Spectral Dependence of the Energy Transfer from Photosynthetic Complexes to Monolayer Graphene
Abstract
1. Introduction
2. Results
2.1. Spectroscopic Characterization of Materials
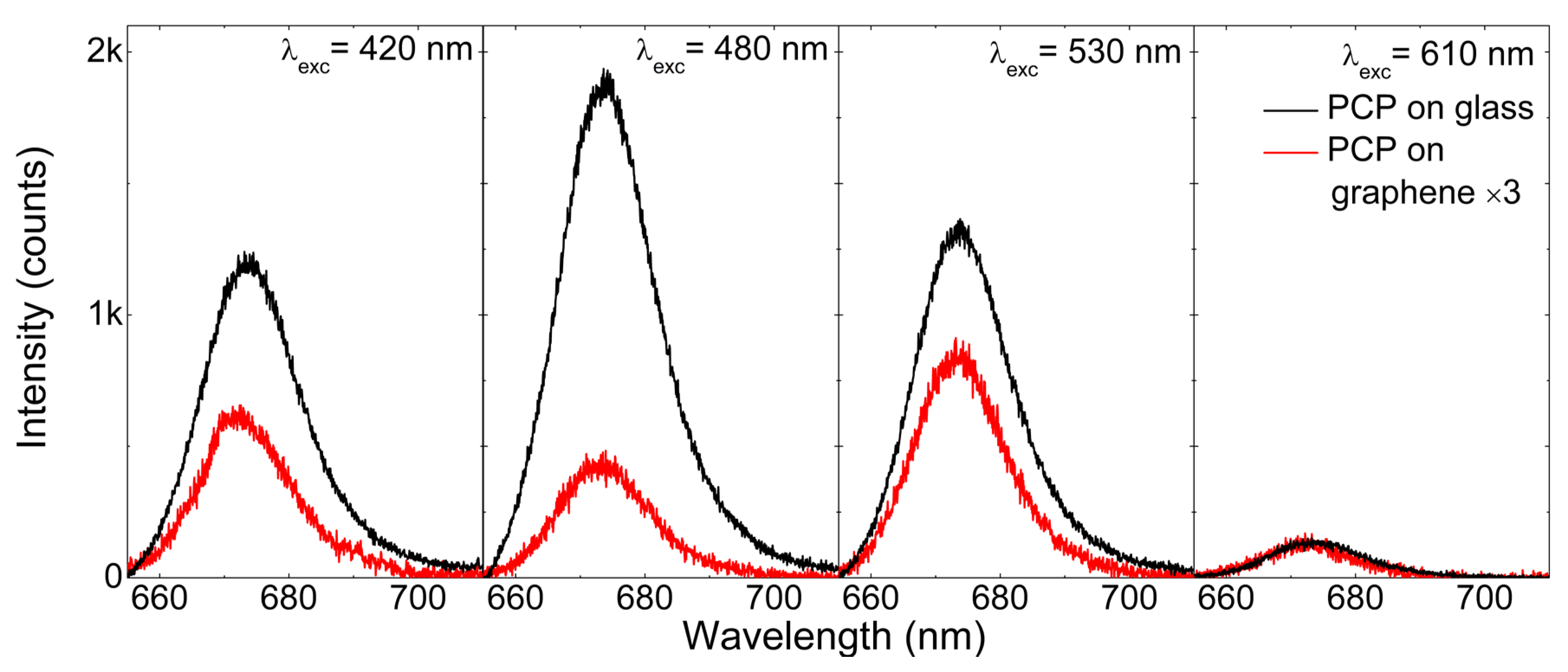
2.2. Excitation Wavelength Dependence of the PCP Emission
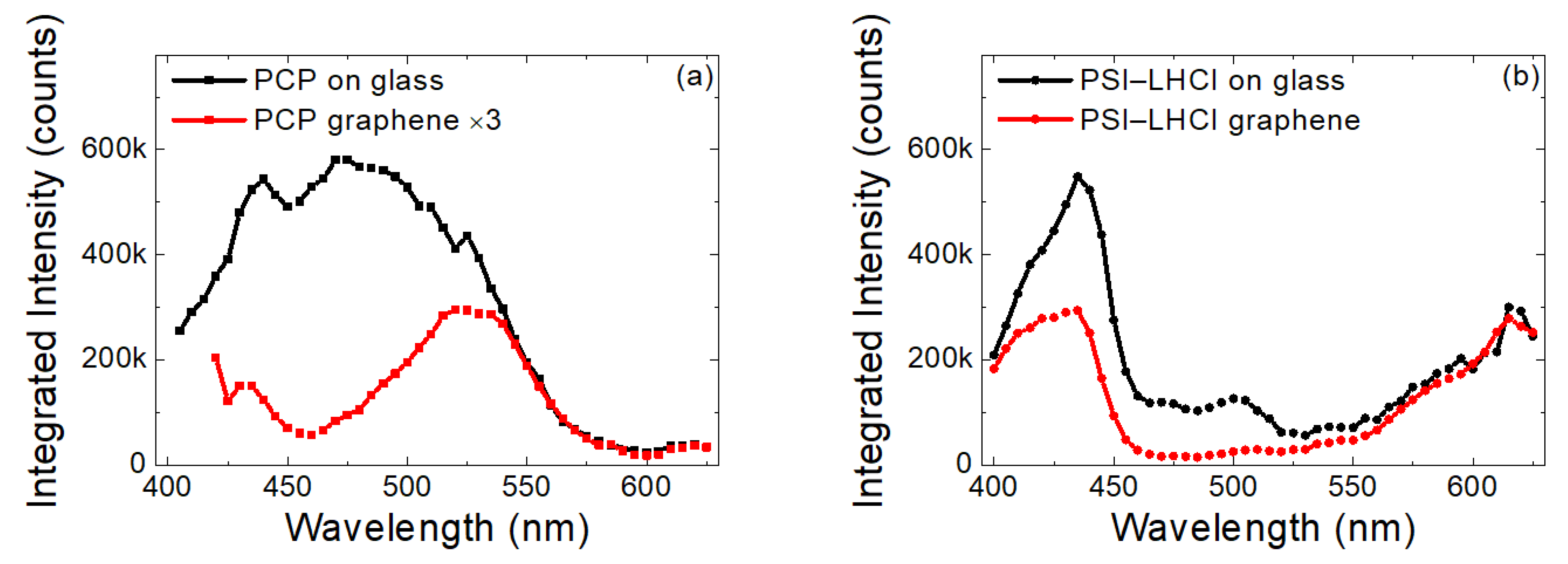
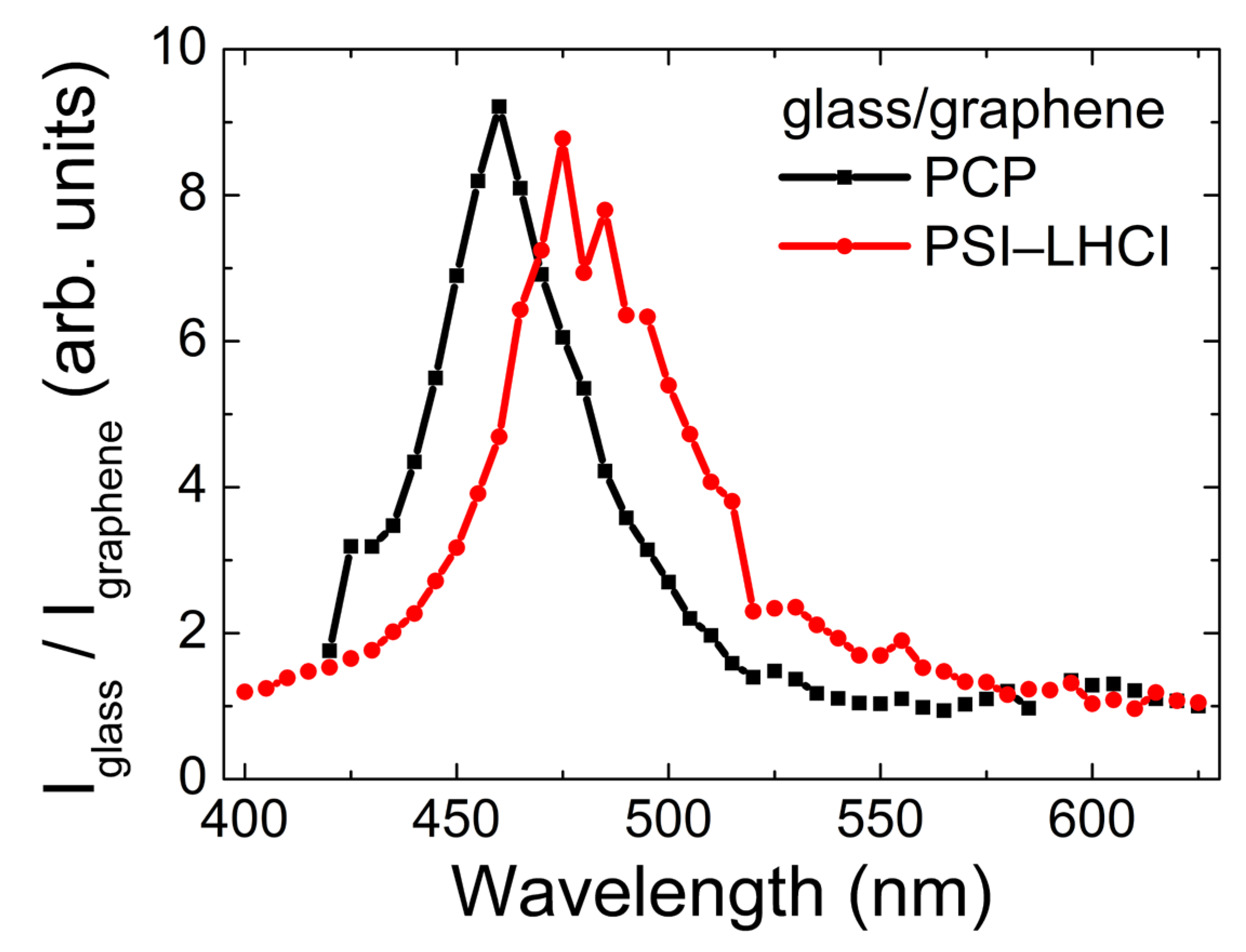
2.3. Excitation Wavelength Dependence of Emission Intensity of PCP and PSI–LHCI
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine Structure Constant Defines Visual Transparency of Graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [PubMed]
- Sprinkle, M.; Siegel, D.; Hu, Y.; Hicks, J.; Tejeda, A.; Taleb-Ibrahimi, A.; Le Fèvre, P.; Bertran, F.; Vizzini, S.; Enriquez, H.; et al. First Direct Observation of a Nearly Ideal Graphene Band Structure. Phys. Rev. Lett. 2009, 103, 226803. [Google Scholar] [CrossRef] [PubMed]
- Gaudreau, L.; Tielrooij, K.J.; Prawiroatmodjo, G.E.D.K.; Osmond, J.; de Abajo, F.J.G.; Koppens, F.H.L. Universal Distance-Scaling of Nonradiative Energy Transfer to Graphene. Nano Lett. 2013, 13, 2030–2035. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Berciaud, S.; Nuckolls, C.; Heinz, T.F.; Brus, L.E. Energy Transfer from Individual Semiconductor Nanocrystals to Graphene. ACS Nano 2010, 4, 2964–2968. [Google Scholar] [CrossRef]
- Swathi, R.S.; Sebastian, K.L. Long Range Resonance Energy Transfer from a Dye Molecule to Graphene Has (Distance)−4 Dependence. J. Chem. Phys. 2009, 130, 086101. [Google Scholar] [CrossRef]
- Kaminska, I.; Wiwatowski, K.; Mackowski, S. Efficiency of Energy Transfer Decreases with the Number of Graphene Layers. RSC Adv. 2016, 6, 102791–102796. [Google Scholar] [CrossRef]
- Mackowski, S.; Kamińska, I. Dependence of the Energy Transfer to Graphene on the Excitation Energy. Appl. Phys. Lett. 2015, 107, 023110. [Google Scholar] [CrossRef]
- Federspiel, F.; Froehlicher, G.; Nasilowski, M.; Pedetti, S.; Mahmood, A.; Doudin, B.; Park, S.; Lee, J.-O.; Halley, D.; Dubertret, B.; et al. Distance Dependence of the Energy Transfer Rate from a Single Semiconductor Nanostructure to Graphene. Nano Lett. 2015, 15, 1252–1258. [Google Scholar] [CrossRef]
- Blankenship, R.E.; Tiede, D.M.; Barber, J.; Brudvig, G.W.; Fleming, G.; Ghirardi, M.; Gunner, M.R.; Junge, W.; Kramer, D.M.; Melis, A.; et al. Comparing Photosynthetic and Photovoltaic Efficiencies and Recognizing the Potential for Improvement. Science 2011, 332, 805–809. [Google Scholar] [CrossRef]
- Cogdell, R. Can Photosynthesis Provide a “biological Blueprint” for the Design of Novel Solar Cells? Trends Biotechnol. 1998, 16, 521–527. [Google Scholar] [CrossRef]
- Mackowski, S. Hybrid Nanostructures for Efficient Light Harvesting. J. Phys. Condens. Matter 2010, 22, 193102. [Google Scholar] [CrossRef]
- Kargul, J.; Janna Olmos, J.D.; Krupnik, T. Structure and Function of Photosystem I and Its Application in Biomimetic Solar-to-Fuel Systems. J. Plant Physiol. 2012, 169, 1639–1653. [Google Scholar] [CrossRef]
- Janna Olmos, J.D.; Kargul, J. A Quest for the Artificial Leaf. Int. J. Biochem. Cell Biol. 2015, 66, 37–44. [Google Scholar] [CrossRef]
- Janna Olmos, J.D.; Kargul, J. Oxygenic Photosynthesis: Translation to Solar Fuel Technologies. Acta Soc. Bot. Pol. 2014, 83, 423–440. [Google Scholar] [CrossRef][Green Version]
- LeBlanc, G.; Winter, K.M.; Crosby, W.B.; Jennings, G.K.; Cliffel, D.E. Integration of Photosystem I with Graphene Oxide for Photocurrent Enhancement. Adv. Energy Mater. 2014, 4, 1301953. [Google Scholar] [CrossRef]
- LeBlanc, G.; Gizzie, E.; Yang, S.; Cliffel, D.E.; Jennings, G.K. Photosystem I Protein Films at Electrode Surfaces for Solar Energy Conversion. Langmuir 2014, 30, 10990–11001. [Google Scholar] [CrossRef]
- Kiliszek, M.; Harputlu, E.; Szalkowski, M.; Kowalska, D.; Unlu, C.G.; Haniewicz, P.; Abram, M.; Wiwatowski, K.; Niedziółka-Jönsson, J.; Maćkowski, S.; et al. Orientation of Photosystem I on Graphene through Cytochrome C553 Leads to Improvement in Photocurrent Generation. J. Mater. Chem. A 2018, 6, 18615–18626. [Google Scholar] [CrossRef]
- Szalkowski, M.; Harputlu, E.; Kiliszek, M.; Unlu, C.G.; Maćkowski, S.; Ocakoglu, K.; Kargul, J.; Kowalska, D. Plasmonic Enhancement of Photocurrent Generation in a Photosystem I-Based Hybrid Electrode. J. Mater. Chem. C 2020, 8, 5807–5814. [Google Scholar] [CrossRef]
- Ocakoglu, K.; Krupnik, T.; van den Bosch, B.; Harputlu, E.; Gullo, M.P.; Olmos, J.D.J.; Yildirimcan, S.; Gupta, R.K.; Yakuphanoglu, F.; Barbieri, A.; et al. Photosystem I-Based Biophotovoltaics on Nanostructured Hematite. Adv. Funct. Mater. 2014, 24, 7467–7477. [Google Scholar] [CrossRef]
- Gizzie, E.A.; LeBlanc, G.; Jennings, G.K.; Cliffel, D.E. Electrochemical Preparation of Photosystem I–Polyaniline Composite Films for Biohybrid Solar Energy Conversion. ACS Appl. Mater. Interfaces 2015, 7, 9328–9335. [Google Scholar] [CrossRef]
- Torella, J.P.; Gagliardi, C.J.; Chen, J.S.; Bediako, D.K.; Colón, B.; Way, J.C.; Silver, P.A.; Nocera, D.G. Efficient Solar-to-Fuels Production from a Hybrid Microbial–Water-Splitting Catalyst System. Proc. Natl. Acad. Sci. USA 2015, 112, 2337–2342. [Google Scholar] [CrossRef] [PubMed]
- Osella, S.; Kargul, J.; Izzo, M.; Trzaskowski, B. Architecture and Function of Biohybrid Solar Cell and Solar-to-Fuel Nanodevices. In Theory and Simulation in Physics for Materials Applications; Levchenko, E.V., Dappe, Y.J., Ori, G., Eds.; Springer Series in Materials Science; Springer International Publishing: Cham, Switzerland, 2020; Volume 296, pp. 227–274. ISBN 978-3-030-37789-2. [Google Scholar]
- Goyal, A.; Szewczyk, S.; Burdziński, G.; Abram, M.; Kargul, J.; Gibasiewicz, K. Competition between Intra-Protein Charge Recombination and Electron Transfer Outside Photosystem I Complexes Used for Photovoltaic Applications. Photochem. Photobiol. Sci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Sokol, K.P.; Robinson, W.E.; Warnan, J.; Kornienko, N.; Nowaczyk, M.M.; Ruff, A.; Zhang, J.Z.; Reisner, E. Bias-Free Photoelectrochemical Water Splitting with Photosystem II on a Dye-Sensitized Photoanode Wired to Hydrogenase. Nat. Energy 2018, 3, 944–951. [Google Scholar] [CrossRef]
- Utschig, L.M.; Soltau, S.R.; Mulfort, K.L.; Niklas, J.; Poluektov, O.G. Z-Scheme Solar Water Splitting via Self-Assembly of Photosystem I-Catalyst Hybrids in Thylakoid Membranes. Chem. Sci. 2018, 9, 8504–8512. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Reisner, E. Advancing Photosystem II Photoelectrochemistry for Semi-Artificial Photosynthesis. Nat. Rev. Chem. 2020, 4, 6–21. [Google Scholar] [CrossRef]
- Villarreal, C.C.; Monge, S.; Aguilar, D.; Tames, A.; Araya, N.; Aguilar, M.; Ramakrishna, S.; Thavasi, V.; Song, Z.; Mulchandani, A.; et al. Bio-Sensitized Solar Cells Built from Renewable Carbon Sources. Mater. Today Energy 2022, 23, 100910. [Google Scholar] [CrossRef]
- Izzo, M.; Jacquet, M.; Fujiwara, T.; Harputlu, E.; Mazur, R.; Wróbel, P.; Góral, T.; Unlu, C.G.; Ocakoglu, K.; Miyagishima, S.; et al. Development of a Novel Nanoarchitecture of the Robust Photosystem I from a Volcanic Microalga Cyanidioschyzon Merolae on Single Layer Graphene for Improved Photocurrent Generation. Int. J. Mol. Sci. 2021, 22, 8396. [Google Scholar] [CrossRef]
- Badura, A.; Esper, B.; Ataka, K.; Grunwald, C.; Wöll, C.; Kuhlmann, J.; Heberle, J.; Rögner, M. Light-Driven Water Splitting for (Bio-)Hydrogen Production: Photosystem 2 as the Central Part of a Bioelectrochemical Device. Photochem. Photobiol. 2006, 82, 1385–1390. [Google Scholar] [CrossRef]
- Kondo, M.; Iida, K.; Dewa, T.; Tanaka, H.; Ogawa, T.; Nagashima, S.; Nagashima, K.V.P.; Shimada, K.; Hashimoto, H.; Gardiner, A.T.; et al. Photocurrent and Electronic Activities of Oriented-His-Tagged Photosynthetic Light-Harvesting/Reaction Center Core Complexes Assembled onto a Gold Electrode. Biomacromolecules 2012, 13, 432–438. [Google Scholar] [CrossRef]
- Kim, J.S.; Park, J.H.; Lee, J.H.; Jo, J.; Kim, D.-Y.; Cho, K. Control of the Electrode Work Function and Active Layer Morphology via Surface Modification of Indium Tin Oxide for High Efficiency Organic Photovoltaics. Appl. Phys. Lett. 2007, 91, 112111. [Google Scholar] [CrossRef]
- Yu, P.; Chang, C.-H.; Chiu, C.-H.; Yang, C.-S.; Yu, J.-C.; Kuo, H.-C.; Hsu, S.-H.; Chang, Y.-C. Efficiency Enhancement of GaAs Photovoltaics Employing Antireflective Indium Tin Oxide Nanocolumns. Adv. Mater. 2009, 21, 1618–1621. [Google Scholar] [CrossRef]
- DuBow, J.B.; Burk, D.E.; Sites, J.R. Efficient Photovoltaic Heterojunctions of Indium Tin Oxides on Silicon. Appl. Phys. Lett. 1976, 29, 494–496. [Google Scholar] [CrossRef]
- Heimer, T.A.; Bignozzi, C.A.; Meyer, G.J. Molecular Level Photovoltaics: The Electrooptical Properties of Metal Cyanide Complexes Anchored to Titanium Dioxide. J. Phys. Chem. 1993, 97, 11987–11994. [Google Scholar] [CrossRef]
- Kay, A.; Grätzel, M. Low Cost Photovoltaic Modules Based on Dye Sensitized Nanocrystalline Titanium Dioxide and Carbon Powder. Sol. Energy Mater. Sol. Cells 1996, 44, 99–117. [Google Scholar] [CrossRef]
- Li, Y.; Hagen, J.; Schaffrath, W.; Otschik, P.; Haarer, D. Titanium Dioxide Films for Photovoltaic Cells Derived from a Sol–Gel Process. Sol. Energy Mater. Sol. Cells 1999, 56, 167–174. [Google Scholar] [CrossRef]
- Feifel, S.C.; Lokstein, H.; Hejazi, M.; Zouni, A.; Lisdat, F. Unidirectional Photocurrent of Photosystem I on π-System-Modified Graphene Electrodes: Nanobionic Approaches for the Construction of Photobiohybrid Systems. Langmuir 2015, 31, 10590–10598. [Google Scholar] [CrossRef]
- Osella, S.; Kiliszek, M.; Harputlu, E.; Unlu, C.G.; Ocakoglu, K.; Trzaskowski, B.; Kargul, J. Role of Metal Centers in Tuning the Electronic Properties of Graphene-Based Conductive Interfaces. J. Phys. Chem. C 2019, 123, 8623–8632. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Radiative Decay Engineering: Biophysical and Biomedical Applications. Anal. Biochem. 2001, 298, 1–24. [Google Scholar] [CrossRef]
- Czechowski, N.; Lokstein, H.; Kowalska, D.; Ashraf, K.; Cogdell, R.J.; Mackowski, S. Large Plasmonic Fluorescence Enhancement of Cyanobacterial Photosystem I Coupled to Silver Island Films. Appl. Phys. Lett. 2014, 105, 043701. [Google Scholar] [CrossRef]
- Szalkowski, M.; Ashraf, K.U.; Lokstein, H.; Mackowski, S.; Cogdell, R.J.; Kowalska, D. Silver Island Film Substrates for Ultrasensitive Fluorescence Detection of (Bio)Molecules. Photosynth. Res. 2016, 127, 103–108. [Google Scholar] [CrossRef]
- Maćkowski, S.; Czechowski, N.; Ashraf, K.U.; Szalkowski, M.; Lokstein, H.; Cogdell, R.J.; Kowalska, D. Origin of Bimodal Fluorescence Enhancement Factors of Chlorobaculum Tepidum Reaction Centers on Silver Island Films. FEBS Lett. 2016, 590, 2558–2565. [Google Scholar] [CrossRef]
- Kowalska, D.; Krajnik, B.; Olejnik, M.; Twardowska, M.; Czechowski, N.; Hofmann, E.; Mackowski, S. Metal-Enhanced Fluorescence of Chlorophylls in Light-Harvesting Complexes Coupled to Silver Nanowires. Sci. World J. 2013, 2013, 670412. [Google Scholar] [CrossRef]
- Friebe, V.M.; Delgado, J.D.; Swainsbury, D.J.K.; Gruber, J.M.; Chanaewa, A.; van Grondelle, R.; von Hauff, E.; Millo, D.; Jones, M.R.; Frese, R.N. Plasmon-Enhanced Photocurrent of Photosynthetic Pigment Proteins on Nanoporous Silver. Adv. Funct. Mater. 2016, 26, 285–292. [Google Scholar] [CrossRef]
- Furuya, R.; Omagari, S.; Tan, Q.; Lokstein, H.; Vacha, M. Enhancement of the Photocurrent of a Single Photosystem I Complex by the Localized Plasmon of a Gold Nanorod. J. Am. Chem. Soc. 2021, 143, 13167–13174. [Google Scholar] [CrossRef] [PubMed]
- Förster, T. Zwischenmolekulare Energiewanderung Und Fluoreszenz. Ann. Phys. 1948, 437, 55–75. [Google Scholar] [CrossRef]
- Sahoo, H. Förster Resonance Energy Transfer—A Spectroscopic Nanoruler: Principle and Applications. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 20–30. [Google Scholar] [CrossRef]
- Olmos, J.D.J.; Becquet, P.; Gront, D.; Sar, J.; Dąbrowski, A.; Gawlik, G.; Teodorczyk, M.; Pawlak, D.; Kargul, J. Biofunctionalisation of P-Doped Silicon with Cytochrome C553 Minimises Charge Recombination and Enhances Photovoltaic Performance of the All-Solid-State Photosystem I-Based Biophotoelectrode. RSC Adv. 2017, 7, 47854–47866. [Google Scholar] [CrossRef]
- Hofmann, E.; Wrench, P.M.; Sharples, F.P.; Hiller, R.G.; Welte, W.; Diederichs, K. Structural Basis of Light Harvesting by Carotenoids: Peridinin-Chlorophyll-Protein from Amphidinium Carterae. Science 1996, 272, 1788–1791. [Google Scholar] [CrossRef]
- Polívka, T.; Hofmann, E. Structure-Function Relationship in Peridinin-Chlorophyll Proteins. In The Structural Basis of Biological Energy Generation; Hohmann-Marriott, M.F., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2014; Volume 39, pp. 39–58. ISBN 978-94-017-8741-3. [Google Scholar]
- Song, P.S.; Koka, P.; Prézelin, B.B.; Haxo, F.T. Molecular Topology of the Photosynthetic Light-Harvesting Pigment Complex, Peridinin-Chlorophyll a-Protein, from Marine Dinoflagellates. Biochemistry 1976, 15, 4422–4427. [Google Scholar] [CrossRef]
- Kleima, F.J.; Hofmann, E.; Gobets, B.; van Stokkum, I.H.M.; van Grondelle, R.; Diederichs, K.; van Amerongen, H. Förster Excitation Energy Transfer in Peridinin-Chlorophyll-a-Protein. Biophys. J. 2000, 78, 344–353. [Google Scholar] [CrossRef]
- Qin, X.; Suga, M.; Kuang, T.; Shen, J.-R. Photosynthesis. Structural Basis for Energy Transfer Pathways in the Plant PSI-LHCI Supercomplex. Science 2015, 348, 989–995. [Google Scholar] [CrossRef]
- Mazor, Y.; Borovikova, A.; Nelson, N. The Structure of Plant Photosystem I Super-Complex at 2.8 Å Resolution. eLife 2015, 4, e07433. [Google Scholar] [CrossRef]
- Haniewicz, P.; Abram, M.; Nosek, L.; Kirkpatrick, J.; El-Mohsnawy, E.; Olmos, J.D.J.; Kouřil, R.; Kargul, J.M. Molecular Mechanisms of Photoadaptation of Photosystem I Supercomplex from an Evolutionary Cyanobacterial/Algal Intermediate. Plant Physiol. 2018, 176, 1433–1451. [Google Scholar] [CrossRef]
- Ihalainen, J.A.; Jensen, P.E.; Haldrup, A.; van Stokkum, I.H.M.; van Grondelle, R.; Scheller, H.V.; Dekker, J.P. Pigment Organization and Energy Transfer Dynamics in Isolated Photosystem I (PSI) Complexes from Arabidopsis thaliana Depleted of the PSI-G, PSI-K, PSI-L, or PSI-N Subunit. Biophys. J. 2002, 83, 2190–2201. [Google Scholar] [CrossRef]
- Swathi, R.S.; Sebastian, K.L. Resonance Energy Transfer from a Dye Molecule to Graphene. J. Chem. Phys. 2008, 129, 054703. [Google Scholar] [CrossRef]
- Twardowska, M.; Chomicki, D.; Kamińska, I.; Niedziółka-Jönsson, J.; Maćkowski, S. Fluorescence Imaging of Hybrid Nanostructures Composed of Natural Photosynthetic Complexes and Reduced Graphene Oxide. Nanospectroscopy 2015, 1. [Google Scholar] [CrossRef][Green Version]
- Schulte, T.; Johanning, S.; Hofmann, E. Structure and Function of Native and Refolded Peridinin-Chlorophyll-Proteins from Dinoflagellates. Eur. J. Cell Biol. 2010, 89, 990–997. [Google Scholar] [CrossRef]
- Blankenship, R.E. Molecular Mechanisms of Photosynthesis; Wiley-Blackwell: Hoboken, NJ, USA, 2014; ISBN 978-1-4051-8976-7. [Google Scholar]
- Mackowski, S.; Wörmke, S.; Maier, A.J.; Brotosudarmo, T.H.P.; Harutyunyan, H.; Hartschuh, A.; Govorov, A.O.; Scheer, H.; Bräuchle, C. Metal-Enhanced Fluorescence of Chlorophylls in Single Light-Harvesting Complexes. Nano Lett. 2008, 8, 558–564. [Google Scholar] [CrossRef]
- Ben-Shem, A.; Frolow, F.; Nelson, N. Crystal Structure of Plant Photosystem I. Nature 2003, 426, 630–635. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szalkowski, M.; Surrente, A.; Wiwatowski, K.; Yang, Z.; Zhang, N.; Olmos, J.D.J.; Kargul, J.; Plochocka, P.; Maćkowski, S. Spectral Dependence of the Energy Transfer from Photosynthetic Complexes to Monolayer Graphene. Int. J. Mol. Sci. 2022, 23, 3493. https://doi.org/10.3390/ijms23073493
Szalkowski M, Surrente A, Wiwatowski K, Yang Z, Zhang N, Olmos JDJ, Kargul J, Plochocka P, Maćkowski S. Spectral Dependence of the Energy Transfer from Photosynthetic Complexes to Monolayer Graphene. International Journal of Molecular Sciences. 2022; 23(7):3493. https://doi.org/10.3390/ijms23073493
Chicago/Turabian StyleSzalkowski, Marcin, Alessandro Surrente, Kamil Wiwatowski, Zhuo Yang, Nan Zhang, Julian D. Janna Olmos, Joanna Kargul, Paulina Plochocka, and Sebastian Maćkowski. 2022. "Spectral Dependence of the Energy Transfer from Photosynthetic Complexes to Monolayer Graphene" International Journal of Molecular Sciences 23, no. 7: 3493. https://doi.org/10.3390/ijms23073493
APA StyleSzalkowski, M., Surrente, A., Wiwatowski, K., Yang, Z., Zhang, N., Olmos, J. D. J., Kargul, J., Plochocka, P., & Maćkowski, S. (2022). Spectral Dependence of the Energy Transfer from Photosynthetic Complexes to Monolayer Graphene. International Journal of Molecular Sciences, 23(7), 3493. https://doi.org/10.3390/ijms23073493







