Therapeutic Potential of Combining IL-6 and TNF Blockade in a Mouse Model of Allergic Asthma
Abstract
:1. Introduction
2. Results
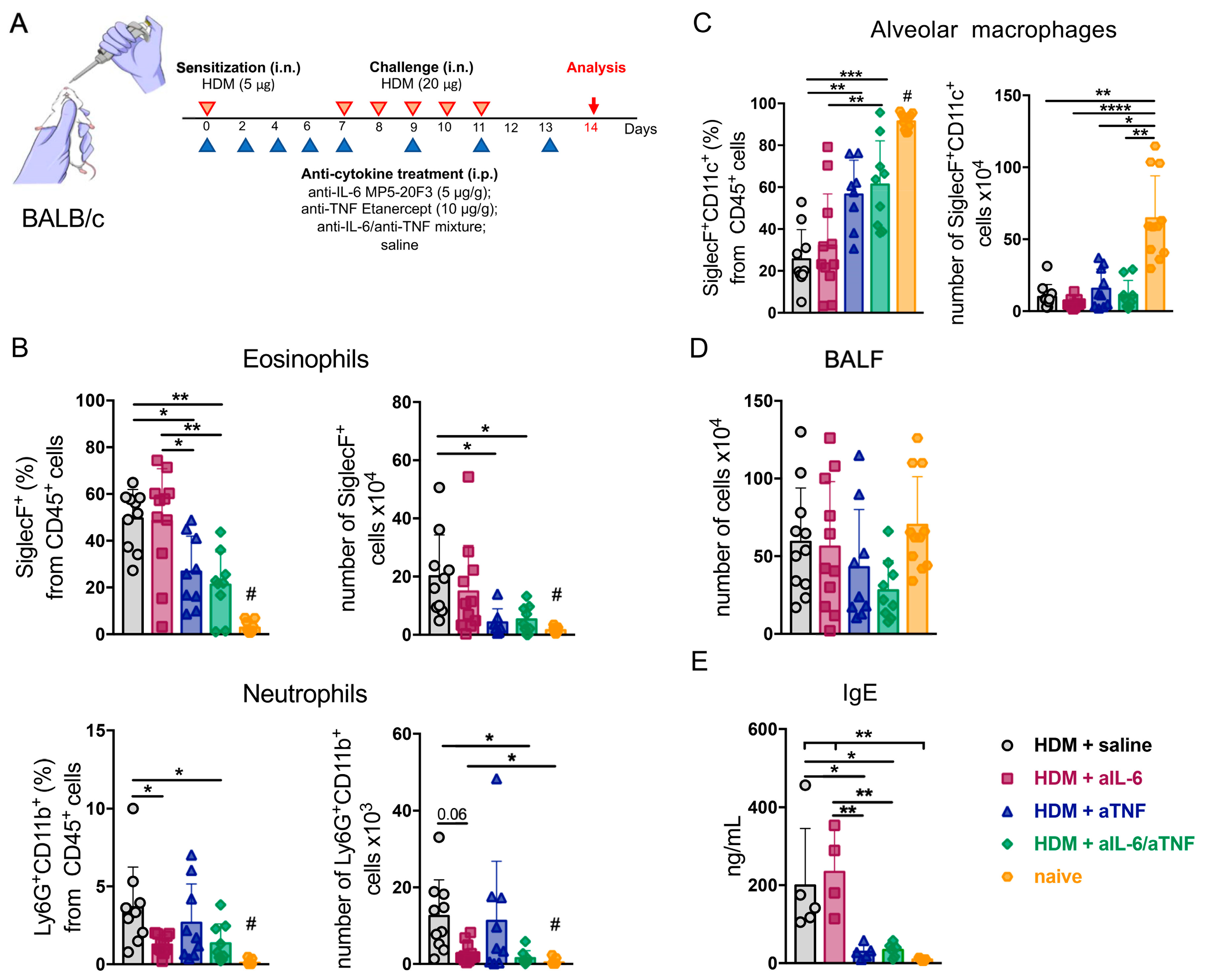
2.1. Combined Inhibition of IL-6 and TNF in Mice with Acute HDM-Induced Asthma Results in Decreased Granulocyte Infiltrate in the Airways
2.2. Simultaneous Ablation of IL-6 and TNF Significantly Reduced Th2- and Th1-Mediated Inflammation in the Lungs
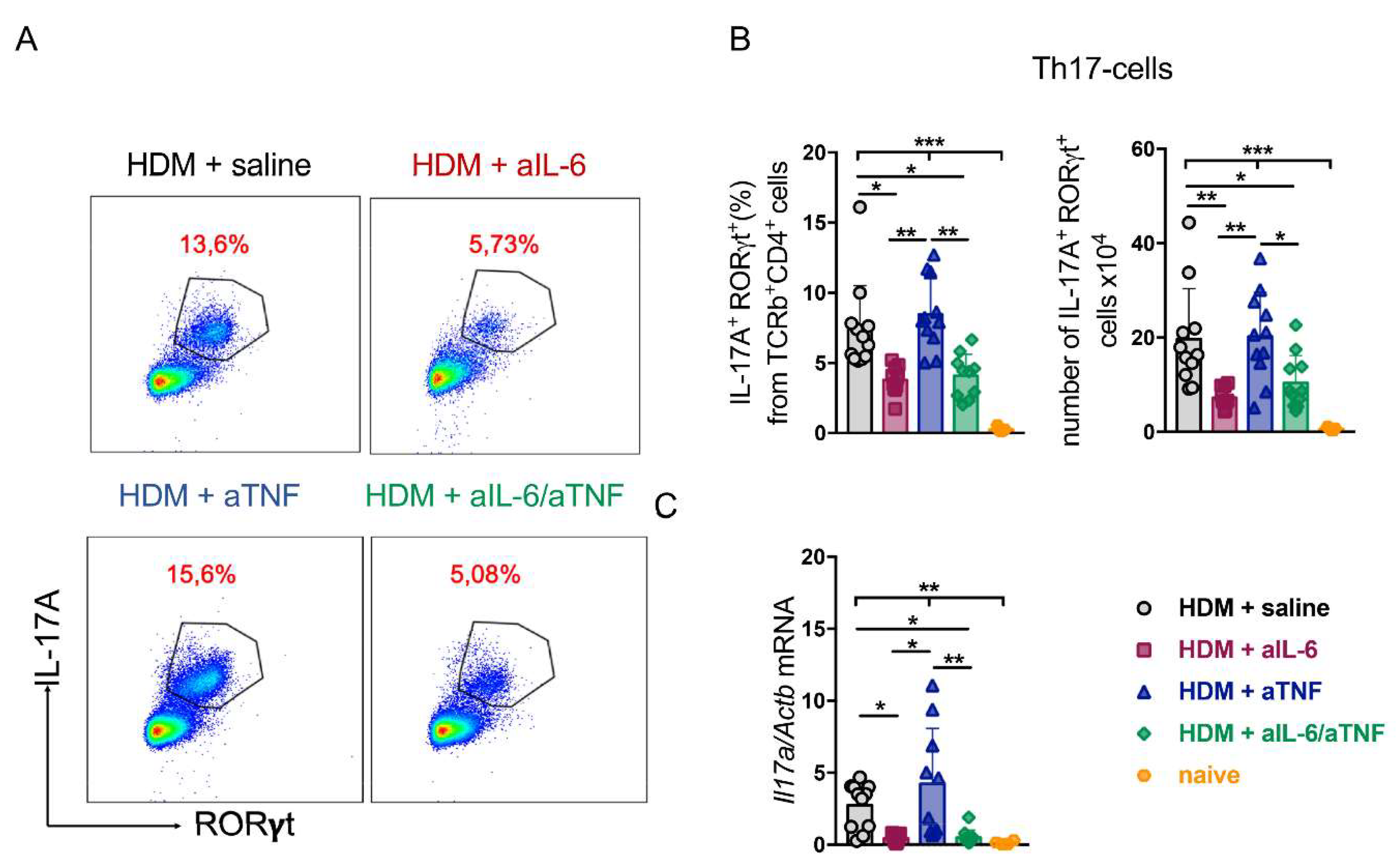
2.3. Unlike Anti-TNF Monotherapy, Anti-IL-6 Treatment and Combined Pharmacological Inhibition of TNF and IL-6 Suppressed Th17-Response in the Lungs
2.4. Simultaneous TNF/IL-6 Inhibition Prevented Lung Tissue Remodeling in Severe HDM-Induced Asthma
3. Discussion
4. Materials and Methods
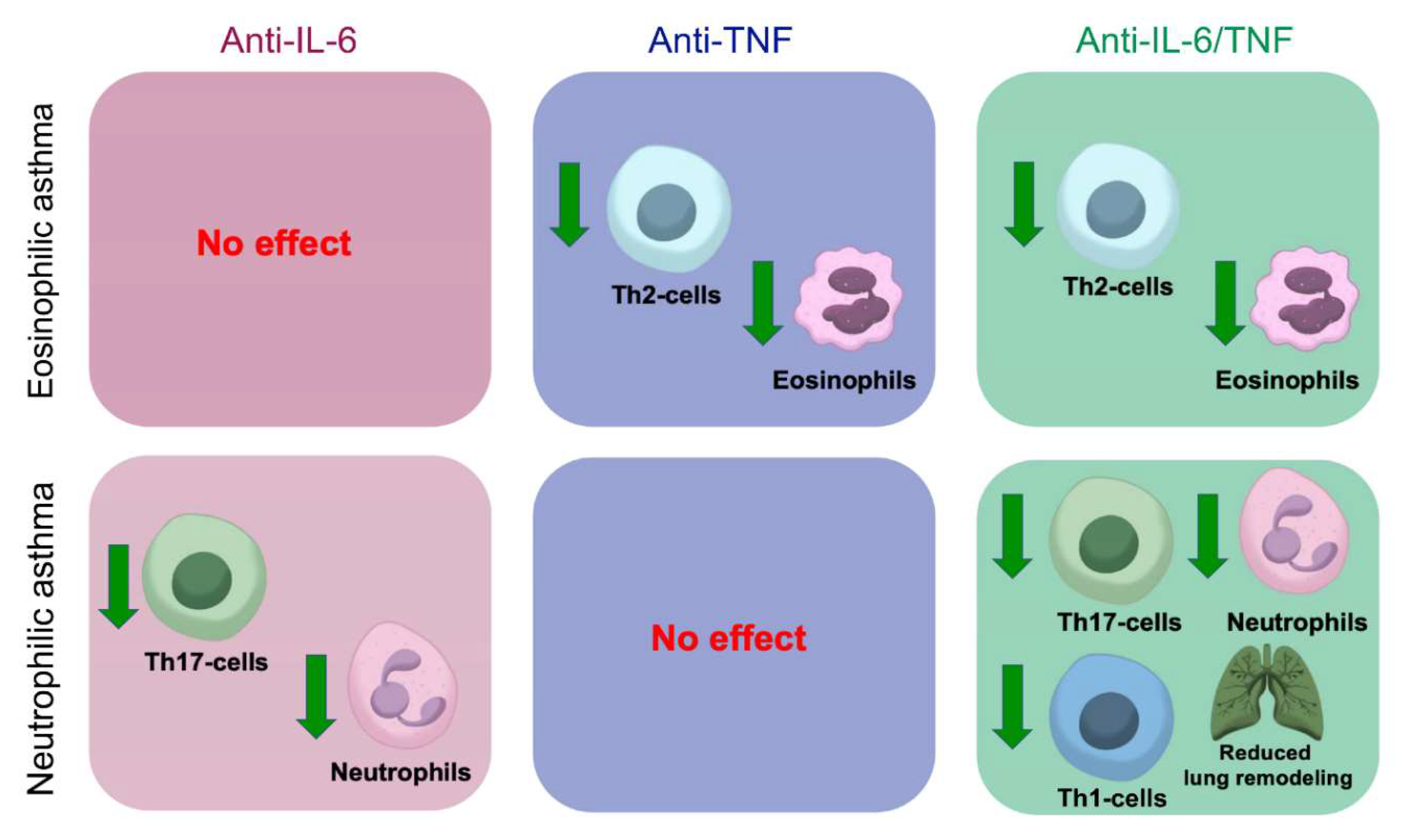
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rhee, H.; Love, T.; Harrington, D.; Grape, A. Common allergies in urban adolescents and their relationships with asthma control and healthcare utilization. Allergy Asthma Clin. Immunol. 2018, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T. Innate and adaptive immune responses in asthma. Nat. Med. 2012, 18, 673–683. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H.; Fahy, J.V. The Cytokines of Asthma. Immunity 2019, 50, 975–991. [Google Scholar] [CrossRef]
- Chesne, J.; Braza, F.; Mahay, G.; Brouard, S.; Aronica, M.; Magnan, A. IL-17 in severe asthma. Where do we stand? Am. J. Respir. Crit. Care Med. 2014, 190, 1094–1101. [Google Scholar] [CrossRef]
- Liles, W.C.; Dale, D.C.; Klebanoff, S.J. Glucocorticoids inhibit apoptosis of human neutrophils. Blood 1995, 86, 3181–3188. [Google Scholar] [CrossRef] [Green Version]
- Saffar, A.S.; Ashdown, H.; Gounni, A.S. The molecular mechanisms of glucocorticoids-mediated neutrophil survival. Curr. Drug Targets 2011, 12, 556–562. [Google Scholar] [CrossRef] [Green Version]
- Rabe, K.F.; Nair, P.; Brusselle, G.; Maspero, J.F.; Castro, M.; Sher, L.; Zhu, H.; Hamilton, J.D.; Swanson, B.N.; Khan, A.; et al. Efficacy and Safety of Dupilumab in Glucocorticoid-Dependent Severe Asthma. N. Engl. J. Med. 2018, 378, 2475–2485. [Google Scholar] [CrossRef]
- Busse, W.W.; Maspero, J.F.; Rabe, K.F.; Papi, A.; Wenzel, S.E.; Ford, L.B.; Pavord, I.D.; Zhang, B.; Staudinger, H.; Pirozzi, G.; et al. Liberty Asthma QUEST: Phase 3 Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate Dupilumab Efficacy/Safety in Patients with Uncontrolled, Moderate-to-Severe Asthma. Adv. Ther. 2018, 35, 737–748. [Google Scholar] [CrossRef] [Green Version]
- Wenzel, S.; Ford, L.; Pearlman, D.; Spector, S.; Sher, L.; Skobieranda, F.; Wang, L.; Kirkesseli, S.; Rocklin, R.; Bock, B.; et al. Dupilumab in persistent asthma with elevated eosinophil levels. N. Engl. J. Med. 2013, 368, 2455–2466. [Google Scholar] [CrossRef]
- Niessen, N.M.; Gibson, P.G.; Baines, K.J.; Barker, D.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Jenkins, C.; et al. Sputum TNF markers are increased in neutrophilic and severe asthma and are reduced by azithromycin treatment. Allergy 2021, 76, 2090–2101. [Google Scholar] [CrossRef]
- Lukacs, N.W.; Strieter, R.M.; Chensue, S.W.; Widmer, M.; Kunkel, S.L. TNF-alpha mediates recruitment of neutrophils and eosinophils during airway inflammation. J. Immunol. 1995, 154, 5411–5417. [Google Scholar] [PubMed]
- Oyoshi, M.K.; Barthel, R.; Tsitsikov, E.N. TRAF1 regulates recruitment of lymphocytes and, to a lesser extent, neutrophils, myeloid dendritic cells and monocytes to the lung airways following lipopolysaccharide inhalation. Immunology 2007, 120, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Beutler, B.; Keppler, D. Tumor necrosis factor alpha stimulates leukotriene production in vivo. Eur. J. Immunol. 1988, 18, 2085–2088. [Google Scholar] [CrossRef] [PubMed]
- Amrani, Y.; Chen, H.; Panettieri, R.A., Jr. Activation of tumor necrosis factor receptor 1 in airway smooth muscle: A potential pathway that modulates bronchial hyper-responsiveness in asthma? Respir. Res. 2000, 1, 49–53. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.L.; Zhang, R.G.; Chen, F.Y.; Qiu, Z.E.; Chen, L.; Huang, Z.X.; Huang, J.; Zhu, Y.X.; Zhao, L.; Zhou, W.L. Cellular Mechanism Underlying the Facilitation of Contractile Response Induced by Tumor Necrosis Factor-alpha in Mouse Tracheal Smooth Muscle. Am. J. Pathol. 2022, 192, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Lechner, A.; Henkel, F.D.R.; Hartung, F.; Bohnacker, S.; Alessandrini, F.; Gubernatorova, E.O.; Drutskaya, M.S.; Angioni, C.; Schreiber, Y.; Haimerl, P.; et al. Macrophages acquire a TNF-dependent inflammatory memory in allergic asthma. J. Allergy Clin. Immunol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E.; Barnes, P.J.; Bleecker, E.R.; Bousquet, J.; Busse, W.; Dahlen, S.E.; Holgate, S.T.; Meyers, D.A.; Rabe, K.F.; Antczak, A.; et al. A randomized, double-blind, placebo-controlled study of tumor necrosis factor-alpha blockade in severe persistent asthma. Am. J. Respir. Crit. Care Med. 2009, 179, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Keane, J.; Gershon, S.; Wise, R.P.; Mirabile-Levens, E.; Kasznica, J.; Schwieterman, W.D.; Siegel, J.N.; Braun, M.M. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 2001, 345, 1098–1104. [Google Scholar] [CrossRef]
- Wolfe, F.; Michaud, K. Lymphoma in rheumatoid arthritis: The effect of methotrexate and anti-tumor necrosis factor therapy in 18,572 patients. Arthritis Rheum. 2004, 50, 1740–1751. [Google Scholar] [CrossRef]
- Korn, T.; Hiltensperger, M. Role of IL-6 in the commitment of T cell subsets. Cytokine 2021, 146, 155654. [Google Scholar] [CrossRef]
- Wei, Q.; Liao, J.; Jiang, M.; Liu, J.; Liang, X.; Nong, G. Relationship between Th17-mediated immunity and airway inflammation in childhood neutrophilic asthma. Allergy Asthma Clin. Immunol. 2021, 17, 4. [Google Scholar] [CrossRef]
- Xie, Y.; Abel, P.W.; Casale, T.B.; Tu, Y. TH17 cells and corticosteroid insensitivity in severe asthma. J. Allergy Clin. Immunol. 2022, 149, 467–479. [Google Scholar] [CrossRef]
- Chu, D.K.; Al-Garawi, A.; Llop-Guevara, A.; Pillai, R.A.; Radford, K.; Shen, P.; Walker, T.D.; Goncharova, S.; Calhoun, W.J.; Nair, P.; et al. Therapeutic potential of anti-IL-6 therapies for granulocytic airway inflammation in asthma. Allergy Asthma Clin. Immunol. 2015, 11, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bissonnette, E.Y.; Lauzon-Joset, J.F.; Debley, J.S.; Ziegler, S.F. Cross-Talk Between Alveolar Macrophages and Lung Epithelial Cells is Essential to Maintain Lung Homeostasis. Front. Immunol. 2020, 11, 583042. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.R.; Sur, S. IgE and eosinophils as therapeutic targets in asthma. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 42–49. [Google Scholar] [CrossRef]
- Ozdemir, C.; Kucuksezer, U.C.; Akdis, M.; Akdis, C.A. The concepts of asthma endotypes and phenotypes to guide current and novel treatment strategies. Expert Rev. Respir. Med. 2018, 12, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Halwani, R.; Sultana, A.; Vazquez-Tello, A.; Jamhawi, A.; Al-Masri, A.A.; Al-Muhsen, S. Th-17 regulatory cytokines IL-21, IL-23, and IL-6 enhance neutrophil production of IL-17 cytokines during asthma. J. Asthma 2017, 54, 893–904. [Google Scholar] [CrossRef]
- Ray, A.; Kolls, J.K. Neutrophilic Inflammation in Asthma and Association with Disease Severity. Trends Immunol. 2017, 38, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.T.; Hagner, S.; Ruchti, F.; Radzikowska, U.; Tan, G.; Altunbulakli, C.; Eljaszewicz, A.; Moniuszko, M.; Akdis, M.; Akdis, C.A.; et al. Tight junction, mucin, and inflammasome-related molecules are differentially expressed in eosinophilic, mixed, and neutrophilic experimental asthma in mice. Allergy 2019, 74, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.E.; Ferris, M.; Pociask, D.; Brody, A.R. Tumor necrosis factor-alpha induces transforming growth factor-beta1 expression in lung fibroblasts through the extracellular signal-regulated kinase pathway. Am. J. Respir. Cell Mol. Biol. 2005, 32, 342–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, M.; Li, H.; Sun, L.; Brigstock, D.R.; Gao, R. Interleukin-6 participates in human pancreatic stellate cell activation and collagen I production via TGF-beta1/Smad pathway. Cytokine 2021, 143, 155536. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F. Diagnosis and Management of Severe Asthma. Semin. Respir. Crit. Care Med. 2018, 39, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Brightling, C.E.; Gupta, S.; Hollins, F.; Sutcliffe, A.; Amrani, Y. Immunopathogenesis of severe asthma. Curr. Pharm. Des. 2011, 17, 667–673. [Google Scholar] [CrossRef]
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014, 43, 343–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gubernatorova, E.O.; Namakanova, O.A.; Gorshkova, E.A.; Medvedovskaya, A.D.; Nedospasov, S.A.; Drutskaya, M.S. Novel Anti-Cytokine Strategies for Prevention and Treatment of Respiratory Allergic Diseases. Front. Immunol. 2021, 12, 601842. [Google Scholar] [CrossRef] [PubMed]
- Albers, F.C.; Papi, A.; Taille, C.; Bratton, D.J.; Bradford, E.S.; Yancey, S.W.; Kwon, N. Mepolizumab reduces exacerbations in patients with severe eosinophilic asthma, irrespective of body weight/body mass index: Meta-analysis of MENSA and MUSCA. Respir. Res. 2019, 20, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjermer, L.; Lemiere, C.; Maspero, J.; Weiss, S.; Zangrilli, J.; Germinaro, M. Reslizumab for Inadequately Controlled Asthma With Elevated Blood Eosinophil Levels: A Randomized Phase 3 Study. Chest 2016, 150, 789–798. [Google Scholar] [CrossRef] [Green Version]
- Chipps, B.E.; Hirsch, I.; Trudo, F.; Alacqua, M.; Zangrilli, J.G. Benralizumab efficacy for patients with fixed airflow obstruction and severe, uncontrolled eosinophilic asthma. Ann. Allergy Asthma Immunol. 2020, 124, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Menzies-Gow, A.; Corren, J.; Bourdin, A.; Chupp, G.; Israel, E.; Wechsler, M.E.; Brightling, C.E.; Griffiths, J.M.; Hellqvist, A.; Bowen, K.; et al. Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma. N. Engl. J. Med. 2021, 384, 1800–1809. [Google Scholar] [CrossRef]
- Whitehead, G.S.; Thomas, S.Y.; Shalaby, K.H.; Nakano, K.; Moran, T.P.; Ward, J.M.; Flake, G.P.; Nakano, H.; Cook, D.N. TNF is required for TLR ligand-mediated but not protease-mediated allergic airway inflammation. J. Clin. Invest. 2017, 127, 3313–3326. [Google Scholar] [CrossRef] [Green Version]
- Berry, M.A.; Hargadon, B.; Shelley, M.; Parker, D.; Shaw, D.E.; Green, R.H.; Bradding, P.; Brightling, C.E.; Wardlaw, A.J.; Pavord, I.D. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N. Engl. J. Med. 2006, 354, 697–708. [Google Scholar] [CrossRef] [Green Version]
- Holgate, S.T.; Wenzel, S.; Postma, D.S.; Weiss, S.T.; Renz, H.; Sly, P.D. Asthma. Nat. Rev. Dis. Primers. 2015, 1, 15025. [Google Scholar] [CrossRef]
- Erin, E.M.; Leaker, B.R.; Nicholson, G.C.; Tan, A.J.; Green, L.M.; Neighbour, H.; Zacharasiewicz, A.S.; Turner, J.; Barnathan, E.S.; Kon, O.M.; et al. The effects of a monoclonal antibody directed against tumor necrosis factor-alpha in asthma. Am. J. Respir. Crit. Care Med. 2006, 174, 753–762. [Google Scholar] [CrossRef]
- Taille, C.; Poulet, C.; Marchand-Adam, S.; Borie, R.; Dombret, M.C.; Crestani, B.; Aubier, M. Monoclonal Anti-TNF-alpha Antibodies for Severe Steroid-Dependent Asthma: A Case Series. Open Respir. Med. J. 2013, 7, 21–25. [Google Scholar] [CrossRef]
- Flynn, J.L.; Goldstein, M.M.; Chan, J.; Triebold, K.J.; Pfeffer, K.; Lowenstein, C.J.; Schreiber, R.; Mak, T.W.; Bloom, B.R. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 1995, 2, 561–572. [Google Scholar] [CrossRef] [Green Version]
- Deveci, F.; Muz, M.H.; Ilhan, N.; Kirkil, G.; Turgut, T.; Akpolat, N. Evaluation of the anti-inflammatory effect of infliximab in a mouse model of acute asthma. Respirology 2008, 13, 488–497. [Google Scholar] [CrossRef]
- Kim, J.; McKinley, L.; Natarajan, S.; Bolgos, G.L.; Siddiqui, J.; Copeland, S.; Remick, D.G. Anti-tumor necrosis factor-alpha antibody treatment reduces pulmonary inflammation and methacholine hyper-responsiveness in a murine asthma model induced by house dust. Clin. Exp. Allergy 2006, 36, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Busse, P.J.; Zhang, T.F.; Schofield, B.; Kilaru, S.; Patil, S.; Li, X.M. Decrease in airway mucous gene expression caused by treatment with anti-tumor necrosis factor alpha in a murine model of allergic asthma. Ann. Allergy Asthma Immunol. 2009, 103, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Gubernatorova, E.O.; Gorshkova, E.A.; Polinova, A.I.; Drutskaya, M.S. IL-6: Relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. 2020, 53, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Dienz, O.; Rincon, M. The effects of IL-6 on CD4 T cell responses. Clin. Immunol. 2009, 130, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Ochando, J.; Yopp, A.; Bromberg, J.S.; Ding, Y. IL-6 plays a unique role in initiating c-Maf expression during early stage of CD4 T cell activation. J. Immunol. 2005, 174, 2720–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, D.C.; Restifo, N.P. Suppressors of cytokine signaling (SOCS) in T cell differentiation, maturation, and function. Trends Immunol. 2009, 30, 592–602. [Google Scholar] [CrossRef] [Green Version]
- Kimura, A.; Kishimoto, T. IL-6: Regulator of Treg/Th17 balance. Eur. J. Immunol. 2010, 40, 1830–1835. [Google Scholar] [CrossRef] [PubMed]
- Gubernatorova, E.O.; Gorshkova, E.A.; Namakanova, O.A.; Zvartsev, R.V.; Hidalgo, J.; Drutskaya, M.S.; Tumanov, A.V.; Nedospasov, S.A. Non-redundant Functions of IL-6 Produced by Macrophages and Dendritic Cells in Allergic Airway Inflammation. Front. Immunol. 2018, 9, 2718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, M.B.; Deshpande, D.A.; Chou, J.; Cui, W.; Smith, S.; Langefeld, C.; Hastie, A.T.; Bleecker, E.R.; Hawkins, G.A. IL-6 trans-signaling increases expression of airways disease genes in airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Esty, B.; Harb, H.; Bartnikas, L.M.; Charbonnier, L.M.; Massoud, A.H.; Leon-Astudillo, C.; Visner, G.; Subramaniam, M.; Phipatanakul, W.; Chatila, T.A. Treatment of severe persistent asthma with IL-6 receptor blockade. J. Allergy Clin. Immunol. Pract. 2019, 7, 1639–1642.e4. [Google Scholar] [CrossRef]
- Revez, J.A.; Bain, L.M.; Watson, R.M.; Towers, M.; Collins, T.; Killian, K.J.; O’Byrne, P.M.; Gauvreau, G.M.; Upham, J.W.; Ferreira, M.A. Effects of interleukin-6 receptor blockade on allergen-induced airway responses in mild asthmatics. Clin. Transl. Immunol. 2019, 8, 1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busse, W.W.; Brusselle, G.G.; Korn, S.; Kuna, P.; Magnan, A.; Cohen, D.; Bowen, K.; Piechowiak, T.; Wang, M.M.; Colice, G. Tralokinumab did not demonstrate oral corticosteroid-sparing effects in severe asthma. Eur. Respir. J. 2019, 53, 1800948. [Google Scholar] [CrossRef]
- Hanania, N.A.; Korenblat, P.; Chapman, K.R.; Bateman, E.D.; Kopecky, P.; Paggiaro, P.; Yokoyama, A.; Olsson, J.; Gray, S.; Holweg, C.T.; et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): Replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir. Med. 2016, 4, 781–796. [Google Scholar] [CrossRef]
- Steinke, J.W. Anti-interleukin-4 therapy. Immunol. Allergy Clin. N. Am. 2004, 24, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.L.; Leigh, R. Use of biologicals as immunotherapy in asthma and related diseases. Expert Rev. Clin. Immunol. 2008, 4, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Leon, L.R.; White, A.A.; Kluger, M.J. Role of IL-6 and TNF in thermoregulation and survival during sepsis in mice. Am. J. Physiol. 1998, 275, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Kern, L.; Mittenbuhler, M.J.; Vesting, A.J.; Ostermann, A.L.; Wunderlich, C.M.; Wunderlich, F.T. Obesity-Induced TNFalpha and IL-6 Signaling: The Missing Link between Obesity and Inflammation-Driven Liver and Colorectal Cancers. Cancers Basel 2018, 11, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genovese, M.C.; Burmester, G.R.; Hagino, O.; Thangavelu, K.; Iglesias-Rodriguez, M.; John, G.S.; Gonzalez-Gay, M.A.; Mandrup-Poulsen, T.; Fleischmann, R. Interleukin-6 receptor blockade or TNFalpha inhibition for reducing glycaemia in patients with RA and diabetes: Post hoc analyses of three randomised, controlled trials. Arthritis Res. Ther. 2020, 22, 206. [Google Scholar] [CrossRef]
- Tarentini, E.; Odorici, G.; Righi, V.; Paganelli, A.; Giacomelli, L.; Mirisola, V.; Mucci, A.; Benassi, L.; D’Aversa, E.; Lasagni, C.; et al. Integrated metabolomic analysis and cytokine profiling define clusters of immuno-metabolic correlation in new-onset psoriasis. Sci. Rep. 2021, 11, 10472. [Google Scholar] [CrossRef]
- Shen, H.; Goldstein, D.R. IL-6 and TNF-alpha synergistically inhibit allograft acceptance. J. Am. Soc. Nephrol. 2009, 20, 1032–1040. [Google Scholar] [CrossRef] [Green Version]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-alpha and IFN-gamma triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. BioRxiv 2020, 184, 149–168. [Google Scholar] [CrossRef]
- Brebner, K.; Hayley, S.; Zacharko, R.; Merali, Z.; Anisman, H. Synergistic effects of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha: Central monoamine, corticosterone, and behavioral variations. Neuropsychopharmacology 2000, 22, 566–580. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Y.; Giannopoulou, E.G.; Chan, C.H.; Park, S.H.; Gong, S.; Chen, J.; Hu, X.; Elemento, O.; Ivashkiv, L.B. Synergistic activation of inflammatory cytokine genes by interferon-gamma-induced chromatin remodeling and toll-like receptor signaling. Immunity 2013, 39, 454–469. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, C.M.; Hessel, E.M. Functions of T cells in asthma: More than just T(H)2 cells. Nat. Rev. Immunol. 2010, 10, 838–848. [Google Scholar] [CrossRef]
- Cosmi, L.; Liotta, F.; Maggi, E.; Romagnani, S.; Annunziato, F. Th17 cells: New players in asthma pathogenesis. Allergy 2011, 66, 989–998. [Google Scholar] [CrossRef]
- Choy, D.F.; Hart, K.M.; Borthwick, L.A.; Shikotra, A.; Nagarkar, D.R.; Siddiqui, S.; Jia, G.; Ohri, C.M.; Doran, E.; Vannella, K.M.; et al. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci. Transl. Med. 2015, 7, 301ra129. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Zhou, Y.; Bao, W.; Fu, Q.; Hao, H.; Han, L.; Zhang, X.; Tian, X.; Zhang, M. STAT3 and IL-6 Contribute to Corticosteroid Resistance in an OVA and Ozone-induced Asthma Model with Neutrophil Infiltration. Front. Mol. Biosci. 2021, 8, 717962. [Google Scholar] [CrossRef] [PubMed]
- Ritter, K.; Sodenkamp, J.C.; Holscher, A.; Behrends, J.; Holscher, C. IL-6 is not Absolutely Essential for the Development of a TH17 Immune Response after an Aerosol Infection with Mycobacterium Tuberculosis H37rv. Cells 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, Z.; Deng, Y.; Zha, S.; Yu, L.; Li, D.; Liang, Z.; Yang, K.; Liu, S.; Chen, R. Prevention of IL-6 signaling ameliorates toluene diisocyanate-induced steroid-resistant asthma. Allergol. Int. 2022, 71, 73–82. [Google Scholar] [CrossRef]
- Urbano, P.C.M.; Aguirre-Gamboa, R.; Ashikov, A.; van Heeswijk, B.; Krippner-Heidenreich, A.; Tijssen, H.; Li, Y.; Azevedo, V.F.; Smits, L.J.T.; Hoentjen, F.; et al. TNF-alpha-induced protein 3 (TNFAIP3)/A20 acts as a master switch in TNF-alpha blockade-driven IL-17A expression. J. Allergy Clin. Immunol. 2018, 142, 517–529. [Google Scholar] [CrossRef] [Green Version]
- Hough, K.P.; Curtiss, M.L.; Blain, T.J.; Liu, R.M.; Trevor, J.; Deshane, J.S.; Thannickal, V.J. Airway Remodeling in Asthma. Front. Med. Lausanne 2020, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Mauad, T.; Bel, E.H.; Sterk, P.J. Asthma therapy and airway remodeling. J. Allergy Clin. Immunol. 2007, 120, 997–1009. [Google Scholar] [CrossRef]
- Broide, D.H. Immunologic and inflammatory mechanisms that drive asthma progression to remodeling. J. Allergy Clin. Immunol. 2008, 121, 560–570. [Google Scholar] [CrossRef] [Green Version]
- Halwani, R.; Al-Muhsen, S.; Al-Jahdali, H.; Hamid, Q. Role of transforming growth factor-beta in airway remodeling in asthma. Am. J. Respir. Cell Mol. Biol. 2011, 44, 127–133. [Google Scholar] [CrossRef]
- Doherty, T.; Broide, D. Cytokines and growth factors in airway remodeling in asthma. Curr. Opin. Immunol. 2007, 19, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Reeves, S.R.; Kolstad, T.; Lien, T.Y.; Elliott, M.; Ziegler, S.F.; Wight, T.N.; Debley, J.S. Asthmatic airway epithelial cells differentially regulate fibroblast expression of extracellular matrix components. J. Allergy Clin. Immunol. 2014, 134, 663–670.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, D.E. The role of the epithelium in airway remodeling in asthma. Proc. Am. Thorac. Soc. 2009, 6, 678–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammit, A.J.; Moir, L.M.; Oliver, B.G.; Hughes, J.M.; Alkhouri, H.; Ge, Q.; Burgess, J.K.; Black, J.L.; Roth, M. Effect of IL-6 trans-signaling on the pro-remodeling phenotype of airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, 199–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMillan, S.J.; Xanthou, G.; Lloyd, C.M. Manipulation of allergen-induced airway remodeling by treatment with anti-TGF-beta antibody: Effect on the Smad signaling pathway. J. Immunol. 2005, 174, 5774–5780. [Google Scholar] [CrossRef] [Green Version]
- Frangogiannis, N. Transforming growth factor-beta in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef]
- Epstein Shochet, G.; Brook, E.; Bardenstein-Wald, B.; Shitrit, D. TGF-beta pathway activation by idiopathic pulmonary fibrosis (IPF) fibroblast derived soluble factors is mediated by IL-6 trans-signaling. Respir. Res. 2020, 21, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakir, J.; Shannon, J.; Molet, S.; Fukakusa, M.; Elias, J.; Laviolette, M.; Boulet, L.P.; Hamid, Q. Airway remodeling-associated mediators in moderate to severe asthma: Effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J. Allergy Clin. Immunol. 2003, 111, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Hansel, T.T.; Kropshofer, H.; Singer, T.; Mitchell, J.A.; George, A.J. The safety and side effects of monoclonal antibodies. Nat. Rev. Drug Discov. 2010, 9, 325–338. [Google Scholar] [CrossRef]
- Efimov, G.A.; Kruglov, A.A.; Khlopchatnikova, Z.V.; Rozov, F.N.; Mokhonov, V.V.; Rose-John, S.; Scheller, J.; Gordon, S.; Stacey, M.; Drutskaya, M.S.; et al. Cell-type-restricted anti-cytokine therapy: TNF inhibition from one pathogenic source. Proc. Natl. Acad. Sci. USA 2016, 113, 3006–3011. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.C.; Zhu, Q.Q.; Li, J.; Liu, A.J.; Huang, G.R. Aerosol Inhalation-mediated Delivery of an Adeno-associated Virus 5-expressed Antagonistic Interleukin-4 Mutant Ameliorates Experimental Murine Asthma. Arch. Med. Res. 2019, 50, 384–392. [Google Scholar] [CrossRef]
- Nials, A.T.; Uddin, S. Mouse models of allergic asthma: Acute and chronic allergen challenge. Dis. Model. Mech. 2008, 1, 213–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gueders, M.M.; Paulissen, G.; Crahay, C.; Quesada-Calvo, F.; Hacha, J.; Van Hove, C.; Tournoy, K.; Louis, R.; Foidart, J.M.; Noel, A.; et al. Mouse models of asthma: A comparison between C57BL/6 and BALB/c strains regarding bronchial responsiveness, inflammation, and cytokine production. Inflamm. Res. 2009, 58, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Haspeslagh, E.; Debeuf, N.; Hammad, H.; Lambrecht, B.N. Murine Models of Allergic Asthma. Methods Mol. Biol. 2017, 1559, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Kim, H.; Bak, Y.; Shim, D.; Kwon, K.W.; Kim, C.H.; Yoon, J.H.; Shin, S.J. An Alternative Dendritic Cell-Induced Murine Model of Asthma Exhibiting a Robust Th2/Th17-Skewed Response. Allergy Asthma Immunol. Res. 2020, 12, 537–555. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namakanova, O.A.; Gorshkova, E.A.; Zvartsev, R.V.; Nedospasov, S.A.; Drutskaya, M.S.; Gubernatorova, E.O. Therapeutic Potential of Combining IL-6 and TNF Blockade in a Mouse Model of Allergic Asthma. Int. J. Mol. Sci. 2022, 23, 3521. https://doi.org/10.3390/ijms23073521
Namakanova OA, Gorshkova EA, Zvartsev RV, Nedospasov SA, Drutskaya MS, Gubernatorova EO. Therapeutic Potential of Combining IL-6 and TNF Blockade in a Mouse Model of Allergic Asthma. International Journal of Molecular Sciences. 2022; 23(7):3521. https://doi.org/10.3390/ijms23073521
Chicago/Turabian StyleNamakanova, Olga A., Ekaterina A. Gorshkova, Ruslan V. Zvartsev, Sergei A. Nedospasov, Marina S. Drutskaya, and Ekaterina O. Gubernatorova. 2022. "Therapeutic Potential of Combining IL-6 and TNF Blockade in a Mouse Model of Allergic Asthma" International Journal of Molecular Sciences 23, no. 7: 3521. https://doi.org/10.3390/ijms23073521
APA StyleNamakanova, O. A., Gorshkova, E. A., Zvartsev, R. V., Nedospasov, S. A., Drutskaya, M. S., & Gubernatorova, E. O. (2022). Therapeutic Potential of Combining IL-6 and TNF Blockade in a Mouse Model of Allergic Asthma. International Journal of Molecular Sciences, 23(7), 3521. https://doi.org/10.3390/ijms23073521




