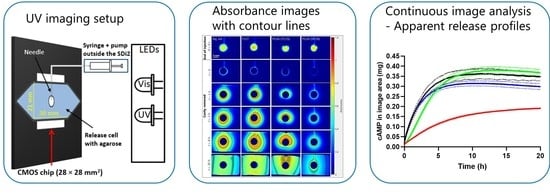
Methodological Considerations in Development of UV Imaging for Characterization of Intra-Tumoral Injectables Using cAMP as a Model Substance
Abstract
:1. Introduction
2. Results and Discussion
2.1. Initial Experiments—Development of In Vitro Release Setup for Static Conditions
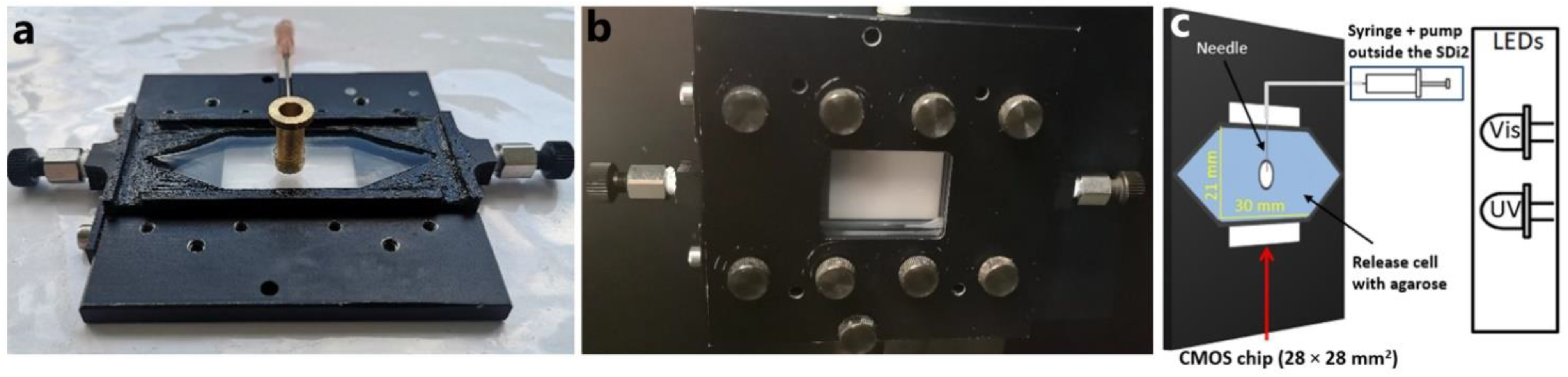
2.1.1. Release Cell
2.1.2. Matrix and Injection
2.1.3. Temperature Control
2.2. Instrument Performance and Image Analysis
2.2.1. Resolution
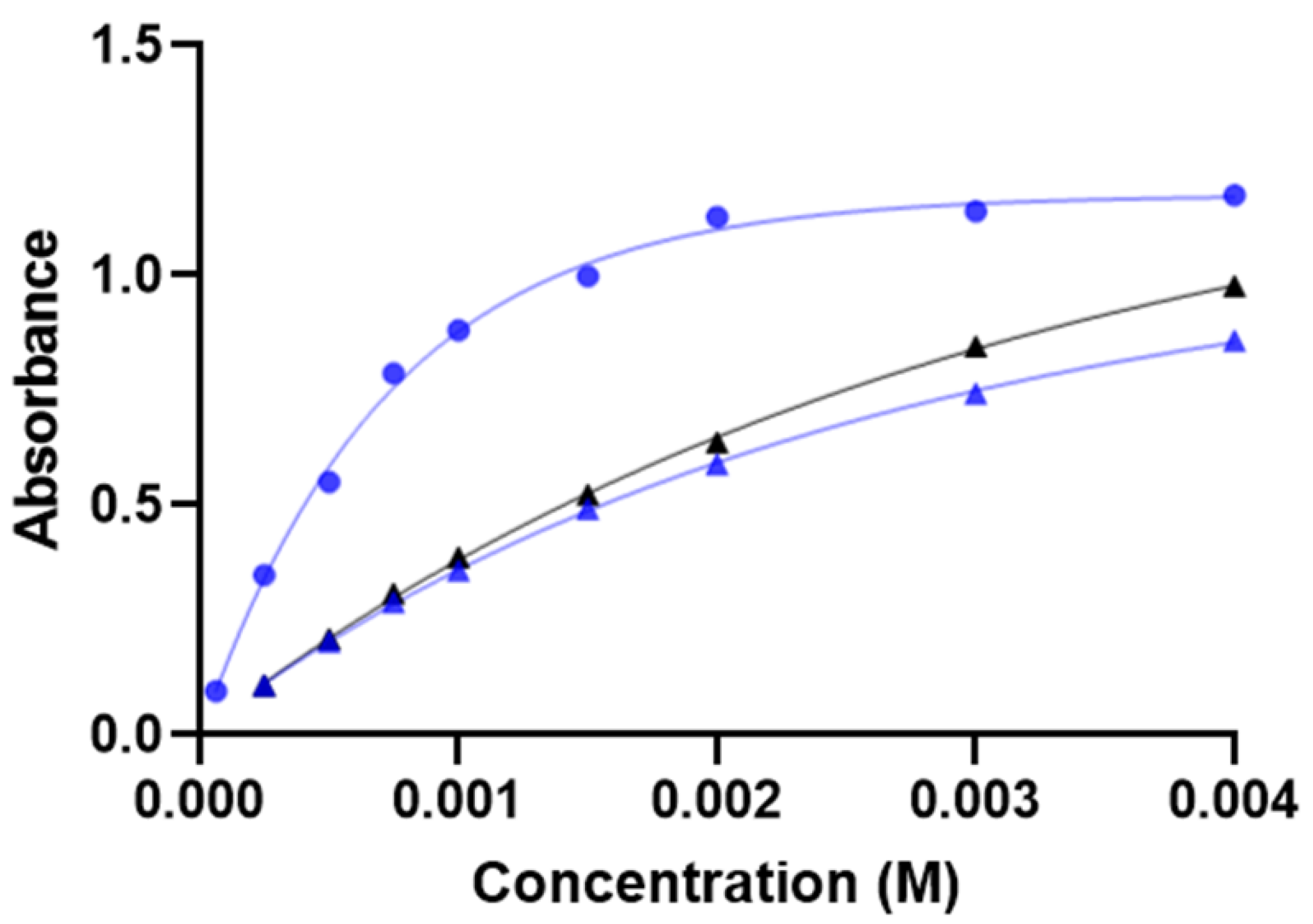
2.2.2. Measuring Range
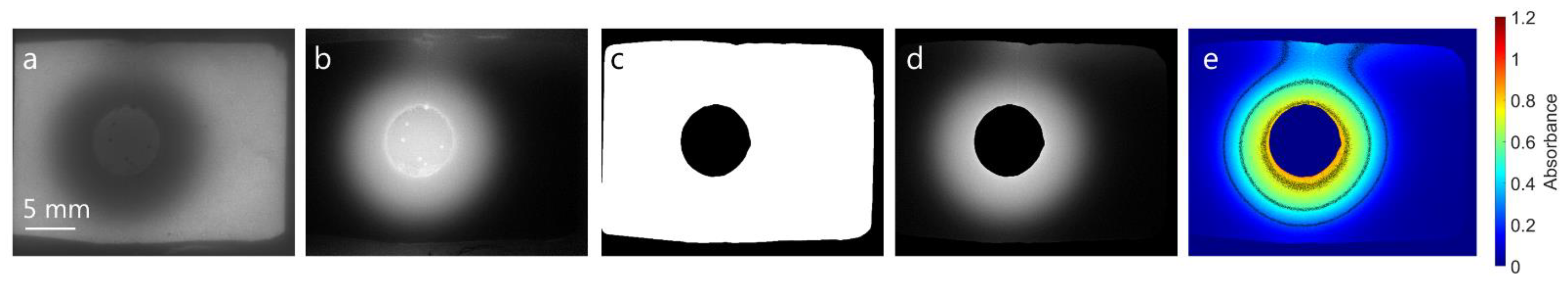
2.2.3. Image Analysis
2.3. UV Imaging Release Experiments
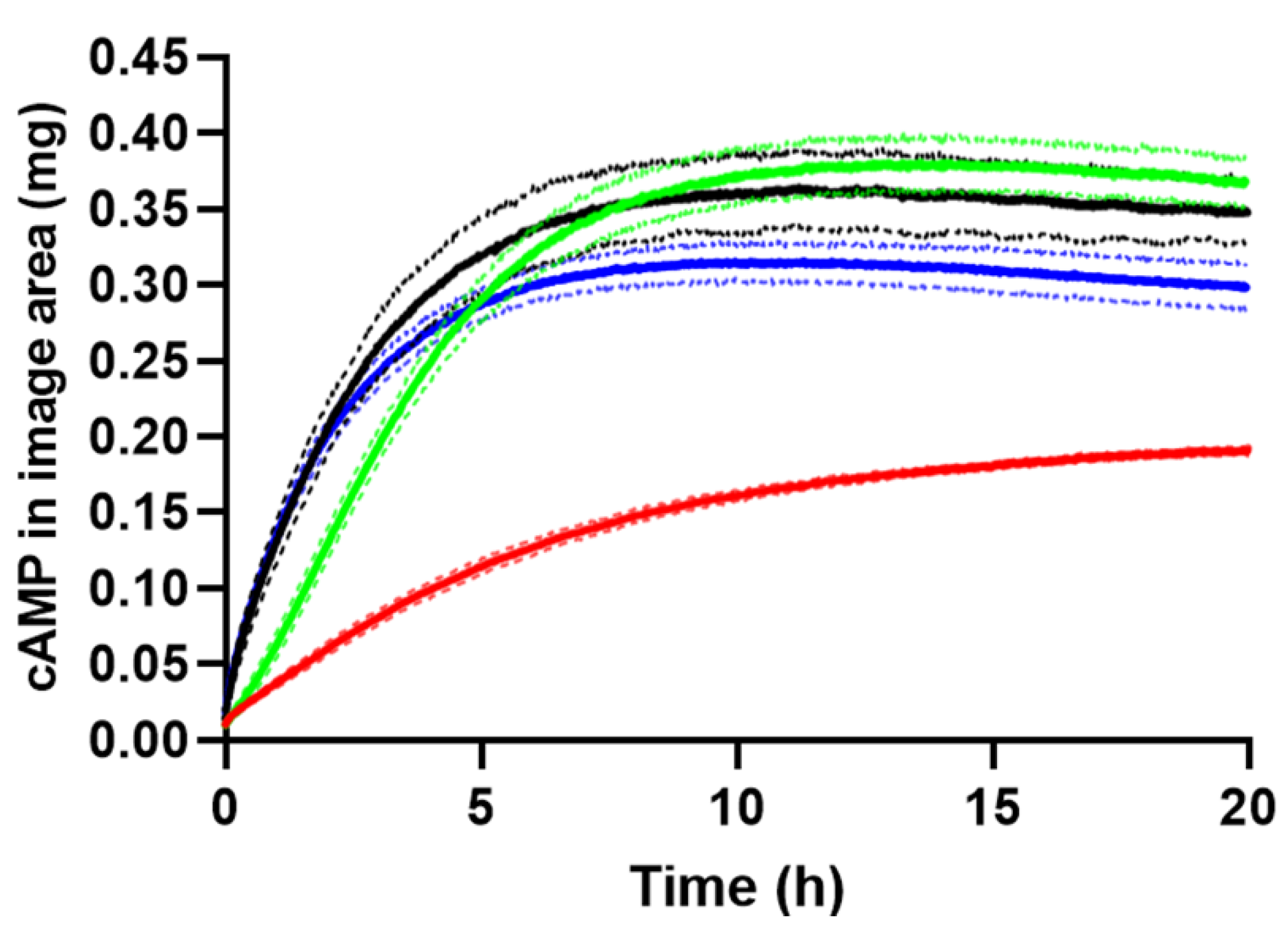
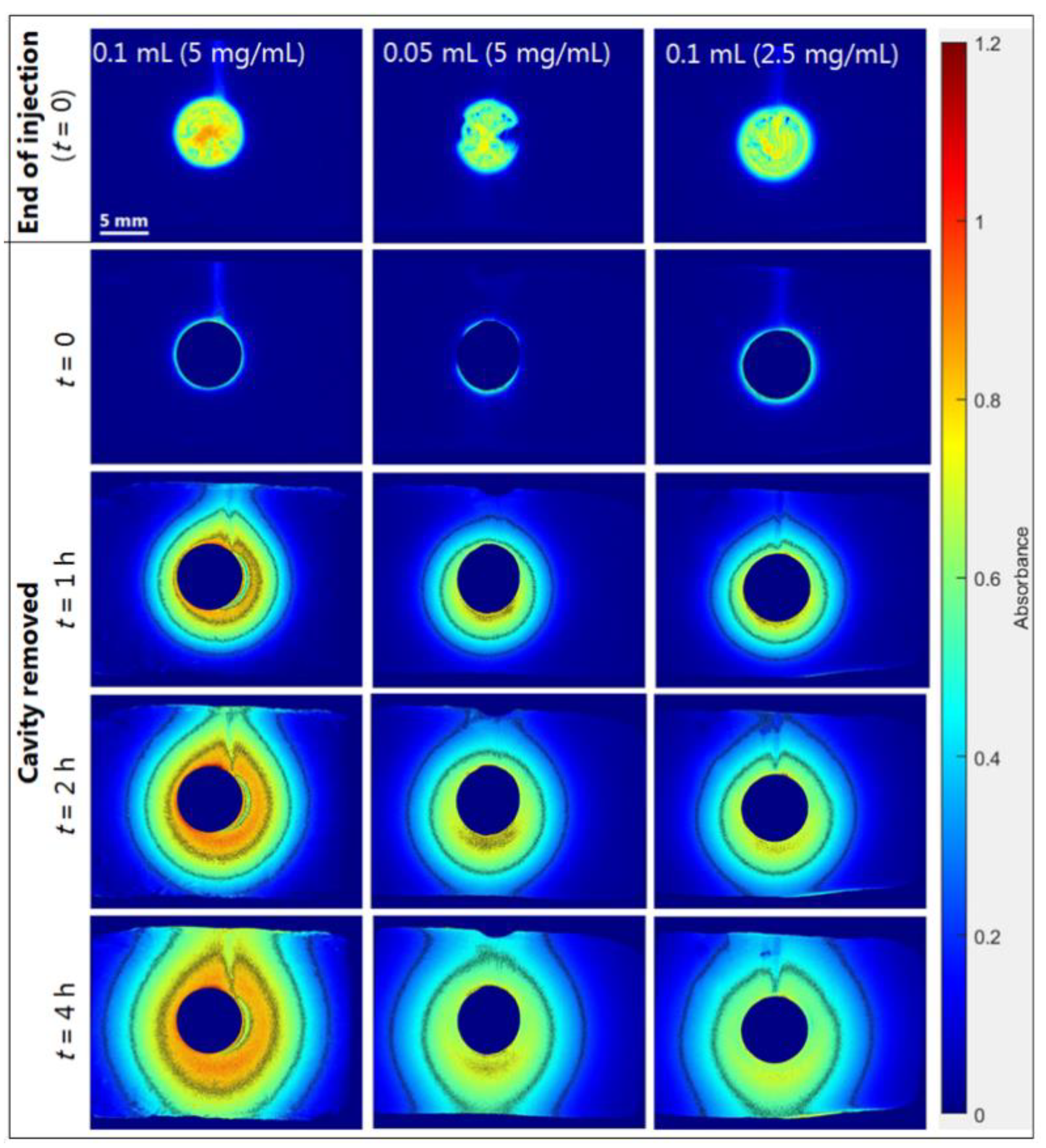
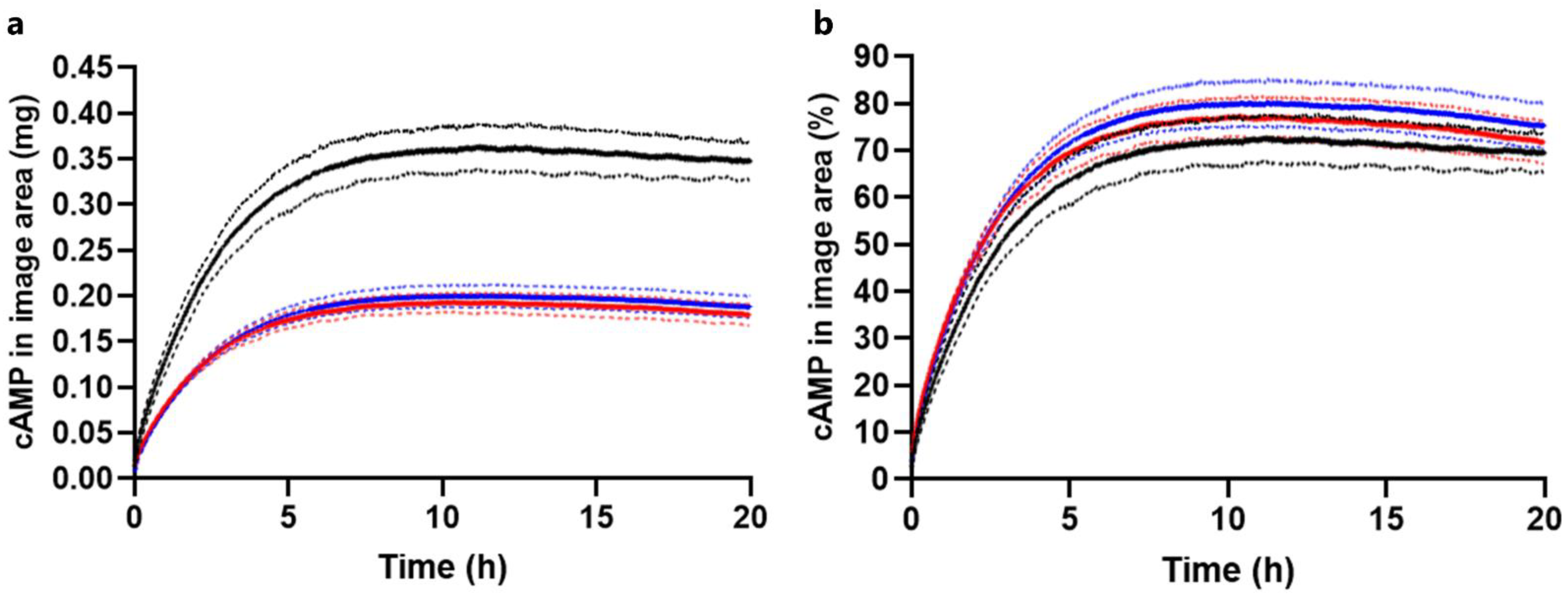
2.3.1. Drug Load Study
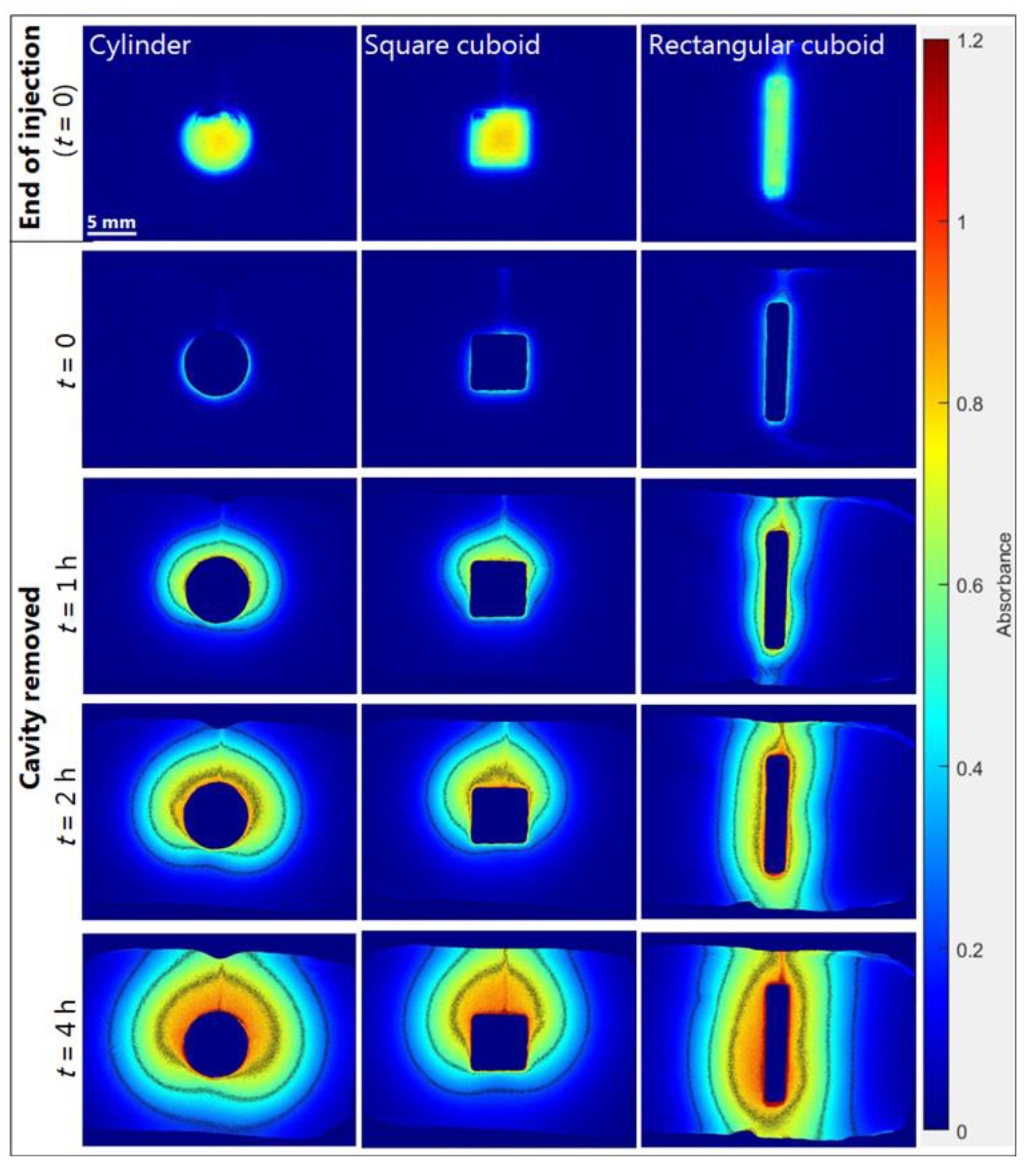
2.3.2. Effect of Formulation Geometry on Release
3. Materials and Methods
3.1. Materials
Preparation of cAMP Formulations
3.2. UV Imaging System
3.3. UV–Vis Imaging Release Experiments
HPLC Analysis
3.4. Instrument Performance and Image Analysis
3.4.1. Determination of Resolution
3.4.2. Calibration Curves
3.4.3. Image Analysis of Release Experiments
3.5. cAMP Diffusion and Distribution in Agarose and Pluronic F127 Gels
3.6. Diffusion and Distribution Analysis
3.7. Taylor Dispersion Analysis (TDA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shae, D.; Becker, K.W.; Christov, P.; Yun, D.S.; Lytton-Jean, A.K.R.; Sevimli, S.; Ascano, M.; Kelley, M.; Johnson, D.B.; Balko, J.M.; et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat. Nanotechnol. 2019, 14, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.S. Improving cancer immunotherapy through nanotechnology. Nat. Rev. Cancer 2019, 19, 587–602. [Google Scholar] [CrossRef]
- Leach, D.G.; Dharmaraj, N.; Piotrowski, S.L.; Lopez-Silva, T.L.; Lei, Y.L.; Sikora, A.G.; Young, S.; Hartgerink, J.D. STINGel: Controlled release of a cyclic dinucleotide for enhanced cancer immunotherapy. Biomaterials 2018, 163, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Motedayen Aval, L.; Pease, J.E.; Sharma, R.; Pinato, D.J. Challenges and Opportunities in the Clinical Development of STING Agonists for Cancer Immunotherapy. J. Clin. Med. 2020, 9, 3323. [Google Scholar] [CrossRef]
- Hernandez, C.; Gawlik, N.; Goss, M.; Zhou, H.; Jeganathan, S.; Gilbert, D.; Exner, A.A. Macroporous acrylamide phantoms improve prediction of in vivo performance of in situ forming implants. J. Control. Release 2016, 243, 225–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arvanitis, C.D.; Bazan-Peregrino, M.; Rifai, B.; Seymour, L.W.; Coussios, C.C. Cavitation-enhanced extravasation for drug delivery. Ultrasound Med. Biol. 2011, 37, 1838–1852. [Google Scholar] [CrossRef]
- Paris, J.L.; Mannaris, C.; Cabanas, M.V.; Carlisle, R.; Manzano, M.; Vallet-Regi, M.; Coussios, C.C. Ultrasound-mediated cavitation-enhanced extravasation of mesoporous silica nanoparticles for controlled-release drug delivery. Chem. Eng. J. 2018, 340, 2–8. [Google Scholar] [CrossRef]
- Klose, D.; Azaroual, N.; Siepmann, F.; Vermeersch, G.; Siepmann, J. Towards more realistic in vitro release measurement techniques for biodegradable microparticles. Pharm. Res. 2009, 26, 691–699. [Google Scholar] [CrossRef]
- Kozak, J.; Rabiskova, M.; Lamprecht, A. In-vitro drug release testing of parenteral formulations via an agarose gel envelope to closer mimic tissue firmness. Int. J. Pharm. 2021, 594, 120142. [Google Scholar] [CrossRef]
- Gietz, U.; Arvinte, T.; Mader, E.; Oroszlan, P.; Merkle, H.P. Sustained release of injectable zinc-recombinant hirudin suspensions: Development and validation of in vitro release model. Eur. J. Pharm. Biopharm. 1998, 45, 259–264. [Google Scholar] [CrossRef]
- Li, Z.; Mu, H.; Larsen, S.W.; Jensen, H.; Ostergaard, J. Initial Leuprolide Acetate Release from Poly(d,l-lactide-co-glycolide) In Situ Forming Implants as Studied by Ultraviolet-Visible Imaging. Mol. Pharm. 2020, 17, 4522–4532. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.H.; Kapoor, Y.; Alleyne, C.; Walsh, E.; Leithead, A.; Habulihaz, B.; Salituro, G.M.; Bak, A.; Rhodes, T. Development of a Convenient In Vitro Gel Diffusion Model for Predicting the In Vivo Performance of Subcutaneous Parenteral Formulations of Large and Small Molecules. AAPS PharmSciTech 2017, 18, 2203–2213. [Google Scholar] [CrossRef] [PubMed]
- James, J.; Murukeshan, V.M.; Woh, L.S. Integrated photoacoustic, ultrasound and fluorescence platform for diagnostic medical imaging-proof of concept study with a tissue mimicking phantom. Biomed. Opt. Express. 2014, 5, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, L.W.; Fausett, L.; Mueller, A.; Odéen, H.; Payne, A.; Christensen, D.A.; Parker, D.L. Development and characterization of a tissue mimicking psyllium husk gelatin phantom for ultrasound and magnetic resonance imaging. Int. J. Hyperth. 2020, 37, 283–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostergaard, J. UV imaging in pharmaceutical analysis. J. Pharm. Biomed. Anal. 2018, 147, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ostergaard, J. Application of UV Imaging in Formulation Development. Pharm. Res. 2017, 34, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Hanley, S.L.; Pygall, S.R.; Avalle, P.; Williams, H.D.; Melia, C.D. In Vitro Physical and Imaging Techniques to Evaluate Drug Release Mechanisms from Hydrophilic Matrix Tablets. In Hydrophilic Matrix Tablets for Oral Controlled Release; Timmins, P., Pygall, S.R., Melia, C.D., Eds.; Springer: New York, NY, USA, 2014; pp. 165–189. [Google Scholar]
- Brown, B.; Ward, A.; Fazili, Z.; Ostergaard, J.; Asare-Addo, K. Application of UV dissolution imaging to pharmaceutical systems. Adv. Drug Deliv. Rev. 2021, 177, 113949. [Google Scholar] [CrossRef]
- Ye, F.; Yaghmur, A.; Jensen, H.; Larsen, S.W.; Larsen, C.; Ostergaard, J. Real-time UV imaging of drug diffusion and release from Pluronic F127 hydrogels. Eur. J. Pharm. Sci. 2011, 43, 236–243. [Google Scholar] [CrossRef]
- Jensen, S.S.; Jensen, H.; Møller, E.H.; Cornett, C.; Siepmann, F.; Siepmann, J.; Østergaard, J. In vitro release studies of insulin from lipid implants in solution and in a hydrogel matrix mimicking the subcutis. Eur. J. Pharm. Sci. 2016, 81, 103–112. [Google Scholar] [CrossRef]
- Preem, L.; Bock, F.; Hinnu, M.; Putrinš, M.; Sagor, K.; Tenson, T.; Meos, A.; Østergaard, J.; Kogermann, K.; Bock; et al. Monitoring of Antimicrobial Drug Chloramphenicol Release from Electrospun Nano- and Microfiber Mats Using UV Imaging and Bacterial Bioreporters. Pharmaceutics 2019, 11, 487. [Google Scholar] [CrossRef] [Green Version]
- Ward, A.; Walton, K.; Box, K.; Østergaard, J.; Gillie, L.J.; Conway, B.R.; Asare-Addo, K. Variable-focus microscopy and UV surface dissolution imaging as complementary techniques in intrinsic dissolution rate determination. Int. J. Pharm. 2017, 530, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Etherson, K.; Dunn, C.; Matthews, W.; Pamelund, H.; Barragat, C.; Sanderson, N.; Izumi, T.; Mathews, C.D.C.; Halbert, G.; Wilson, C.; et al. An interlaboratory investigation of intrinsic dissolution rate determination using surface dissolution. Eur. J. Pharm. Biopharm. 2020, 150, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, K.; Patel, M.M.; Mehta, P.J. Long-Acting Injectables: Current Perspectives and Future Promise. Crit. Rev. Ther. Drug Carr. Syst. 2019, 36, 137–181. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.; Østergaard, J.; Yaghmur, A.; Jensen, H.; Larsen, C. Use of in vitro release models in the design of sustained and localized drug delivery systems for subcutaneous and intra-articular administration. J. Drug Deliv. Sci. Technol. 2013, 23, 315–324. [Google Scholar] [CrossRef]
- D’Souza, S. Injectables. In In Vitro Drug Release Testing of Special Dosage Forms, 1st ed.; Fotaki, N., Klein, S., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 55–85. [Google Scholar]
- Reginald, W.B.; Earl, W.S.; Robison, G.A. Cyclic AMP.; Academic Press: New York, NY, USA, 1971; pp. 17–47. [Google Scholar]
- Hansch, C.; David, H.; Albert, L. Exploring QSAR.; American Chemical Society: Washington, DC, USA, 1995; p. 73. [Google Scholar]
- Kaczmarek, P.; Jezowska-Bojczuk, M. Coordination properties of 3′, 5′-cyclic adenosine monophosphate towards copper(II) ions. Inorg. Chim. Acta 2005, 358, 2073–2076. [Google Scholar] [CrossRef]
- Tetko, I.V.; Gasteiger, J.; Todeschini, R.; Mauri, A.; Livingstone, D.; Ertl, P.; Palyulin, V.A.; Radchenko, E.V.; Zefirov, N.S.; Makarenko, A.S.; et al. Virtual computational chemistry laboratory--design and description. J. Comput.-Aided Mol. Des. 2005, 19, 453–463. [Google Scholar] [CrossRef]
- Advanced Chemistry Development (ACD/Labs) I. Advanced Chemistry Development (ACD/Labs) Software V11.02. Toronto, ON, Canada. 2021. Available online: www.acdlabs.com (accessed on 23 July 2021).
- Sun, Y.; Chapman, A.; Larsen, S.W.; Jensen, H.; Petersen, N.J.; Goodall, D.M.; Østergaard, J. UV-vis Imaging of Piroxicam Supersaturation, Precipitation, and Dissolution in a Flow-Through Setup. Anal. Chem. 2018, 90, 6413–6418. [Google Scholar] [CrossRef]
- Jensen, S.S.; Jensen, H.; Goodall, D.M.; Ostergaard, J. Performance characteristics of UV imaging instrumentation for diffusion, dissolution and release testing studies. J. Pharm. Biomed. Anal. 2016, 131, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Østergaard, J. UV/Vis Spectrophotometry and UV Imaging. In Analytical Techniques in the Pharmaceutical Sciences; Müllertz, A., Perrie, Y., Rades, T., Eds.; Springer: New York, NY, USA, 2016; pp. 3–27. [Google Scholar]
- Hines, D.J.; Kaplan, D.L. Poly(lactic-co-glycolic) acid-controlled-release systems: Experimental and modeling insights. Crit. Rev. Ther. Drug Carr. Syst. 2013, 30, 257–276. [Google Scholar] [CrossRef]
- Graham, P.D.; Brodbeck, K.J.; McHugh, A.J. Phase inversion dynamics of PLGA solutions related to drug delivery. J. Control. Release 1999, 58, 233–245. [Google Scholar] [CrossRef]
- Jin, S.; Xia, X.; Huang, J.; Yuan, C.; Zuo, Y.; Li, Y.; Li, J. Recent advances in PLGA-based biomaterials for bone tissue regeneration. Acta Biomater. 2021, 127, 56–79. [Google Scholar] [CrossRef] [PubMed]
- Oppong, F.; Li, Z.; Fakhrabadi, E.A.; Raorane, T.; Giri, P.M.; Liberatore, M.W.; Sarver, J.G.; Trabbic, C.J.; Hosier, C.E.; Erhardt, P.W.; et al. Investigating the Potential to Deliver and Maintain Plasma and Brain Levels of a Novel Practically Insoluble Methuosis Inducing Anticancer Agent 5-Methoxy MOMIPP Through an Injectable In Situ Forming Thermoresponsive Hydrogel Formulation. J. Pharm. Sci. 2020, 109, 2719–2728. [Google Scholar] [CrossRef] [PubMed]
- Bode, C.; Kranz, H.; Siepmann, F.; Siepmann, J. Coloring of PLGA implants to better understand the underlying drug release mechanisms. Int. J. Pharm. 2019, 569, 118563. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.S.; Kastellorizios, M.; Tipnis, N.; Zou, Y.; Wang, Y.; Choi, S.; Burgess, D.J. Effect of implant formation on drug release kinetics of in situ forming implants. Int. J. Pharm. 2021, 592, 120105. [Google Scholar] [CrossRef]
- Zhang, Q.; Fassihi, R. Release rate determination from in situ gel forming PLGA implant: A novel ‘shape-controlled basket in tube’ method. J. Pharm. Pharmacol. 2020, 72, 1038–1048. [Google Scholar] [CrossRef]
- Kalicharan, R.W.; Baron, P.; Oussoren, C.; Bartels, L.W.; Vromans, H. Spatial distribution of oil depots monitored in human muscle using MRI. Int. J. Pharm. 2016, 505, 52–60. [Google Scholar] [CrossRef]
- Schmolka, I.R. Artificial skin. I. Preparation and properties of pluronic F-127 gels for treatment of burns. J. Biomed. Mater. Res. 1972, 6, 571–582. [Google Scholar] [CrossRef]
- Pion-Inc. Sirius SDi2—Technical Specification Billerica, MA, USA. Available online: https://pion-inc.com/resource/192/download (accessed on 18 December 2020).
- Østergaard, J.; Meng-Lund, E.; Larsen, S.; Larsen, C.; Petersson, K.; Lenke, J.; Jensen, H. Real-time UV imaging of nicotine release from transdermal patch. Pharm. Res. 2010, 27, 2614–2623. [Google Scholar] [CrossRef]
- Stephen. Natural-Order Filename Sort: MATLAB Central File Exchange. 2021. Available online: https://www.mathworks.com/matlabcentral/fileexchange/47434-natural-order-filename-sort (accessed on 17 November 2021).
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarendon: Oxford, UK, 1975; p. 421. [Google Scholar]
- Ye, F.; Larsen, S.W.; Yaghmur, A.; Jensen, H.; Larsen, C.; Ostergaard, J. Real-time UV imaging of piroxicam diffusion and distribution from oil solutions into gels mimicking the subcutaneous matrix. Eur. J. Pharm. Sci. 2012, 46, 72–78. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bock, F.; Bøtker, J.P.; Larsen, S.W.; Lu, X.; Østergaard, J. Methodological Considerations in Development of UV Imaging for Characterization of Intra-Tumoral Injectables Using cAMP as a Model Substance. Int. J. Mol. Sci. 2022, 23, 3599. https://doi.org/10.3390/ijms23073599
Bock F, Bøtker JP, Larsen SW, Lu X, Østergaard J. Methodological Considerations in Development of UV Imaging for Characterization of Intra-Tumoral Injectables Using cAMP as a Model Substance. International Journal of Molecular Sciences. 2022; 23(7):3599. https://doi.org/10.3390/ijms23073599
Chicago/Turabian StyleBock, Frederik, Johan Peter Bøtker, Susan Weng Larsen, Xujin Lu, and Jesper Østergaard. 2022. "Methodological Considerations in Development of UV Imaging for Characterization of Intra-Tumoral Injectables Using cAMP as a Model Substance" International Journal of Molecular Sciences 23, no. 7: 3599. https://doi.org/10.3390/ijms23073599
APA StyleBock, F., Bøtker, J. P., Larsen, S. W., Lu, X., & Østergaard, J. (2022). Methodological Considerations in Development of UV Imaging for Characterization of Intra-Tumoral Injectables Using cAMP as a Model Substance. International Journal of Molecular Sciences, 23(7), 3599. https://doi.org/10.3390/ijms23073599