Melissa officinalis: Composition, Pharmacological Effects and Derived Release Systems—A Review
Abstract
:1. Introduction
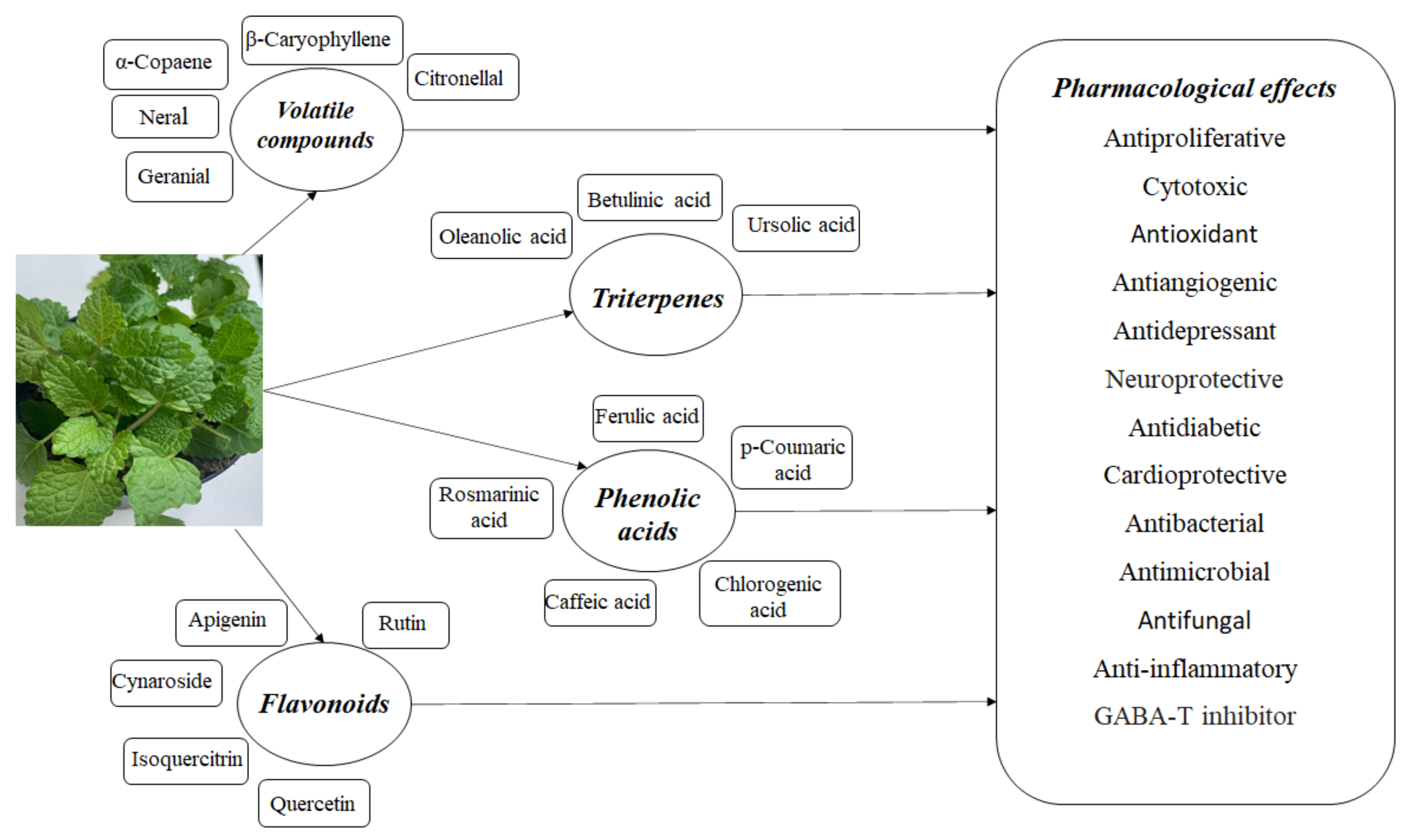
2. Phytochemical Composition
2.1. Volatile Compounds
2.2. Triterpenes
2.3. Polyphenolic Compounds
2.4. Other Components
3. Pharmacological Studies
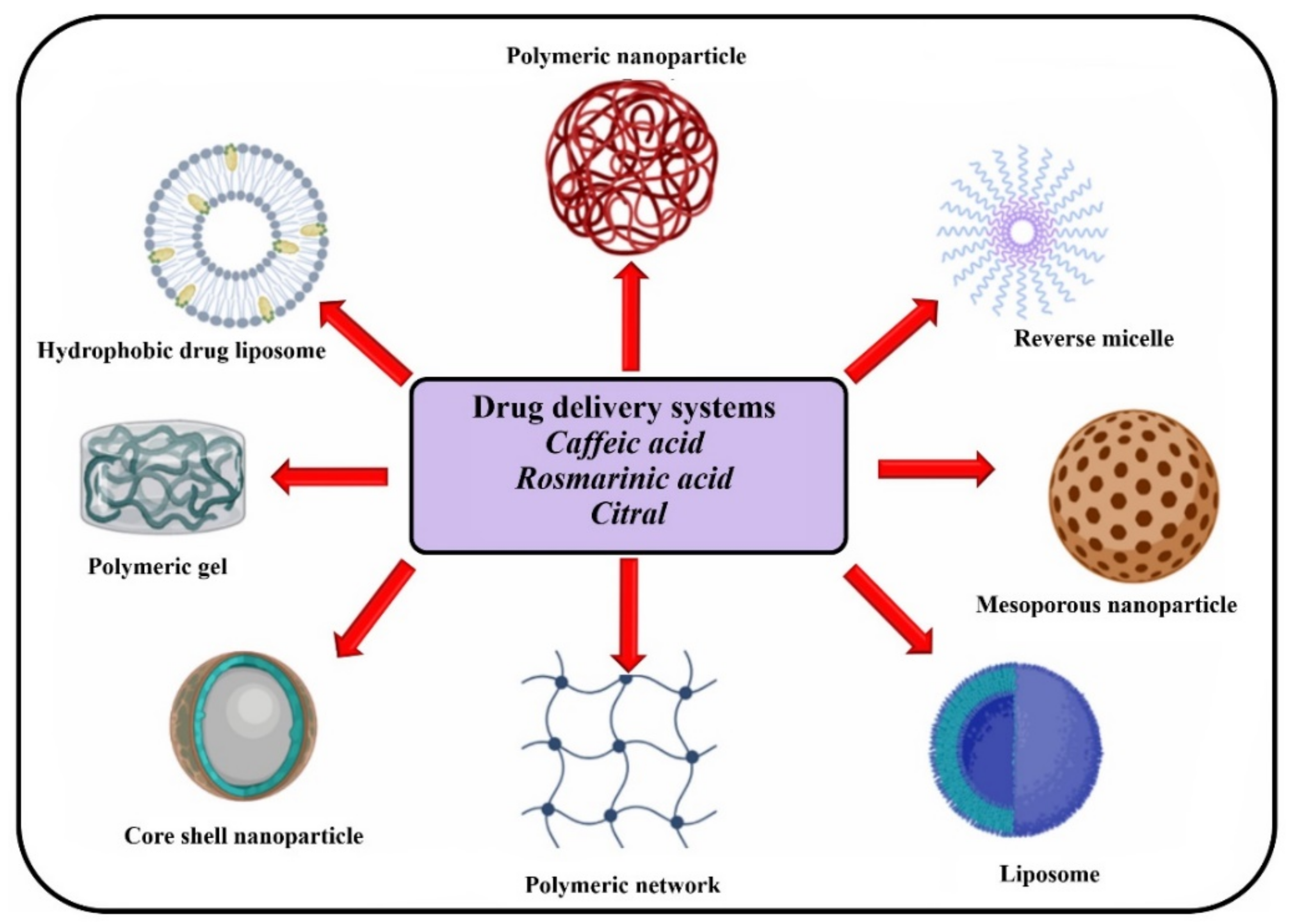
4. Controlled Release Systems
4.1. Drug Delivery Systems Based on Pure Components Which Are Available in Melissa officinalis
4.2. Drug Delivery Systems Based on Melissa officinalis Extracts
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Süntar, I. Importance of ethnopharmacological studies in drug discovery: Role of medicinal plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Bonifácio, B.V.; Silva, P.B.; Ramos, M.A.; Negri, K.M.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Xu, J.; Fang, H.; Li, Z.; Li, M. Advances and challenges in medicinal plant breeding. Plant Sci. 2020, 298, 110573. [Google Scholar] [CrossRef] [PubMed]
- Ghiulai, R.; Avram, S.; Stoian, D.; Pavel, I.Z.; Coricovac, D.; Oprean, C.; Vlase, L.; Farcas, C.; Mioc, M.; Minda, D.; et al. Lemon Balm Extracts Prevent Breast Cancer Progression In Vitro and In Ovo on Chorioallantoic Membrane Assay. Evid.-Based Complement. Altern. Med. 2020, 2020, 6489159. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Naime, W.A.; Fahim, J.R.; Fouad, M.A.; Kamel, M.S. Antibacterial, antifungal, and GC-MS studies of Melissa officinalis. S. Afr. J. Bot. 2019, 124, 228–234. [Google Scholar] [CrossRef]
- Mokhtarzadeh, S.; Demirci, B.; Goger, G.; Khawar, K.M.; Kirimer, N. Characterization of volatile components in Melissa officinalis L. under in vitro conditions. J. Essent. Oil Res. 2017, 29, 299–303. [Google Scholar] [CrossRef]
- Moradkhani, H.; Sargsyan, E.; Bibak, H.; Naseri, B.; Sadat-Hosseini, M.; Fayazi-Barjin, A.; Meftahizade, H. Melissa officinalis L., a valuable medicine plant: A review. J. Med. Plants Res. 2010, 4, 2753–2759. [Google Scholar]
- Miraj, S.; Rafieian-Kopaei, M.; Kiani, S. Melissa officinalis L: A Review Study with an Antioxidant Prospective. J. Evid.-Based Integr. Med. 2017, 22, 385–394. [Google Scholar] [CrossRef]
- Zarei, A.; Ashtiyani, S.C.; Taheri, S.; Rasekh, F. Comparison between effects of different doses of Melissa officinalis and atorvastatin on the activity of liver enzymes in hypercholesterolemia rats. Avicenna J. Phytomed. 2014, 4, 15–23. [Google Scholar] [CrossRef]
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis L.—A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 188, 204–228. [Google Scholar] [CrossRef]
- Bağdat, R.B.; Coşge, B. The essential oil of Lemon balm (Melissa officinalis L.), its components and using fields. J. Fac. Agric. OMU 2006, 21, 116–121. [Google Scholar]
- Nouri, A.; Mirabzadeh, M.; Safari, N.; Ebadi, M.T. Evaluation of Essential Oil Composition and Rosmarinic Acid Content in Lemon Balm (Melissa officinalis L.) Cultivated in South of Iran. J. Med. Plants By-Prod. 2020, 9, 159–166. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R.; Bogucka-Kocka, A.; Szymczak, G. Volatile Constituents of Melissa officinalis Leaves Determined by Plant Age. Nat. Prod. Commun. 2014, 9, 703–706. [Google Scholar] [CrossRef] [Green Version]
- Souihi, M.; Amri, I.; Souissi, A.; Hosni, K.; Ben Brahim, N.; Annabi, M. Essential oil and fatty acid composition of Melissa officinalis L. Prog. Nutr. 2020, 22, 253–258. [Google Scholar] [CrossRef]
- Abdellatif, F.; Boudjella, H.; Zitouni, A.; Hassani, A. Chemical composition and antimicrobial activity of the essential oil from leaves of Algerian Melissa officinalis L. EXCLI J. 2014, 13, 772. [Google Scholar] [CrossRef] [PubMed]
- Seidler-Łożykowska, K.; Zawirska-Wojtasiak, R.; Wojtowicz, E.; Bocianowski, J. Essential oil content and its composition in herb of lemon balm (Melissa officinalis L.) breeding strains. J. Essent. Oil Res. 2017, 29, 351–356. [Google Scholar] [CrossRef]
- Taherpour, A.; Maroofi, H.; Rafie, Z.; Larijani, K. Chemical composition analysis of the essential oil of Melissa officinalis L. from Kurdistan, Iran by HS/SPME method and calculation of the biophysicochemical coefficients of the components. Nat. Prod. Res. 2012, 26, 152–160. [Google Scholar] [CrossRef]
- Ahl, H.A.S.-A.; Sabra, A.S.; Gendy, A.S.; Astatkie, T. Essential Oil Content and Concentration of Constituents of Lemon Balm (Melissa officinalis L.) at Different Harvest Dates. J. Essent. Oil Bear. Plants 2018, 21, 1410–1417. [Google Scholar] [CrossRef]
- Kowalski, R.; Kowalska, G.; Jankowska, M.; Nawrocka, A.; Kałwa, K.; Pankiewicz, U.; Włodarczyk-Stasiak, M. Secretory structures and essential oil composition of selected industrial species of lamiaceae. Acta Sci. Pol. Hortorum Cultus 2019, 18, 53–69. [Google Scholar] [CrossRef]
- Kittler, J.; Krüger, H.; Lohwasser, U.; Ulrich, D.; Zeiger, B.; Schütze, W.; Böttcher, C.; Gudi, G.; Kästner, U.; Marthe, F. Evaluation of 28 balm and lemon balm (Melissa officinalis) accessions for content and composition of essential oil and content of rosmarinic acid. Genet. Resour. Crop Evol. 2018, 65, 745–757. [Google Scholar] [CrossRef]
- Basta, A.; Tzakou, O.; Couladis, M. Composition of the leaves essential oil of Melissa officinalis s. l. from Greece. Flavour Fragr. J. 2005, 20, 642–644. [Google Scholar] [CrossRef]
- Barakat, S.A.; Hudaib, M.; Burns, D.T. Composition of Volatile Oil and Methanolic Extract of Jordanian Melissa officinalis L. and Actions against Human Cancer Cell Lines. Orient. J. Chem. 2016, 32, 2355–2362. [Google Scholar] [CrossRef] [Green Version]
- Thimmappa, R.; Geisler, K.; Louveau, T.; O’Maille, P.; Osbourn, A. Triterpene Biosynthesis in Plants. Annu. Rev. Plant Biol. 2014, 65, 225–257. [Google Scholar] [CrossRef] [PubMed]
- Mencherini, T.; Picerno, P.; Scesa, C.; Aquino, R. Triterpene, Antioxidant, and Antimicrobial Compounds from Melissa officinalis. J. Nat. Prod. 2007, 70, 1889–1894. [Google Scholar] [CrossRef]
- Abdel-Naime, W.; Fahim, J.; Abdelmohsen, U.; Fouad, M.; Al-Footy, K.; Abdel-Lateff, A.; Kamel, M. New antimicrobial triterpene glycosides from lemon balm (Melissa officinalis). S. Afr. J. Bot. 2019, 125, 161–167. [Google Scholar] [CrossRef]
- Mencherini, T.; Picerno, P.; Russo, P.; Meloni, M.; Aquino, R. Composition of the Fresh Leaves and Stems of Melissa officinalis and Evaluation of Skin Irritation in a Reconstituted Human Epidermis Model. J. Nat. Prod. 2009, 72, 1512–1515. [Google Scholar] [CrossRef]
- Ibarra, A.; Feuillere, N.; Roller, M.; Lesburgere, E.; Beracochea, D. Effects of chronic administration of Melissa officinalis L. extract on anxiety-like reactivity and on circadian and exploratory activities in mice. Phytomedicine 2010, 17, 397–403. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Dias, M.I.; Sousa, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic profiles of cultivated, in vitro cultured and commercial samples of Melissa officinalis L. infusions. Food Chem. 2013, 136, 1–8. [Google Scholar] [CrossRef]
- Fierascu, I.; Georgiev, M.I.; Ortan, A.; Fierascu, R.C.; Avramescu, S.M.; Ionescu, D.; Sutan, A.; Brinzan, A.; Ditu, L.M. Phyto-mediated metallic nano-architectures via Melissa officinalis L.: Synthesis, characterization and biological properties. Sci. Rep. 2017, 7, 12428. [Google Scholar] [CrossRef] [Green Version]
- Gayibova, S.; Ivanišová, E.; Árvay, J.; Hŕstková, M.; Slávik, M.; Petrová, J.; Hleba, L.; Tóth, T.; Kačániová, M.; Aripov, T. In vitro screening of antioxidant and antimicrobial activities of medicinal plants growing in Slovakia. J. Microbiol. Biotechnol. Food Sci. 2019, 8, 1281–1289. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miron, T.; Herrero, M.; Ibanez, E. Enrichment of antioxidant compounds from lemon balm (Melissa officinalis) by pressurized liquid extraction and enzyme-assisted extraction. J. Chromatogr. A 2013, 1288, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, R.P.; Boligon, A.A.; Appel, A.S.; Fachinetto, R.; Ceron, C.S.; Tanus-Santos, J.E.; Athayde, M.L.; da Rocha, J.B.T. Chemical composition, antioxidant and anticholinesterase activity of Melissa officinalis. Ind. Crop. Prod. 2014, 53, 34–45. [Google Scholar] [CrossRef]
- Spiridon, I.; Colceru, S.; Anghel, N.; Teaca, C.A.; Bodirlau, R.; Armatu, A. Antioxidant capacity and total phenolic contents of oregano (Origanum vulgare), lavender (Lavandula angustifolia) and lemon balm (Melissa officinalis) from Romania. Nat. Prod. Res. 2011, 25, 1657–1661. [Google Scholar] [CrossRef] [PubMed]
- Ashori, A.; Hamzeh, Y.; Amani, F. Lemon Balm (Melissa officinalis) Stalk: Chemical Composition and Fiber Morphology. J. Polym. Environ. 2011, 19, 297–300. [Google Scholar] [CrossRef]
- Komes, D.; Belščak-Cvitanović, A.; Horžić, D.; Rusak, G.; Likić, S.; Berendika, M. Phenolic composition and antioxidant properties of some traditionally used medicinal plants affected by the extraction time and hydrolysis. Phytochem. Anal. 2011, 22, 172–180. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Sousa, M.J.; Ferreira, I.C. Systematic comparison of nutraceuticals and antioxidant potential of cultivated, in vitro cultured and commercial Melissa officinalis samples. Food Chem. Toxicol. 2012, 50, 1866–1873. [Google Scholar] [CrossRef]
- Moacă, E.A.; Farcaş, C.; Ghiţu, A.; Coricovac, D.; Popovici, R.; Cărăba-Meiţă, N.L.; Ardelean, F.; Antal, D.S.; Dehelean, C.; Avram, Ş. A Comparative Study of Melissa officinalis Leaves and Stems Ethanolic Extracts in terms of Antioxidant, Cytotoxic, and Antiproliferative Potential. Evid.-Based Complement. Alternat. Med. 2018, 2018, 7860456. [Google Scholar] [CrossRef] [Green Version]
- Encalada, M.A.; Hoyos, K.M.; Rehecho, S.; Berasategi, I.; De Ciriano, M.G.; Ansorena, D.; Astiasarán, I.; Navarro-Blasco, I.; Cavero, R.Y.; Calvo, M.I. Anti-proliferative Effect of Melissa officinalis on Human Colon Cancer Cell Line. Mater. Veg. 2011, 66, 328–334. [Google Scholar] [CrossRef]
- Magalhães, D.B.; Castro, I.; Lopes-Rodrigues, V.; Pereira, J.M.; Barros, L.; Ferreira, I.C.F.R.; Xavier, C.P.R.; Vasconcelos, M.H. Melissa officinalis L. ethanolic extract inhibits the growth of a lung cancer cell line by interfering with the cell cycle and inducing apoptosis. Food Funct. 2018, 9, 3134–3142. [Google Scholar] [CrossRef] [Green Version]
- Pereira, R.P.; Fachinetto, R.; Prestes, A.; Puntel, R.; Da Silva, G.N.S.; Heinzmann, B.M.; Boschetti, T.K.; Athayde, M.L.; Bürger, M.E.; Morel, A.F.; et al. Antioxidant Effects of Different Extracts from Melissa officinalis, Matricaria recutita and Cymbopogon citratus. Neurochem. Res. 2009, 34, 973–983. [Google Scholar] [CrossRef]
- Ehsani, A.; Alizadeh, O.; Hashemi, M.; Afshari, A.; Aminzare, M. Phytochemical, antioxidant and antibacterial properties of Melissa officinalis and Dracocephalum moldavica essential oils. Vet. Res. Forum Int. Q. J. 2017, 8, 223–229. [Google Scholar]
- Sedighi, M.; Faghihi, M.; Rafieian-Kopaei, M.; Rasoulian, B.; Nazari, A. Cardioprotective effect of ethanolic leaf extract of Melissa officinalis L. against regional ischemia-induced arrhythmia and heart injury after five days of reperfusion in rats. Iran. J. Pharm. Res. IJPR 2019, 18, 1530–1542. [Google Scholar] [CrossRef] [PubMed]
- Hasanein, P.; Riahi, H. Antinociceptive and Antihyperglycemic Effects of Melissa officinalis Essential Oil in an Experimental Model of Diabetes. Med. Princ. Pract. 2015, 24, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Ghazizadeh, J.; Hamedeyazdan, S.; Torbati, M.; Farajdokht, F.; Fakhari, A.; Mahmoudi, J.; Araj-khodaei, M.; Sadigh-Eteghad, S. Melissa officinalis L. hydro-alcoholic extract inhibits anxiety and depression through prevention of central oxidative stress and apoptosis. Exp. Physiol. 2020, 105, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Bayat, M.; Tameh, A.A.; Ghahremani, M.H.; Akbari, M.; Mehr, S.E.; Khanavi, M.; Hassanzadeh, G. Neuroprotective properties of Melissa officinalis after hypoxic-ischemic injury both in vitro and in vivo. DARU J. Pharm. Sci. 2012, 20, 42. [Google Scholar] [CrossRef] [Green Version]
- Awad, R.; Muhammad, A.; Durst, T.; Trudeau, V.L.; Arnason, J.T. Bioassay-guided fractionation of lemon balm (Melissa officinalis L.) using an in vitro measure of GABA transaminase activity. Phytother. Res. 2009, 23, 1075–1081. [Google Scholar] [CrossRef]
- Cunha, F.; Tintino, S.R.; Figueredo, F.; Barros, L.; Duarte, A.E.; Gomez, M.C.V.; Coronel, C.C.; Rolón, M.; Leite, N.; Sobral-Souza, C.E.; et al. HPLC-DAD phenolic profile, cytotoxic and anti-kinetoplastidae activity of Melissa officinalis. Pharm. Biol. 2016, 54, 1664–1670. [Google Scholar] [CrossRef] [Green Version]
- Rastegarian, A.; Abedi, H.; Jahromi, H.K.; Zarei, S.; Nematollahi, A.; Mansouri, E.; Sameni, H. Analgesic Effect of Intrathecal Melissa officinalis in the Rat Model of Hot-Water and Formalin-Induced Pain. J. Acupunct. Meridian Stud. 2020, 13, 19–24. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Safaei, A. Hypnotic effect of Coriandrum sativum, Ziziphus jujuba, Lavandula angustifolia and Melissa officinalis extracts in mice. Res. Pharm. Sci. 2015, 10, 477–484. [Google Scholar]
- Chung, M.J.; Cho, S.-Y.; Bhuiyan, M.J.H.; Kim, K.H.; Lee, S.-J. Anti-diabetic effects of lemon balm (Melissa officinalis) essential oil on glucose- and lipid-regulating enzymes in type 2 diabetic mice. Br. J. Nutr. 2010, 104, 180–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, Y.; Lee, D.; Ahn, J.; Lee, M.; Shin, S.S.; Yoon, M. The herbal extract ALS-L1023 from Melissa officinalis reduces weight gain, elevated glucose levels and beta-cell loss in Otsuka Long-Evans Tokushima fatty rats. J. Ethnopharmacol. 2020, 264, 113360. [Google Scholar] [CrossRef] [PubMed]
- Gürbüz, P.; Martinez, A.; Pérez, C.; Martínez-González, L.; Göger, F.; Ayran, İ. Potential anti-Alzheimer effects of selected Lamiaceae plants through polypharmacology on glycogen synthase kinase-3β, β-secretase, and casein kinase 1δ. Ind. Crop. Prod. 2019, 138, 111431. [Google Scholar] [CrossRef]
- Aubert, P.; Guinobert, I.; Blondeau, C.; Bardot, V.; Ripoche, I.; Chalard, P.; Neunlist, M. Basal and Spasmolytic Effects of a Hydroethanolic Leaf Extract of Melissa officinalis L. on Intestinal Motility: An Ex Vivo Study. J. Med. Food 2019, 22, 653–662. [Google Scholar] [CrossRef] [Green Version]
- Astani, A.; Reichling, J.; Schnitzler, P. Melissa officinalis Extract Inhibits Attachment of Herpes Simplex Virus in vitro. Chemotherapy 2012, 58, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Araújo, S.G.; Lima, W.G.; Pinto, M.E.A.; Morais, M.; de Sá, N.P.; Johann, S.; Rosa, C.A.; Lima, L.A.R.D.S. Pharmacological prospection in-vitro of Lamiaceae species against human pathogenic fungi associated to invasive infections. Biocatal. Agric. Biotechnol. 2019, 21, 101345. [Google Scholar] [CrossRef]
- El Ouadi, Y.; Manssouri, M.; Bouyanzer, A.; Majidi, L.; Bendaif, H.; Elmsellem, H.; Shariati, M.; Melhaoui, A.; Hammouti, B. Essential oil composition and antifungal activity of Melissa officinalis originating from north-Est Morocco, against postharvest phytopathogenic fungi in apples. Microb. Pathog. 2017, 107, 321–326. [Google Scholar] [CrossRef]
- Patora, J.; Klimek, B. Flavonoids from lemon balm (Melissa officinalis L., Lamiaceae). Acta Pol. Pharm. Drug Res. 2002, 59, 139–144. [Google Scholar]
- Astani, A.; Navid, M.H.; Schnitzler, P. Attachment and Penetration of Acyclovir-resistant Herpes Simplex Virus Are Inhibited by Melissa officinalis Extract. Phytother. Res. 2014, 28, 1547–1552. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.Y.Y.; Fantus, I.G. Oral antihyperglycemic therapy for type 2 diabetes mellitus. Can. Med. Assoc. J. 2005, 172, 213–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutter, J.D.; Mendes, M.; Franco, O.H. Cardioprotective drugs. In The ESC Textbook of Preventive Cardiology; Oxford University Press: Oxford, UK, 2017; p. 251. [Google Scholar] [CrossRef]
- UpToDate. Pharmacology of Medicines for Treatment of Adults with Generalized Anxiety Disorder (GAD). Available online: https://www.uptodate.com/contents/image/print?imageKey=PSYCH%2F77409 (accessed on 9 January 2022).
- Barks, J.D.; Liu, Y.-Q.; Shangguan, Y.; Silverstein, F.S. Phenobarbital Augments Hypothermic Neuroprotection. Pediatr. Res. 2010, 67, 532–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- UpToDate. Dosing and Selected Characteristics of Analgesic Drugs for TREATMENT of Persistent Pain in Older Adults. Available online: https://www.uptodate.com/contents/image?imageKey=ANEST%2F95621&topicKey=PALC%2F86302&source=see_link (accessed on 9 January 2022).
- Drugs.com. Chloral Hydrate Dosage. Available online: https://www.drugs.com/dosage/chloral-hydrate.html (accessed on 9 January 2022).
- Erhorn, S. Triclofos. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Newcastle upon Tyne, UK, 2007; pp. 1–4. [Google Scholar]
- Oral Sedation. In Sedation, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 7, pp. 95–119. [CrossRef]
- Southwell, I. Backhousia citriodora F. Muell. (Lemon Myrtle), an Unrivalled Source of Citral. Foods 2021, 10, 1596. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Ficai, D.; Ficai, A.; Truşcă, R.-D.; Ilie, C.-I.; Oprea, O.-C.; Andronescu, E. Innovative Antimicrobial Chitosan/ZnO/Ag NPs/Citronella Essential Oil Nanocomposite—Potential Coating for Grapes. Foods 2020, 9, 1801. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Oprea, O.-C.; Ficai, A.; Ene, V.-L.; Vasile, B.-S.; Andronescu, E.; Holban, A.-M. Antibacterial Biodegradable Films Based on Alginate with Silver Nanoparticles and Lemongrass Essential Oil–Innovative Packaging for Cheese. Nanomaterials 2021, 11, 2377. [Google Scholar] [CrossRef]
- Zheng, Y.; Shang, Y.; Li, M.; Li, Y.; Ouyang, W. Antifungal Activities of cis-trans Citral Isomers against Trichophyton rubrum with ERG6 as a Potential Target. Molecules 2021, 26, 4263. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Oprea, O.; Ficai, A.; Trusca, R.-D.; Andronescu, E.; Holban, A. Biodegradable Alginate Films with ZnO Nanoparticles and Citronella Essential Oil—A Novel Antimicrobial Structure. Pharmaceutics 2021, 13, 1020. [Google Scholar] [CrossRef]
- Luu-Dam, N.A.; Tabanca, N.; Estep, A.S.; Nguyen, D.H.; Kendra, P.E. Insecticidal and Attractant Activities of Magnolia citrata Leaf Essential Oil against Two Major Pests from Diptera: Aedes aegypti (Culicidae) and Ceratitis capitata (Tephritidae). Molecules 2021, 26, 2311. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Sikora, V.; Semerdjieva, I.B.; Kačániová, M.; Astatkie, T.; Dincheva, I. Grinding and Fractionation during Distillation Alter Hemp Essential Oil Profile and Its Antimicrobial Activity. Molecules 2020, 25, 3943. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, X.; Wang, B.; Lin, Z.; Ye, M.; Ma, R.; Zheng, M.; Xiang, H.; Xu, P. Six herbs essential oils suppressing inflammatory responses via inhibiting COX-2/TNF-α/IL-6/NF-κB activation. Microchem. J. 2020, 156, 104769. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Dhibi, S.; Dhifi, W.; Ben Saad, R.; Brini, F.; Hfaidh, N.; Mnif, W. Essential oil from halophyte Lobularia maritima: Protective effects against CCl4-induced hepatic oxidative damage in rats and inhibition of the production of proinflammatory gene expression by lipopolysaccharide-stimulated RAW 264.7 macrophages. RSC Adv. 2019, 9, 36758–36770. [Google Scholar] [CrossRef] [Green Version]
- Zheljazkov, V.; Cantrell, C.; Semerdjieva, I.; Radoukova, T.; Stoyanova, A.; Maneva, V.; Kačániová, M.; Astatkie, T.; Borisova, D.; Dincheva, I.; et al. Essential Oil Composition and Bioactivity of Two Juniper Species from Bulgaria and Slovakia. Molecules 2021, 26, 3659. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Silva, C.; Fernandes, M.X.; Câmara, J.S. Prediction of Terpenoid Toxicity Based on a Quantitative Structure—Activity Relationship Model. Foods 2019, 8, 628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plata-Rueda, A.; Rolim, G.D.S.; Wilcken, C.F.; Zanuncio, J.C.; Serrão, J.E.; Martínez, L.C. Acute Toxicity and Sublethal Effects of Lemongrass Essential Oil and Their Components against the Granary Weevil, Sitophilus granarius. Insects 2020, 11, 379. [Google Scholar] [CrossRef]
- Colombo, E.; Biocotino, M.; Frapporti, G.; Randazzo, P.; Christodoulou, M.; Piccoli, G.; Polito, L.; Seneci, P.; Passarella, D. Nanolipid-Trehalose Conjugates and Nano-Assemblies as Putative Autophagy Inducers. Pharmaceutics 2019, 11, 422. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Xie, T.; Tian, X.; Han, N.; Liu, X.; Chen, H.; Qi, J.; Gao, F.; Li, W.; Wu, Q.; et al. Betulinic Acid-Nitrogen Heterocyclic Derivatives: Design, Synthesis, and Antitumor Evaluation in Vitro. Molecules 2020, 25, 948. [Google Scholar] [CrossRef] [Green Version]
- Kil, H.W.; Rho, T.; Yoon, K.D. Phytochemical Study of Aerial Parts of Leea asiatica. Molecules 2019, 24, 1733. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.M.; Alvarado, H.L.; Abrego, G.; Martins-Gomes, C.; Garduño-Ramirez, M.L.; García, M.L.; Calpena, A.C.; Souto, E.B. In Vitro Cytotoxicity of Oleanolic/Ursolic Acids-Loaded in PLGA Nanoparticles in Different Cell Lines. Pharmaceutics 2019, 11, 362. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Maharjan, A.; Jin, M.; Park, T.; Amatya, R.; Yang, J.; Min, K.A.; Shin, M.C. Potential Albumin-Based Antioxidant Nanoformulations for Ocular Protection against Oxidative Stress. Pharmaceutics 2019, 11, 297. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues Pereira, J.; Suassuna Bezerra, G.; Alves Furtado, A.; de Carvalho, T.G.; Costa da Silva, V.; Lins Bispo Monteiro, A.; Bernardo Guerra, G.C.; de Araújo Júnior, R.F.; Sant’Ana, A.E.G.; Fernandes-Pedrosa, M.D.F.; et al. Chitosan Film Containing Mansoa hirsute Fraction for Wound Healing. Pharmaceutics 2020, 12, 484. [Google Scholar] [CrossRef]
- Aguilar, L.E.; Jang, S.R.; Park, C.H.; Lee, K.M. Supramolecular Caffeic Acid and Bortezomib Nanomedicine: Prodrug Inducing Reactive Oxygen Species and Inhibiting Cancer Cell Survival. Pharmaceutics 2020, 12, 1082. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Oyarzo, D.; Plaza, T.; Recio-Sánchez, G.; Abdalla, D.S.; Salazar, L.A.; Hernández-Montelongo, J. Use of nPSi-βCD Composite Microparticles for the Controlled Release of Caffeic Acid and Pinocembrin, Two Main Polyphenolic Compounds Found in a Chilean Propolis. Pharmaceutics 2019, 11, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sergio, L.; Boari, F.; Pieralice, M.; Linsalata, V.; Cantore, V.; Di Venere, D. Bioactive Phenolics and Antioxidant Capacity of Some Wild Edible Greens as Affected by Different Cooking Treatments. Foods 2020, 9, 1320. [Google Scholar] [CrossRef] [PubMed]
- Dupas de Matos, A.; Longo, E.; Chiotti, D.; Pedri, U.; Eisenstecken, D.; Sanoll, C.; Robatscher, P.; Boselli, E. Pinot Blanc: Impact of the Winemaking Variables on the Evolution of the Phenolic, Volatile and Sensory Profiles. Foods 2020, 9, 499. [Google Scholar] [CrossRef] [Green Version]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A Review of the Anti-Inflammatory Effects of Rosmarinic Acid on Inflammatory Diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Rassu, G.; Gavini, E.; Sorrenti, M.; Catenacci, L.; Giunchedi, P. Nose-to-Brain Delivery of Antioxidants as a Potential Tool for the Therapy of Neurological Diseases. Pharmaceutics 2020, 12, 1246. [Google Scholar] [CrossRef]
- Odrobińska, J.; Skonieczna, M.; Neugebauer, D. PEG Graft Polymer Carriers of Antioxidants: In Vitro Evaluation for Transdermal Delivery. Pharmaceutics 2020, 12, 1178. [Google Scholar] [CrossRef]
- Grimaudo, M.A.; Concheiro, A.; Alvarez-Lorenzo, C. Crosslinked Hyaluronan Electrospun Nanofibers for Ferulic Acid Ocular Delivery. Pharmaceutics 2020, 12, 274. [Google Scholar] [CrossRef] [Green Version]
- López-Yerena, A.; Perez, M.; Vallverdú-Queralt, A.; Escribano-Ferrer, E. Insights into the Binding of Dietary Phenolic Compounds to Human Serum Albumin and Food-Drug Interactions. Pharmaceutics 2020, 12, 1123. [Google Scholar] [CrossRef]
- Chiu, J.Z.S.; Hold, I.; Newman, T.A.C.; Horsfield, J.A.; McDowell, A. Chlorogenic Acid Supplementation Benefits Zebrafish Embryos Exposed to Auranofin. Pharmaceutics 2020, 12, 1199. [Google Scholar] [CrossRef]
- Żyżelewicz, D.; Kulbat-Warycha, K.; Oracz, J.; Żyżelewicz, K. Polyphenols and Other Bioactive Compounds of Sideritis Plants and Their Potential Biological Activity. Molecules 2020, 25, 3763. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Emerging Strategies to Protect the Skin from Ultraviolet Rays Using Plant-Derived Materials. Antioxidants 2020, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Park, B.R.; Moon, S.M.; Shin, S.H.; Kim, J.S.; Kim, D.K.; Kim, C.S. Cynaroside protects human periodontal ligament cells from lipopolysaccharide-induced damage and inflammation through suppression of NF-κB activation. Arch. Oral Biol. 2020, 120, 104944. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.-H.; Guo, J.; Chen, S.-H.X.; Yuan, X.-R.; Zhang, T.; Ding, Y. Antioxidant and Compositional HPLC Analysis of Three Common Bamboo Leaves. Molecules 2020, 25, 409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mota, A.; Duarte, N.; Serra, A.; Ferreira, A.; Bronze, M.; Custódio, L.; Gaspar, M.; Simões, S.; Rijo, P.; Ascensão, L.; et al. Further Evidence of Possible Therapeutic Uses of Sambucus nigra L. Extracts by the Assessment of the In Vitro and In Vivo Anti-Inflammatory Properties of Its PLGA and PCL-Based Nanoformulations. Pharmaceutics 2020, 12, 1181. [Google Scholar] [CrossRef]
- Wasan, E.K.; Zhao, J.; Poteet, J.; Mohammed, M.A.; Syeda, J.; Orlowski, T.; Soulsbury, K.; Cawthray, J.; Bunyamin, A.; Zhang, C.; et al. Development of a UV-Stabilized Topical Formulation of Nifedipine for the Treatment of Raynaud Phenomenon and Chilblains. Pharmaceutics 2019, 11, 594. [Google Scholar] [CrossRef] [Green Version]
- Mikulášová, M.; Chovanová, R.; Vaverková, Š. Synergism between antibiotics and plant extracts or essential oils with efflux pump inhibitory activity in coping with multidrug-resistant staphylococci. Phytochem. Rev. 2016, 15, 651–662. [Google Scholar] [CrossRef]
- Ulrich-Merzenich, G.; Welslau, L.; Aziz-Kalbhenn, H.; Kelber, O.; Shcherbakova, A. Synergy quantifications to identify individual contributions of combination partners to the overall activity—The example of STW 5. Phytomedicine 2019, 60, 153013. [Google Scholar] [CrossRef]
- Zeraatpishe, A.; Oryan, S.; Bagheri, M.H.; Pilevarian, A.A.; Malekirad, A.A.; Baeeri, M.; Abdollahi, M. Effects of Melissa officinalis L. on oxidative status and DNA damage in subjects exposed to long-term low-dose ionizing radiation. Toxicol. Ind. Health 2011, 27, 205–212. [Google Scholar] [CrossRef]
- Jain, N.; Valli, K.S.; Devi, V.K. Importance of novel drug delivery systems in herbal medicines. Pharmacogn. Rev. 2010, 4, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiparissides, C.; Kammona, O. Nanotechnology advances in controlled drug delivery systems. Phys. Status Solidi C—Curr. Top. Solid State Phys. 2008, 5, 3828–3833. [Google Scholar] [CrossRef]
- Balmayor, E.R.; Azevedo, H.S.; Reis, R.L. Controlled Delivery Systems: From Pharmaceuticals to Cells and Genes. Pharm. Res. 2011, 28, 1241–1258. [Google Scholar] [CrossRef]
- Tong, Q.; Qiu, N.; Ji, J.; Ye, L.; Zhai, G. Research Progress in Bioinspired Drug Delivery Systems. Expert Opin. Drug Deliv. 2020, 17, 1269–1288. [Google Scholar] [CrossRef] [PubMed]
- Heng, P.W.S. Controlled release drug delivery systems. Pharm. Dev. Technol. 2018, 23, 833. [Google Scholar] [CrossRef] [PubMed]
- Ruman, U.; Fakurazi, S.; Masarudin, M.J.; Hussein, M.Z. Nanocarrier-Based Therapeutics and Theranostics Drug Delivery Systems for Next Generation of Liver Cancer Nanodrug Modalities. Int. J. Nanomed. 2020, Volume 15, 1437–1456. [Google Scholar] [CrossRef] [Green Version]
- Goyal, A.; Kumar, S.; Nagpal, M.; Singh, I.; Arora, S. Potential of Novel Drug Delivery Systems for Herbal Drugs. Indian J. Pharm. Educ. Res. 2011, 45, 225–235. [Google Scholar]
- Uddin, A.; Saraf, S. Applications of novel drug delivery system for herbal formulations. Fitoterapia 2010, 81, 680–689. [Google Scholar] [CrossRef]
- Garnett, M. Nanomedicines: Delivering drugs using bottom up nanotechnology. Int. J. Nanosci. 2005, 4, 855–861. [Google Scholar] [CrossRef]
- Hallan, S.S.; Sguizzato, M.; Drechsler, M.; Mariani, P.; Montesi, L.; Cortesi, R.; Björklund, S.; Ruzgas, T.; Esposito, E. The Potential of Caffeic Acid Lipid Nanoparticulate Systems for Skin Application: In Vitro Assays to Assess Delivery and Antioxidant Effect. Nanomaterials 2021, 11, 171. [Google Scholar] [CrossRef]
- Fathi, M.; Mirlohi, M.; Varshosaz, J.; Madani, G. Novel Caffeic Acid Nanocarrier: Production, Characterization, and Release Modeling. J. Nanomater. 2013, 2013, 434632. [Google Scholar] [CrossRef]
- Sguizzato, M.; Mariani, P.; Ferrara, F.; Drechsler, M.; Hallan, S.S.; Huang, N.; Simelière, F.; Khunti, N.; Cortesi, R.; Marchetti, N.; et al. Nanoparticulate Gels for Cutaneous Administration of Caffeic Acid. Nanomaterials 2020, 10, 961. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.B.; Amorim, M.; Fonte, P.; Madureira, R.; Ferreira, D.; Pintado, M.; Sarmento, B. Natural extracts into chitosan nanocarriers for rosmarinic acid drug delivery. Pharm. Biol. 2015, 53, 642–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, S.B.; Ferreira, D.; Pintado, M.; Sarmento, B. Chitosan-based nanoparticles for rosmarinic acid ocular delivery—In vitro tests. Int. J. Biol. Macromol. 2016, 84, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Wani, T.U.; Raza, S.N.; Khan, N.A. Rosmarinic Acid Loaded Chitosan Nanoparticles for Wound Healing in Rats. Int. J. Pharm. Sci. Res. 2019, 10, 1138–1147. [Google Scholar]
- Chung, C.H.; Jung, W.; Keum, H.; Kim, T.W.; Jon, S. Nanoparticles Derived from the Natural Antioxidant Rosmarinic Acid Ameliorate Acute Inflammatory Bowel Disease. ACS Nano 2020, 14, 6887–6896. [Google Scholar] [CrossRef]
- Bhatt, R.; Singh, D.; Prakash, A.; Mishra, N. Development, characterization and nasal delivery of rosmarinic acid-loaded solid lipid nanoparticles for the effective management of Huntington’s disease. Drug Deliv. 2013, 22, 931–939. [Google Scholar] [CrossRef]
- Arriagada, F.; Günther, G.; Morales, J. Nanoantioxidant–Based Silica Particles as Flavonoid Carrier for Drug Delivery Applications. Pharmaceutics 2020, 12, 302. [Google Scholar] [CrossRef] [Green Version]
- Nordin, N.; Yeap, S.K.; Zamberi, N.R.; ABU, N.; Mohamad, N.E.; Rahman, H.; How, C.W.; Masarudin, M.J.; Abdullah, R.; Alitheen, N.B. Characterization and toxicity of citral incorporated with nanostructured lipid carrier. PeerJ 2018, 6, e3916. [Google Scholar] [CrossRef] [Green Version]
- Nordin, N.; Yeap, S.K.; Rahman, H.S.; Zamberi, N.R.; Mohamad, N.E.; Abu, N.; Masarudin, M.J.; Abdullah, R.; Alitheen, N.B. Antitumor and Anti-Metastatic Effects of Citral-Loaded Nanostructured Lipid Carrier in 4T1-Induced Breast Cancer Mouse Model. Molecules 2020, 25, 2670. [Google Scholar] [CrossRef]
- Ailincai, D.; Mititelu, L.T.; Marin, L. Drug delivery systems based on biocompatible imino-chitosan hydrogels for local anticancer therapy. Drug Deliv. 2018, 25, 1080–1090. [Google Scholar] [CrossRef] [Green Version]
- Ailincai, D.; Mititelu-Tartau, L.; Marin, L. Citryl-imine-PEG-ylated chitosan hydrogels—Promising materials for drug delivery applications. Int. J. Biol. Macromol. 2020, 162, 1323–1337. [Google Scholar] [CrossRef] [PubMed]
- Usach, I.; Margarucci, E.; Manca, M.L.; Caddeo, C.; Aroffu, M.; Petretto, G.L.; Manconi, M.; Peris, J.-E. Comparison between Citral and Pompia Essential Oil Loaded in Phospholipid Vesicles for the Treatment of Skin and Mucosal Infections. Nanomaterials 2020, 10, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Sotto, A.; Paolicelli, P.; Nardoni, M.; Abete, L.; Garzoli, S.; Di Giacomo, S.; Mazzanti, G.; Casadei, M.A.; Petralito, S. SPC Liposomes as Possible Delivery Systems for Improving Bioavailability of the Natural Sesquiterpene β-Caryophyllene: Lamellarity and Drug-Loading as Key Features for a Rational Drug Delivery Design. Pharmaceutics 2018, 10, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suri, S.S.; Fenniri, H.; Singh, B. Nanotechnology-based drug delivery systems. J. Occup. Med. Toxicol. 2007, 2, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasnain, M.S.; Nayak, A.K. Chitosan as responsive polymer for drug delivery applications. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Woodhead Publishing: Sawston, UK, 2018; Volume 1, pp. 581–605. [Google Scholar]
- Vanti, G.; Ntallis, S.G.; Panagiotidis, C.A.; Dourdouni, V.; Patsoura, C.; Bergonzi, M.C.; Lazari, D.; Bilia, A.R. Glycerosome of Melissa officinalis L. Essential Oil for Effective Anti-HSV Type 1. Molecules 2020, 25, 3111. [Google Scholar] [CrossRef]
- Rechia, L.M.; Morona, J.B.D.J.; Zepon, K.M.; Soldi, V.; Kanis, L.A. Mechanical properties and total hydroxycinnamic derivative release of starch/glycerol/Melissa officinalis extract films. Braz. J. Pharm. Sci. 2010, 46, 491–497. [Google Scholar] [CrossRef]
- Sani, I.K.; Pirsa, S.; Tagi, S. Preparation of chitosan/zinc oxide/Melissa officinalis essential oil nanocomposite film and evaluation of physical, mechanical and antimicrobial properties by response surface method. Polym. Test. 2019, 79, 106004. [Google Scholar] [CrossRef]
- Sani, I.K.; Marand, S.A.; Alizadeh, M.; Amiri, S.; Asdagh, A. Thermal, Mechanical, Microstructural and Inhibitory Characteristics of Sodium Caseinate Based Bioactive Films Reinforced by ZnONPs/Encapsulated Melissa officinalis Essential Oil. J. Inorg. Organomet. Polym. Mater. 2020, 31, 261–271. [Google Scholar] [CrossRef]
- Serra, E.; Saubade, F.; Ligorio, C.; Whitehead, K.; Sloan, A.; Williams, D.W.; Hidalgo-Bastida, A.; Verran, J.; Malic, S. Methylcellulose Hydrogel with Melissa officinalis Essential Oil as a Potential Treatment for Oral Candidiasis. Microorganisms 2020, 8, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najafi-Soulari, S.; Shekarchizadeh, H.; Kadivar, M. Encapsulation optimization of lemon balm antioxidants in calcium alginate hydrogels. J. Biomater. Sci. Polym. Ed. 2016, 27, 1631–1644. [Google Scholar] [CrossRef] [PubMed]
- Santamaria-Echart, A.; Fernandes, I.; Barreiro, F.; Retegi, A.; Arbelaiz, A.; Corcuera, M.A.; Eceiza, A. Development of waterborne polyurethane-ureas added with plant extracts: Study of different incorporation routes and their influence on particle size, thermal, mechanical and antibacterial properties. Prog. Org. Coat. 2018, 117, 76–90. [Google Scholar] [CrossRef] [Green Version]
- Sani, I.K.; Alizadeh, M.; Pirsa, S.; Kia, E.M. Impact of operating parameters and wall material components on the characteristics of microencapsulated Melissa officinalis essential oil. Flavour Fragr. J. 2019, 34, 104–112. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Andronescu, E.; Oprea, A.E.; Holban, A.M.; Socol, G.; Grumezescu, V.; Chifiriuc, M.C.; Iordache, F.; Maniu, H. MAPLE fabricated magnetite@Melissa officinalis and poly lactic acid: Chitosan coated surfaces with anti-staphylococcal properties. J. Sol-Gel Sci. Technol. 2015, 73, 612–619. [Google Scholar] [CrossRef]
- Ahmeda, A.; Zangeneh, A.; Zangeneh, M.M. Preparation, formulation, and chemical characterization of silver nanoparticles using Melissa officinalis leaf aqueous extract for the treatment of acute myeloid leukemia in vitro and in vivo conditions. Appl. Organomet. Chem. 2020, 34, e5378. [Google Scholar] [CrossRef]
- De Jesus Ruíz-Baltazar, A.; Reyes-López, S.Y.; Larrañaga, D.; Estevez, M.; Pérez, R. Green synthesis of silver nanoparticles using a Melissa officinalis leaf extract with antibacterial properties. Results Phys. 2017, 7, 2639–2643. [Google Scholar] [CrossRef]
- Ruíz-Baltazar, A.D.J.; Reyes-López, S.Y.; Silva-Holguin, P.N.; Larrañaga, D.; Estevez, M.; Pérez, R. Novel biosynthesis of Ag-hydroxyapatite: Structural and spectroscopic characterization. Results Phys. 2018, 9, 593–597. [Google Scholar] [CrossRef]
- Scuteri, D.; Rombolá, L.; Tridico, L.; Mizoguchi, H.; Watanabe, C.; Sakurada, T.; Sakurada, S.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Neuropharmacological Properties of the Essential Oil of Bergamot for the Clinical Management of Pain-Related BPSDs. Curr. Med. Chem. 2019, 26, 3764–3774. [Google Scholar] [CrossRef]
- De Jesús Ruíz-Baltazar, Á. Green Composite Based on Silver Nanoparticles Supported on Diatomaceous Earth: Kinetic Adsorption Models and Antibacterial Effect. J. Clust. Sci. 2018, 29, 509–519. [Google Scholar] [CrossRef]
- Lopalco, A.; Denora, N. Nanoformulations for Drug Delivery: Safety, Toxicity, and Efficacy. Comput. Toxicol. Methods Protoc. 2018, 1800, 347–365. [Google Scholar]
- Boraschi, D.; Li, D.; Li, Y.; Italiani, P. In Vitro and In Vivo Models to Assess the Immune-Related Effects of Nanomaterials. Int. J. Environ. Res. Public Health 2021, 18, 11769. [Google Scholar] [CrossRef] [PubMed]
- Lorscheidt, S.; Lamprecht, A. Safety assessment of nanoparticles for drug delivery by means of classic in vitro assays and beyond. Expert Opin. Drug Deliv. 2016, 13, 1545–1558. [Google Scholar] [CrossRef] [PubMed]
| Component Name | Concentration of the Components of the Essential Oil, % | Reference |
|---|---|---|
| Majority components | ||
| (E)-Caryophyllene | 1.06–6.8 | [12,13,14] |
| Caryophyllene oxide | 1.3–43.55 | [12,13,14,15,16] |
| Citronellal | 0.4–20.3 | [12,13,15,16,17] |
| Geranial (citral A) | 6.22–51.21 | [14,15,16,17,18] |
| Geranyl acetate | 0.5–19.3 | [12,13,14,17] |
| Neral (citral B) | 4.28–35.02 | [12,13,14,15,16,17,18] |
| α-Cadinol | 5.64 | [14] |
| α-Copaene | 0.1–7.02 | [12,15,16] |
| β-Caryophyllene | 1.3–29.14 | [14,15,16,17] |
| Minority components (<5%) | ||
| (2E)-Nonen-1-al | 0.2 | [12] |
| (E)-Nerolidol | 0.2 | [12] |
| (E)-α-Bergamotene | 1.24 | [14] |
| (E)-β-Ionone | 0.9 | [12] |
| (E)-β-Ocimene | 0.1–0.5 | [12,13] |
| (E-E)-Geranyl linalool | 1.59 | [14] |
| (Z)-β-Ocimene | 0.1 | [15] |
| 1,2-Benzenedicarboxilic acid, butyl 2-methylopropyl ester | 0.6 | [13] |
| 1,8-Dehydro-cineol | 0.1 | [13] |
| 14-Hydroxy-9-epi-(E)-caryophyllene | 0.2 | [13] |
| 1-Octen-3-ol | 0.2–0.3 | [12,13,15] |
| 3,5-Heptadienal,2-ethylidene-6-methyl | 0.4 | [13] |
| 3-Octanone | 0.2 | [17] |
| 6-Methyl-5-hepten-2-ol | 0.2–1.7 | [12,13,15] |
| Benzene acetaldehyde | 0.3 | [12] |
| Camphene | 0.38–1.38 | [14,16] |
| Camphor | 0.1–0.4 | [13,15] |
| Carvacrol | 0.3–1 | [12,13] |
| Caryophyllenol | 0.5–2.23 | [14] |
| cis-2H-3a-Methyl-octahydro-Inden-2-one | 4.7 | [17] |
| Cis-Chrysanthenol | 0.7–1.7 | [12,13,15] |
| Cis-Rose oxide | 0.1–0.2 | [12,15] |
| Citronellol | 0.4–1.88 | [12,14] |
| Citronellyl acetate | 0.1 | [12] |
| Dihydrocitronellol acetate | 0.3 | [15] |
| Geraniol | 0.6–0.7 | [12,13,15] |
| Germacrene D | 0.2–2.0 | [12,13,14] |
| Humulene epoxide II | 0.2–1.29 | [13,14] |
| iso Aromadendren epoxide | 0.46 | [14] |
| Isogeranial | 1.4–2.0 | [13] |
| Isomenthol | 2.4 | [15] |
| Linalool | 0.3–0.5 | [12,15] |
| Linalool + trans-Sabinene hydrate | 0.5–0.8 | [13] |
| Menthol | 0.3 | [15] |
| Methyl citronellate | 0.5–2.78 | [12,13,16] |
| Methyl eugenol | 0.1 | [12] |
| Methyl geranate | 0.2–0.4 | [12,13,17] |
| Myrcene | 0.1–0.3 | [13,15] |
| n-Eicosane | 0.6 | [15] |
| Nerol | 0.2 | [15] |
| Neryl acetate | 0.1 | [12] |
| n-Heneicosane | 0.4 | [15] |
| n-Nonanal | 0.1–0.4 | [12,15] |
| para-Mentha-1(7),8-diene | 0.1 | [13] |
| p-Cymene | 0.1 | [12,13] |
| Phytol | 3.64 | [14] |
| Rosefuran epoxide | 0.6–0.7 | [13] |
| Sabinene | 0.4 | [13] |
| Thymol | 0.1–3.1 | [12,13,14] |
| t-Muurolol | 0.59 | [14] |
| trans-Limonene oxide | 0.6 | [13] |
| trans-para-Mentha-1(7),8-dien-2-ol | 2.3 | [17] |
| Trans-Rose oxide | 0.1 | [12,15] |
| Valencene | 0.1 | [15] |
| α-Humulene | 0.2–2.6 | [12,13,15,16] |
| α-Calacorene | 0.76 | [14] |
| α-Cubebene | 0.42–1.23 | [14] |
| β-Cubebene | 0.1 | [15] |
| β-Pinene oxide | 1.1 | [13] |
| β-sesquiphellandrene | 0.97 | [14] |
| γ-Cadinene | 0.76–1.77 | [14] |
| γ-Terpinene | 0.3–0.5 | [12,13] |
| Component Name | Content *, μg/g | Part of Plant | Reference |
|---|---|---|---|
| Betulinic acid | 12.85–169.88 | aerial parts | [4] |
| Oleanolic acid | 915.03–6151.67 | aerial parts | [4] |
| Ursolic acid | 3577.00–11,234.97 | aerial parts | [4] |
| 23-Sulfate ester of niga-ichigoside F1 | n.a. | leaves and stems | [25] |
| 3β,16β,23-Trihydroxy-13,28-epoxyurs-11-ene-3-O-β-D-glucopyranoside | n.a. | dried leaves and stems | [24] |
| 3,23-Disulfate ester of 2α,3β,19α,23-tetrahydroxyurs-12-en-28-oicacid | n.a. | dried leaves and stems | [24] |
| 3,23-Disulfate ester of 2α,3β,19α,23-tetrahydroxyurs-12-en-28-oicacid 28-O-β-D-glucopyranoside | n.a. | dried leaves and stems | [24] |
| 3,23-Disulfate ester of2α,3β,23,29-tetrahydroxyolean-12-en-28-oicacid | n.a. | dried leaves and stems | [24] |
| 3,23-disulfate ester of 3β-23,29-trihydroxyolean-12-en-28-oic acid | n.a. | dried leaves and stems | [24] |
| 3,23-Disulfate ester of 2α,3β-23,29-tetrahydroxyolean-12-ene-28-oicacid | n.a. | dried leaves and stems | [24] |
| 23-sulfate ester of 2α,3β,19 α,23-tetrahydroxyurs-12-en-28-oic acid | n.a. | fresh leaves and stems | [26] |
| 23-sulfate ester of 2α,3β,19 α,23-tetrahydroxyurs-12-en-28-oic acid 28-O-β- D-glucopyranoside | n.a. | fresh leaves and stems | [26] |
| Melissioside A | n.a. | leaves and stems | [25] |
| Melissioside B | n.a. | leaves and stems | [25] |
| Melissioside C | n.a. | leaves and stems | [25] |
| Group Name | Compound Name | Content *, μg/g | Part of Plant | Reference |
|---|---|---|---|---|
| Phenolic acids | Caffeic acid | 39.38–860.72 | Dried leaves | [4] |
| Caftaric acid | 1.85–344.34 | Dried leaves | [4] | |
| Chlorogenic acid | 0.62–75.529 | Dried leaves | [4,29] | |
| Ferulic acid | 1.03–45.489 | Dried leaves | [4,29] | |
| Gentisic acid | 10.40–60.48 | Dried leaves | [4] | |
| p-Coumaric acid | 1.06–20.72 | Dried leaves | [4] | |
| 13.37 ± 2.84 | Aerialparts | [30] | ||
| Rosmarinic acid | 3515.60–86,637.60 | Dried leaves | [4] | |
| 6914.1 ± 779 | Aerial parts | [30] | ||
| Flavonoids | Apigenin | 0.66–84.53 | Dried leaves | [4,29] |
| 41.71 ± 20.6 | Aerial parts | [30] | ||
| Cynaroside | 408.13 ± 30.0 | Aerial parts | [30] | |
| Daidzein | 51.25 ± 8.07 | Aerial parts | [30] | |
| Hyperoside | 3.30–16.240 | Dried leaves | [4] | |
| Isoquercetin | 6.82–162.40 | Dried leaves | [4] | |
| Kaempherol | 21.84 | Dried leaves | [4] | |
| Luteolin | 0.81–26.32 | Dried leaves | [4] | |
| Myricetin | 3.45–17.92 | Dried leaves | [4] | |
| Quercetin | 153.46 | Dried leaves | [29] | |
| Quercetrol | 5.72–33.60 | Dried leaves | [4] | |
| Rutin | 8.11–1462.99 | Dried leaves | [4,29] |
| Effect | Model | Dosage or Concentration | Tested Systems | Results | Type of Extract | Reference |
|---|---|---|---|---|---|---|
| Antiproliferative | in vitro | 20, 100, 250 μg/mL | Breast cancer cells MDA-MB- 231 and healthy HaCat cells | Inhibitory effect on migration and proliferation of both types of cells | ethanolic extract | [38] |
| in vitro | 50% | Human Colon Cancer Cell Line (HCT-116) | The 50 % ethanolic extract showed significant differences after 72 h of treatment, reducing cell proliferation to values close to 40% | ethanolic and aqueous extracts | [39] | |
| Antitumor | in vitro | Different concentration | Human tumor cell lines: MCF-7, AGS and NCI-H460 | Obtained revealed that the ethanolic extract presented the highest cell growth inhibitory potential in all the human tumor cell lines tested | ethanolic, methanolic, hydro-methanolic, hydro-ethanolic and aqueous extracts) | [40] |
| Antioxidant | in vitro | Different concentration | Encephalic tissue from male Wistar rats | Effective agent in the prevention of various neurological diseases associated with oxidative stress | ethanolic, methanolic and aqueous extracts | [41] |
| in vitro | 1, 2.5, 5 and 10 mg/mL | DPPH radical scavenging activity assay, β-carotene bleaching test and ABTS assay | Good antioxidant activity | essential oil | [42] | |
| Antiangiogenic | in vitro, in ovo | 50 μg/mL | Two breast cancer cell lines, MCF-7 And MDA-MB-231 | Highest cell inhibitory activity was exhibited by the 96% ethanolic extract | ethanolic extracts and methanolic extracts | [4] |
| Cardioprotective | in vivo | 25, 50 and 100 mg/kg b.w. * (4.23/8.46/16.91 mg/kg b.w. *) | Rats | Antioxidant and cardio-protective effects against arrhythmias induced by ischemia and ischemia-reperfusion | ethanolic leaf extract | [43] |
| Antinociceptive Antihyperglycemic | in vivo | 0.01, 0.02 and 0.04 mg/day (0.0063/0.0126/0.0252 mg/kg b.w. *) | Male adult Wistar rats | Long-term oral administration of essential oil (at an effective dose of 0.04 mg/day) can suppress chemical hyperalgesia in diabetic rats | essential oil | [44] |
| Anxiolytic Antidepressant | in vivo | 50, 75 and 150 mg/kg b.w./day * (3.91/5.86/11.72 mg/kg b.w. *) | Albino BALB/c male mice | Hydro-alcoholic extract (75 and 150 mg/kg) significantly reversed anxiety- and depressive-like behaviors | hydro-alcoholic extract | [45] |
| Neuroprotective | in vitro | 5, 10, 50, 100, 500 μg/mL | Cortical neuronal Culture system | Protective effects on neurons in the brain | balm oil | [46] |
| in vivo | 50, 100, 200 and 400 mg/kg b.w. * (8.35/16.71/33.41/66.83 mg/kg b.w. *) | Male rats | Treatment with 100 mg/kg of oil attenuated the increased caspase-3 like protease activity significantly | balm oil | [46] | |
| GABA-T inhibitor | in vitro | 0–4 mg/mL | Rat brain | Phytochemical characterization of the crude extract determined rosmarinic acid as the major compound responsible for activity (40% inhibition at 100 μg/mL) since it represented approximately 1.5% of the dry mass of the leaves | methanol extract | [47] |
| Anti-kinetoplastidae | in vitro | 31.25, 62.5, 125, 250 μg/mL | T. cruzi, L. brasiliensi, L. infantum | A potential source of natural product featuring anti-Leishmania and anti-Trypanosoma activity | ethanol extract | [48] |
| Analgesic | in vivo | 5, 10, 20 mg/kg b.w. * (0.87/1.73/3.46 mg/kg b.w. *) | Male Wistar rats | Intrathecal administration could significantly improve hot-water and formalin-induced pain in male Wistar rats | hydro-alcoholic extract | [49] |
| Hypnotic | in vivo | 200, 400 and 800 mg/kg b.w. * (14.81/29.61/59.23 mg/kg b.w. *) | Male Swiss mice | Extracts may be useful for insomnia | hydro-alcoholic extracts | [50] |
| Antidiabetic | in vivo | 0.0125 mg/d | db/db mice | Anti-hyperglycaemic agent | essential oil | [51] |
| in vivo | 0.4%, 0.8% (w/w) | Otsuka Long-Evans Tokushima fatty rats | An effective therapeutic strategy to treat human obesity and type 2 diabetes | herbal extract | [52] | |
| Anti-Alzheimer | in vitro | 8.8 mg/mL | GSK-3Β, CK-1δ, and BACE-1 | Best activity for ck-1δ inhibitory activity with maximum inhibitory concentration values at half (IC50) below 250 μg/mL | methanol extract | [53] |
| Antispasmodic | ex vivo | 1, 5, 10, 25, and 50 mg/mL | Different segments of the gastrointestinal tract of mice | Site- and dose-dependent effects on the contractile activity of the gastrointestinal tract | hydro-ethanolic leaf extract | [54] |
| Antiviral | in vitro | 1.5–150 μg/mL | RC-37 cells | High virucidal activity against HSV-1 | aqueous extract | [55] |
| Antifungal | in vitro | 15.5–2000 μg/mL | Human Pathogenic fungi | Good antifungal activity | ethanol extracts | [56] |
| 0.25–2 μL/mL | Phytopathogenic fungi in apples | essential oil | [57] | |||
| Antibacterial | in vitro | 10 and 15 mg/mL | E. coli, L. monocytogenes, S. aureus and S. typhimurium | A significant antimicrobial effect | essential oil | [42] |
| Substance | Activity | Reference |
|---|---|---|
| Geranial (citral A) | Antibacterial, antifungal | [69,70] |
| Neral (citral B) | Antibacterial, antifungal | [71,72] |
| Citronellal | Antimicrobial, insecticidal | [73,74] |
| β-Caryophyllene | Anti-inflammatory, antioxidant, antibacterial | [75,76] |
| α-Cadinol | Antifungal, hepatoprotective | [77,78] |
| Geranyl acetate | Antibacterial, insecticidal | [79,80] |
| Betulinic acid | Antiviral, anti-inflammatory, anticancer | [81,82] |
| Oleanolic acid | Antiviral, hepatoprotective | [83,84] |
| Ursolic acid | Antibacterial, antioxidant | [85,86] |
| Caffeic acid | Antioxidant, anti-inflammatory | [87,88] |
| Caftaric acid | Antioxidant | [89,90] |
| Rosmarinic acid | Antioxidant, anti-inflammatory | [91,92] |
| Ferulic acid | Antioxidant | [93,94] |
| Chlorogenic acid | Antidiabetic, antioxidant | [95,96] |
| p-Coumaric acid | Antioxidant, anti-inflammatory | [97,98] |
| Cynaroside | Antioxidant, anti-inflammatory | [99,100] |
| Rutin | Antioxidant, anti-inflammatory | [101,102] |
| Type of Delivery System | Delivery System | Effect | Reference | |
|---|---|---|---|---|
| Type of Carrier | Active Agent | |||
| Organic/Inorganic Nanoparticles | poloxamer, soybean lecithin ethosome | caffeic acid | antioxidant | [116] |
| chitosan, sodium alginate | [117] | |||
| poloxamer | [118] | |||
| chitosan | rosmarinic acid * | antioxidant | [119,120] | |
| rosmarinic acid | antimicrobial | [121] | ||
| PEG-containing amine | anti-inflammatory | [122] | ||
| glycerol monostearate, soya lecithin, hydrogenated soya phosphatidyl choline | therapeutic | [123] | ||
| mesoporous silica | antioxidant | [124] | ||
| nanostructured lipid | citral | anticancer | [125,126] | |
| Hydrogels | chitosan | citral | anticancer | [127] |
| imine-PEG-ylated chitosan | local therapy | [128] | ||
| Vesicles | soybean phospholipids | citral | antimicrobial | [129] |
| ethosome | caffeic acid | antioxidant | [116] | |
| soybean phosphatidyl-choline liposomes | β-caryophyllene | antiproliferative | [130] | |
| Type of Delivery System | Delivery System | Effect | Reference | |
|---|---|---|---|---|
| Type of Carrier | Type of Extract | |||
| Glycerosomes | phosphatidylcholine and cholesterol | essential oil | anti-herpetic | [134] |
| Films | starch and glycerol | Hydroalcoholic extract | anti-herpetic | [135] |
| chitosan and zinc oxide nanoparticles | essential oil | antimicrobial | [136,137] | |
| sodium caseinate and zinc oxide nanoparticles | ||||
| Hydrogel | methylcellulose | essential oil | antimicrobial for candida albicans in the oral cavity | [138] |
| calcium alginate | aqueous extract | antioxidant | [139] | |
| Dispersion | water-based polyurethane-urea | infusion | antimicrobial | [140] |
| Nanocapsule | isolated whey proteins and sodium caseinate | essential oil | n.a. | [141] |
| nanoparticles of magnetite, essential oil, polylactic acid, chitosan | essential oil | anti-staphylococcal | [142] | |
| Nanoparticle | silver, gold and gold-silver | ethanolic extract | antimicrobial | [29] |
| silver | aqueous extract | antioxidant and cytotoxic for the acute myeloid leukemia | [143] | |
| silver | infusion | antibacterial | [144] | |
| Nanocomposite | silver-hydroxyapatite | infusion | antibacterial | [145] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrisor, G.; Motelica, L.; Craciun, L.N.; Oprea, O.C.; Ficai, D.; Ficai, A. Melissa officinalis: Composition, Pharmacological Effects and Derived Release Systems—A Review. Int. J. Mol. Sci. 2022, 23, 3591. https://doi.org/10.3390/ijms23073591
Petrisor G, Motelica L, Craciun LN, Oprea OC, Ficai D, Ficai A. Melissa officinalis: Composition, Pharmacological Effects and Derived Release Systems—A Review. International Journal of Molecular Sciences. 2022; 23(7):3591. https://doi.org/10.3390/ijms23073591
Chicago/Turabian StylePetrisor, Gabriela, Ludmila Motelica, Luminita Narcisa Craciun, Ovidiu Cristian Oprea, Denisa Ficai, and Anton Ficai. 2022. "Melissa officinalis: Composition, Pharmacological Effects and Derived Release Systems—A Review" International Journal of Molecular Sciences 23, no. 7: 3591. https://doi.org/10.3390/ijms23073591
APA StylePetrisor, G., Motelica, L., Craciun, L. N., Oprea, O. C., Ficai, D., & Ficai, A. (2022). Melissa officinalis: Composition, Pharmacological Effects and Derived Release Systems—A Review. International Journal of Molecular Sciences, 23(7), 3591. https://doi.org/10.3390/ijms23073591









