Curcumin in Retinal Diseases: A Comprehensive Review from Bench to Bedside
Abstract
1. Introduction
- -
- Curcuminoids (95% of the standardized extract): curcumin, demethoxicurcumin and bisdemethoxycurcumin
- -
- Volatile oils: tumerone, atlantone and zingiberene
2. Literature Search
3. Molecular Mechanisms
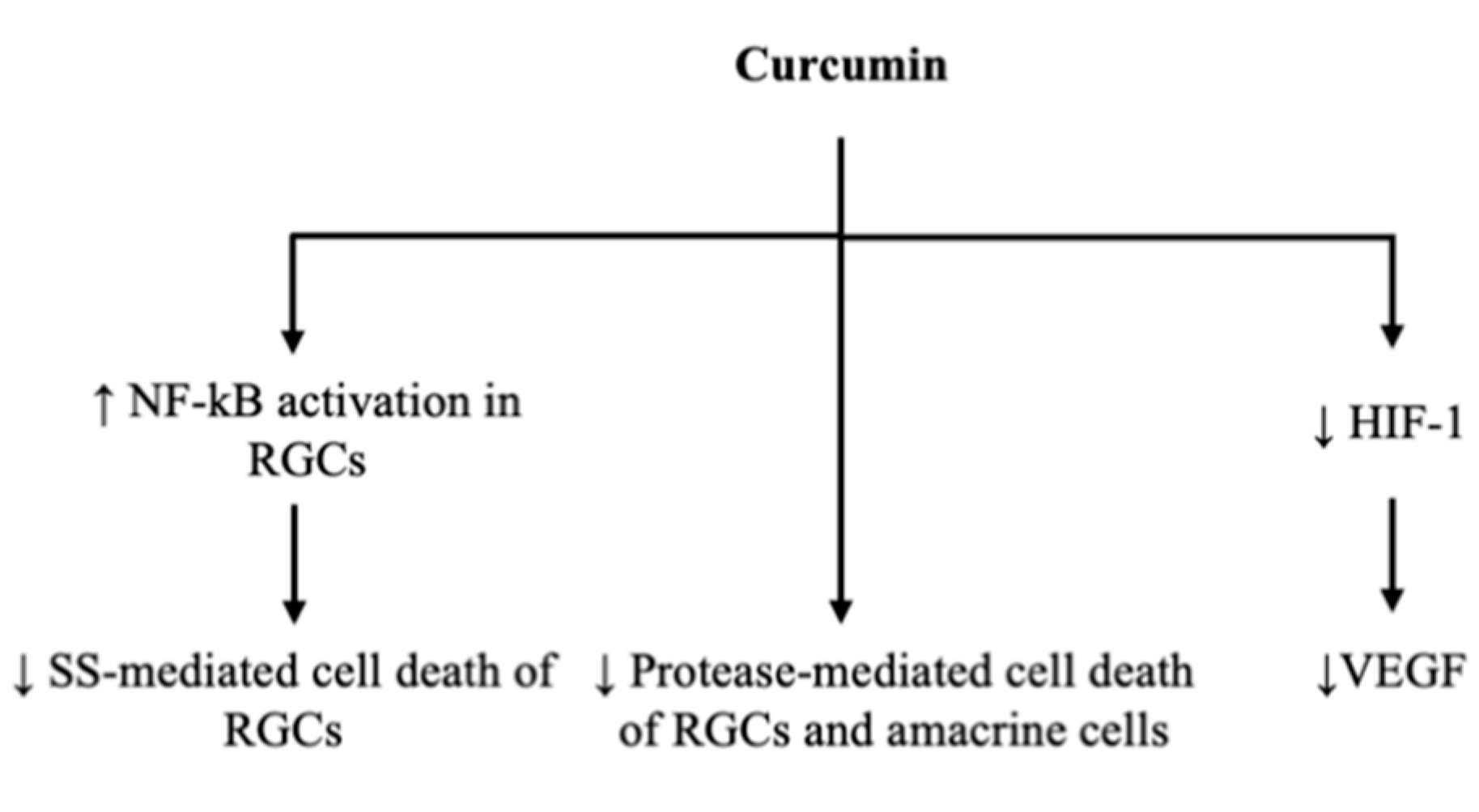
3.1. Gene Transcription to Reduce Ganglion Cell Apoptosis
3.2. Vascular Endothelial Growth Factor Reduction
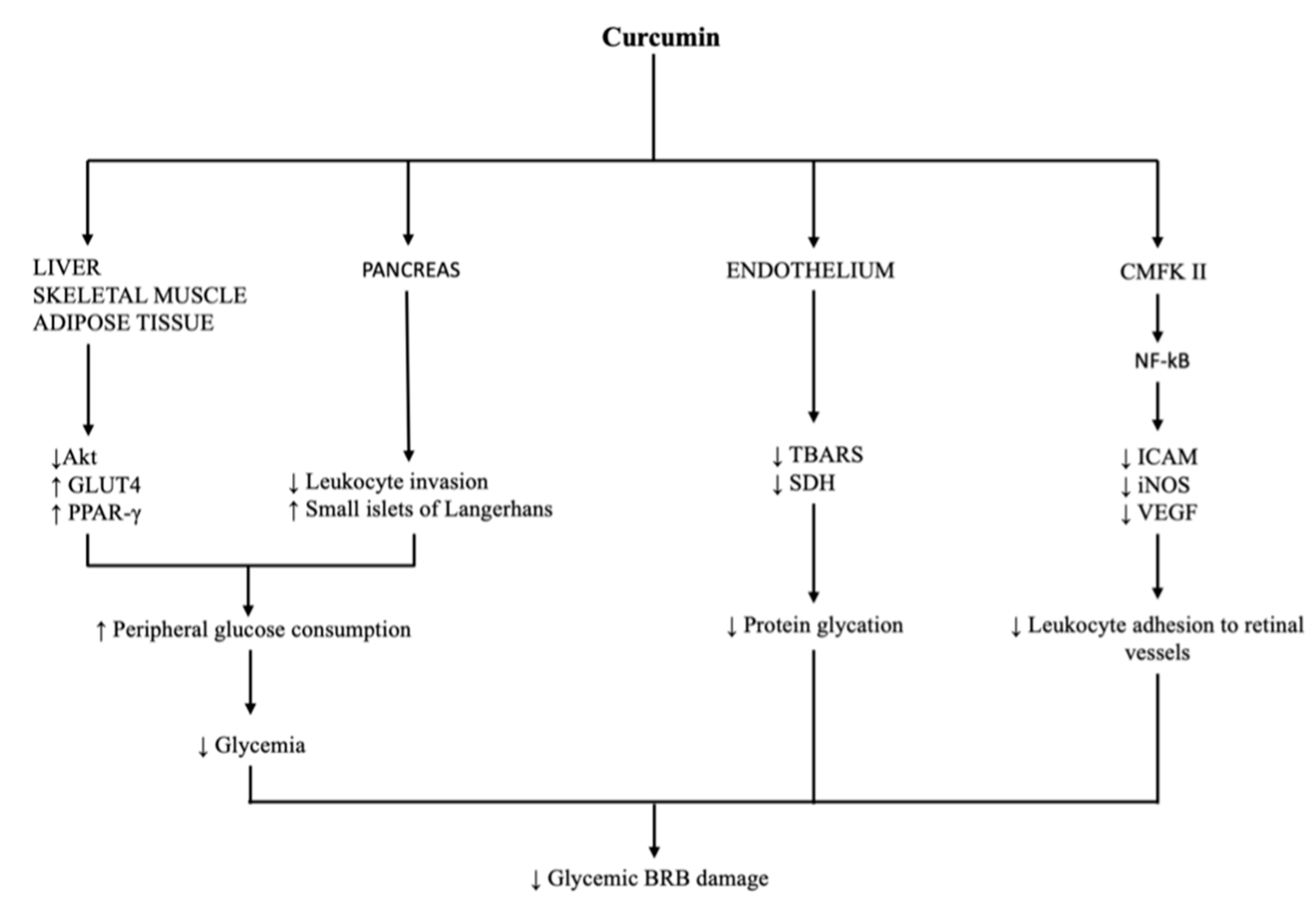
3.3. Glucose Level
3.4. Vascular Dysfunction
4. Pharmacokinetics
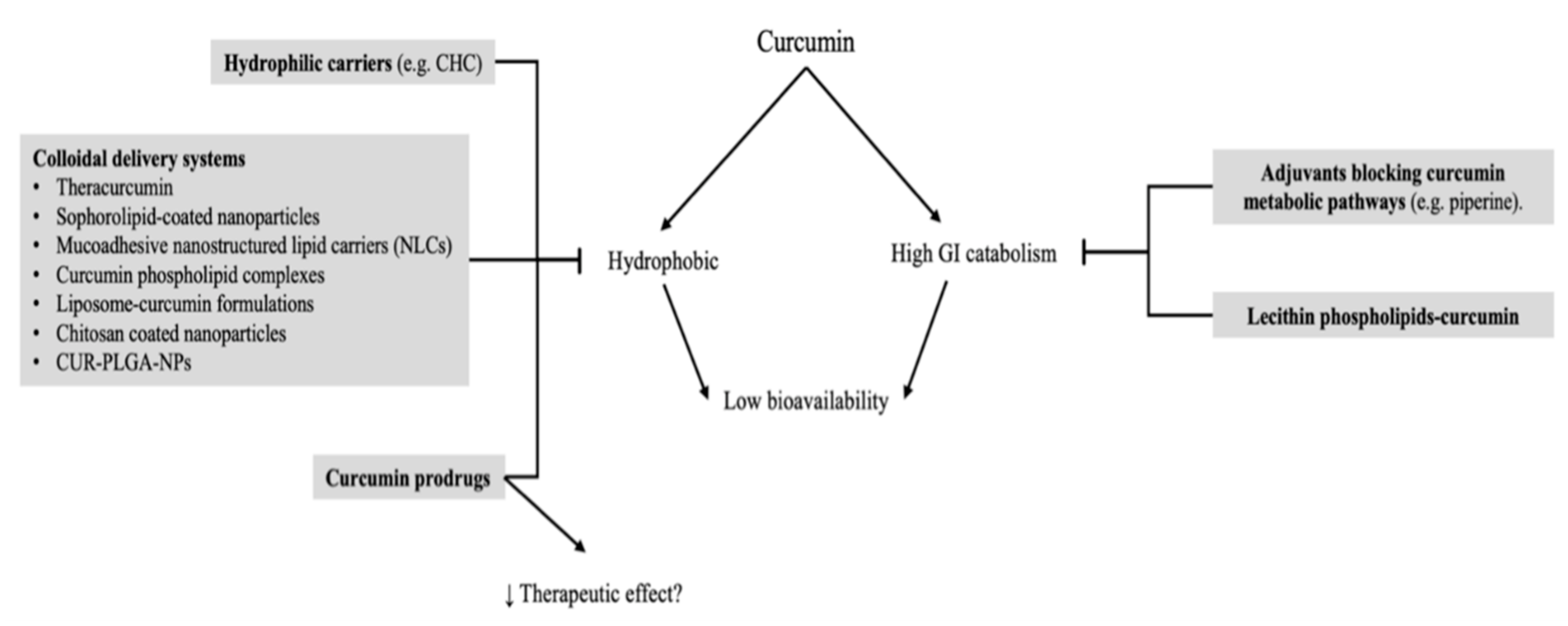
5. Carriers and Bioavailability
6. Safety Profile in Humans
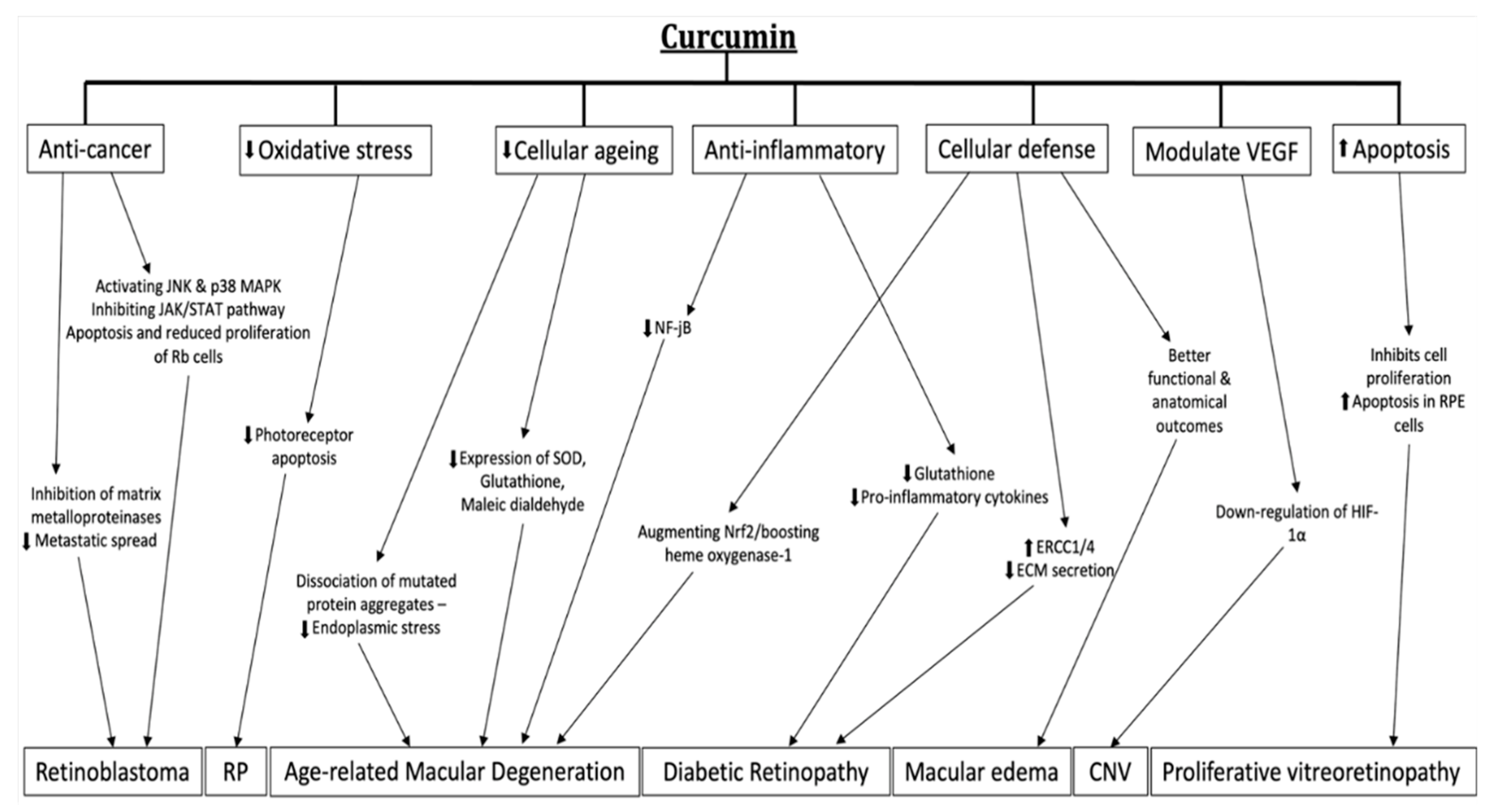
7. Evidence in Treatment of Retinal Diseases
7.1. Age-Related Macular Degeneration and Choroidal Neovascularization
7.2. Diabetic Retinopathy
7.3. Retinitis Pigmentosa
7.4. Macular Edema
7.5. Proliferative Vitreoretinopathy
7.6. Retinoblastoma
8. Human Clinical Trials on Curcumin
9. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beatty, S.; Koh, H.-H.; Phil, M.; Henson, D.; Boulton, M. The Role of Oxidative Stress in the Pathogenesis of Age-Related Macular Degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Arnal, E.; Miranda, M.; Johnsen-Soriano, S.; Alvarez-Nölting, R.; Díaz-Llopis, M.; Araiz, J.; Cervera, E.; Bosch-Morell, F.; Romero, F.J. Beneficial Effect of Docosahexanoic Acid and Lutein on Retinal Structural, Metabolic, and Functional Abnormalities in Diabetic Rats. Curr. Eye Res. 2009, 34, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qin, Y.J.; Yip, Y.W.Y.; Chan, K.P.; Chu, K.O.; Chu, W.K.; Ng, T.K.; Pang, C.P.; Chan, S.O. Green tea catechins are potent anti-oxidants that ameliorate sodium iodate-induced retinal degeneration in rats. Sci. Rep. 2016, 6, 29546. Available online: http://europepmc.org/abstract/MED/27383468 (accessed on 3 July 2021). [PubMed]
- Di Marco, S.; Carnicelli, V.; Franceschini, N.; Di Paolo, M.; Piccardi, M.; Bisti, S.; Falsini, B. Saffron: A Multitask Neuroprotective Agent for Retinal Degenerative Diseases. Antioxidants 2019, 8, 224. Available online: https://pubmed.ncbi.nlm.nih.gov/31319529 (accessed on 4 June 2021).
- Martínez-Solís, I.; Acero, N.; Bosch-Morell, F.; Castillo, E.; González-Rosende, M.E.; Muñoz-Mingarro, D.; Ortega, T.; Sanahuja, M.A.; Villagrasa, V. Neuroprotective Potential of Ginkgo biloba in Retinal Diseases. Planta Medica 2019, 85, 1292–1303. [Google Scholar] [CrossRef]
- Radomska-Leśniewska, D.M.; Osiecka-Iwan, A.; Hyc, A.; Góźdź, A.; Dąbrowska, A.M.; Skopiński, P. Therapeutic potential of curcumin in eye diseases. Central Eur. J. Immunol. 2019, 44, 181–189. [Google Scholar] [CrossRef]
- Pescosolido, N.; Giannotti, R.; Plateroti, A.M.; Pascarella, A.; Nebbioso, M. Curcumin: Therapeutical Potential in Ophthalmology. Planta Medica 2013, 80, 249–254. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Meng, X.; Li, S.; Gan, R.-Y.; Li, Y.; Li, H.-B. Bioactivity, Health Benefits, and Related Molecular Mechanisms of Curcumin: Current Progress, Challenges, and Perspectives. Nutrients 2018, 10, 1553. [Google Scholar] [CrossRef]
- Lu, J.; Zhu, W.; Wu, Y.; Meng, Y.-F.; Wang, J.-Y.; Xu, M.; Tao, J.-J. Effect of curcumin on aging retinal pigment epithelial cells. Drug Des. Dev. Ther. 2015, 9, 5337–5344. [Google Scholar] [CrossRef][Green Version]
- Woo, J.M.; Shin, D.-Y.; Lee, S.J.; Joe, Y.; Zheng, M.; Yim, J.H.; Callaway, Z.; Chung, H.T. Curcumin protects retinal pigment epithelial cells against oxidative stress via induction of heme oxygenase-1 expression and reduction of reactive oxygen. Mol. Vis. 2012, 18, 901–908. [Google Scholar]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Bramer, W.M.; Rethlefsen, M.L.; Kleijnen, J.; Franco, O.H. Optimal database combinations for literature searches in systematic reviews: A prospective exploratory study. Syst. Rev. 2017, 6, 245. [Google Scholar] [PubMed]
- Chung, J.-G.; Lu, H.-F.; Lai, K.-C.; Hsu, S.-C.; Lin, H.-J.; Yang, M.-D.; Chen, Y.-L.; Fan, M.-J.; Yang, J.-S.; Cheng, P.-Y.; et al. Curcumin induces apoptosis through FAS and FADD, in caspase-3-dependent and -independent pathways in the N18 mouse-rat hybrid retina ganglion cells. Oncol. Rep. 2009, 22, 97–104. [Google Scholar] [CrossRef]
- Lu, H.F.; Yang, J.S.; Lai, K.C.; Hsu, S.C.; Hsueh, S.C.; Chen, Y.L.; Chiang, J.-H.; Lu, C.-C.; Lo, C.; Yang, M.-D.; et al. Curcumin-induced DNA damage and inhibited dna repair genes expressions in mouse-rat hybrid retina neuroblastoma cells ganglion cells (n18). Neurochem. Res. 2009, 34, 1491–1497. [Google Scholar]
- Chung, S.C.; Lin, H.-J.; Su, C.-C.; Lu, H.-F.; Yang, J.-S.; Hsu, S.-C.; Ip, S.-W.; Wu, J.-J.; Li, Y.-C.; Ho, C.-C.; et al. Curcumin blocks migration and invasion of mouse-rat hybrid retina ganglion cells (N18) through the inhibition of MMP-2, -9, FAK, Rho A and Rock-1 gene expression. Oncol. Rep. 2010, 23, 665–670. [Google Scholar] [CrossRef]
- Burugula, B.; Ganesh, B.S.; Chintala, S.K. Curcumin Attenuates Staurosporine-Mediated Death of Retinal Ganglion Cells. Investig. Opthalmology Vis. Sci. 2011, 52, 4263–4273. [Google Scholar] [CrossRef]
- Duh, E.; Aiello, L.P. Vascular endothelial growth factor and diabetes: The agonist versus antagonist paradox. Diabetes 1999, 48, 1899–1906. [Google Scholar] [CrossRef]
- Ferrara, M.; Allegrini, D.; Sorrentino, T.; Sborgia, G.; Parmeggiani, F.; Borgia, A.; Romano, M.R. Curcumin-Based Treatment for Macular Edema from Uncommon Etiologies: Efficacy and Safety Assessment. J. Med. Food 2020, 23, 834–840. [Google Scholar] [CrossRef]
- Boehm, B.O.; Lang, G.; Volpert, O.; Jehle, P.M.; Kurkhaus, A.; Rosinger, S.; Bouck, N. Low content of the natural ocular anti-angiogenic agent pigment epithelium-derived factor (PEDF) in aqueous humor predicts progression of diabetic retinopathy. Diabetologia 2003, 46, 394–400. [Google Scholar] [CrossRef]
- Stuart, A.; Ford, J.A.; Duckworth, S.; Jones, C.; Pereira, A. Anti-VEGF therapies in the treatment of choroidal neovascularisation secondary to non-age-related macular degeneration: A systematic review. BMJ Open 2015, 5, e007746. [Google Scholar] [CrossRef]
- Aiello, L.P.; Northrup, J.M.; Keyt, B.A.; Takagi, H.; Iwamoto, M.A. Hypoxic Regulation of Vascular Endothelial Growth Factor in Retinal Cells. Arch. Ophthalmol. 1995, 113, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Robbins, S.G.; Rajaratnam, V.S.; Penn, J.S. Evidence for upregulation and redistribution of vascular endothelial growth factor (VEGF) receptors flt-1 and flk-1 in the oxygen-injured rat retina. Growth Factors 1998, 16, 1–9. [Google Scholar] [PubMed]
- Miller, J.W.; Adamis, A.P.; Aiello, L.P. Vascular Endothelial Growth Factor in Ocular Neovascularization and Proliferative Diabetic Retinopathy. Diabetes Metab. Rev. 1997, 13, 37–50. [Google Scholar] [PubMed]
- Ida, H.; Tobe, T.; Nambu, H.; Matsumura, M.; Uyama, M.; Campochiaro, P.A. RPE cells modulate subretinal neovascularization, but do not cause regression in mice with sustained expression of VEGF. Investig. Opthalmology Vis. Sci. 2003, 44, 5430–5437. [Google Scholar] [CrossRef][Green Version]
- Forsythe, J.A.; Jiang, B.H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor. Mol. Cell. Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef]
- Bae, M.-K.; Kim, S.-H.; Jeong, J.-W.; Lee, Y.M.; Kim, H.-S.; Kim, S.-R.; Yun, I.; Bae, S.-K.; Kim, K.-W. Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF. Oncol. Rep. 2006, 15, 1557–1562. [Google Scholar] [CrossRef]
- Choi, H.; Chun, Y.-S.; Kim, S.-W.; Kim, M.-S.; Park, J.-W. Curcumin Inhibits Hypoxia-Inducible Factor-1 by Degrading Aryl Hydrocarbon Receptor Nuclear Translocator: A Mechanism of Tumor Growth Inhibition. Mol. Pharmacol. 2006, 70, 1664–1671. [Google Scholar] [CrossRef]
- Premanand, C.; Rema, M.; Sameer, M.Z.; Sujatha, M.; Balasubramanyam, M. Effect of Curcumin on Proliferation of Human Retinal Endothelial Cells under In Vitro Conditions. Investig. Opthalmology Vis. Sci. 2006, 47, 2179–2184. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Kanwar, M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr. Metab. 2007, 4, 8. [Google Scholar] [CrossRef]
- Mrudula, T.; Suryanarayana, P.; Srinivas, P.; Reddy, G.B. Effect of curcumin on hyperglycemia-induced vascular endothelial growth factor expression in streptozotocin-induced diabetic rat retina. Biochem. Biophys. Res. Commun. 2007, 361, 528–532. [Google Scholar] [CrossRef]
- Ciolino, H.P.; Daschner, P.J.; Yeh, G.C. Dietary flavonols quercetin and kaempferol are ligands of the aryl hydrocarbon receptor that affect CYP1A1 transcription differentially. Biochem. J. 1999, 340, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Bardbori, A.; Bengtsson, J.; Rannug, U.; Rannug, A.; Wincent, E. Quercetin, Resveratrol, and Curcumin Are Indirect Activators of the Aryl Hydrocarbon Receptor (AHR). Chem. Res. Toxicol. 2012, 25, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Hammond, C.L.; Roztocil, E.; Gupta, V.; Feldon, S.E.; Woeller, C.F. More than Meets the Eye: The Aryl Hydrocarbon Receptor is an Environmental Sensor, Physiological Regulator and a Therapeutic Target in Ocular Disease. Front. Toxicol. 2022, 4, 791082. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, T.; Balasubashini, M.M.S.; Menon, V.P. Photo-Irradiated Curcumin Supplementation in Streptozotocin-Induced Diabetic Rats: Effect on Lipid Peroxidation. Therapie 2004, 59, 639–644. [Google Scholar] [CrossRef]
- Arun, N.; Nalini, N. Efficacy of turmeric on blood sugar and polyol pathway in diabetic albino rats. Mater. Veg. 2002, 57, 41–52. [Google Scholar] [CrossRef]
- El-Moselhy, M.A.; Taye, A.; Sharkawi, S.S.; El-Sisi, S.F.; Ahmed, A.F. The antihyperglycemic effect of curcumin in high fat diet fed rats. Role of TNF-α and free fatty acids. Food Chem. Toxicol. 2011, 49, 1129–1140. [Google Scholar] [CrossRef]
- Gutierres, V.O.; Campos, M.L.; Arcaro, C.A.; Assis, R.P.; Baldan-Cimatti, H.M.; Peccinini, R.G.; Paula-Gomes, S.; Kettelhut, I.C.; Baviera, A.M.; Brunetti, I.L. Curcumin Pharmacokinetic and Pharmacodynamic Evidences in Streptozotocin-Diabetic Rats Support the Antidiabetic Activity to Be via Metabolite(s). Evid. Based Complement. Altern. Med. 2015, 2015, 678218. [Google Scholar] [CrossRef]
- Chougala, M.B.; Bhaskar, J.J.; Rajan, M.; Salimath, P.V. Effect of curcumin and quercetin on lysosomal enzyme activities in streptozotocin-induced diabetic rats. Clin. Nutr. 2012, 31, 749–755. [Google Scholar] [CrossRef]
- Seo, K.-I.; Choi, M.-S.; Jung, U.J.; Kim, H.-J.; Yeo, J.; Jeon, S.-M.; Lee, M.-K. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Mol. Nutr. Food Res. 2008, 52, 995–1004. [Google Scholar]
- Wojcik, M.; Krawczyk, M.; Wojcik, P.; Cypryk, K.; Wozniak, L.A. Molecular Mechanisms Underlying Curcumin-Mediated Therapeutic Effects in Type 2 Diabetes and Cancer. Oxidative Med. Cell. Longev. 2018, 2018, 9698258. [Google Scholar] [CrossRef]
- Nishiyama, T.; Mae, T.; Kishida, H.; Tsukagawa, M.; Mimaki, Y.; Kuroda, M.; Sashida, Y.; Takahashi, K.; Kawada, T.; Nakagawa, A.K.; et al. Curcuminoids and Sesquiterpenoids in Turmeric (Curcuma longa L.) Suppress an Increase in Blood Glucose Level in Type 2 Diabetic KK-Ay Mice. J. Agric. Food Chem. 2005, 53, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Chanpoo, M.; Petchpiboonthai, H.; Panyarachun, B.; Anupunpisit, V. Effect of curcumin in the amelioration of pancreatic islets in streptozotocin-induced diabetic mice. J. Med Assoc. Thail. 2010, 93, S152–S159. [Google Scholar]
- Hussain, H.E.M.A. Hypoglycemic, hypolipidemic and antioxidant properties of combination of Curcumin from Curcuma longa, Linn, and partially purified product from Abroma augusta, Linn. in streptozotocin induced diabetes. Indian J. Clin. Biochem. 2002, 17, 33–43. Available online: https://pubmed.ncbi.nlm.nih.gov/23105348 (accessed on 3 September 2021).
- Murugan, P.; Pari, L. Influence of tetrahydrocurcumin on erythrocyte membrane bound enzymes and antioxidant status in experimental type 2 diabetic rats. J. Ethnopharmacol. 2007, 113, 479–486. [Google Scholar] [CrossRef] [PubMed]
- El-Azab, M.F.; Attia, F.M.; El-Mowafy, A.M. Novel role of curcumin combined with bone marrow transplantation in reversing experimental diabetes: Effects on pancreatic islet regeneration, oxidative stress, and inflammatory cytokines. Eur. J. Pharmacol. 2011, 658, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Soetikno, V.; Sari, F.R.; Veeraveedu, P.T.; Thandavarayan, R.A.; Harima, M.; Sukumaran, V.; Lakshmanan, A.P.; Suzuki, K.; Kawachi, H.; Watanabe, K. Curcumin ameliorates macrophage infiltration by inhibiting NF-κB activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutr. Metab. 2011, 8, 35. [Google Scholar]
- Zheng, L.; Du, Y.; Miller, C.; Gubitosi-Klug, R.A.; Kern, T.S.; Ball, S.; Berkowitz, B.A. Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes. Diabetologia 2007, 50, 1987–1996. [Google Scholar]
- Joussen, A.M.; Poulaki, V.; Qin, W.; Kirchhof, B.; Mitsiades, N.; Wiegand, S.J.; Rudge, J.; Yancopoulos, G.D.; Adamis, A.P. Retinal Vascular Endothelial Growth Factor Induces Intercellular Adhesion Molecule-1 and Endothelial Nitric Oxide Synthase Expression and Initiates Early Diabetic Retinal Leukocyte Adhesion in Vivo. Am. J. Pathol. 2002, 160, 501–509. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Barber, A.J.; Khin, S.; Lieth, E.; Tarbell, J.M.; Gardner, T.W. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: Vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes 1998, 47, 1953–1959. [Google Scholar] [CrossRef]
- Meffert, M.K.; Chang, J.M.; Wiltgen, B.J.; Fanselow, M.S.; Baltimore, D. NF-kappa B functions in synaptic signaling and behavior. Nat. Neurosci. 2003, 6, 1072–1078. [Google Scholar]
- Fan, W.; Cooper, N.G.F. Glutamate-induced NFkappaB activation in the retina. Investig. Opthalmology Vis. Sci. 2009, 50, 917–925. [Google Scholar] [CrossRef][Green Version]
- Chiu, J.; Khan, Z.A.; Farhangkhoee, H.; Chakrabarti, S. Curcumin prevents diabetes-associated abnormalities in the kidneys by inhibiting p300 and nuclear factor-kappaB. Nutrition 2009, 25, 964–972. [Google Scholar] [PubMed]
- Yousif, M.H.M.; Akhtar, S.; Walther, T.; Benter, I.F. Role of Ca2+/calmodulin-dependent protein kinase II in development of vascular dysfunction in diabetic rats with hypertension. Cell Biochem. Funct. 2008, 26, 256–263. [Google Scholar] [PubMed]
- Benter, I.F.; Yousif, M.H.M.; Canatan, H.; Akhtar, S. Inhibition of Ca/calmodulin-dependent protein kinase II, RAS-GTPase and 20-hydroxyeicosatetraenoic acid attenuates the development of diabetes-induced vascular dysfunction in the rat carotid artery. Pharmacol. Res. 2005, 52, 252–257. [Google Scholar] [CrossRef]
- Yousif, M.H.M.; Benter, I.F.; Akhtar, S. Inhibition of calcium/calmodulin-dependent protein kinase II normalizes diabetes-induced abnormal vascular reactivity in the rat perfused mesenteric vascular bed. Auton. Autacoid Pharmacol. 2003, 23, 27–33. [Google Scholar] [CrossRef]
- Li, J.; Wang, P.; Ying, J.; Chen, Z.; Yu, S. Curcumin Attenuates Retinal Vascular Leakage by Inhibiting Calcium/Calmodulin-Dependent Protein Kinase II Activity in Streptozotocin-Induced Diabetes. Cell. Physiol. Biochem. 2016, 39, 1196–1208. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Vareed, S.K.; Kakarala, M.; Ruffin, M.; Crowell, J.A.; Normolle, D.P.; Djuric, Z.; Brenner, D.E. Pharmacokinetics of Curcumin Conjugate Metabolites in Healthy Human Subjects. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1411–1417. [Google Scholar] [CrossRef]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Klickovic, U.; Doberer, D.; Gouya, G.; Aschauer, S.; Weisshaar, S.; Storka, A.; Bilban, M.; Wolzt, M. Human Pharmacokinetics of High Dose Oral Curcumin and Its Effect on Heme Oxygenase-1 Expression in Healthy Male Subjects. BioMed Res. Int. 2014, 2014, 458592. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Otsuka, Y.; Otsuka, K.; Sato, M.; Nishimura, T.; Mori, Y.; Kawaguchi, M.; Hatano, E.; Kodama, Y.; Matsumoto, S.; et al. A phase I study investigating the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin®) in cancer patients. Cancer Chemother. Pharmacol. 2013, 71, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Storka, A.; Vcelar, B.; Klickovic, U.; Gouya, G.; Weisshaar, S.; Aschauer, S.; Bolger, G.; Helson, L.; Wolzt, M. Safety, tolerability and pharmacokinetics of liposomal curcumin in healthy humans. Int. J. Clin. Pharmacol. Ther. 2015, 53, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Fança-Berthon, P.; Tenon, M.; Le Bouter-Banon, S.; Manfré, A.; Maudet, C.; Dion, A.; Chevallier, H.; Laval, J.; van Breemen, R.B. Pharmacokinetics of a Single Dose of Turmeric Curcuminoids Depends on Formulation: Results of a Human Crossover Study. J. Nutr. 2021, 151, 1802–1816. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Sunagawa, Y.; Takahashi, K.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Wada, H.; Katanasaka, Y.; Kakeya, H.; Fujita, M.; et al. Innovative Preparation of Curcumin for Improved Oral Bioavailability. Biol. Pharm. Bull. 2011, 34, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Van Den Berg, H.; Arthur, J.; Bast, A.; Dainty, J.; Faulks, R.M.; Gärtner, C.; Haenen, G.; Hollman, P.; Holst, B.; et al. Bioavailability and metabolism. Mol. Aspects Med. 2002, 23, 39–100. [Google Scholar]
- Jamwal, R. Bioavailable curcumin formulations: A review of pharmacokinetic studies in healthy volunteers. J. Integr. Med. 2018, 16, 367–374. [Google Scholar] [CrossRef]
- Jäger, R.; Lowery, R.P.; Calvanese, A.V.; Joy, J.M.; Purpura, M.; Wilson, J.M. Comparative absorption of curcumin formulations. Nutr. J. 2014, 13, 11. [Google Scholar] [CrossRef]
- Peng, S.; Li, Z.; Zou, L.; Liu, W.; Liu, C.; McClements, D.J. Enhancement of Curcumin Bioavailability by Encapsulation in Sophorolipid-Coated Nanoparticles: An in Vitro and in Vivo Study. J. Agric. Food Chem. 2018, 66, 1488–1497. [Google Scholar] [CrossRef]
- Chanburee, S.; Tiyaboonchai, W. Mucoadhesive nanostructured lipid carriers (NLCs) as potential carriers for improving oral delivery of curcumin. Drug Dev. Ind. Pharm. 2017, 43, 432–440. [Google Scholar] [CrossRef]
- Tian, C.; Asghar, S.; Wu, Y.; Chen, Z.; Jin, X.; Yin, L.; Huang, L.; Ping, Q.; Xiao, Y. Improving intestinal absorption and oral bioavailability of curcumin via taurocholic acid-modified nanostructured lipid carriers. Int. J. Nanomed. 2017, 12, 7897–7911. [Google Scholar] [CrossRef]
- Cuomo, F.; Perugini, L.; Marconi, E.; Messia, M.C.; Lopez, F. Enhanced Curcumin Bioavailability through Nonionic Surfactant/Caseinate Mixed Nanoemulsions. J. Food Sci. 2019, 84, 2584–2591. [Google Scholar] [CrossRef] [PubMed]
- De Souza Ferreira, S.B.; Bruschi, M.L. Improving the bioavailability of curcumin: Is micro/nanoencapsulation the key? Ther. Deliv. 2019, 10, 83–86. [Google Scholar] [PubMed]
- Ipar, V.S.; Dsouza, A.; Devarajan, P.V. Enhancing Curcumin Oral Bioavailability through Nanoformulations. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 459–480. [Google Scholar] [CrossRef]
- Chen, H.; Wu, J.; Sun, M.; Guo, C.; Yu, A.; Cao, F.; Zhao, L.; Tan, Q.; Zhai, G. N-trimethyl chitosan chloride-coated liposomes for the oral delivery of curcumin. J. Liposome Res. 2011, 22, 100–109. [Google Scholar] [CrossRef]
- Takahashi, M.; Uechi, S.; Takara, K.; Asikin, Y.; Wada, K. Evaluation of an Oral Carrier System in Rats: Bioavailability and Antioxidant Properties of Liposome-Encapsulated Curcumin. J. Agric. Food Chem. 2009, 57, 9141–9146. [Google Scholar] [CrossRef]
- Ratnatilaka Na Bhuket, P.; El-Magboub, A.; Haworth, I.S.; Rojsitthisak, P. Enhancement of Curcumin Bioavailability via the Prodrug Approach: Challenges and Prospects. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 341–353. [Google Scholar]
- Saheb, M.; Fereydouni, N.; Nemati, S.; Barreto, G.E.; Johnston, T.P.; Sahebkar, A. Chitosan-based delivery systems for curcumin: A review of pharmacodynamic and pharmacokinetic aspects. J. Cell. Physiol. 2019, 234, 12325–12340. [Google Scholar] [CrossRef]
- Xie, X.; Tao, Q.; Zou, Y.; Zhang, F.; Guo, M.; Wang, Y.; Wang, H.; Zhou, Q.; Yu, S. PLGA Nanoparticles Improve the Oral Bioavailability of Curcumin in Rats: Characterizations and Mechanisms. J. Agric. Food Chem. 2011, 59, 9280–9289. [Google Scholar] [CrossRef]
- Schiborr, C.; Kocher, A.; Behnam, D.; Jandasek, J.; Toelstede, S.; Frank, J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol. Nutr. Food Res. 2014, 58, 516–527. [Google Scholar] [CrossRef]
- Cheng, A.L.; Hsu, C.-H.; Lin, J.K.; Hsu, M.M.; Ho, Y.-F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.-R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar] [PubMed]
- Lao, C.D.; Ruffin, M.T., 4th; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement Altern. Med. 2006, 6, 10. [Google Scholar]
- Vassilev, Z.P.; Ruigómez, A.; Soriano-Gabarró, M.; Rodriguez, L.A.G. Diabetes, Cardiovascular Morbidity, and Risk of Age-Related Macular Degeneration in a Primary Care Population. Investig. Opthalmology Vis. Sci. 2015, 56, 1585–1592. [Google Scholar] [CrossRef]
- Mukhtar, S.; Ambati, B.K. The value of nutritional supplements in treating Age-Related Macular Degeneration: A review of the literature. Int. Ophthalmol. 2019, 39, 2975–2983. [Google Scholar] [CrossRef]
- Subhi, Y.; Henningsen, G.Ø.; Larsen, C.T.; Sørensen, M.S.; Sørensen, T.L. Foveal Morphology Affects Self-Perceived Visual Function and Treatment Response in Neovascular Age-Related Macular Degeneration: A Cohort Study. PLoS ONE 2014, 9, e91227. [Google Scholar] [CrossRef][Green Version]
- Howell, J.C.; Chun, E.; Farrell, A.N.; Hur, E.Y.; Caroti, C.M.; Iuvone, P.M.; Haque, R. Global microRNA expression profiling: Curcumin (diferuloylmethane) alters oxidative stress-responsive microRNAs in human ARPE-19 cells. Mol. Vis. 2013, 19, 544–560. [Google Scholar]
- Park, S.I.; Lee, E.H.; Kim, S.R.; Jang, Y.P. Anti-apoptotic effects of Curcuma longa L. extract and its curcuminoids against blue light-induced cytotoxicity in A2E-laden human retinal pigment epithelial cells. J. Pharm. Pharmacol. 2017, 69, 334–340. [Google Scholar]
- Mandal, N.A.; Patlolla, J.M.; Zheng, L.; Agbaga, M.-P.; Tran, J.-T.A.; Wicker, L.; Kasus-Jacobi, A.; Elliott, M.H.; Rao, C.V.; Anderson, R.E. Curcumin protects retinal cells from light-and oxidant stress-induced cell death. Free Radic. Biol. Med. 2009, 46, 672–679. [Google Scholar] [CrossRef]
- Li, Y.; Zou, X.; Cao, K.; Xu, J.; Yue, T.; Dai, F.; Zhou, B.; Lu, W.; Feng, Z.; Liu, J. Curcumin analog 1, 5-bis (2-trifluoromethylphenyl)-1, 4-pentadien-3-one exhibits enhanced ability on Nrf2 activation and protection against acrolein-induced ARPE-19 cell toxicity. Toxicol. Appl. Pharmacol. 2013, 272, 726–735. [Google Scholar] [CrossRef]
- Xie, P.; Zhang, W.; Yuan, S.; Chen, Z.; Yang, Q.; Yuan, D.; Wang, F.; Liu, Q. Suppression of Experimental Choroidal Neovascularization by Curcumin in Mice. PLoS ONE 2012, 7, e53329. [Google Scholar] [CrossRef]
- Allegrini, D.; Raimondi, R.; Angi, M.; Ricciardelli, G.; Montericcio, A.; Borgia, A.; Romano, M.R. Curcuma-Based Nutritional Supplement in Patients with Neovascular Age-Related Macular Degeneration. J. Med. Food 2021, 24, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef]
- Gupta, S.K.; Kumar, B.; Nag, T.C.; Agrawal, S.S.; Agrawal, R.; Agrawal, P.; Saxena, R.; Srivastava, S. Curcumin Prevents Experimental Diabetic Retinopathy in Rats through Its Hypoglycemic, Antioxidant, and Anti-Inflammatory Mechanisms. J. Ocul. Pharmacol. Ther. 2011, 27, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; George, B.; Chen, S.; Feng, B.; Li, X.; Chakrabarti, S. Genotoxic stress and activation of novel DNA repair enzymes in human endothelial cells and in the retinas and kidneys of streptozotocin diabetic rats. Diabetes Metab. Res. Rev. 2012, 28, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Khimmaktong, W.; Petpiboolthai, H.; Sriya, P.; Anupunpisit, V. Effects of curcumin on restoration and improvement of microvasculature characteristic in diabetic rat’s choroid of eye. J. Med Assoc. Thail. 2014, 97, S39–S46. [Google Scholar]
- Emoto, Y.; Yoshizawa, K.; Uehara, N.; Kinoshita, Y.; Yuri, T.; Shikata, N.; Tsubura, A. Curcumin suppresses N-methyl-N-nitrosourea-induced photoreceptor apoptosis in Sprague-Dawley rats. In Vivo 2013, 27, 583–590. [Google Scholar]
- Vasireddy, V.; Chavali, V.R.M.; Joseph, V.T.; Kadam, R.; Lin, J.H.; Jamison, J.A.; Kompella, U.B.; Reddy, G.B.; Ayyagari, R. Rescue of Photoreceptor Degeneration by Curcumin in Transgenic Rats with P23H Rhodopsin Mutation. PLoS ONE 2011, 6, e21193. [Google Scholar] [CrossRef]
- Starace, V.; Battista, M.; Brambati, M.; Cavalleri, M.; Bertuzzi, F.; Amato, A.; Lattanzio, R.; Bandello, F.; Cicinelli, M.V. The role of inflammation and neurodegeneration in diabetic macular edema. Ther. Adv. Ophthalmol. 2021, 13, 25158414211055963. [Google Scholar] [CrossRef]
- Idrees, S.; Sridhar, J.; Kuriyan, A.E. Proliferative Vitreoretinopathy: A Review. Int. Ophthalmol. Clin. 2019, 59, 221–240. [Google Scholar] [CrossRef]
- Sun, Y.; You, Z.-P. Curcumin inhibits human retinal pigment epithelial cell proliferation. Int. J. Mol. Med. 2014, 34, 1013–1019. [Google Scholar] [CrossRef]
- Alex, A.F.; Spitznas, M.; Tittel, A.P.; Kurts, C.; Eter, N. Inhibitory Effect of Epigallocatechin Gallate (EGCG), Resveratrol, and Curcumin on Proliferation of Human Retinal Pigment Epithelial Cells In Vitro. Curr. Eye Res. 2010, 35, 1021–1033. [Google Scholar] [CrossRef] [PubMed]
- An, J.-B.; Ma, J.-X.; Liu, D.-Y.; Gao, Y.-J.; Sheng, M.-Y.; Wang, H.-X.; Liu, L.-Y. The effect of curcumin on DNA content, mitochondrial transmembrane potential and calcium of rabbit cultured retinal pigment epithelial cells. Zhonghua Yan Ke Za Zhi Chin. J. Ophthalmol. 2009, 45, 210–215. [Google Scholar]
- Jiang, H.; Luo, J.; Lei, H. The roles of mouse double minute 2 (MDM2) oncoprotein in ocular diseases: A review. Exp. Eye Res. 2022, 217, 108910. [Google Scholar] [CrossRef] [PubMed]
- Bar-Sela, G.; Epelbaum, R.; Schaffer, M. Curcumin as an Anti-Cancer Agent: Review of the Gap between Basic and Clinical Applications. Curr. Med. Chem. 2010, 17, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, S.; Thirumalai, K.; Danda, R.; Krishnakumar, S. Effect of Curcumin on miRNA Expression in Human Y79 Retinoblastoma Cells. Curr. Eye Res. 2012, 37, 421–428. [Google Scholar] [CrossRef]
- Yu, X.; Zhong, J.; Yan, L.; Li, J.; Wang, H.; Wen, Y.; Zhao, Y. Curcumin exerts antitumor effects in retinoblastoma cells by regulating the JNK and p38 MAPK pathways. Int. J. Mol. Med. 2016, 38, 861–868. [Google Scholar] [CrossRef]
- Li, Y.; Sun, W.; Han, N.; Zou, Y.; Yin, D. Curcumin inhibits proliferation, migration, invasion and promotes apoptosis of retinoblastoma cell lines through modulation of miR-99a and JAK/STAT pathway. BMC Cancer 2018, 18, 1230. [Google Scholar] [CrossRef]
- Baum, L.; Lam, C.W.K.; Cheung, S.K.-K.; Kwok, T.; Lui, V.; Tsoh, J.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; et al. Six-Month Randomized, Placebo-Controlled, Double-Blind, Pilot Clinical Trial of Curcumin in Patients With Alzheimer Disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. [Google Scholar] [CrossRef]
- Thota, R.; Rosato, J.I.; Dias, C.B.; Burrows, T.L.; Martins, R.; Garg, M.L. Dietary Supplementation with Curcumin Reduce Circulating Levels of Glycogen Synthase Kinase-3β and Islet Amyloid Polypeptide in Adults with High Risk of Type 2 Diabetes and Alzheimer’s Disease. Nutrients 2020, 12, 1032. [Google Scholar] [CrossRef]
- Baum, L.; Cheung, S.K.; Mok, V.C.; Lam, L.C.; Leung, V.P.; Hui, E.; Ng, C.C.; Chow, M.; Ho, P.C.; Lam, S. Curcumin effects on blood lipid profile in a 6-month human study. Pharmacol. Res. 2007, 56, 509–514. [Google Scholar] [CrossRef]
- Rainey-Smith, S.R.; Brown, B.M.; Sohrabi, H.R.; Shah, T.; Goozee, K.G.; Gupta, V.B.; Martins, R.N. Curcumin and cognition: A randomised, placebo-controlled, double-blind study of community-dwelling older adults. Br. J. Nutr. 2016, 115, 2106–2113. [Google Scholar] [PubMed]
- Sugawara, J.; Akazawa, N.; Miyaki, A.; Choi, Y.; Tanabe, Y.; Imai, T.; Maeda, S. Effect of endurance exercise training and curcumin intake on central arterial hemodynamics in postmenopausal women: Pilot study. Am. J. Hypertens. 2012, 25, 651–656. [Google Scholar] [CrossRef] [PubMed]
| Authors | Year of Publication | Sample (n=) | Duration | Main Findings |
|---|---|---|---|---|
| Baum et al. [108] | 2008 | 34 | 6 months | Curcumin may have the ability to disaggregate Ab deposits |
| Thota et al. [109] | 2020 | 29 | 12 weeks | Better glycemic control through a decrease in insulin-resistance fasting serum insulin |
| Baum et al. [110] | 2007 | 36 | 6 months | No effect of curcumin on blood serum profiles |
| Rainey-Smith et al. [111] | 2016 | 96 | 12 months | Curcumin treatment group had no cognitive decline at six months compared to placebo group |
| Sugarawa et al. [112] | 2012 | 45 | 8 weeks | Higher decrease in aortic systolic blood pressure, HR-corrected aortic AP and AIx in the group taking curcumin and practicing regular endurance exercise |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allegrini, D.; Raimondi, R.; Borgia, A.; Sorrentino, T.; Montesano, G.; Tsoutsanis, P.; Cancian, G.; Verma, Y.; De Rosa, F.P.; Romano, M.R. Curcumin in Retinal Diseases: A Comprehensive Review from Bench to Bedside. Int. J. Mol. Sci. 2022, 23, 3557. https://doi.org/10.3390/ijms23073557
Allegrini D, Raimondi R, Borgia A, Sorrentino T, Montesano G, Tsoutsanis P, Cancian G, Verma Y, De Rosa FP, Romano MR. Curcumin in Retinal Diseases: A Comprehensive Review from Bench to Bedside. International Journal of Molecular Sciences. 2022; 23(7):3557. https://doi.org/10.3390/ijms23073557
Chicago/Turabian StyleAllegrini, Davide, Raffaele Raimondi, Alfredo Borgia, Tania Sorrentino, Giovanni Montesano, Panos Tsoutsanis, Giuseppe Cancian, Yash Verma, Francesco Paolo De Rosa, and Mario R. Romano. 2022. "Curcumin in Retinal Diseases: A Comprehensive Review from Bench to Bedside" International Journal of Molecular Sciences 23, no. 7: 3557. https://doi.org/10.3390/ijms23073557
APA StyleAllegrini, D., Raimondi, R., Borgia, A., Sorrentino, T., Montesano, G., Tsoutsanis, P., Cancian, G., Verma, Y., De Rosa, F. P., & Romano, M. R. (2022). Curcumin in Retinal Diseases: A Comprehensive Review from Bench to Bedside. International Journal of Molecular Sciences, 23(7), 3557. https://doi.org/10.3390/ijms23073557







