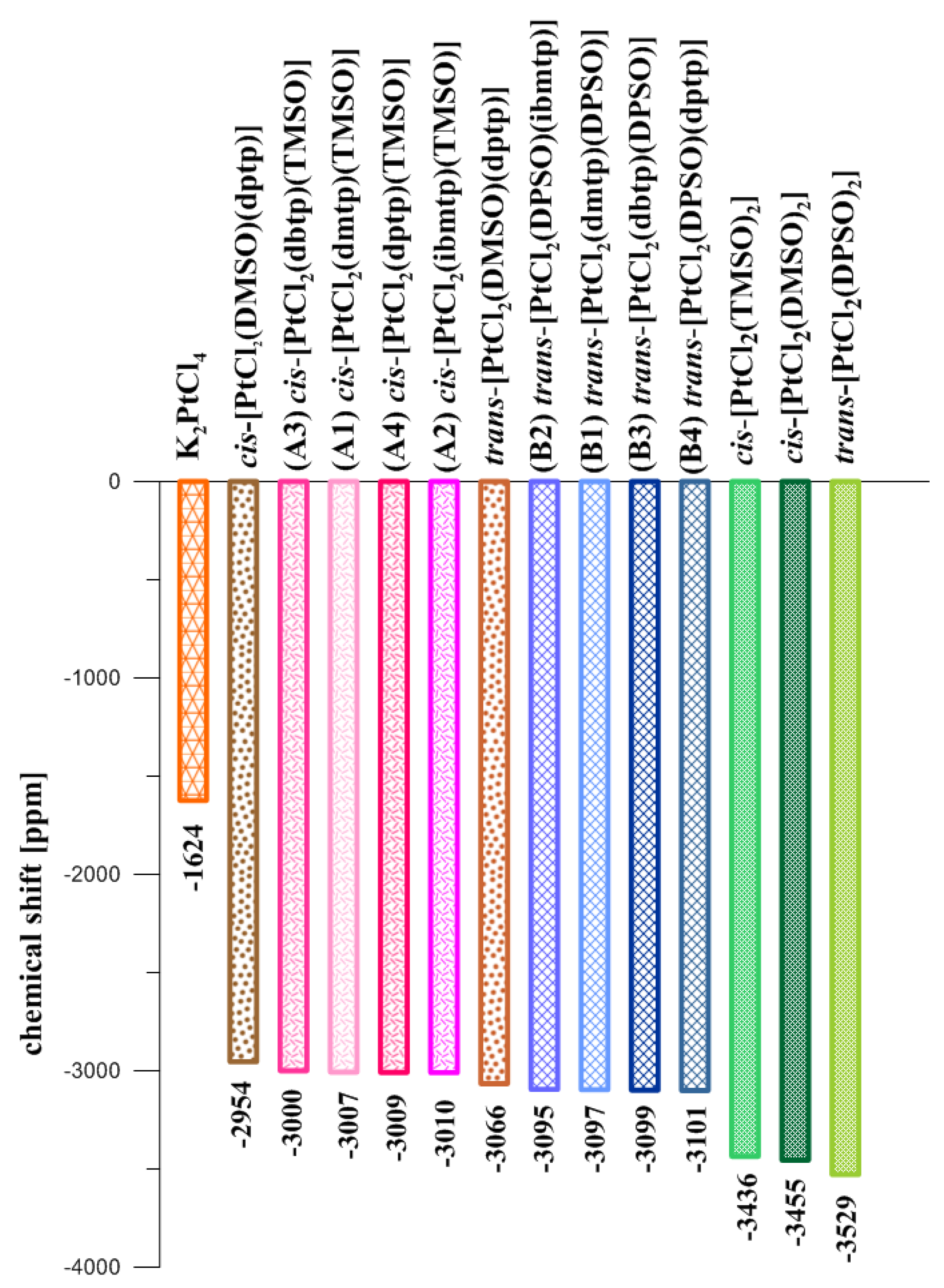
2.2. Multinuclear 1H, 13C, 15N, and 195Pt NMR Spectroscopy
3. Materials and Methods
3.1. Materials and Instrumentation
3.2. Instrumentation
3.3. X-Ray Structure Determination
3.4. Computational Methods
3.5. Syntheses of Complexes
3.5.1. cis-[PtCl2(TMSO)2]
3.5.2. cis-[PtCl2(DPSO)2]
3.5.3. cis-[PtCl2(dstp) (TMSO)] (A1–A4) and trans-[PtCl2(dstp) (DPSO] (B1–B4)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, W.Y.; Abd-El-Aziz, A.S. Molecular Design of Photofunctional Polymers and Materials; RSC Publishing: London, UK, 2012. [Google Scholar]
- Sigel, A.; Sigel, H. Metal Ions in Biological Systems; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Wilson, J.J.; Lippard, S.J. Synthetic Methods for the Preparation of Platinum Anticancer Complexes. Chem. Rev. 2014, 114, 4470–4495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melnik, M.; Mikus, P. Stereochemistry of Pt(η2-P2L)Cl2 derivatives. Rev. Inorg. Chem. 2019, 39, 199–210. [Google Scholar] [CrossRef]
- Peloso, A. Kinetics of nickel, palladium and platinum complexes. Coord. Chem. Rev. 1973, 10, 123–181. [Google Scholar] [CrossRef]
- Lippert, B. Cisplatin; Chemistry and Biochemistry of a Leading Anticancer Drug Wiley-VCH: Weinheim, Germany, 1999. [Google Scholar]
- Cvitkovic, E. Cumulative toxicities from cisplatin therapy and current cytoprotective measures. Cancer Treat. Rev. 1998, 24, 265–281. [Google Scholar] [CrossRef]
- Priqueler, J.R.L.; Butler, I.S.; Rochon, F.D. An Overview of 195Pt Nuclear Magnetic Resonance Spectroscopy. Appl. Spectrosc. Rev. 2006, 41, 185–226. [Google Scholar] [CrossRef]
- Tessier, C.; Rochon, F.D. Multinuclear NMR study and crystal structures of complexes of the types cis- and trans-Pt(Ypy)2X2, where Ypy=pyridine derivative and X=Cl and I. Inorg. Chim. Acta 1999, 295, 25–38. [Google Scholar] [CrossRef]
- Łakomska, I.; Szłyk, E.; Sitkowski, J.; Kozerski, L.; Wietrzyk, J.; Pełczyńska, M.; Nasulewicz, A.; Opolski, A. Multinuclear NMR spectroscopy and antitumor activity of novel platinum(II) complexes with 5,7-disubstituted-1,2,4-triazolo[1,5-a]pyrimidines. J. Inorg. Biochem. 2004, 98, 167–172. [Google Scholar] [CrossRef]
- Łakomska, I.; Babinska, M.; Wojtczak, A.; Kozakiewicz, A.; Sitkowski, J.; Jarzęcki, A. Experimental and theoretical investigation of the complexation of 5-methyl-7-isobutyl-1,2,4-triazolo[1,5-a]pyrimidine with platinum(II) ions. New J. Chem. 2017, 41, 7775–7782. [Google Scholar] [CrossRef]
- Szłyk, E.; Łakomska, I.; Surdykowski, A.; Głowiak, T.; Pazderski, L.; Sitkowski, J.; Kozerski, L. The X-ray structure and spectroscopy of platinum(II) complexes with 1,2,4-triazolo[1,5-a]pyrimidines and dimethylsulfoxide. Inorg. Chim. Acta 2002, 333, 93–99. [Google Scholar] [CrossRef]
- Jolley, J.; Cross, W.I.; Pritchard, R.G.; McAuliffe, C.A.; Nolan, K.B. Synthesis and characterisation of mercaptoimidazole, mercaptopyrimidine and mercaptopyridine complexes of platinum(II) and platinum(III). The crystal and molecular structures of tetra(2-mercaptobenzimidazole)- and tetra(2-mercaptoimidazole)platinum(II) chloride. Inorg. Chim. Acta 2001, 315, 36–43. [Google Scholar]
- Łakomska, I.; Hoffmann, K.; Muzioł, T.; Sitkowski, J. Multinuclear magnetic resonance and X-ray characterization of platinum(II) complexes with substituted-1,2,4-triazolo[1,5-a]pyrimidines. J. Mol. Struct. 2014, 1056–1057, 146–151. [Google Scholar] [CrossRef]
- Hoffmann, K.; Wiśniewska, J.; Wojtczak, A.; Sitkowski, J.; Denslow, A.; Wietrzyk, J.; Jakubowski, M.; Łakomska, I. Rational design of dicarboxylato platinum(II) complexes with purine-mimetic ligands as novel anticancer agents. J. Inorg. Biochem. 2017, 172, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Łakomska, I.; Hoffmann, K.; Wojtczak, A.; Sitkowski, J.; Maj, E.; Wietrzyk, J. Cytotoxic malonate platinum(II) complexes with 1,2,4-triazolo[1,5-a]pyrimidine derivatives: Structural characterization and mechanism of the suppression of tumor cell growth. J. Inorg. Biochem. 2014, 141, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Łakomska, I.; Babinska, M.; Wojtczak, A.; Sitkowski, J. Synthesis, characterization and in vitro cytotoxicity of three types of platinum(II) complexes containing 5,7-diethyl-1,2,4-triazolo[1,5-a]pyrimidine. Inorg. Chim. Acta 2016, 453, 516–521. [Google Scholar] [CrossRef]
- Łakomska, I. Molecular structure and antitumor activity of platinum(II) complexes containing purine analogs. Inorg. Chim. Acta. 2009, 362, 669–681. [Google Scholar] [CrossRef]
- Bülow, C.; Haas, K. Synthetische Versuche zur Darstellung von Derivaten des heterokondensierten, heterocyclischen 1.3-Triazo-7.0′-pyrimidins. Ber. Der Dtsch. Chem. Ges. 1909, 42, 4638–4644. [Google Scholar] [CrossRef] [Green Version]
- Haasnoot, J.G. Mononuclear, oligonuclear and polynuclear metal coordination compounds with 1,2,4-triazole derivatives as ligands. Coord. Chem. Rev. 2000, 200, 131–185. [Google Scholar] [CrossRef]
- Furrer, J. A Robust, sensitive, and versatile HMBC experiment for rapid structure elucidation by NMR: IMPACT-HMBC. Chem. Commun. 2010, 46, 3396–3398. [Google Scholar] [CrossRef]
- Kupce, E.; Freeman, R. Fast multidimensional NMR by polarization sharing. Magn. Reson. Chem. 2007, 45, 2–4. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CrysAlisPro Version 1.171.41.120; A Software Package; Rigaku OD: Neu-Isenburg, Germany, 2021.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Figgen, D.; Peterson, K.A.; Dolg, M.; Stoll, H. Energy-consistent pseudopotentials and correlation consistent basis sets for the 5d elements Hf-Pt. J. Chem. Phys. 2009, 130, 164108. [Google Scholar] [CrossRef] [PubMed]
- Ditchfield, R. Self-consistent perturbation theory of diamagnetism: I. A gauge-invariant LCAO method for NMR chemical shifts. Mol. Phys. 1974, 27, 789. [Google Scholar] [CrossRef]
- Gauss, J.J. Effects of electron correlation in the calculation of nuclear magnetic resonance chemical shifts. Chem. Phys. 1993, 99, 3629. [Google Scholar] [CrossRef]
- Feller, D.J. The role of databases in support of computational chemistry calculations. Comput. Chem. 1996, 17, 1571–1586. [Google Scholar] [CrossRef]
- Cossi, M.; Scalmani, G.; Rega, N.; Barone, V. New Developments in the Polarizable Continuum Model for QuantumMechanical and Classical Calculations on Molecules in Solution. J. Chem. Phys. 2002, 117, 43. [Google Scholar] [CrossRef]
- Price, J.H.; Williamson, A.S.; Schramm, R.S.; Wayland, B.B. Palladium(II) and Platinum(II) Alkyl Sulfoxide Complexes. Examples of Sulfur-Bonded, Mixed Sulfur- and Oxygen-Bonded, and Totally Oxygen-Bonded Complexes. Inorg. Chem. 1972, 11, 1280–1284. [Google Scholar] [CrossRef]

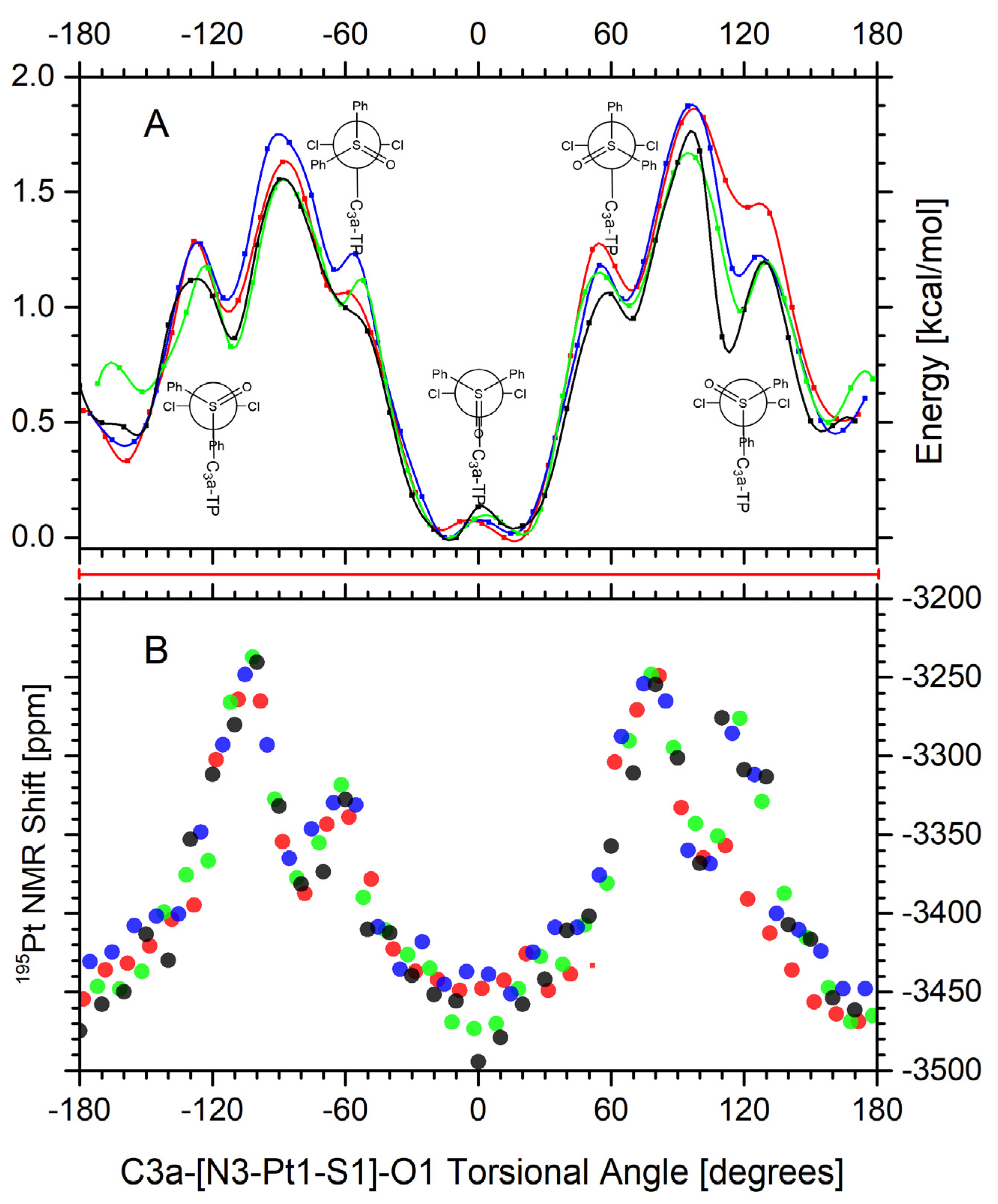
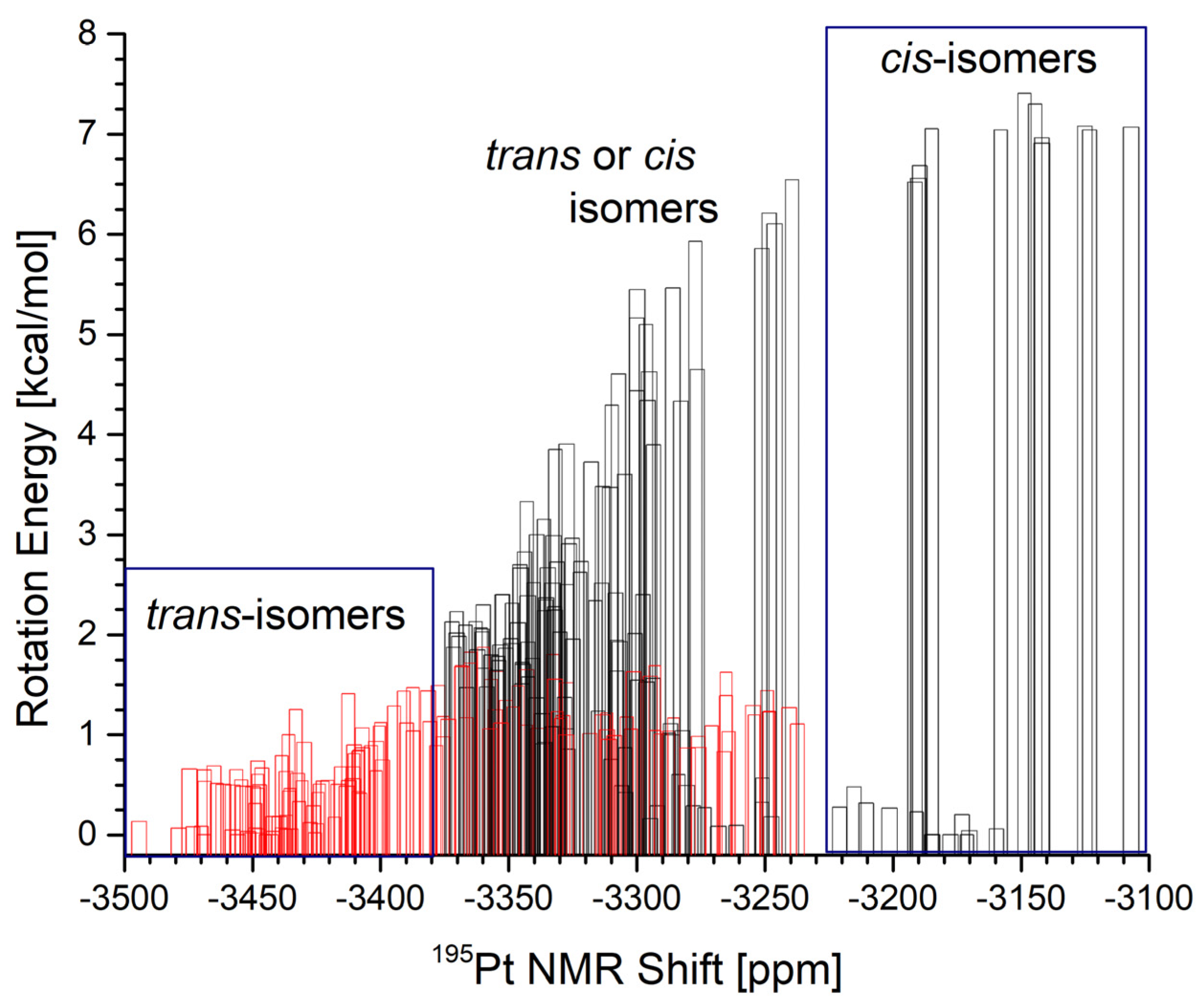
| (A1) | (A3) | (B3) | ||||
|---|---|---|---|---|---|---|
| Bond Lengths [Å] | ||||||
| Exp. | Calc. | Exp. | Calc. | Exp. | Calc. | |
| Pt1-N3 | 2.017 (4) | 2.043 | 2.020 (6) | 2.046 | 2.033 (6) | 2.052 |
| Pt1-S1 | 2.199 (14) | 2.256 | 2.199 (2) | 2.274 | 2.215 (18) | 2.267 |
| Pt1-Cl1 | 2.301 (12) | 2.343 | 2.307 (2) | 2.349 | 2.281 (2) | 2.357 |
| Pt1-Cl2 | 2.306 (15) | 2.351 | 2.299 (2) | 2.342 | 2.295 (2) | 2.349 |
| Angles [°] | ||||||
| Exp. | Calc. | Exp. | Calc. | Exp. | Calc. | |
| N3-Pt1-S1 | 89.65 (13) | 91.5 | 89.28 (18) | 89.33 | 175.29 (16) | 175.6 |
| N3-Pt1-Cl1 | 178.63 (11) | 178.7 | 88.55 (19) | 87.47 | 88.28 (18) | 87.6 |
| S1-Pt1-Cl1 | 90.60 (5) | 91.5 | 177.30 (8) | 176.28 | 92.86 (7) | 94.5 |
| N3-Pt1-Cl2 | 88.56 (13) | 87.9 | 179.17 (19) | 177.19 | 87.58 (18) | 88.0 |
| S1-Pt1-Cl2 | 175.51 (5) | 179.3 | 90.60 (9) | 93.43 | 91.32 (8) | 89.8 |
| Cl1-Pt1-Cl2 | 91.29 (5) | 90.8 | 91.54 (9) | 89.75 | 175.82 (9) | 175.2 |
| (A2) | (A4) | (B1) | (B2) | (B4) | |
|---|---|---|---|---|---|
| Bond Lengths [Å] | |||||
| Pt1-N3 | 2.042 | 2.043 | 2.053 | 2.054 | 2.052 |
| Pt1-S1 | 2.259 | 2.258 | 2.267 | 2.266 | 2.267 |
| Pt1-Cl1 | 2.351 | 2.349 | 2.348 | 2.350 | 2.355 |
| Pt1-Cl2 | 2.341 | 2.343 | 2.355 | 2.355 | 2.355 |
| Angles [°] | |||||
| N3-Pt1-S1 | 91.5 | 91.4 | 175.4 | 175.4 | 175.7 |
| N3-Pt1-Cl1 | 87.7 | 87.8 | 87.4 | 87.3 | 87.8 |
| S1-Pt1-Cl1 | 178.6 | 179.1 | 94.5 | 94.6 | 90.5 |
| N3-Pt1-Cl2 | 178.5 | 178.5 | 87.9 | 88.0 | 87.7 |
| S1-Pt1-Cl2 | 89.9 | 89.9 | 90.1 | 90.0 | 90.5 |
| Cl1-Pt1-Cl2 | 90.9 | 90.9 | 174.8 | 175.1 | 174.6 |
| (A1) | (A2) | (A3) | (A4) | |||||
|---|---|---|---|---|---|---|---|---|
| Expt. | Calc. | Expt. | Calc. | Expt. | Calc. | Expt. | Calc. | |
| δH2 | 8.53 (+0.13) | 8.82 | 8.56 (+0.10) | 8.83 | 8.56 (+0.14) | 8.86 | 8.66 (+0.07) | 8.88 |
| δH6 | 7.07 (+0.26) | 7.55 | 6.95 (+0.22) | 7.52 | 7.16 (+0.16) | 7.74 | 7.79 (+0.07) | 8.30 |
| δC2 | 153.0 (−2.2) | 153.10 | 152.9 (−2.3) | 152.72 | 152.1 (−2.1) | 151.56 | 153.7 (−1.1) | 153.79 |
| δC3a | 151.9 (−3.2) | 151.34 | 151.8 (−3.6) | 151.87 | 152.5 (−3.4) | 151.90 | 153.0 (−2.3) | 152.63 |
| δC5 | 168.7 (+4.0) | 168.86 | 168.6 (+3.9) | 168.21 | 179.1 (+3.4) | 179.50 | 164.4 (+1.9) | 163.00 |
| δC6 | 113.4 (+2.6) | 110.44 | 113.2 (+2.7) | 111.24 | 106.0 (+2.6) | 106.01 | 108.8 (+1.6) | 106.64 |
| δC7 | 148.1 (+1.4) | 149.74 | 151.1 (+1.4) | 152.49 | 158.7 (+1.3) | 160.73 | 149.3 (+0.9) | 150.21 |
| δN1 | −110.6 (+1.6) | −116.91 | −110.7 (+1.3) | −118.69 | −106.6 (+0.4) | −113.26 | −110.3 (+1.0) | −117.28 |
| δN3 | −226.2 (−72.2) | −207.31 | −226.7 (−72.7) | −206.98 | −228.0 (−70.9) | −208.19 | −225.5 (−64.6) | −207.80 |
| δN4 | −114.5 (−1.1) | −125.52 | −114.4 (−1.0) | −126.62 | −114.7 (+0.2) | −130.09 | −122.2 (−0.4) | −137.47 |
| δN8 | −157.2 (−2.3) | −165.06 | −157.9 (−2.4) | −165.27 | −160.3 (−2.2) | −166.95 | −162.0(−2.2) | −170.60 |
| δPt | −3010 | −3209.32 | −3007 | −3202.16 | −3000 | −3186.18 | −3009 | −3195.93 |
| (B1) | (B2) | (B3) | (B4) | |||||
|---|---|---|---|---|---|---|---|---|
| Expt. | Calc. | Expt. | Calc. | Expt. | Calc. | Expt. | Calc. | |
| δH2 | 8.57 (+0.17) | 8.87 | 8.55 (+0.09) | 8.86 | 8.54 (+0.12) | 8.92 | 8.65 (+0.06) | 8.97 |
| δH6 | 7.01 (+0.20) | 7.57 | 6.93 (+0.20) | 7.55 | 7.15 (+0.15) | 7.74 | 7.79 (+0.07) | 8.36 |
| δC2 | 152.9 (−2.3) | 152.81 | 152.8 (−2.4) | 152.69 | 151.8 (−2.4) | 152.13 | 153.6 (−1.2) | 153.59 |
| δC3a | 151.8 (−3.3) | 151.62 | 152.1 (−3.3) | 151.72 | 152.5 (−3.4) | 152.33 | 152.9 (−2.4) | 152.77 |
| δC5 | 168.8 (+4.1) | 168.44 | 168.6 (+3.9) | 168.37 | 179.0 (+3.3) | 180.05 | 164.2 (+1.7) | 163.33 |
| δC6 | 113.4 (+2.6) | 110.50 | 113.1 (+2.6) | 111.24 | 106.0 (+2.6) | 103.99 | 108.7 (+1.5) | 106.82 |
| δC7 | 148.0 (+1.3) | 149.77 | 151.0 (+1.3) | 152.67 | 158.7 (+1.3) | 161.84 | 149.3 (+0.9) | 150.65 |
| δN1 | −110.6 (+1.6) | −117.15 | −110.7 (+1.3) | −117.15 | −106.0 (+1.0) | −113.19 | −110.0 (+1.3) | −117.58 |
| δN3 | −226.6 (−72.6) | −213.13 | −227.1 (−73.1) | −213.80 | −227.7 (−70.6) | −217.63 | −225.4 (−64.5) | −213.41 |
| δN4 | −113.7(−0.3) | −126.29 | −113.6 (−0.2) | −126.25 | −115.1 (−0.2) | −125.87 | −122.0 (+0.2) | −136.29 |
| δN8 | −157.4 (−2.5) | −163.81 | −158.1 (−2.6) | −164.62 | −160.4 (−2.3) | 165.46 | −162.0 (−2.2) | 169.46 |
| δPt | −3097 | −3465.9 | −3095 | −3466.9 | −3099 | 3445.3 | −3101 | 3442.8 |
| Compound | cis-[PtCl2(dmtp)(TMSO)] (A1) | cis-[PtCl2(dbtp)(TMSO)] (A3) | trans-[PtCl2(dbtp (DPSO)] (B3) |
|---|---|---|---|
| Empirical formula | C11H16Cl2N4OPtS | C17H28Cl2N4OPtS | C25H30Cl2N4OPtS |
| Formula weight | 518.33 | 602.48 | 700.58 |
| Temperature; K | 293 (2) | 293 (2) | 293 (2) |
| Wavelength; Å | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Monoclinic | Monoclinic | Triclinic |
| Space group | P21/n | P21/n | P-1 |
| Unit cell dimensions; Å, ° | a = 10.7446 (3) | a = 12.3610 (16) | a = 9.3248 (7) |
| b = 8.3993 (3) | b = 10.1722 (10) | b = 10.8972 (8) | |
| c = 18.0425 (6) | c = 17.871 (2) | c = 14.6506 (12) | |
| α = 90 | α =90 | α =88.183 (6) | |
| β = 98.946 (3) | β = 94.347 (10) | β = 73.143 (7) | |
| γ = 90 | γ = 90 | γ = 77.059 (6) | |
| Volume; Å3 | 1608.48 (10) | 2240.6 (5) | 1387.67 (19) |
| Z | 4 | 4 | 2 |
| Density (calculated); Mg/m3 | 2.140 | 1.786 | 1.677 |
| Absorption coefficient; mm−1 | 9.185 | 6.608 | 5.348 |
| F (000) | 984 | 1176 | 688 |
| Crystal size; mm | 0.340 × 0.209 × 0.136 | 0.609 × 0.579 × 0.194 | 0.312 × 0.237 × 0.086 |
| Theta range for data collection | 2.285 to 28.462°. | 2.305 to 28.486°. | 2.342 to 28.516°. |
| Index ranges | −13 < = h < = 14, −10 < = k < = 10, −23 < = l < = 21 | −15 < = h < = 15, −12 < = k < = 13, −21 < = l < = 23 | −12 < = h < = 12, −13 < = k < = 13, −19 < = l < = 13 |
| Reflections collected | 10,499 | 15,074 | 9764 |
| Independent reflections | 3696 [R(int) = 0.0474] | 5184 [R(int) = 0.0716] | 6129 [R(int) = 0.0389] |
| Completeness to theta = 25.242° | 100.0 % | 99.9 % | 99.9 % |
| Max. and min. transmission | 0.429 and 0.135 | 0.377 and 0.060 | 0.657 and 0.366 |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 3696/0/181 | 5184/0/235 | 6129/0/307 |
| Goodness-of-fit on F2 | 0.995 | 1.052 | 1.063 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0314, wR2 = 0.0497 | R1 = 0.0541, wR2 = 0.1178 | R1 = 0.0412, wR2 = 0.0744 |
| R indices (all data) | R1 = 0.0524, wR2 = 0.0539 | R1 = 0.0816, wR2 = 0.1347 | R1 = 0.0597, wR2 = 0.0918 |
| Largest diff. peak and hole; e.Å−3 | 1.203 and −0.791 | 2.862 and −1.726 | d −0.768 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubowski, M.; Łakomska, I.; Kaszuba, A.; Wojtczak, A.; Sitkowski, J.; Jarzęcki, A.A. Factors Affecting the Stability of Platinum(II) Complexes with 1,2,4-Triazolo[1,5-a]pyrimidine Derivatives and Tetrahydrothiophene-1-Oxide or Diphenyl Sulfoxide. Int. J. Mol. Sci. 2022, 23, 3656. https://doi.org/10.3390/ijms23073656
Jakubowski M, Łakomska I, Kaszuba A, Wojtczak A, Sitkowski J, Jarzęcki AA. Factors Affecting the Stability of Platinum(II) Complexes with 1,2,4-Triazolo[1,5-a]pyrimidine Derivatives and Tetrahydrothiophene-1-Oxide or Diphenyl Sulfoxide. International Journal of Molecular Sciences. 2022; 23(7):3656. https://doi.org/10.3390/ijms23073656
Chicago/Turabian StyleJakubowski, Mateusz, Iwona Łakomska, Adriana Kaszuba, Andrzej Wojtczak, Jerzy Sitkowski, and Andrzej A. Jarzęcki. 2022. "Factors Affecting the Stability of Platinum(II) Complexes with 1,2,4-Triazolo[1,5-a]pyrimidine Derivatives and Tetrahydrothiophene-1-Oxide or Diphenyl Sulfoxide" International Journal of Molecular Sciences 23, no. 7: 3656. https://doi.org/10.3390/ijms23073656
APA StyleJakubowski, M., Łakomska, I., Kaszuba, A., Wojtczak, A., Sitkowski, J., & Jarzęcki, A. A. (2022). Factors Affecting the Stability of Platinum(II) Complexes with 1,2,4-Triazolo[1,5-a]pyrimidine Derivatives and Tetrahydrothiophene-1-Oxide or Diphenyl Sulfoxide. International Journal of Molecular Sciences, 23(7), 3656. https://doi.org/10.3390/ijms23073656






