Metallodrugs: Mechanisms of Action, Molecular Targets and Biological Activity
Funding
Conflicts of Interest
References
- Rosenberg, B. Chapter 2—Cisplatin: Its history and possible mechanisms of action. In Cisplatin: Current Status and New Developments; Prestayko, A.W., Crooke, S.T., Carter, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 1980; pp. 9–20. [Google Scholar]
- Florea, A.-M.; Büsselberg, D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef] [PubMed]
- Komeda, S.; Casini, A. Next-generation anticancer metallodrugs. Curr. Top. Med. Chem. 2012, 12, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.I.; Ooi, K.K.; Tiekink, E.R.T. Gold-based medicine: A paradigm shift in anti-cancer therapy? Molecules 2018, 23, 1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thota, S.; Rodrigues, D.A.; Crans, D.C.; Barreiro, E.J. Ru(II) compounds: Next-generation anticancer metallotherapeutics? J. Med. Chem. 2018, 61, 5805–5821. [Google Scholar] [CrossRef]
- Jamieson, E.R.; Lippard, S.J. Structure, recognition, and processing of cisplatin−DNA adducts. Chem. Rev. 1999, 99, 2467–2498. [Google Scholar] [CrossRef]
- Xiong, X.; Liu, L.-Y.; Mao, Z.-W.; Zou, T. Approaches towards understanding the mechanism-of-action of metallodrugs. Coord. Chem. Rev. 2022, 453, 214311. [Google Scholar] [CrossRef]
- Merlino, A. Recent advances in protein metalation:structural studies. Chem. Commun. 2021, 57, 1295–1307. [Google Scholar] [CrossRef]
- Marloye, M.; Berger, G.; Gelbcke, M.; Dufrasne, F. A survey of the mechanisms of action of anticancer transition metal complexes. Future Med. Chem. 2016, 18, 2263–2286. [Google Scholar] [CrossRef]
- Annunziata, A.; Liberti, D.; Bedini, E.; Cucciolito, M.E.; Loreto, D.; Monti, D.M.; Merlino, A.; Ruffo, F. Square-planar vs. trigonal bipyramidal geometry in Pt(II) complexes containing triazole-based glucose ligands as potential anticancer agents. Int. J. Mol. Sci. 2021, 22, 8704. [Google Scholar] [CrossRef]
- De Palo, A.; Draca, D.; Murrali, M.G.; Zacchini, S.; Pampaloni, G.; Mijatovic, S.; Maksimovic-Ivanic, D.; Marchetti, F. A comparative analysis of the in vitro anticancer activity of iridium(III) {η5-C5Me4R} complexes with variable R groups. Int. J. Mol. Sci. 2021, 22, 7422. [Google Scholar] [CrossRef]
- Vessières, A.; Quissac, E.; Lemaire, N.; Alentorn, A.; Domeracka, P.; Pigeon, P.; Sanson, M.; Idbaih, A.; Verreault, M. Heterogeneity of response to iron-based metallodrugs in glioblastoma is associated with differences in chemical structures and driven by fas expression dynamics and transcriptomic subtypes. Int. J. Mol. Sci. 2021, 22, 10404. [Google Scholar] [CrossRef] [PubMed]
- Butsch, K.; Haseloer, A.; Schmitz, S.; Ott, I.; Schur, J.; Klein, A. FeIII, CuII and ZnII complexes of the rigid 9-oxido-phenalenone ligand—Spectroscopy, electrochemistry, and cytotoxic properties. Int. J. Mol. Sci. 2021, 22, 3976. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, A.; Cucciolito, M.E.; Esposito, R.; Imbimbo, P.; Petruk, G.; Ferraro, G.; Pinto, V.; Tuzi, A.; Monti, D.M.; Merlino, A.; et al. A highly efficient and selective antitumor agent based on a glucoconjugated carbene platinum(II) complex. Dalton Trans. 2019, 48, 7794–7800. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, A.; Cucciolito, M.E.; Esposito, R.; Ferraro, G.; Monti, D.M.; Merlino, A.; Ruffo, F. Five-coordinate Pt(II) compounds as potential anticancer agents. Eur. J. Inorg. Chem. 2020, 11, 918–929. [Google Scholar] [CrossRef]
- Szefler, B.; Czeleń, P.; Krawczyk, P. The affinity of carboplatin to b-vitamins and nucleobases. Int. J. Mol. Sci. 2021, 22, 3634. [Google Scholar] [CrossRef]
- Anghel, N.; Müller, J.; Serricchio, M.; Jelk, J.; Bütikofer, P.; Boubaker, G.; Imhof, D.; Ramseier, J.; Desiatkina, O.; Păunescu, E.; et al. Cellular and molecular targets of nucleotide-tagged trithiolato-bridged arene ruthenium complexes in the protozoan parasites toxoplasma gondii and trypanosoma brucei. Int. J. Mol. Sci. 2021, 22, 10787. [Google Scholar] [CrossRef]
- Petruk, G.; Monti, D.M.; Ferraro, G.; Pica, A.; D’Elia, L.; Pane, F.; Amoresano, A.; Furrer, J.; Kowalski, K.; Merlino, A. Encapsulation of the dinuclear trithiolato-bridged arene ruthenium complex diruthenium-1 in an apoferritin nanocage: Structure and cytotoxicity. Chem. Med. Chem. 2019, 14, 594–602. [Google Scholar] [CrossRef]
- Monti, D.M.; Ferraro, G.; Merlino, A. Ferritin-based anticancer metallodrug delivery: Crystallographic, analytical and cytotoxicity studies. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 101997. [Google Scholar] [CrossRef]
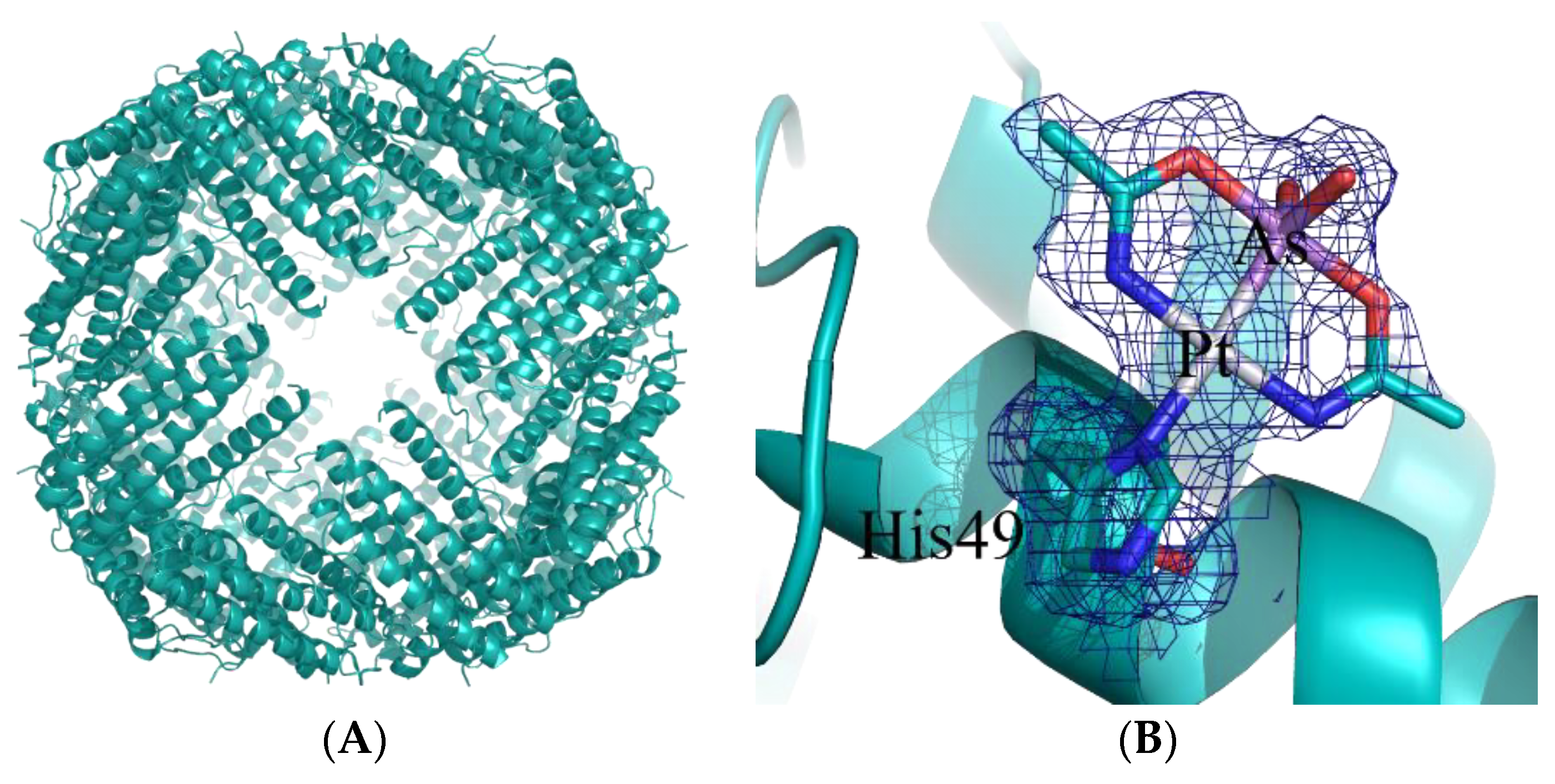
- Ferraro, G.; Pratesi, A.; Cirri, D.; Imbimbo, P.; Monti, D.M.; Messori, L.; Merlino, A. Arsenoplatin-ferritin nanocage: Structure and cytotoxicity. Int. J. Mol. Sci. 2021, 22, 1874. [Google Scholar] [CrossRef]
- Miodragović, Đ.U.; Quentzel, J.A.; Kurutz, J.W.; Stern, C.L.; Ahn, R.W.; Kandela, I.; Mazar, A.; O’Halloran, T.V. Robust structure and reactivity of aqueous arsenous acid–platinum (II) anticancer complexes. Angew. Chem. Int. Ed. 2013, 52, 10749–10752. [Google Scholar] [CrossRef] [Green Version]
- Miodragović, Đ.; Merlino, A.; Swindell, E.P.; Bogachkov, A.; Ahn, R.W.; Abuhadba, S.; Ferraro, G.; Marzo, T.; Mazar, A.P.; Messori, L.; et al. Arsenoplatin-1 is a dual pharmacophore anticancer agent. J. Am. Chem. Soc. 2019, 141, 6453–6457. [Google Scholar] [CrossRef] [PubMed]
- Loreto, D.; Ferraro, G.; Merlino, A. Unusual structural features in the adduct of dirhodium tetraacetate with lysozyme. Int. J. Mol. Sci. 2021, 22, 1496. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.; Woo, W.S. Effects of Rh2(O2CC2H5)4L2 on the replication of ehrlich tumor cells in vivo. Korean Biochem. J. 1976, 9, 175–180. [Google Scholar]
- Bear, J.L. Rhodium compounds for antitumor use. In Proceedings of 9th International Conference of the International Precious Metals Institute, New York, NY, USA; Zysk, E.D., Bonucci, J.A., Eds.; International Precious Metals Institute: Allentown, PA, USA, 1986; pp. 337–344. [Google Scholar]
- Erck, A.; Rainen, L.; Whileyman, J.; Chang, I.M.; Kimball, A.P.; Bear, J.L. Studies of rhodium(II) carboxylates as potential antitumor agents. Proc. Soc. Exp. Biol. Med. 1974, 145, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, G.G.; Pratesi, A.; Messori, L.; Merlino, A. Protein interactions of dirhodium tetraacetate:a structural study. Dalton Trans. 2020, 49, 2412–2416. [Google Scholar] [CrossRef]
- Loreto, D.; Merlino, A. The interaction of rhodium compounds with proteins: A structural overview. Coord. Chem. Rev. 2021, 442, 213999. [Google Scholar] [CrossRef]
- Messori, L.; Marzo, T.; Fernandes Sanches, R.N.; Rehman, H.-U.; de Oliveira Silva, D.; Merlino, A. Unusual structural features in the lysozyme derivative of tetrakis(acetato)chlorido diruthenium(II,III) complex. Angew. Chem. Int. Ed. 2014, 53, 6172–6175. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Abe, S.; Koshiyama, T.; Ohki, T.; Hikage, T.; Watanabe, Y. Elucidation of metal-ion accumulation induced by hydrogen bonds on protein surfaces by using porous lysozyme crystals containing RhIII ions as the model surfaces. Chem. Eur. J. 2010, 16, 2730–2740. [Google Scholar] [CrossRef]
- Manna, S.L.; Florio, D.; Iacobucci, I.; Napolitano, F.; Benedictis, I.D.; Malfitano, A.M.; Monti, M.; Ravera, M.; Gabano, E.; Marasco, D. A comparative study of the effects of platinum (II) complexes on β-amyloid aggregation: Potential neurodrug applications. Int. J. Mol. Sci. 2021, 22, 3015. [Google Scholar] [CrossRef]
- Shoghi-Jadid, K.; Small, G.W.; Agdeppa, E.D.; Kepe, V.; Ercoli, L.M.; Siddarth, P.; Read, S.; Satyamurthy, N.; Petric, A.; Huang, S.-C. Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with alzheimer disease. Am. J. Geriatr. Psychiatry 2002, 10, 24–35. [Google Scholar] [CrossRef]
- Tabbì, G.; Cucci, L.M.; Pinzino, C.; Munzone, A.; Marzo, T.; Pizzanelli, S.; Satriano, C.; Magrì, A.; La Mendola, D. Peptides Derived from angiogenin regulate cellular copper uptake. Int. J. Mol. Sci. 2021, 22, 9530. [Google Scholar] [CrossRef] [PubMed]
- Magrì, A.; La Mendola, D.; Rizzarelli, E. Nerve growth factor peptides bind copper(II) with high affinity: A thermodynamic approach to unveil overlooked neurotrophin roles. Int. J. Mol. Sci. 2021, 22, 5085. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Xu, Z. Three decades of research on angiogenin: A review and perspective. Acta Biochim. Biophys. Sin. 2016, 48, 399–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofroniew, M.V.; Howe, C.L.; Mobley, W.C. Nerve growth factor signaling, neuroprotection, and neural repair. Annu. Rev. Neurosci. 2001, 24, 1217–1281. [Google Scholar] [CrossRef]
- Marzo, T.; La Mendola, D. The effects on angiogenesis of relevant inorganic chemotherapeutics. Curr. Top. Med. Chem. 2021, 21, 73–86. [Google Scholar] [CrossRef]
- Cucci, L.M.; Satriano, C.; Marzo, T.; La Mendola, D. Angiogenin and copper crossing in wound healing. Int. J. Mol. Sci. 2021, 22, 10704. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraro, G.; Merlino, A. Metallodrugs: Mechanisms of Action, Molecular Targets and Biological Activity. Int. J. Mol. Sci. 2022, 23, 3504. https://doi.org/10.3390/ijms23073504
Ferraro G, Merlino A. Metallodrugs: Mechanisms of Action, Molecular Targets and Biological Activity. International Journal of Molecular Sciences. 2022; 23(7):3504. https://doi.org/10.3390/ijms23073504
Chicago/Turabian StyleFerraro, Giarita, and Antonello Merlino. 2022. "Metallodrugs: Mechanisms of Action, Molecular Targets and Biological Activity" International Journal of Molecular Sciences 23, no. 7: 3504. https://doi.org/10.3390/ijms23073504
APA StyleFerraro, G., & Merlino, A. (2022). Metallodrugs: Mechanisms of Action, Molecular Targets and Biological Activity. International Journal of Molecular Sciences, 23(7), 3504. https://doi.org/10.3390/ijms23073504





