The Role of CCL2/CCR2 Axis in Cerebral Ischemia-Reperfusion Injury and Treatment: From Animal Experiments to Clinical Trials
Abstract
1. Introduction
2. CCL2 and CCR2 Signaling Pathway
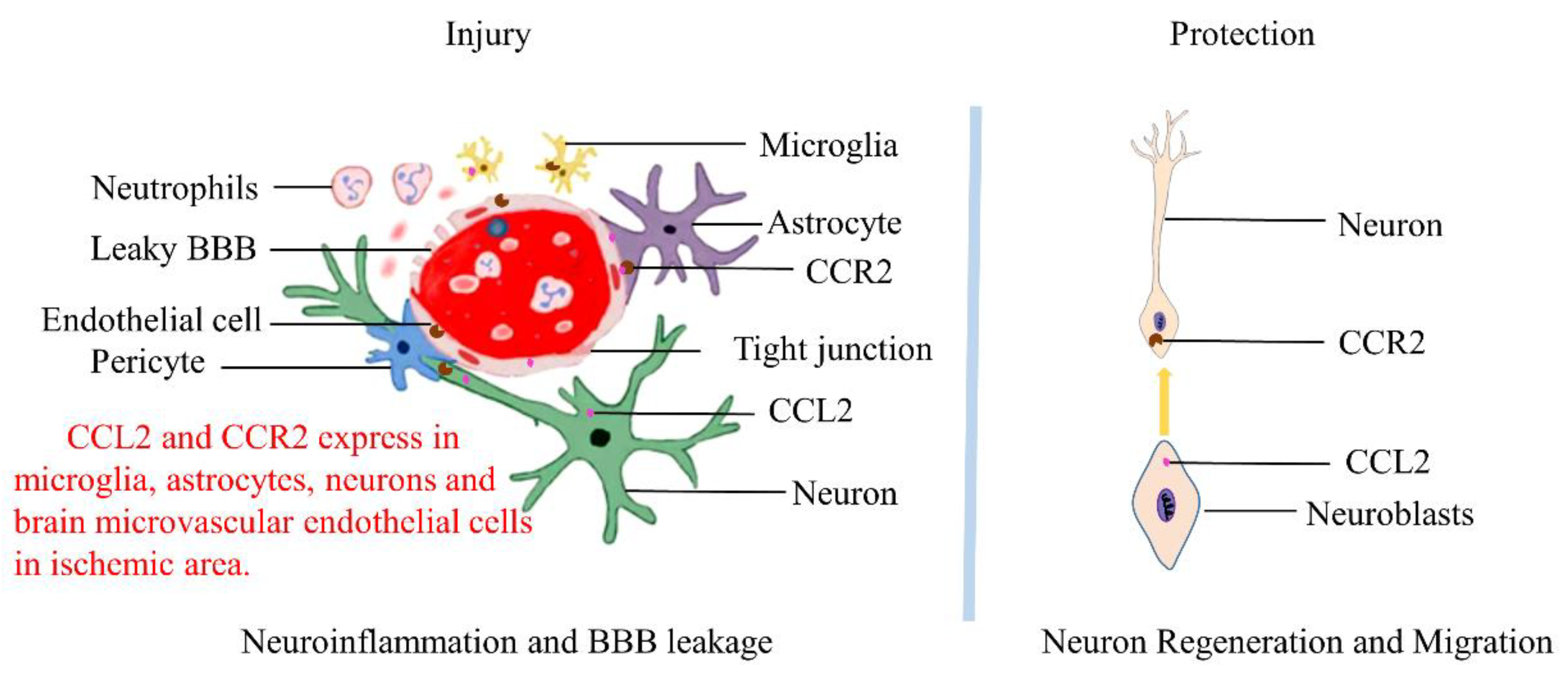
3. Expression of CCL2/CCR2 in the Central Nervous System
4. CCL2/CCR2 Axis and Cerebral Ischemia-Reperfusion Injury and Treatment
4.1. Experimental Research
4.1.1. CCL2/CCR2 Signaling Pathway and Cerebral Ischemia-Reperfusion Injury
4.1.2. The Potential Application of the CCL2/CCR2 Signaling Pathway in the Treatment of Cerebral Ischemia-Reperfusion
4.2. Clinical Studies
4.2.1. CCL2/CCR2 Signaling Pathway and Cerebral Ischemia-Reperfusion Injury
4.2.2. The Potential Application of the CCL2/CCR2 Signaling Pathway in the Treatment of Cerebral Ischemia-Reperfusion
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.; Chu, S.F.; Liu, D.D.; Zhang, Z.; Kong, L.L.; Zhou, X.; Chen, N.H. Chemokines play complex roles in cerebral ischemia. Neurochem. Int. 2018, 112, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Jäkel, S.; Dimou, L. Glial Cells and Their Function in the Adult Brain: A Journey through the History of Their Ablation. Front. Cell Neurosci. 2017, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Réaux-Le Goazigo, A.; Van Steenwinckel, J.; Rostène, W.; Mélik Parsadaniantz, S. Current status of chemokines in the adult CNS. Prog. Neurobiol. 2013, 104, 67–92. [Google Scholar] [CrossRef]
- Takahashi, M.; Galligan, C.; Tessarollo, L.; Yoshimura, T. Monocyte chemoattractant protein-1 (MCP-1), not MCP-3, is the primary chemokine required for monocyte recruitment in mouse peritonitis induced with thioglycollate or zymosan A. J. Immunol. 2009, 183, 3463–3471. [Google Scholar] [CrossRef]
- Anderson, C.D.; Lee, J.M.; Kamatani, Y.; Hopewell, J.C.; Worrall, B.B.; Bernhagen, J.; Sudlow, C.L.M.; Malik, R.; Dichgans, M. Genetically Determined Levels of Circulating Cytokines and Risk of Stroke. Circulation 2019, 139, 256–268. [Google Scholar]
- Gao, H.H.; Gao, L.B.; Wen, J.M. Correlations of MCP-1 -2518A>G polymorphism and serum levels with cerebral infarction risk: A meta-analysis. DNA Cell Biol. 2014, 33, 522–530. [Google Scholar] [CrossRef]
- Arakelyan, A.; Zakharyan, R.; Hambardzumyan, M.; Petrkova, J.; Olsson, M.C.; Petrek, M.; Boyajyan, A. Functional genetic polymorphisms of monocyte chemoattractant protein 1 and C-C chemokine receptor type 2 in ischemic stroke. J. Interferon Cytokine Res. 2014, 34, 100–105. [Google Scholar] [CrossRef]
- Giannakopoulou, E.; Ragia, G.; Marousi, S.; Ellul, J.; Manolopoulos, V.G.; Tavridou, A. Association of monocyte chemoattractant protein-1 -2518A>G polymorphism with occurrence, severity, and outcome in ischemic stroke. Neurol. Sci. 2013, 34, 1315–1320. [Google Scholar] [CrossRef]
- Bonifačić, D.; Toplak, A.; Benjak, I.; Tokmadžić, V.S.; Lekić, A.; Kučić, N. Monocytes and monocyte chemoattractant protein 1 (MCP-1) as early predictors of disease outcome in patients with cerebral ischemic stroke. Wien Klin Wochenschr. 2016, 128, 20–27. [Google Scholar] [CrossRef]
- Cai, G.; Zhang, B.; Weng, W.; Shi, G.; Huang, Z. The associations between the MCP-1 -2518 A/G polymorphism and ischemic heart disease and ischemic stroke: A meta-analysis of 28 research studies involving 21,524 individuals. Mol. Biol. Rep. 2015, 42, 997–1012. [Google Scholar] [CrossRef]
- Bianconi, V.; Sahebkar, A.; Atkin, S.L.; Pirro, M. The regulation and importance of monocyte chemoattractant protein-1. Curr. Opin. Hematol. 2018, 25, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Mulet, M.; Blasco-Ibáñez, J.M.; Kirstein, M.; Crespo, C.; Nacher, J.; Varea, E. Phenotypic characterization of MCP-1 expressing neurons in the rat cerebral cortex. J. Chem. Neuroanat. 2020, 106, 101785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Luo, J. Role of MCP-1 and CCR2 in alcohol neurotoxicity. Pharmacol. Res. 2019, 139, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Huma, Z.E.; Sanchez, J.; Lim, H.D.; Bridgford, J.L.; Huang, C.; Parker, B.J.; Pazhamalil, J.G.; Porebski, B.T.; Pfleger, K.D.G.; Lane, J.R.; et al. Key determinants of selective binding and activation by the monocyte chemoattractant proteins at the chemokine receptor CCR2. Sci. Signal. 2017, 10, eaai8529. [Google Scholar] [CrossRef]
- Haller, H.; Bertram, A.; Nadrowitz, F.; Menne, J. Monocyte chemoattractant protein-1 and the kidney. Curr. Opin. Nephrol. Hypertens. 2016, 25, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, F.; Wang, Y.; Long, J. Agomelatine prevents macrophage infiltration and brain endothelial cell damage in a stroke mouse model. Aging 2021, 13, 13548–13559. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Jing, G.; Wang, J.J.; Sheibani, N.; Zhang, S.X. ATF4 is a novel regulator of MCP-1 in microvascular endothelial cells. J. Inflamm. 2015, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Liao, Y.C.; Wang, Y.F.; Lin, I.F.; Wang, S.J.; Fuh, J.L. Plasma MCP-1 and Cognitive Decline in Patients with Alzheimer’s Disease and Mild Cognitive Impairment: A Two-year Follow-up Study. Sci. Rep. 2018, 8, 1280. [Google Scholar] [CrossRef] [PubMed]
- Che, X.; Ye, W.; Panga, L.; Wu, D.C.; Yang, G.Y. Monocyte chemoattractant protein-1 expressed in neurons and astrocytes during focal ischemia in mice. Brain Res. 2001, 902, 171–177. [Google Scholar] [CrossRef]
- Popiolek-Barczyk, K.; Ciechanowska, A.; Ciapała, K.; Pawlik, K.; Oggioni, M.; Mercurio, D.; De Simoni, M.G.; Mika, J. The CCL2/CCL7/CCL12/CCR2 pathway is substantially and persistently upregulated in mice after traumatic brain injury, and CCL2 modulates the complement system in microglia. Mol. Cell Probes 2020, 54, 101671. [Google Scholar] [CrossRef]
- Al-Hakeim, H.K.; Almulla, A.F.; Maes, M. The Neuroimmune and Neurotoxic Fingerprint of Major Neurocognitive Psychosis or Deficit Schizophrenia: A Supervised Machine Learning Study. Neurotoxic. Res. 2020, 37, 753–771. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, S.; Wu, X.; Wang, Y.; Jiang, D. Correlation between MCP-1-2518A/G polymorphism and the risk of Alzheimer’s disease. J. Neural. Transm. 2018, 125, 1781–1786. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Dong, Q.; Xu, L.; Zou, W.; Li, H. The MCP-1 A-2518G polymorphism increases the risk of Alzheimer’s disease: A case-control study. Neurosci. Lett. 2021, 749, 135710. [Google Scholar] [CrossRef] [PubMed]
- Santaella, A.; Kuiperij, H.B.; van Rumund, A.; Esselink, R.A.J.; van Gool, A.J.; Bloem, B.R.; Verbeek, M.M. Cerebrospinal fluid monocyte chemoattractant protein 1 correlates with progression of Parkinson’s disease. NPJ Parkinsons Dis. 2020, 6, 21. [Google Scholar] [CrossRef]
- Santaella, A.; Kuiperij, H.B.; van Rumund, A.; Esselink, R.A.J.; van Gool, A.J.; Bloem, B.R.; Verbeek, M.M. Inflammation biomarker discovery in Parkinson’s disease and atypical parkinsonisms. BMC Neurol. 2020, 20, 26. [Google Scholar] [CrossRef]
- Çoban, A.; Düzel, B.; Tüzün, E.; Tamam, Y. Investigation of the prognostic value of adipokines in multiple sclerosis. Mult. Scler. Relat. Disord. 2017, 15, 11–14. [Google Scholar] [CrossRef]
- Hong, S.; Lee, E.E.; Martin, A.S.; Soontornniyomkij, B.; Soontornniyomkij, V.; Achim, C.L.; Reuter, C.; Irwin, M.R.; Eyler, L.T.; Jeste, D.V. Abnormalities in chemokine levels in schizophrenia and their clinical correlates. Schizophr. Res. 2017, 181, 63–69. [Google Scholar] [CrossRef]
- Zakharyan, R.; Boyajyan, A.; Arakelyan, A.; Melkumova, M.; Mrazek, F.; Petrek, M. Monocyte chemoattractant protein-1 in schizophrenia: 2518A/G genetic variant and protein levels in Armenian population. Cytokine 2012, 58, 351–354. [Google Scholar] [CrossRef]
- Worthmann, H.; Dengler, R.; Schumacher, H.; Schwartz, A.; Eisert, W.G.; Lichtinghagen, R.; Weissenborn, K. Monocyte chemotactic protein-1 as a potential biomarker for early anti-thrombotic therapy after ischemic stroke. Int. J. Mol. Sci. 2012, 13, 8670–8678. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and foe for ischemic stroke. J Neuroinflamm. 2019, 16, 142. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Zheng, L.N.; Wei, J.F.; Hou, X.L.; Yu, S.Z.; Zhang, W.W.; Jing, J.M. Expression of CCL2 and CCR2 in the hippocampus and the interventional roles of propofol in rat cerebral ischemia/reperfusion. Exp. Ther. Med. 2014, 8, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Pedragosa, J.; Miró-Mur, F.; Otxoa-de-Amezaga, A.; Justicia, C.; Ruíz-Jaén, F.; Ponsaerts, P.; Pasparakis, M.; Planas, A.M. CCR2 deficiency in monocytes impairs angiogenesis and functional recovery after ischemic stroke in mice. J. Cereb. Blood Flow Metab. 2020, 40 (Suppl. S1), S98–S116. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, D.; Faustino, J.; Derugin, N.; Wendland, M.; Lizasoain, I.; Moro, M.A.; Vexler, Z.S. Reduced infarct size and accumulation of microglia in rats treated with WIN 55,212-2 after neonatal stroke. Neuroscience 2012, 207, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yue, T.L.; Barone, F.C.; Feuerstein, G.Z. Monocyte chemoattractant protein-1 messenger RNA expression in rat ischemic cortex. Stroke 1995, 26, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Inose, Y.; Kato, Y.; Kitagawa, K.; Uchiyama, S.; Shibata, N. Activated microglia in ischemic stroke penumbra upregulate MCP-1 and CCR2 expression in response to lysophosphatidylcholine derived from adjacent neurons and astrocytes. Neuropathology 2015, 35, 209–223. [Google Scholar] [CrossRef]
- Chen, Y.; Hallenbeck, J.M.; Ruetzler, C.; Bol, D.; Thomas, K.; Berman, N.E.; Vogel, S.N. Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. J. Cereb. Blood Flow Metab. 2003, 23, 748–755. [Google Scholar] [CrossRef]
- Hughes, P.M.; Allegrini, P.R.; Rudin, M.; Perry, V.H.; Mir, A.K.; Wiessner, C. Monocyte chemoattractant protein-1 deficiency is protective in a murine stroke model. J. Cereb. Blood Flow Metab. 2002, 22, 308–317. [Google Scholar] [CrossRef]
- Dimitrijevic, O.B.; Stamatovic, S.M.; Keep, R.F.; Andjelkovic, A.V. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke 2007, 38, 1345–1353. [Google Scholar] [CrossRef]
- Tei, N.; Tanaka, J.; Sugimoto, K.; Nishihara, T.; Nishioka, R.; Takahashi, H.; Yano, H.; Matsumoto, S.; Ohue, S.; Watanabe, H.; et al. Expression of MCP-1 and fractalkine on endothelial cells and astrocytes may contribute to the invasion and migration of brain macrophages in ischemic rat brain lesions. J. Neurosci. Res. 2013, 91, 681–693. [Google Scholar] [CrossRef]
- Schilling, M.; Strecker, J.K.; Ringelstein, E.B.; Schäbitz, W.R.; Kiefer, R. The role of CC chemokine receptor 2 on microglia activation and blood-borne cell recruitment after transient focal cerebral ischemia in mice. Brain Res. 2009, 1289, 79–84. [Google Scholar] [CrossRef]
- Schuette-Nuetgen, K.; Strecker, J.K.; Minnerup, J.; Ringelstein, E.B.; Schilling, M. MCP-1/CCR-2-double-deficiency severely impairs the migration of hematogenous inflammatory cells following transient cerebral ischemia in mice. Exp. Neurol. 2012, 233, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic, O.B.; Stamatovic, S.M.; Keep, R.F.; Andjelkovic, A.V. Effects of the chemokine CCL2 on blood-brain barrier permeability during ischemia-reperfusion injury. J. Cereb. Blood Flow Metab. 2006, 26, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Strecker, J.K.; Minnerup, J.; Schütte-Nütgen, K.; Gess, B.; Schäbitz, W.R.; Schilling, M. Monocyte chemoattractant protein-1-deficiency results in altered blood-brain barrier breakdown after experimental stroke. Stroke 2013, 44, 2536–2544. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Coelho, P.M.; Michaud, J.P.; Rivest, S. C-C chemokine receptor type 2 (CCR2) signaling protects neonatal male mice with hypoxic-ischemic hippocampal damage from developing spatial learning deficits. Behav. Brain Res. 2015, 286, 146–151. [Google Scholar] [CrossRef]
- Tsukuda, K.; Mogi, M.; Iwanami, J.; Min, L.J.; Jing, F.; Oshima, K.; Horiuchi, M. Irbesartan attenuates ischemic brain damage by inhibition of MCP-1/CCR2 signaling pathway beyond AT₁ receptor blockade. Biochem. Biophys. Res. Commun. 2011, 409, 275–279. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Liu, R.; Li, B.; Huang, L.; Fan, W.; Tembachako, C.R.; Zheng, X.; Xiong, X.; Miyata, M.; Xu, B.; et al. Propagermanium, a CCR2 inhibitor, attenuates cerebral ischemia/reperfusion injury through inhibiting inflammatory response induced by microglia. Neurochem. Int. 2019, 125, 99–110. [Google Scholar] [CrossRef]
- Sakurai-Yamashita, Y.; Shigematsu, K.; Yamashita, K.; Niwa, M. Expression of MCP-1 in the hippocampus of SHRSP with ischemia-related delayed neuronal death. Cell Mol. Neurobiol. 2006, 26, 823–831. [Google Scholar] [CrossRef]
- Lee, S.; Chu, H.X.; Kim, H.A.; Real, N.C.; Sharif, S.; Fleming, S.B.; Mercer, A.A.; Wise, L.M.; Drummond, G.R.; Sobey, C.G. Effect of a broad-specificity chemokine-binding protein on brain leukocyte infiltration and infarct development. Stroke 2015, 46, 537–544. [Google Scholar] [CrossRef]
- Shan, Y.; Hu, J.; Lv, H.; Cui, X.; Di, W. miR-221 Exerts Neuroprotective Effects in Ischemic Stroke by Inhibiting the Proinflammatory Response. J. Stroke Cerebrovasc. Dis. 2021, 30, 105489. [Google Scholar] [CrossRef]
- Huang, L.; Ma, Q.; Li, Y.; Li, B.; Zhang, L. Inhibition of microRNA-210 suppresses pro-inflammatory response and reduces acute brain injury of ischemic stroke in mice. Exp. Neurol. 2018, 300, 41–50. [Google Scholar] [CrossRef]
- Bernstein, D.L.; Rom, S. Let-7g* and miR-98 Reduce Stroke-Induced Production of Proinflammatory Cytokines in Mouse Brain. Front. Cell Dev. Biol. 2020, 8, 632. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Weng, Y.C.; Chiang, I.C.; Huang, Y.T.; Liao, Y.C.; Chen, Y.C.; Kao, C.Y.; Liu, Y.L.; Lee, T.H.; Chou, W.H. Neutralization of Lipocalin-2 Diminishes Stroke-Reperfusion Injury. Int. J. Mol. Sci. 2020, 21, 6253. [Google Scholar] [CrossRef] [PubMed]
- Nishi, T.; Maier, C.M.; Hayashi, T.; Saito, A.; Chan, P.H. Superoxide dismutase 1 overexpression reduces MCP-1 and MIP-1 alpha expression after transient focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2005, 25, 1312–1324. [Google Scholar] [CrossRef] [PubMed]
- Venkat, P.; Ning, R.; Zacharek, A.; Culmone, L.; Liang, L.; Landschoot-Ward, J.; Chopp, M. Treatment with an Angiopoietin-1 mimetic peptide promotes neurological recovery after stroke in diabetic rats. CNS Neurosci. Ther. 2021, 27, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Wattananit, S.; Tornero, D.; Graubardt, N.; Memanishvili, T.; Monni, E.; Tatarishvili, J.; Miskinyte, G.; Ge, R.; Ahlenius, H.; Lindvall, O.; et al. Monocyte-Derived Macrophages Contribute to Spontaneous Long-Term Functional Recovery after Stroke in Mice. J. Neurosci. 2016, 36, 4182–4195. [Google Scholar] [CrossRef]
- Fang, W.; Zhai, X.; Han, D.; Xiong, X.; Wang, T.; Zeng, X.; He, S.; Liu, R.; Miyata, M.; Xu, B.; et al. CCR2-dependent monocytes/macrophages exacerbate acute brain injury but promote functional recovery after ischemic stroke in mice. Theranostics 2018, 8, 3530–3543. [Google Scholar] [CrossRef]
- Chu, H.X.; Kim, H.A.; Lee, S.; Broughton, B.R.S.; Drummond, G.R.; Sobey, C.G. Evidence of CCR2-independent transmigration of Ly6C(hi) monocytes into the brain after permanent cerebral ischemia in mice. Brain Res. 2016, 1637, 118–127. [Google Scholar] [CrossRef]
- Llovera, G.; Benakis, C.; Enzmann, G.; Cai, R.; Arzberger, T.; Ghasemigharagoz, A.; Mao, X.; Malik, R.; Lazarevic, I.; Liebscher, S.; et al. The choroid plexus is a key cerebral invasion route for T cells after stroke. Acta Neuropathol. 2017, 134, 851–868. [Google Scholar] [CrossRef]
- Jiang, L.; Newman, M.; Saporta, S.; Chen, N.; Sanberg, C.; Sanberg, P.R.; Willing, A.E. MIP-1alpha and MCP-1 Induce Migration of Human Umbilical Cord Blood Cells in Models of Stroke. Curr. Neurovasc. Res. 2008, 5, 118–124. [Google Scholar] [CrossRef][Green Version]
- Minami, M.; Satoh, M. Chemokines and their receptors in the brain: Pathophysiological roles in ischemic brain injury. Life Sci. 2003, 74, 321–327. [Google Scholar] [CrossRef]
- Chang, A.Y.; Li, F.C.; Huang, C.W.; Wu, J.C.; Dai, K.Y.; Chen, C.H.; Li, S.H.; Su, C.H.; Wu, R.W. Interplay between brain stem angiotensins and monocyte chemoattractant protein-1 as a novel mechanism for pressor response after ischemic stroke. Neurobiol. Dis. 2014, 71, 292–304. [Google Scholar] [CrossRef] [PubMed]
- DeMars, K.M.; Yang, C.; Candelario-Jalil, E. Neuroprotective effects of targeting BET proteins for degradation with dBET1 in aged mice subjected to ischemic stroke. Neurochem. Int. 2019, 127, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Dou, B.; Li, S.; Wei, L.; Wang, L.; Zhu, S.; Wang, Z.; Ke, Z.; Chen, K.; Wang, Z. Astragaloside IV suppresses post-ischemic natural killer cell infiltration and activation in the brain: Involvement of histone deacetylase inhibition. Front. Med. 2021, 15, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Tsirka, S.E. Truncation of monocyte chemoattractant protein 1 by plasmin promotes blood-brain barrier disruption. J Cell Sci. 2011, 124, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.J.; Zhou, C.; Gravanis, I.; Rogove, A.D.; Wu, Y.P.; Bogenhagen, D.F.; Tsirka, S.E. Proteolytic activation of monocyte chemoattractant protein-1 by plasmin underlies excitotoxic neurodegeneration in mice. J. Neurosci. 2007, 27, 1738–1745. [Google Scholar] [CrossRef]
- Yao, Y.; Tsirka, S.E. Monocyte chemoattractant protein-1 and the blood-brain barrier. Cell Mol. Life Sci. 2014, 71, 683–697. [Google Scholar] [CrossRef]
- Guo, F.; Xu, D.; Lin, Y.; Wang, G.; Wang, F.; Gao, Q.; Wei, Q.; Lei, S. Chemokine CCL2 contributes to BBB disruption via the p38 MAPK signaling pathway following acute intracerebral hemorrhage. FASEB J. 2020, 34, 1872–1884. [Google Scholar] [CrossRef]
- Xu, D.; Gao, Q.; Wang, F.; Peng, Q.; Wang, G.; Wei, Q.; Lei, S.; Zhao, S.; Zhang, L.; Guo, F. Sphingosine-1-phosphate receptor 3 is implicated in BBB injury via the CCL2-CCR2 axis following acute intracerebral hemorrhage. CNS Neurosci. Ther. 2021, 27, 674–686. [Google Scholar] [CrossRef]
- Ribeiro, F.F.; Xapelli, S. An Overview of Adult Neurogenesis. Adv. Exp. Med. Biol. 2021, 1331, 77–94. [Google Scholar]
- Kim, S.S.; Pyeon, H.J.; Bae, Y.K.; Nam, H.; Kim, C.K.; Lee, S.H.; Joo, K.M. Adult Human Multipotent Neural Cells Could Be Distinguished from Other Cell Types by Proangiogenic Paracrine Effects via MCP-1 and GRO. Stem Cells Int. 2021, 2021, 6737288. [Google Scholar] [CrossRef]
- Hayashi, Y.; Kato, H.; Nonaka, K.; Nakanishi, H. Stem cells from human exfoliated deciduous teeth attenuate mechanical allodynia in mice through distinct from the siglec-9/MCP-1-mediated tissue-repairing mechanism. Sci. Rep. 2021, 11, 20053. [Google Scholar] [CrossRef] [PubMed]
- Mansurov, N.; Chen, W.C.W.; Awada, H.; Huard, J.; Wang, Y.; Saparov, A. A controlled release system for simultaneous delivery of three human perivascular stem cell-derived factors for tissue repair and regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e1164–e1172. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.M.; Neumann, S.; Kuhn, H.G.; Blomgren, K. Caspase inhibition impaired the neural stem/progenitor cell response after cortical ischemia in mice. Oncotarget 2016, 7, 2239–2248. [Google Scholar] [CrossRef] [PubMed]
- Andres, R.H.; Choi, R.; Pendharkar, A.V.; Gaeta, X.; Wang, N.; Nathan, J.K.; Chua, J.Y.; Lee, S.W.; Palmer, T.D.; Steinberg, G.K.; et al. The CCR2/CCL2 interaction mediates the transendothelial recruitment of intravascularly delivered neural stem cells to the ischemic brain. Stroke 2011, 42, 2923–2931. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.S.; Zhang, Z.G.; Zhang, R.L.; Gregg, S.R.; Wang, L.; Yier, T.; Chopp, M. Chemokine ligand 2 (CCL2) induces migration and differentiation of subventricular zone cells after stroke. J. Neurosci. Res. 2007, 85, 2120–2125. [Google Scholar] [CrossRef]
- Yan, Y.P.; Sailor, K.A.; Lang, B.T.; Park, S.W.; Vemuganti, R.; Dempsey, R.J. Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2007, 27, 1213–1224. [Google Scholar] [CrossRef]
- Baba, N.; Wang, F.; Iizuka, M.; Shen, Y.; Yamashita, T.; Takaishi, K.; Tsuru, E.; Matsushima, S.; Miyamura, M.; Fujieda, M.; et al. Induction of regional chemokine expression in response to human umbilical cord blood cell infusion in the neonatal mouse ischemia-reperfusion brain injury model. PLoS ONE 2019, 14, e0221111. [Google Scholar] [CrossRef]
- Laterza, C.; Wattananit, S.; Uoshima, N.; Ge, R.; Pekny, R.; Tornero, D.; Monni, E.; Lindvall, O.; Kokaia, Z. Monocyte depletion early after stroke promotes neurogenesis from endogenous neural stem cells in adult brain. Exp. Neurol. 2017, 297, 129–137. [Google Scholar] [CrossRef]
- Lee, S.H.; Jin, K.S.; Bang, O.Y.; Kim, B.J.; Park, S.J.; Lee, N.H.; Yoo, K.H.; Koo, H.H.; Sung, K.W. Differential Migration of Mesenchymal Stem Cells to Ischemic Regions after Middle Cerebral Artery Occlusion in Rats. PLoS ONE 2015, 10, e0134920. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Cai, J.; Qiu, Y.; Zheng, H.; Lai, X.; Sui, X.; Wang, Y.; Lu, Q.; Zhang, Y.; et al. Targeted homing of CCR2-overexpressing mesenchymal stromal cells to ischemic brain enhances post-stroke recovery partially through PRDX4-mediated blood-brain barrier preservation. Theranostics 2018, 8, 5929–5944. [Google Scholar] [CrossRef]
- Lee, S.; Kim, O.J.; Lee, K.O.; Jung, H.; Oh, S.H.; Kim, N.K. Enhancing the Therapeutic Potential of CCL2-Overexpressing Mesenchymal Stem Cells in Acute Stroke. Int. J. Mol. Sci. 2020, 21, 7795. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Zhang, M.; Wu, R.; Zheng, H.Q.; Zhang, L.Y.; Luo, J.; Li, L.L.; Hu, X.Q. C-C chemokine receptor type 2-overexpressing exosomes alleviated experimental post-stroke cognitive impairment by enhancing microglia/macrophage M2 polarization. World J. Stem Cells 2020, 12, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 20, 107598. [Google Scholar] [CrossRef] [PubMed]
- Sayeed, I.; Won, S.; Lyle, A.N.; Stein, D.G. Progesterone protects endothelial cells after cerebrovascular occlusion by decreasing MCP-1- and CXCL1-mediated macrophage infiltration. Exp. Neurol. 2015, 271, 401–408. [Google Scholar]
- Yu, M.; Zheng, N.; Jiang, D.; Wang, L.; Zhan, Q.; Zhao, J. Chemokine C-C motif ligand 2 suppressed the growth of human brain astrocytes under Ischemic/hypoxic conditions via regulating ERK1/2 pathway. Brain Inj. 2020, 34, 1277–1282. [Google Scholar] [CrossRef]
- Shi, K.; Zou, M.; Jia, D.M.; Shi, S.; Yang, X.; Liu, Q.; Dong, J.F.; Sheth, K.N.; Wang, X.; Shi, F.D. tPA Mobilizes Immune Cells That Exacerbate Hemorrhagic Transformation in Stroke. Circ. Res. 2021, 128, 62–75. [Google Scholar] [CrossRef]
- Strecker, J.K.; Minnerup, J.; Gess, B.; Ringelstein, E.B.; Schäbitz, W.R.; Schilling, M. Monocyte chemoattractant protein-1-deficiency impairs the expression of IL-6, IL-1β and G-CSF after transient focal ischemia in mice. PLoS ONE 2011, 6, e25863. [Google Scholar] [CrossRef]
- Rehni, A.K.; Singh, T.G. Involvement of CCR-2 chemokine receptor activation in ischemic preconditioning and postconditioning of brain in mice. Cytokine 2012, 60, 83–89. [Google Scholar] [CrossRef]
- Garcia-Bonilla, L.; Brea, D.; Benakis, C.; Lane, D.A.; Murphy, M.; Moore, J.; Racchumi, G.; Jiang, X.; Iadecola, C.; Anrather, J. Endogenous Protection from Ischemic Brain Injury by Preconditioned Monocytes. J. Neurosci. 2018, 38, 6722–6736. [Google Scholar] [CrossRef]
- Stowe, A.M.; Wacker, B.K.; Cravens, P.D.; Perfater, J.L.; Li, M.K.; Hu, R.; Freie, A.B.; Stüve, O.; Gidday, J.M. CCL2 upregulation triggers hypoxic preconditioning-induced protection from stroke. J. Neuroinflamm. 2012, 9, 33. [Google Scholar] [CrossRef]
- Lee, J.H.; Wei, Z.Z.; Cao, W.; Won, S.; Gu, X.; Winter, M.; Dix, T.A.; Wei, L.; Yu, S.P. Regulation of therapeutic hypothermia on inflammatory cytokines, microglia polarization, migration and functional recovery after ischemic stroke in mice. Neurobiol. Dis. 2016, 96, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lou, W.; Li, H.; Zhu, Y.; Huang, X. Upregulated C-C Motif Chemokine Ligand 2 Promotes Ischemic Stroke via Chemokine Signaling Pathway. Ann. Vasc. Surg. 2020, 68, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.T.; Chen, A.X.; Song, B.; Fan, J.; Li, W.; Xing, Z.; Peng, S.Y.; Zhang, Q.P.; Dong, L.; Yan, C.; et al. Suppression of microglial activation and monocyte infiltration ameliorates cerebellar hemorrhage induced-brain injury and ataxia. Brain Behav. Immun. 2020, 89, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Alvarez, V.M.; Soto-Rodriguez, G.; Gonzalez-Barrios, J.A.; Martinez-Fong, D.; Brambila, E.; Torres-Soto, M.; Aguilar-Peralta, A.K.; Gonzalez-Vazquez, A.; Tomás-Sanchez, C.; Limón, I.D.; et al. Prophylactic Subacute Administration of Zinc Increases CCL2, CCR2, FGF2, and IGF-1 Expression and Prevents the Long-Term Memory Loss in a Rat Model of Cerebral Hypoxia-Ischemia. Neural Plast. 2015, 2015, 375391. [Google Scholar] [CrossRef] [PubMed]
- 95. Shen, L.; Gan, Q.; Yang, Y.; Reis, C.; Zhang, Z.; Xu, S.; Zhang, T.; Sun, C. Mitophagy in Cerebral Ischemia and Ischemia/Reperfusion Injury. Front. Aging Neurosci. 2021, 13, 687246. [Google Scholar] [CrossRef]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline from the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467, Correction in Stroke 2021, 52, e483–e484. [Google Scholar] [CrossRef]
- Georgakis, M.K.; Malik, R.; Björkbacka, H.; Pana, T.A.; Demissie, S.; Ayers, C.; Elhadad, M.A.; Fornage, M.; Beiser, A.S.; Benjamin, E.J.; et al. Circulating Monocyte Chemoattractant Protein-1 and Risk of Stroke: Meta-Analysis of Population-Based Studies Involving 17 180 Individuals. Circ. Res. 2019, 125, 773–782. [Google Scholar] [CrossRef]
- Arakelyan, A.; Petrkova, J.; Hermanova, Z.; Boyajyan, A.; Lukl, J.; Petrek, M. Serum levels of the MCP-1 chemokine in patients with ischemic stroke and myocardial infarction. Mediat. Inflamm. 2005, 175–179. [Google Scholar] [CrossRef]
- Mechtouff, L.; Bochaton, T.; Paccalet, A.; Crola Da Silva, C.; Buisson, M.; Amaz, C.; Derex, L.; Ong, E.; Berthezene, Y.; Eker, O.F.; et al. Matrix Metalloproteinase-9 and Monocyte Chemoattractant Protein-1 Are Associated with Collateral Status in Acute Ischemic Stroke with Large Vessel Occlusion. Stroke 2020, 51, 2232–2235. [Google Scholar] [CrossRef]
- Fringuello, A.; Tatman, P.D.; Wroblewski, T.; Thompson, J.A.; Yu, X.; Lillehei, K.O.; Kowalski, R.G.; Graner, M.W. Cytokine-Laden Extracellular Vesicles Predict Patient Prognosis after Cerebrovascular Accident. Int. J. Mol. Sci. 2021, 22, 7847. [Google Scholar] [CrossRef]
- Timasheva, Y.R.; Nasibullin, T.R.; Mustafina, O.E. The CXCR2 Gene Polymorphism Is Associated with Stroke in Patients with Essential Hypertension. Cerebrovasc. Dis. Extra 2015, 5, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Farooqui, M.; Ikram, A.; Suriya, S.; Kempuraj, D.; Khan, M.; Tasneem, N.; Qaryouti, D.; Quadri, S.; Adams, H.P.; et al. Cytokines, brain proteins, and growth factors in acute stroke patients: A pilot study. Surg. Neurol. Int. 2021, 12, 366. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, D.R.; Cui, C.; Wen, L.J. Clinical significance of serum MCP-1 and VE-cadherin levels in patients with acute cerebral infarction. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 804–808. [Google Scholar] [PubMed]
- Holmegaard, L.; Stanne, T.M.; Andreasson, U.; Zetterberg, H.; Blennow, K.; Blomstrand, C.; Jood, K.; Jern, C. Proinflammatory protein signatures in cryptogenic and large artery atherosclerosis stroke. Acta Neurol. Scand. 2020, 143, 303–312. [Google Scholar] [CrossRef]
- Losy, J.; Zaremba, J. Monocyte chemoattractant protein-1 is increased in the cerebrospinal fluid of patients with ischemic stroke. Stroke 2001, 32, 2695–2696. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Worthmann, H.; Tryc, A.B.; Goldbecker, A.; Ma, Y.T.; Tountopoulou, A.; Hahn, A.; Dengler, R.; Lichtinghagen, R.; Weissenborn, K. The temporal profile of inflammatory markers and mediators in blood after acute ischemic stroke differs depending on stroke outcome. Cerebrovasc. Dis. 2010, 30, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Grosse, G.M.; Schulz-Schaeffer, W.J.; Teebken, O.E.; Schuppner, R.; Dirks, M.; Worthmann, H.; Lichtinghagen, R.; Maye, G.; Limbourg, F.P.; Weissenborn, K. Monocyte Subsets and Related Chemokines in Carotid Artery Stenosis and Ischemic Stroke. Int. J. Mol. Sci. 2016, 17, 433. [Google Scholar] [CrossRef]
- 108. Landreneau, M.J.; Mullen, M.T.; Messé, S.R.; Cucchiara, B.; Sheth, K.N.; McCullough, L.D.; Kasner, S.E.; Sansing, L.H. Serum Markers After Spontaneous Cerebral Hemorrhage (SMASCH) Investigators. CCL2 and CXCL10 are associated with poor outcome after intracerebral hemorrhage. Ann. Clin. Transl. Neurol. 2018, 5, 962–970. [Google Scholar] [CrossRef]
- Xia, C.; Li, X.Q.; Zhou, Z.H.; Chen, H.S. Identification of cytokines for early prediction of malignant middle cerebral artery infarction. Int. J. Neurosci. 2017, 127, 86–91. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, J.; Li, X.; Xia, C.; Han, Y.; Chen, H. Protein microarray analysis identifies key cytokines associated with malignant middle cerebral artery infarction. Brain Behav. 2017, 7, e00746. [Google Scholar] [CrossRef]
- Hatakeyama, M.; Ninomiya, I.; Kanazawa, M. Angiogenesis and neuronal remodeling after ischemic stroke. Neural Regen. Res. 2020, 15, 16–19. [Google Scholar] [PubMed]
- Kanazawa, M.; Takahashi, T.; Ishikawa, M.; Onodera, O.; Shimohata, T.; Del Zoppo, G.J. Angiogenesis in the ischemic core: A potential treatment target? J. Cereb. Blood Flow Metab. 2019, 39, 753–769. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Xu, Y.; He, L.; Choi, W.; Gonzalez, D.; Jin, S.W.; Simons, M. Role of Venous Endothelial Cells in Developmental and Pathologic Angiogenesis. Circulation 2021, 144, 1308–1322. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, Y.; Zhong, Y.; Ye, Y.; Hu, X.; Gu, L.; Xiong, X. Inflammation-Mediated Angiogenesis in Ischemic Stroke. Front. Cell Neurosci. 2021, 15, 652647. [Google Scholar] [CrossRef]
- Navarro-Sobrino, M.; Hernández-Guillamon, M.; Fernandez-Cadenas, I.; Ribó, M.; Romero, I.A.; Couraud, P.O.; Weksler, B.B.; Montaner, J.; Rosell, A. The angiogenic gene profile of circulating endothelial progenitor cells from ischemic stroke patients. Vasc. Cell 2013, 5, 3. [Google Scholar] [CrossRef]
- Zhu, J.; Yi, X.; Zhang, Y.; Pan, Z.; Zhong, L.; Huang, P. Systems Pharmacology-Based Approach to Comparatively Study the Independent and Synergistic Mechanisms of Danhong Injection and Naoxintong Capsule in Ischemic Stroke Treatment. Evid.-Based Complement. Altern. Med. 2019, 2019, 1056708. [Google Scholar] [CrossRef]
- Grell, A.S.; Mostajeran, M.; Frederiksen, S.D.; Edvinsson, L.; Ansar, S. Cerebrovascular Gene Expression in Spontaneously Hypertensive Rats After Transient Middle Cerebral Artery Occlusion. Neuroscience 2017, 367, 219–232. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, S.; Liao, Q.; Li, X.; Tan, S.; Li, S.; Ke, T. RNA-seq analysis of ischemia stroke and normal brain in a tree shrew model with or without type 2 diabetes mellitus. Metab. Brain Dis. 2021, 36, 1889–1901. [Google Scholar] [CrossRef]
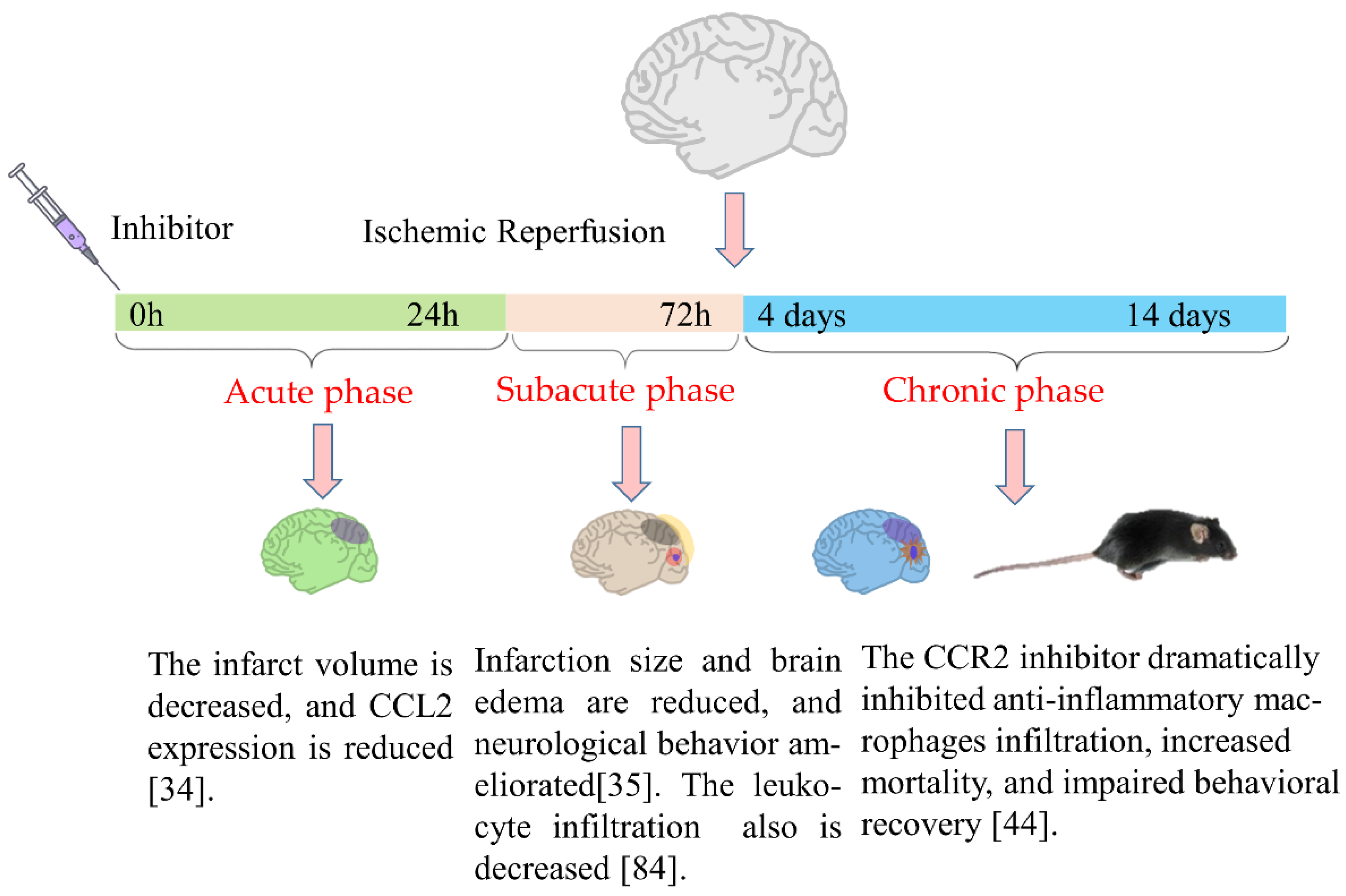
| Number | Species | Animal Model | Gene Type of Animal | Measured Time | Pathological Effects | References |
|---|---|---|---|---|---|---|
| 1 | SD rats | Middle cerebral artery occlusion (MCAO), 90 min | Wildtype (WT) | 24 or 72 h after MCAO | The expression levels of CCL2 and CCR2 were significantly increased, along with microglial activation. | [33] |
| 2 | Rats | MCAO, 160 min | Wildtype (WT) | 1 h, 6 h, 12 h, and 5 days after MCAO | The CCL2 mRNA expression was significantly increased and remained for 5 days. | [34,35] |
| 3 | Mice | MCAO, 120 min | CCL2 transgenic mice | 24 to 48 h after MCAO | The infarction volumes were significantly larger in transgenic mice than in wildtype controls, as well as perivascular accumulation of macrophages and neutrophils. | [36] |
| 4 | Mice | pMCAO | CCL2-deficient mice | 24 h, 36 h, and 2 weeks after MCAO | The infarction volumes, microglia activation, and the accumulation of macrophages were reduced. | [37] |
| 5 | Mice | MCAO, 30 min | CCR2+/+ and CCR2−/− mice | 1 and 5 days after MCAO | CCR2−/− mice had reduced infarct sizes, BBB permeability, and brain edema, compared with CCR2+/+ mice. | [38] |
| 6 | Wistar rats | MCAO, 90 min | Wildtype (WT) | 1, 2, 3, 4, 5, and 7 days after MCAO | The macrophages migrated into the brain parenchyma were dependent on the CCL2/CCR2 signaling pathway. | [39,40] |
| 7 | Mice | MCAO, 60 min | CCL2/CCR2 double-deficient mice | 1, 2, 4, and 7 days after MCAO | The invasion of macrophages and neutrophils was reduced. | [41] |
| 8 | Mice | MCAO, 45 min | Wildtype (WT) and CCL2-deficient mice | 12 or 36 h after MCAO | BBB leakage and cerebral infarction were reduced in CCL2-deficient mice. | [42,43] |
| 9 | Mice | Hypoxic-ischemic (HI), 40 min | Wildtype (WT) and CCR2 knockout mice | 5 weeks and 12 weeks after hypoxia-ischemia | The activation of macrophages and microglia was reduced, along with enhanced long-term spatial learning ability of female mice. | [44] |
| 10 | Mice | MCAO | Wildtype (WT) | 72 h or 2 weeks after MCAO | CCR2 antagonist inhibited the expression of inflammatory cytokines, reduced brain edema and infarction volume, and promoted the recovery of neurological function. | [45,46] |
| 11 | Rat | Transient global ischemia | Wildtype (WT) | 8 h and 1, 2, 4, and 7 days after reperfusion | CCL2 is related to the delayed neuronal death in the pyramidal neurons. | [47] |
| 12 | Mice | MCAO, 60 min | Wildtype (WT) | 23 or 47 h after reperfusion | CCL2 levels were markedly increased in plasma, and microglia and leukocytes infiltrated into the brain. | [48] |
| 13 | Mice | MCAO | Wildtype (WT) | 48 h after MCAO | The mRNA and protein levels of CCL2 were significantly increased, as well as macrophage infiltration and microglial activation | [49] |
| 14 | Mice | MCAO | Wildtype (WT) | 6–24 h after MCAO | The mRNA and protein levels of CCL2 were significantly increased 24 h after MCAO. | [50] |
| 15 | Mice | MCAO, 60 min | Wildtype (WT) | 72 h after MCAO | The expression of CCL2 was significantly increased after MCAO. | [51] |
| Number | Patients | Types of Research | Sample | Cases | Results | References |
|---|---|---|---|---|---|---|
| 1 | Any stroke | Meta-analysis | Blood | 17,180 | Higher CCL2 levels were associated with the risk of stroke. | [97] |
| 2 | Ischemic stroke | Analytical study | Serum | 40 | CCL2 levels were increased in ischemic stroke patients. | [98] |
| 3 | Acute ischemic stroke | Cohort study | Blood | 122 | High CCL2 levels were associated with good collateral status. | [99] |
| 4 | Hemorrhagic stroke | Comprehensive analysis | Plasma EVs | 31 | High CCL2 levels correlated with detrimental outcomes after stroke. | [100] |
| 5 | Acute ischemic stroke | Pilot study | Plasma | The levels of plasma CCL2 in patients >60 years was higher, compared with patients ≤60 years old. | [101] | |
| 6 | Ischemic stroke | Analytical study | Blood | 71 | CCL2 gene polymorphism was associated with stroke. | [102] |
| 7 | Ischemic stroke | Comparative study | Cerebrospinal fluid | 23 | CCL2-mediated neuroinflammation was involved in the early process of brain injury in ischemic stroke. | [105] |
| 8 | Ischemic stroke | Analytical study | Blood | 69 | CCL2 level was independently related to clinical outcome scores at specific timepoints with ischemic stroke. | [107] |
| 9 | Spontaneous intracerebral hemorrhage | Prospective cohort study | Blood | 115 | CCL2 level was associated with poor outcome in patients with intracerebral hemorrhage. | [108] |
| 10 | Malignant middle cerebral artery infarction | Cytokine antibody array | Serums | 8 | The level of CCL2 may serve as a diagnostic biomarker or drug target for malignant middle cerebral artery infarction. | [109,110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, H.; Chen, L.; Tang, J.; Chen, Y.; Wang, L. The Role of CCL2/CCR2 Axis in Cerebral Ischemia-Reperfusion Injury and Treatment: From Animal Experiments to Clinical Trials. Int. J. Mol. Sci. 2022, 23, 3485. https://doi.org/10.3390/ijms23073485
Geng H, Chen L, Tang J, Chen Y, Wang L. The Role of CCL2/CCR2 Axis in Cerebral Ischemia-Reperfusion Injury and Treatment: From Animal Experiments to Clinical Trials. International Journal of Molecular Sciences. 2022; 23(7):3485. https://doi.org/10.3390/ijms23073485
Chicago/Turabian StyleGeng, Huixia, Luna Chen, Jing Tang, Yi’ang Chen, and Lai Wang. 2022. "The Role of CCL2/CCR2 Axis in Cerebral Ischemia-Reperfusion Injury and Treatment: From Animal Experiments to Clinical Trials" International Journal of Molecular Sciences 23, no. 7: 3485. https://doi.org/10.3390/ijms23073485
APA StyleGeng, H., Chen, L., Tang, J., Chen, Y., & Wang, L. (2022). The Role of CCL2/CCR2 Axis in Cerebral Ischemia-Reperfusion Injury and Treatment: From Animal Experiments to Clinical Trials. International Journal of Molecular Sciences, 23(7), 3485. https://doi.org/10.3390/ijms23073485






