GLP1 Exerts Paracrine Activity in the Intestinal Lumen of Human Colon
Abstract
:1. Introduction
2. Results
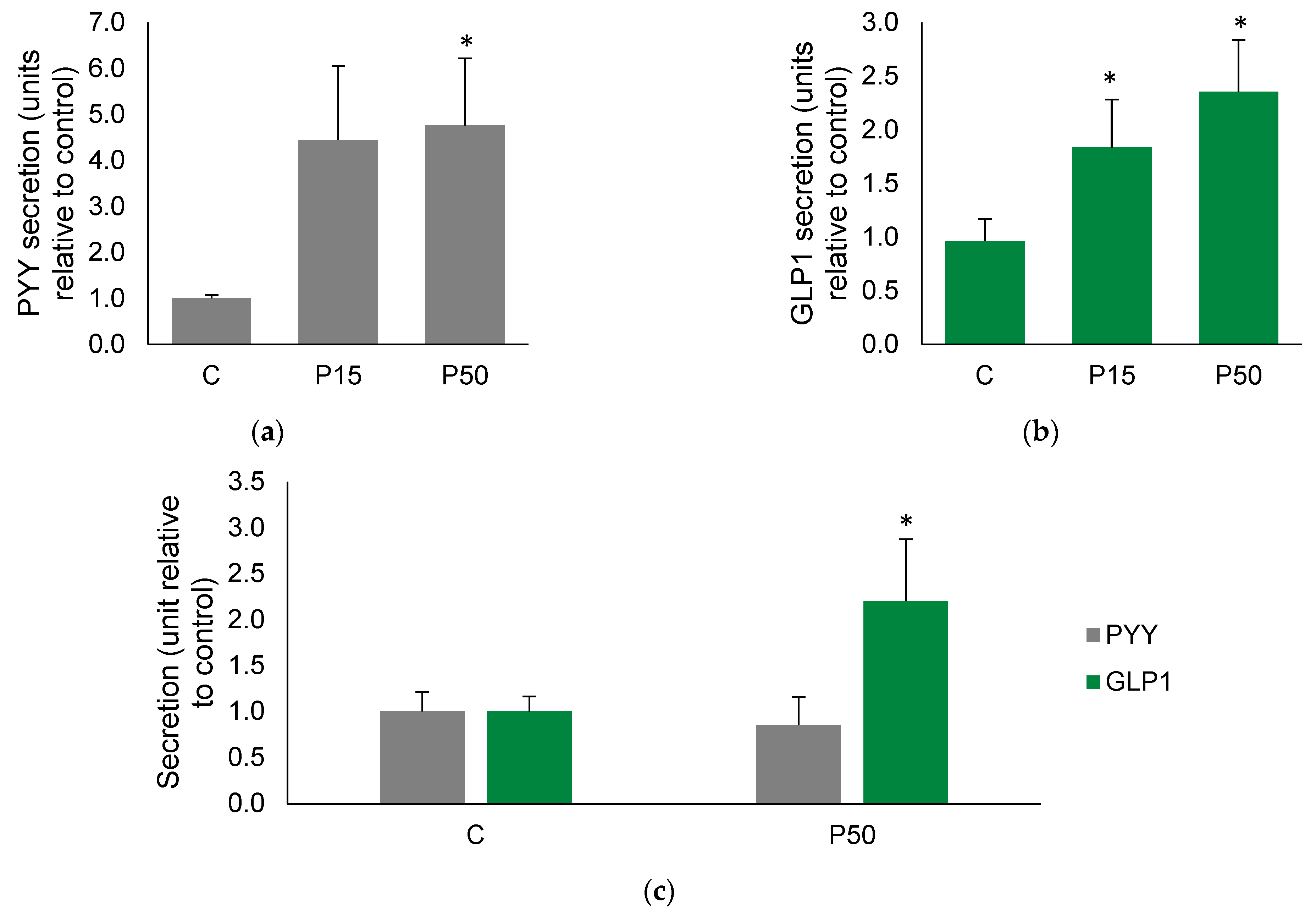
2.1. Meat Peptone Stimulates PYY and GLP1 Secretion in Human Colon
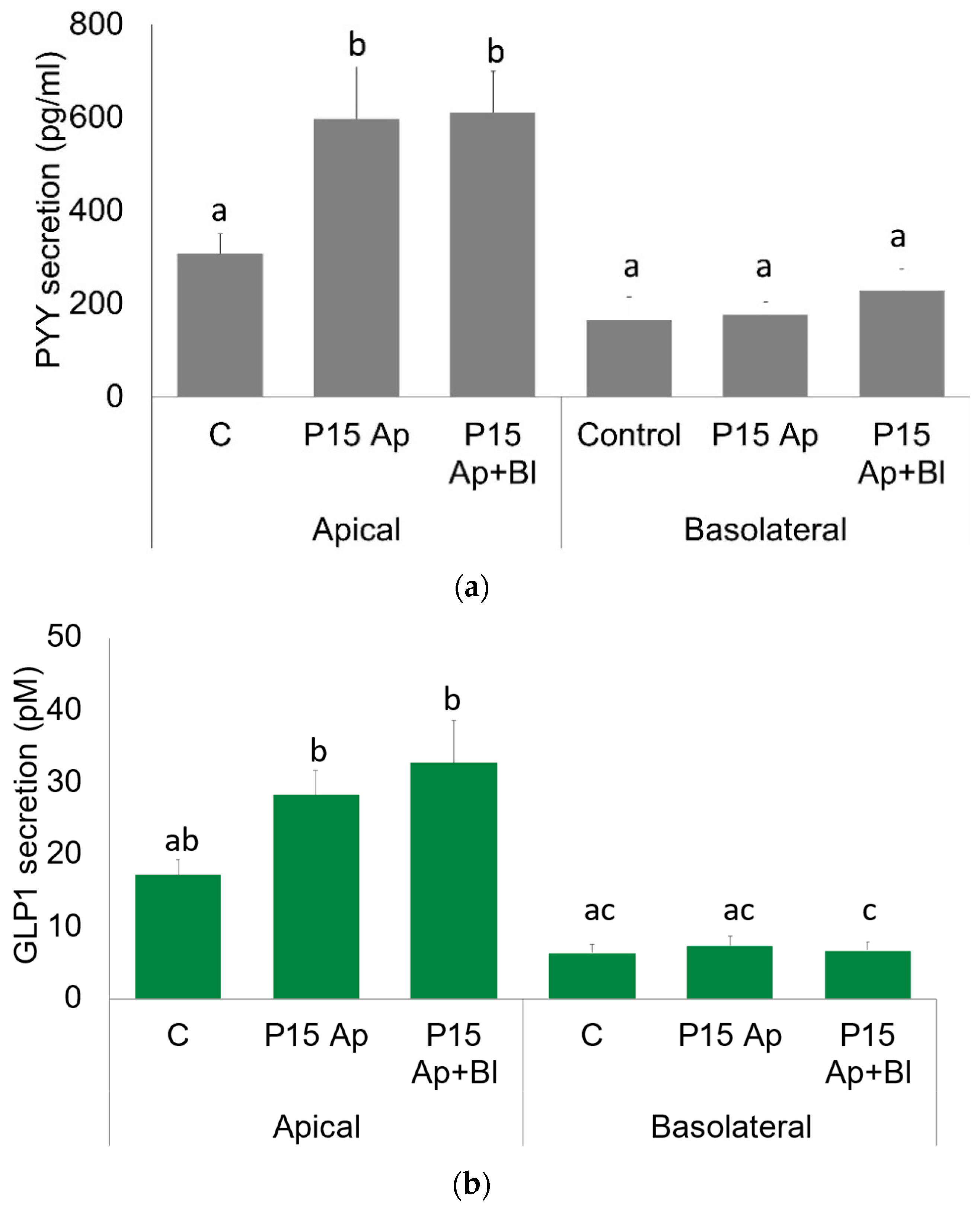
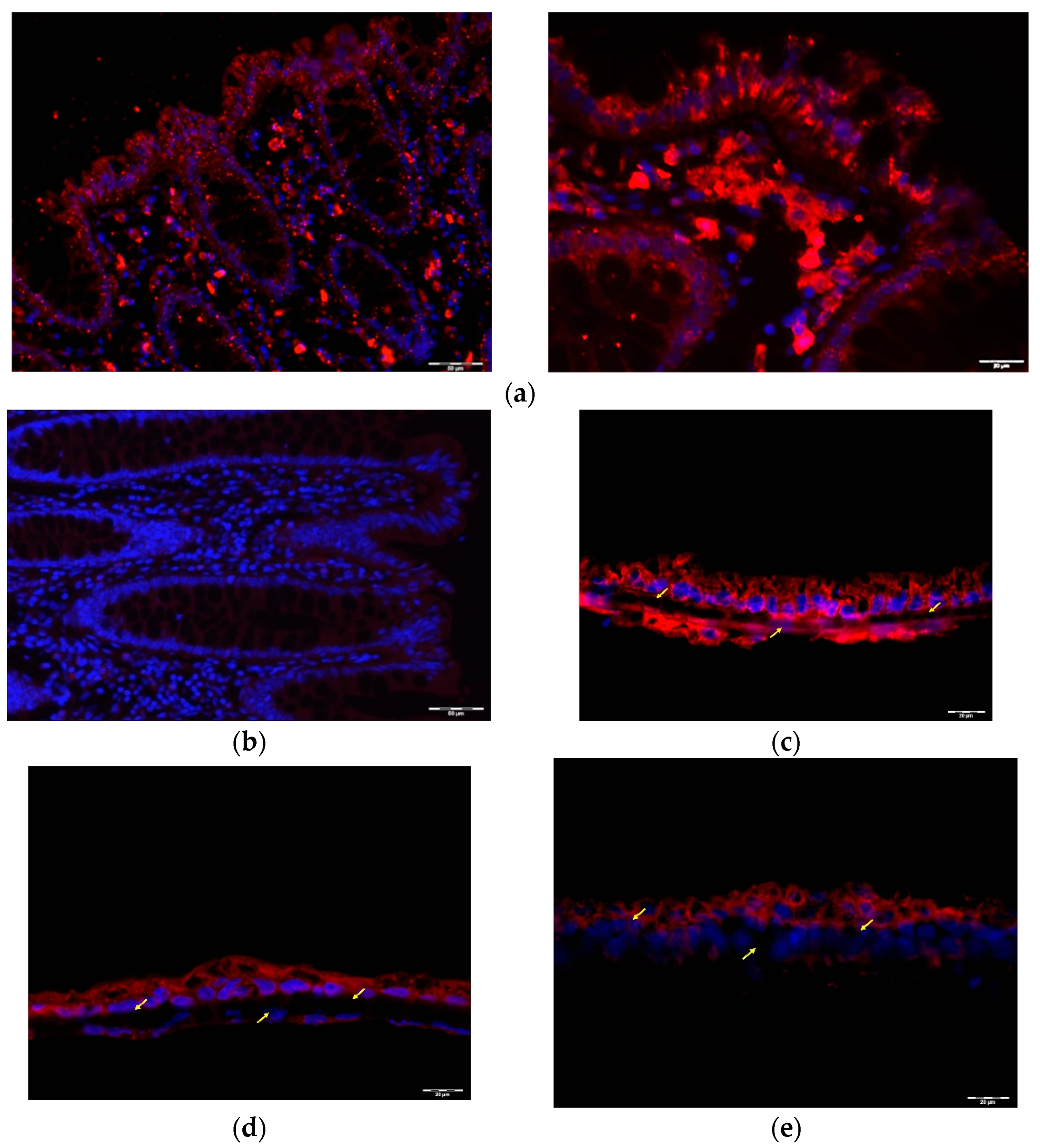
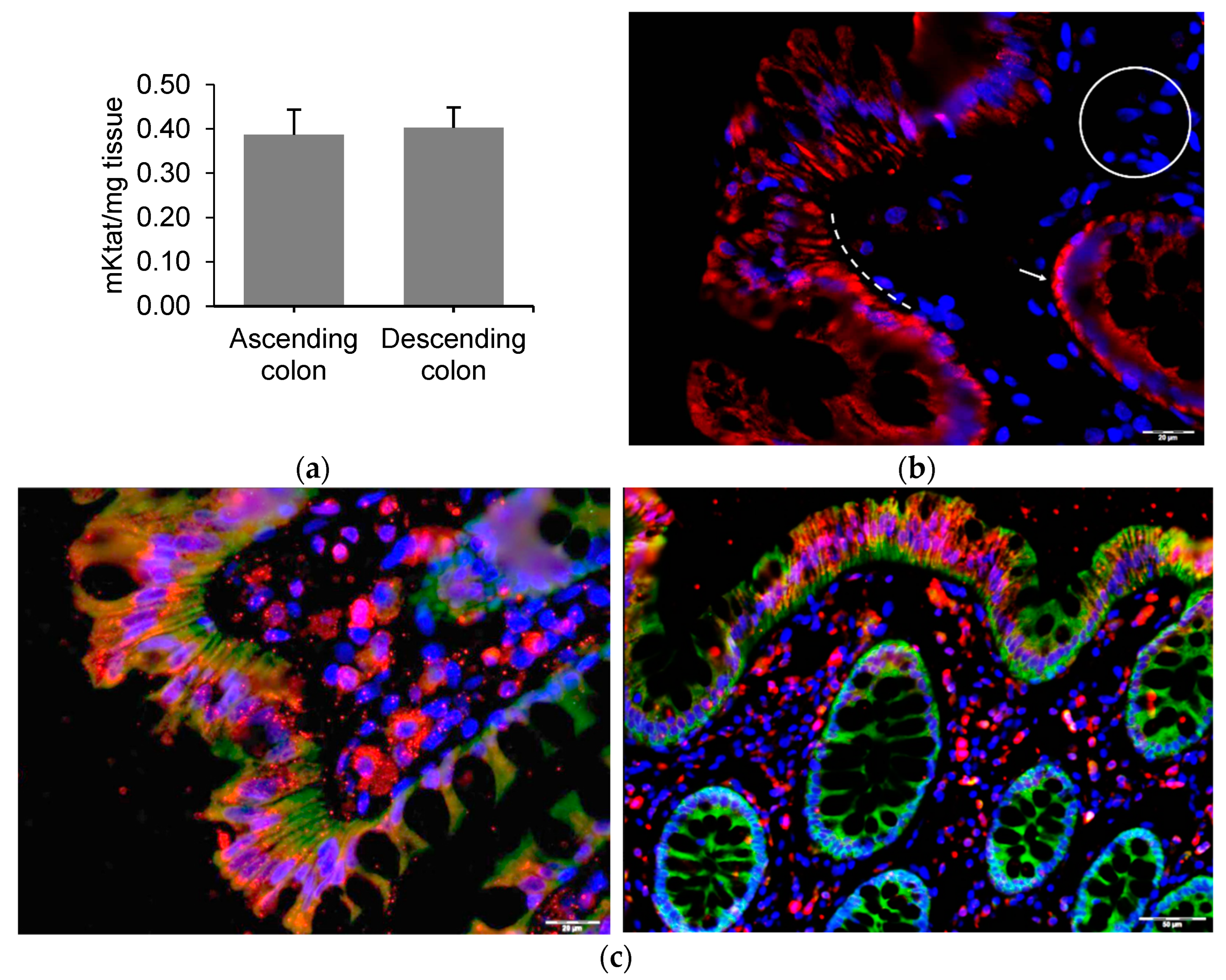
2.2. GLP1 and PYY Are Secreted into the Intestinal Lumen of Human Colon
2.3. GLP1 and PYY Are Secreted into the Intestinal Lumen of Human Colon
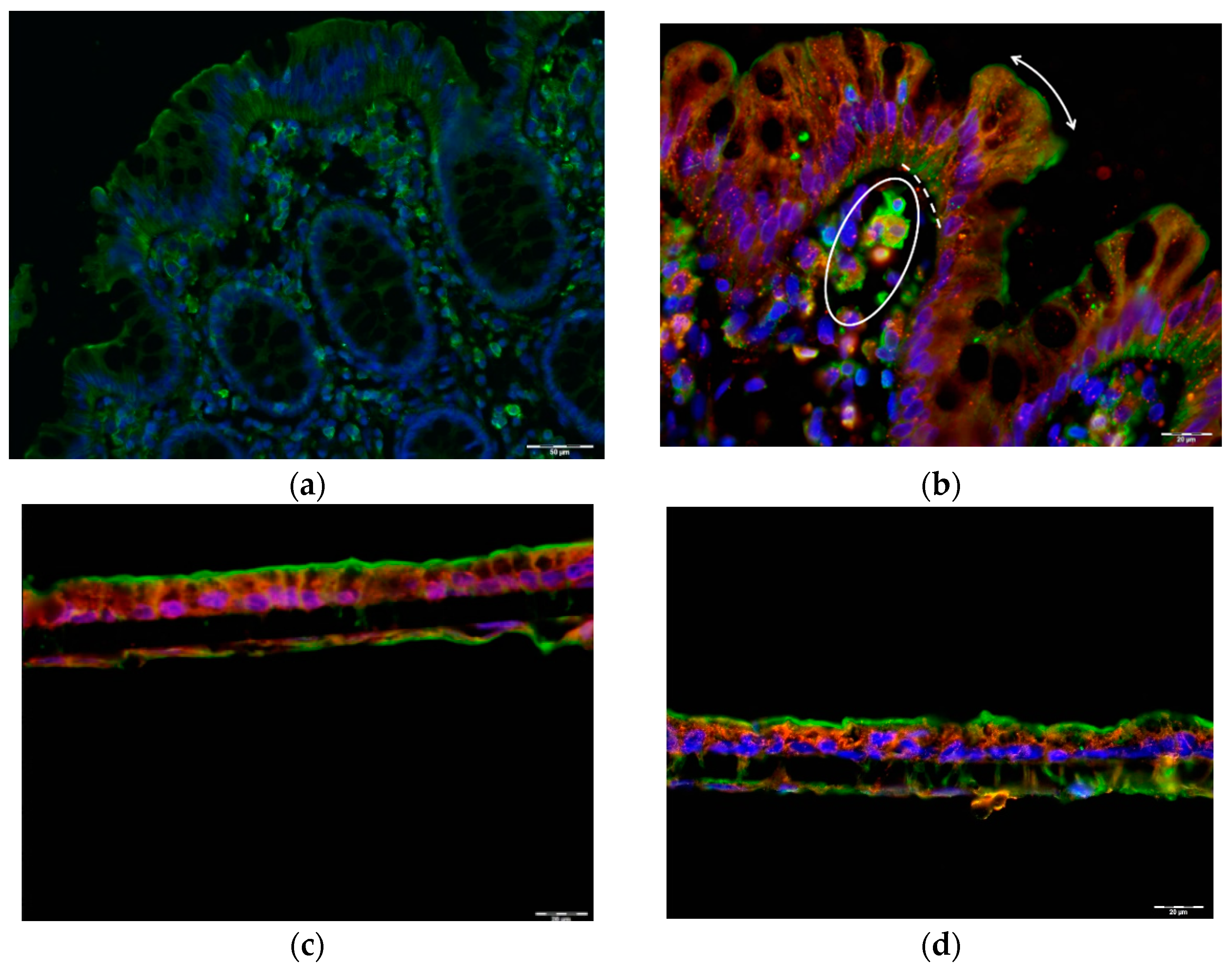
2.4. Alternative GLP1 Receptors May Be Responsible for GLP1 (9–36) Sensing in Human Colon Lumen
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Obtaining Intestinal Mucosa Samples
4.3. Enterohormone Secretion Study in Explants
4.4. Enterohormone Secretion Study in an Ussing Chamber
4.5. Enterohormone Quantification
4.6. DPP4 Activity Measurements
4.7. Cell Culture
4.8. Immunofluorescence Staining
4.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahlman, H.; Nilsson, O. The gut as the largest endocrine organ in the body. Ann. Oncol. 2001, 12, S63–S68. [Google Scholar] [CrossRef] [PubMed]
- Steinert, R.E.; Feinle-Bisset, C.; Asarian, L.; Horowitz, M.; Beglinger, C.; Geary, N. Ghrelin, CCK, GLP-1, and PYY(3-36): Secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol. Rev. 2017, 97, 411–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosele, J.I.; Macià, A.; Motilva, M. Metabolic and Microbial Modulation of the Large Intestine Ecosystem by Non-Absorbed Diet Phenolic Compounds: A Review. Molecules 2015, 20, 17429–17468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greiner, T.U.; Bäckhed, F. Microbial regulation of GLP-1 and L-cell biology. Mol. Metab. 2016, 5, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Halim, M.A.; Degerblad, M.; Sundbom, M.; Karlbom, U.; Holst, J.J.; Webb, D.-L.; Hellström, P.M. Glucagon-Like Peptide-1 Inhibits Prandial Gastrointestinal Motility Through Myenteric Neuronal Mechanisms in Humans. J. Clin. Endocrinol. Metab. 2018, 103, 575–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, L.; Mesgarzadeh, S.; Ramesh, K.S.; Huey, E.L.; Liu, Y.; Gray, L.A.; Aitken, T.J.; Chen, Y.; Beutler, L.R.; Ahn, J.S.; et al. Genetic Identification of Vagal Sensory Neurons That Control Feeding. Cell 2019, 179, 1129–1143.e23. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.J.; Gethmann, A.; Nauck, M.A.; Götze, O.; Schmitz, F.; Deacon, C.F.; Gallwitz, B.; Schmidt, W.E.; Holst, J.J. The glucagon-like peptide-1 metabolite GLP-1-(9-36) amide reduces postprandial glycemia independently of gastric emptying and insulin secretion in humans. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1118–E1123. [Google Scholar] [CrossRef] [Green Version]
- Paternoster, S.; Falasca, M. Dissecting the physiology and pathophysiology of glucagon-like peptide-1. Front. Endocrinol. 2018, 9, 584. [Google Scholar] [CrossRef] [Green Version]
- Guglielmi, V.; Sbraccia, P. GLP-1 receptor independent pathways: Emerging beneficial effects of GLP-1 breakdown products. Eat. Weight Disord. 2017, 22, 231–240. [Google Scholar] [CrossRef]
- Cantini, G.; Mannucci, E.; Luconi, M. Perspectives in GLP-1 Research: New Targets, New Receptors. Trends Endocrinol. Metab. 2016, 27, 427–438. [Google Scholar] [CrossRef]
- Guida, C.; Miranda, C.; Asterholm, I.W.; Basco, D.; Benrick, A.; Chanclon, B.; Chibalina, M.; Harris, M.; Kellard, J.; McCulloch, L.; et al. GLP-1(9-36) mediates the glucagonostatic effect of GLP-1 by promiscuous activation of the glucagon receptor. bioRxiv 2019, 1, 785667. [Google Scholar] [CrossRef]
- Stevens, L.J.; van Lipzig, M.M.H.; Erpelinck, S.L.A.; Pronk, A.; van Gorp, J.; Wortelboer, H.M.; van de Steeg, E. A higher throughput and physiologically relevant two-compartmental human ex vivo intestinal tissue system for studying gastrointestinal processes. Eur. J. Pharm. Sci. 2019, 137, 104989. [Google Scholar] [CrossRef] [PubMed]
- Gribble, F.M.; Reimann, F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat. Rev. Endocrinol. 2019, 15, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.M.; O’Brien, R.; Brosnan, E.; Ross, R.P.; Stanton, C.; Buckley, J.M.; O’Malley, D. Glucagon-Like Peptide-1 Secreting L-Cells Coupled to Sensory Nerves Translate Microbial Signals to the Host Rat Nervous System. Front. Cell. Neurosci. 2020, 14, 1–13. [Google Scholar] [CrossRef]
- Cordier-Bussat, M.; Bernard, C.; Levenez, F.; Klages, N.; Laser-Ritz, B.; Philippe, J.; Chayvialle, J.A.; Cuber, J.C. Peptones stimulate both the secretion of the incretin hormone glucagon- like peptide 1 and the transcription of the proglucagon gene. Diabetes 1998, 47, 1038–1045. [Google Scholar] [CrossRef]
- Modvig, I.M.; Kuhre, R.E.; Holst, J.J. Peptone-mediated glucagon-like peptide-1 secretion depends on intestinal absorption and activation of basolaterally located Calcium-Sensing Receptors. Physiol. Rep. 2019, 7, e14056. [Google Scholar] [CrossRef]
- Billing, L.J.; Smith, C.A.; Larrau, P.; Goldspink, D.A.; Galvin, S.; Kay, R.G.; Howe, J.D.; Walker, R.; Pruna, M.; Glass, L.; et al. glucagon-like peptide-1 and peptideYY from murine and human colonic enteroendocrine cells. Mol. Metab. 2018, 16, 65–75. [Google Scholar] [CrossRef]
- Schneeberger, K.; Roth, S.; Nieuwenhuis, E.E.S.; Middendorp, S. Intestinal epithelial cell polarity defects in disease: Lessons from microvillus inclusion disease. DMM Dis. Model. Mech. 2018, 11, dmm031088. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.B.; Grillone, L.; Boyce, R.; Crooke, S.T. Altered actin and immunoglobulin Cμ expression in nitrogen mustard-resistant human Burkitt lymphoma cells. J. Cell. Biochem. 1989, 40, 407–415. [Google Scholar] [CrossRef]
- Sanchez, V.; Dong, J.J.; Battley, J.; Jackson, K.N.; Dykes, B.C. Human cytomegalovirus infection of THP-1 derived macrophages reveals strain-specific regulation of actin dynamics. Virology 2012, 433, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Nargis, T.; Chakrabarti, P. Significance of circulatory DPP4 activity in metabolic diseases. IUBMB Life 2018, 70, 112–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, G.P.; Macrae, F.A.; Gibson, P.R.; Alexeyeff, M.; Whitehead, R.H. Brush border hydrolases in normal and neoplastic colonic epithelium. J. Gastroenterol. Hepatol. 1992, 7, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Deacon, C.F. Physiology and Pharmacology of DPP-4 in Glucose Homeostasis and the Treatment of Type 2 Diabetes. Front. Endocrinol. 2019, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Cantini, G.; Di Franco, A.; Mannucci, E.; Luconi, M. Is cleaved glucagon-like peptide 1 really inactive? Effects of GLP-1(9-36) on human adipose stem cells. Mol. Cell. Endocrinol. 2017, 439, 10–15. [Google Scholar] [CrossRef]
- Olivares, M.; Schüppel, V.; Hassan, A.M.; Beaumont, M.; Neyrinck, A.M.; Bindels, L.B.; Benítez-Páez, A.; Sanz, Y.; Haller, D.; Holzer, P.; et al. The potential role of the Dipeptidyl peptidase-4-like activity from the gut microbiota on the Host Health. Front. Microbiol. 2018, 9, 1900. [Google Scholar] [CrossRef]
- Sandoval, D.A.; D’Alessio, D.A. Physiology of proglucagon peptides: Role ofglucagon and GLP-1 in health and disease. Physiol. Rev. 2015, 95, 513–548. [Google Scholar] [CrossRef] [Green Version]
- Buenaventura, T.; Bitsi, S.; Laughlin, W.E.; Burgoyne, T.; Lyu, Z.; Oqua, A.I.; Norman, H.; McGlone, E.R.; Klymchenko, A.S.; Corrêa, I.R.; et al. Agonist-induced membrane nanodomain clustering drives GLP-1 receptor responses in pancreatic beta cells. PLoS Biol. 2019, 17, e3000097. [Google Scholar] [CrossRef]
- Zhang, L.W.; McMahon Tobin, G.A.; Rouse, R.L. Oleic acid and glucose regulate glucagon-like peptide 1 receptor expression in a rat pancreatic ductal cell line. Toxicol. Appl. Pharmacol. 2012, 264, 274–283. [Google Scholar] [CrossRef]
- Cantini, G.; Trabucco, M.; Di Franco, A.; Mannucci, E.; Luconi, M. Glucagon modulates proliferation and differentiation of human adipose precursors. J. Mol. Endocrinol. 2019, 63, 249–260. [Google Scholar] [CrossRef]
- Varin, E.M.; Mulvihill, E.E.; Baggio, L.L.; Koehler, J.A.; Cao, X.; Seeley, R.J.; Drucker, D.J. Distinct Neural Sites of GLP-1R Expression Mediate Physiological versus Pharmacological Control of Incretin Action. Cell Rep. 2019, 27, 3371–3384.e3. [Google Scholar] [CrossRef] [Green Version]
- Helmstädter, J.; Frenis, K.; Filippou, K.; Grill, A.; Dib, M.; Kalinovic, S.; Pawelke, F.; Kus, K.; Kröller-Schön, S.; Oelze, M.; et al. Endothelial GLP-1 (Glucagon-Like Peptide-1) Receptor Mediates Cardiovascular Protection by Liraglutide In Mice With Experimental Arterial Hypertension. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Rosario, W.; D’Alessio, D. An innate disposition for a healthier gut: Glp-1r signaling in intestinal epithelial lymphocytes. Diabetes 2015, 64, 2329–2331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- May, A.T.; Crowe, M.S.; Blakeney, B.A.; Mahavadi, S.; Wang, H.; Grider, J.R.; Murthy, K.S. Identification of expression and function of the glucagon-like peptide-1 receptor in colonic smooth muscle. Peptides 2019, 112, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.L.; Egerod, K.L.; Engelstoft, M.S.; Dmytriyeva, O.; Theodorsson, E.; Patel, B.A.; Schwartz, T.W. Enterochromaffin 5-HT cells—A major target for GLP-1 and gut microbial metabolites. Mol. Metab. 2018, 11, 70–83. [Google Scholar] [CrossRef]
- Lund, M.L.; Sorrentino, G.; Egerod, K.L.; Kroone, C.; Mortensen, B.; Knop, F.K.; Reimann, F.; Gribble, F.M.; Drucker, D.J.; De Koning, E.J.P.; et al. L-cell differentiation is induced by bile acids through GpBAR1 and paracrine GLP-1 and serotonin signaling. Diabetes 2020, 69, 614–623. [Google Scholar] [CrossRef]
- Jepsen, S.L.; Grunddal, K.V.; Albrechtsen, N.J.W.; Engelstoft, M.S.; Gabe, M.B.N.; Jensen, E.P.; Ørskov, C.; Poulsen, S.S.; Rosenkilde, M.M.; Pedersen, J.; et al. Paracrine crosstalk between intestinal L- And D-cells controls secretion of glucagon-like peptide-1 in mice. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E1081–E1093. [Google Scholar] [CrossRef]
- Sun, E.W.; Martin, A.M.; de Fontgalland, D.; Sposato, L.; Rabbitt, P.; Hollington, P.; Wattchow, D.A.; Colella, A.D.; Chataway, T.; Wewer Albrechtsen, N.J.; et al. Evidence for Glucagon Secretion and Function Within the Human Gut. Endocrinology 2021, 162, bqab022. [Google Scholar] [CrossRef]
- Lang, S.; Yang, J.; Yang, K.; Gu, L.; Cui, X.; Wei, T.; Liu, J.; Le, Y.; Wang, H.; Wei, R.; et al. Glucagon receptor antagonist upregulates circulating GLP-1 level by promoting intestinal L-cell proliferation and GLP-1 production in type 2 diabetes. BMJ Open Diabetes Res. Care 2020, 8, e001025. [Google Scholar] [CrossRef] [Green Version]
- Foley, J.E.; Jordan, J. Weight neutrality with the DPP-4 inhibitor, vildagliptin: Mechanistic basis and clinical experience. Vasc. Health Risk Manag. 2010, 6, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Cox, H.M.; Pollock, E.L.; Tough, I.R.; Herzog, H. Multiple Y receptors mediate pancreatic polypeptide responses in mouse colon mucosa. Peptides 2001, 22, 445–452. [Google Scholar] [CrossRef]
- Furness, J.B.; Jones, C.; Nurgali, K.; Clerc, N. Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog. Neurobiol. 2004, 72, 143–164. [Google Scholar] [CrossRef] [PubMed]
- Luconi, M.; Cantini, G.; Trabucco, M.; Dicembrini, I.; Mannucci, E. Hormonal Signaling in Biology and Medicine, 1st ed.; Litwack, G., Ed.; Academic Press: Cambridge, MA, USA, 2020; ISBN 9780128138144. [Google Scholar]
- Grau-bové, C.; González-Quilen, C.; Terra, X.; Teresa Blay, M.; Beltrán-Debón, R.; Jorba-Martín, R.; Espina, B.; Pinent, M.; Ardévol, A. Effects of flavanols on enteroendocrine secretion. Biomolecules 2020, 10, 844. [Google Scholar] [CrossRef]
- Pini, A.; Fazi, C.; Nardini, P.; Calvani, M.; Fabbri, S.; Guerrini, A.; Forni, G.; La Marca, G.; Rosa, A.C.; Filippi, L. Effect of Beta 3 Adrenoreceptor Modulation on Patency of the Ductus Arteriosus. Cells 2020, 9, 2625. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grau-Bové, C.; González-Quilen, C.; Cantini, G.; Nardini, P.; Espina, B.; Bani, D.; Terra, X.; Blay, M.; Rodríguez-Gallego, E.; Luconi, M.; et al. GLP1 Exerts Paracrine Activity in the Intestinal Lumen of Human Colon. Int. J. Mol. Sci. 2022, 23, 3523. https://doi.org/10.3390/ijms23073523
Grau-Bové C, González-Quilen C, Cantini G, Nardini P, Espina B, Bani D, Terra X, Blay M, Rodríguez-Gallego E, Luconi M, et al. GLP1 Exerts Paracrine Activity in the Intestinal Lumen of Human Colon. International Journal of Molecular Sciences. 2022; 23(7):3523. https://doi.org/10.3390/ijms23073523
Chicago/Turabian StyleGrau-Bové, Carme, Carlos González-Quilen, Giulia Cantini, Patrizia Nardini, Beatriz Espina, Daniele Bani, Ximena Terra, MTeresa Blay, Esther Rodríguez-Gallego, Michaela Luconi, and et al. 2022. "GLP1 Exerts Paracrine Activity in the Intestinal Lumen of Human Colon" International Journal of Molecular Sciences 23, no. 7: 3523. https://doi.org/10.3390/ijms23073523
APA StyleGrau-Bové, C., González-Quilen, C., Cantini, G., Nardini, P., Espina, B., Bani, D., Terra, X., Blay, M., Rodríguez-Gallego, E., Luconi, M., Ardévol, A., & Pinent, M. (2022). GLP1 Exerts Paracrine Activity in the Intestinal Lumen of Human Colon. International Journal of Molecular Sciences, 23(7), 3523. https://doi.org/10.3390/ijms23073523










