Therapeutic Applications of Adeno-Associated Virus (AAV) Gene Transfer of HLA-G in the Eye
Abstract
:1. Introduction
2. HLA-G and the Eye
2.1. Role of HLA-G in the Eye
HLA-G Tissue Locations in the Eye
2.2. Development of AAV-HLA-G as a Therapeutic
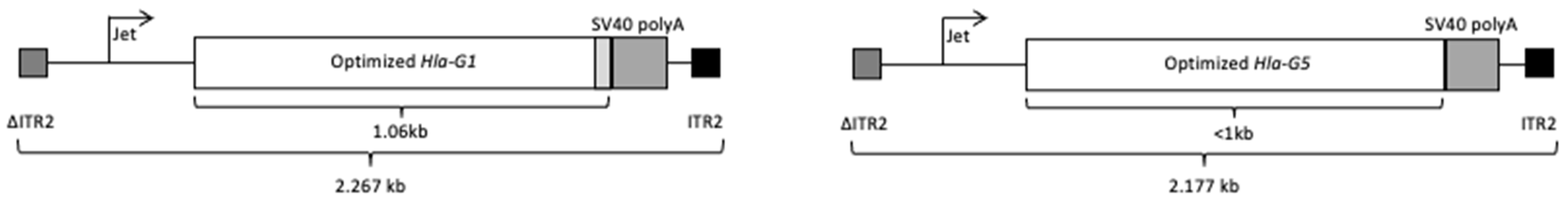
Optimization and In Vitro Validation
2.3. Corneal Applications of AAV-HLA-G
2.3.1. Reduction of Corneal Inflammation, Vascularization, and Fibrosis in a Rabbit Model
2.3.2. Prevention of Corneal Transplant Rejection
2.4. AAV-HLA-G for Treatment of Ocular Graft vs. Host Disease and Potentially Dry Eye
2.5. AAV-HLA-G for Non-Infectious Uveitis
2.6. Limitations and Future Directions
3. Conclusions
4. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stein-Streilein, J.; Streilein, J.W. Anterior Chamber Associated Immune Deviation (ACAID): Regulation, Biological Relevance, and Implications for Therapy. Int. Rev. Immunol. 2002, 21, 123–152. [Google Scholar] [CrossRef] [PubMed]
- Niederkorn, J.Y. See No Evil, Hear No Evil, Do No Evil: The Lessons of Immune Privilege. Nat. Immunol. 2006, 7, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C. Immune Privilege and Angiogenic Privilege of the Cornea. Immune Response Eye 2007, 92, 50–57. [Google Scholar]
- Tan, W.; Zou, J.; Yoshida, S.; Jiang, B.; Zhou, Y. The Role of Inflammation in Age-Related Macular Degeneration. Int. J. Biol. Sci. 2020, 16, 2989–3001. [Google Scholar] [CrossRef]
- Farkouh, A.; Frigo, P.; Czejka, M. Systemic Side Effects of Eye Drops: A Pharmacokinetic Perspective. Clin. Ophthalmol. 2016, 10, 2433–2441. [Google Scholar] [CrossRef] [Green Version]
- Utine, C.A.; Stern, M.; Akpek, E.K. Clinical Review: Topical Ophthalmic Use of Cyclosporin A. Ocul. Immunol. Inflamm. 2010, 18, 352–361. [Google Scholar] [CrossRef]
- Bastola, P.; Song, L.; Gilger, B.C.; Hirsch, M.L. Adeno-Associated Virus Mediated Gene Therapy for Corneal Diseases. Pharmaceutics 2020, 12, 767. [Google Scholar] [CrossRef]
- Li, C.; Samulski, R.J. Engineering Adeno-Associated Virus Vectors for Gene Therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar] [CrossRef]
- Dias, F.C.; Castelli, E.C.; Collares, C.V.; Moreau, P.; Donadi, E.A. The Role of HLA-G Molecule and HLA-G Gene Polymorphisms in Tumors, Viral Hepatitis, and Parasitic Diseases. Front. Immunol. 2015, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Martín-Villa, J.M.; Vaquero-Yuste, C.; Molina-Alejandre, M.; Juarez, I.; Suárez-Trujillo, F.; Lopez-Nares, A.; Palacio-Gruber, J.; Barrera-Gutiérrez, L.; Fernández-Cruz, E.; Rodriguez-Sainz, C.; et al. HLA-G: Too Much or Too Little? Role in Cancer and Autoimmune Disease. Front. Immunol. 2022, 13, 796054. [Google Scholar] [CrossRef]
- Miraldi Utz, V.; Coussa, R.G.; Antaki, F.; Traboulsi, E.I. Gene Therapy for RPE65-Related Retinal Disease. Ophthalmic Genet. 2018, 39, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Prakash, V. Spinraza—A Rare Disease Success Story. Gene Ther. 2017, 24, 497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, M.L.; Conatser, L.M.; Smith, S.M.; Salmon, J.H.; Wu, J.; Buglak, N.E.; Davis, R.; Gilger, B.C. AAV Vector-Meditated Expression of HLA-G Reduces Injury-Induced Corneal Vascularization, Immune Cell Infiltration, and Fibrosis. Sci. Rep. 2017, 7, 17840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilger, B.C.; Conatser, L.M.; Salmon, J.H.; Davis, R.; Hirsch, M.L. AAV Vector-Mediated HLA-G Expression to Prevent Corneal Transplant Rejection. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3317. [Google Scholar]
- Nilles, J.; Roberts, D.; Salmon, J.H.; Hirsch, M.L.; Gilger, B.C. Immunomodulatory Gene Therapy for Treatment of Ocular Graft vs. Host Disease. Investig. Ophthalmol. Vis. Sci. 2021, 62, 798. [Google Scholar]
- Crabtree, E.; Song, L.; Llanga, T.; Bower, J.J.; Cullen, M.; Salmon, J.H.; Hirsch, M.L.; Gilger, B.C. AAV-Mediated Expression of HLA-G1/5 Reduces Severity of Experimental Autoimmune Uveitis. Sci. Rep. 2019, 9, 19864. [Google Scholar] [CrossRef]
- Carosella, E.D.; Rouas-Freiss, N.; Roux, D.T.-L.; Moreau, P.; LeMaoult, J. HLA-G. In Advances in Immunology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 127, pp. 33–144. ISBN 978-0-12-802245-0. [Google Scholar]
- Fons, P.; Chabot, S.; Cartwright, J.E.; Lenfant, F.; L’Faqihi, F.; Giustiniani, J.; Herault, J.-P.; Gueguen, G.; Bono, F.; Savi, P.; et al. Soluble HLA-G1 Inhibits Angiogenesis through an Apoptotic Pathway and by Direct Binding to CD160 Receptor Expressed by Endothelial Cells. Blood 2006, 108, 2608–2615. [Google Scholar] [CrossRef] [Green Version]
- Le Discorde, M.; Moreau, P.; Sabatier, P.; Legeais, J.-M.; Carosella, E.D. Expression of HLA-G in Human Cornea, an Immune-Privileged Tissue. Hum. Immunol. 2003, 64, 1039–1044. [Google Scholar] [CrossRef] [Green Version]
- Robert, P.Y.; Lasalmonie, C.; Cogné, M.; Adenis, J.P.; Drouet, M. HLA-G and Classical HLA Class I Transcripts in Various Components of the Adult Human Eye: HLA Transcripts in the Human Eye. Eur. J. Immunogenet. 1999, 26, 271–274. [Google Scholar] [CrossRef]
- Svendsen, S.G.; Udsen, M.S.; Daouya, M.; Funck, T.; Wu, C.-L.; Carosella, E.D.; LeMaoult, J.; Hviid, T.V.F.; Faber, C.; Nissen, M.H. Expression and Differential Regulation of HLA-G Isoforms in the Retinal Pigment Epithelial Cell Line, ARPE-19. Hum. Immunol. 2017, 78, 414–420. [Google Scholar] [CrossRef]
- Hunt, J.S.; Petroff, M.G.; McIntire, R.H.; Ober, C. HLA-G and Immune Tolerance in Pregnancy. FASEB J. 2005, 19, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Ishitani, A.; Geraghty, D.E. Alternative Splicing of HLA-G Transcripts Yields Proteins with Primary Structures Resembling Both Class I and Class II Antigens. Proc. Natl. Acad. Sci. USA 1992, 89, 3947–3951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, A.W.; Ng, T.F. Negative Regulators That Mediate Ocular Immune Privilege. J. Leukoc. Biol. 2018, 103, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Böhringer, D.; Grotejohann, B.; Ihorst, G.; Reinshagen, H.; Spierings, E.; Reinhard, T. Rejection Prophylaxis in Corneal Transplant. Dtsch. Aerzteblatt Online 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niederkorn, J.Y. High Risk Corneal Allografts and Why They Lose Their Immune Privilege. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 493. [Google Scholar] [CrossRef] [Green Version]
- Keating, A.M.; Jacobs, D.S. Anti-VEGF Treatment of Corneal Neovascularization. Ocul. Surf. 2011, 9, 227–238. [Google Scholar] [CrossRef]
- Chang, J.-H.; Garg, N.K.; Lunde, E.; Han, K.-Y.; Jain, S.; Azar, D.T. Corneal Neovascularization: An Anti-VEGF Therapy Review. Surv. Ophthalmol. 2012, 57, 415–429. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, Y.; Wei, Z.; Cao, K.; Su, G.; Hamrah, P.; Labbe, A.; Liang, Q. Characteristics of Toxic Keratopathy, an In Vivo Confocal Microscopy Study. Transl. Vis. Sci. Technol. 2021, 10, 11. [Google Scholar] [CrossRef]
- Wilson, S.E. Corneal Myofibroblasts and Fibrosis. Exp. Eye Res. 2020, 201, 108272. [Google Scholar] [CrossRef]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167. [Google Scholar] [CrossRef] [Green Version]
- Zhong, W.; Montana, M.; Santosa, S.M.; Isjwara, I.D.; Huang, Y.H.; Han, K.Y.; O’Neil, C.; Wang, A.; Cortina, M.S.; de la Cruz, J.; et al. Angiogenesis and Lymphangiogenesis in Corneal Transplantation—A Review. Surv. Ophthalmol. 2018, 63, 453–479. [Google Scholar] [CrossRef] [PubMed]
- Westeneng, A.; Hettinga, Y.; Lokhorst, H.; Verdonck, L.; Van Dorp, S.; Rothova, A. Ocular Graft-versus-Host Disease after Allogeneic Stem Cell Transplantation. Cornea 2010, 29, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Inamoto, Y.; Valdés-Sanz, N.; Ogawa, Y.; Alves, M.; Berchicci, L.; Galvin, J.; Greinix, H.; Hale, G.A.; Horn, B.; Kelly, D.; et al. Ocular Graft-versus-Host Disease after Hematopoietic Cell Transplantation: Expert Review from the Late Effects and Quality of Life Working Committee of the CIBMTR and Transplant Complications Working Party of the EBMT. Bone Marrow Transplant. 2019, 54, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Cooke, K.R.; Luznik, L.; Sarantopoulos, S.; Hakim, F.T.; Jagasia, M.; Fowler, D.H.; van den Brink, M.R.M.; Hansen, J.A.; Parkman, R.; Miklos, D.B.; et al. The Biology of Chronic Graft-versus-Host Disease: A Task Force Report from the National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2017, 23, 211–234. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, Y.; Kuwana, M.; Yamazaki, K.; Mashima, Y.; Yamada, M.; Mori, T.; Okamoto, S.; Oguchi, Y.; Kawakami, Y. Periductal Area as the Primary Site for T-Cell Activation in Lacrimal Gland Chronic Graft-versus-Host Disease. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1888–1896. [Google Scholar] [CrossRef] [Green Version]
- Abud, T.B.; Amparo, F.; Saboo, U.S.; Di Zazzo, A.; Dohlman, T.H.; Ciolino, J.B.; Hamrah, P.; Dana, R. A Clinical Trial Comparing the Safety and Efficacy of Topical Tacrolimus versus Methylprednisolone in Ocular Graft-versus-Host Disease. Ophthalmology 2016, 123, 1449–1457. [Google Scholar] [CrossRef] [Green Version]
- Inamoto, Y.; Martin, P.J.; Chai, X.; Jagasia, M.; Palmer, J.; Pidala, J.; Cutler, C.; Pavletic, S.Z.; Arora, M.; Jacobsohn, D.; et al. Clinical Benefit of Response in Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2012, 18, 1517–1524. [Google Scholar] [CrossRef] [Green Version]
- Shamloo, K.; Barbarino, A.; Alfuraih, S.; Sharma, A. Graft versus Host Disease-Associated Dry Eye: Role of Ocular Surface Mucins and the Effect of Rebamipide, a Mucin Secretagogue. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4511–4519. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Carrasco, R.; Sánchez-Abarca, L.I.; Nieto-Gómez, C.; García, E.M.; Ramos, T.L.; Velasco, A.; Sánchez-Guijo, F.; Aijón, J.; Hernández-Galilea, E. Assessment of Dry Eye in a GVHD Murine Model: Approximation through Tear Osmolarity Measurement. Exp. Eye Res. 2017, 154, 64–69. [Google Scholar] [CrossRef]
- Perez, V.L.; Barsam, A.; Duffort, S.; Urbieta, M.; Barreras, H.; Lightbourn, C.; Komanduri, K.V.; Levy, R.B. Novel Scoring Criteria for the Evaluation of Ocular Graft-versus-Host Disease in a Preclinical Allogeneic Hematopoietic Stem Cell Transplantation Animal Model. Biol. Blood Marrow Transplant. 2016, 22, 1765–1772. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Llanga, T.; Conatser, L.M.; Zaric, V.; Gilger, B.C.; Hirsch, M.L. Serotype Survey of AAV Gene Delivery via Subconjunctival Injection in Mice. Gene Ther. 2018, 25, 402–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilger, B.C. Immune Relevant Models for Ocular Inflammatory Diseases. ILAR J. 2018, 59, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Thorne, J.E.; Suhler, E.; Skup, M.; Tari, S.; Macaulay, D.; Chao, J.; Ganguli, A. Prevalence of Noninfectious Uveitis in the United States: A Claims-Based Analysis. JAMA Ophthalmol. 2016, 134, 1237. [Google Scholar] [CrossRef] [PubMed]
- Deeg, C.A.; Hauck, S.M.; Amann, B.; Pompetzki, D.; Altmann, F.; Raith, A.; Schmalzl, T.; Stangassinger, M.; Ueffing, M. Equine Recurrent Uveitis—A Spontaneous Horse Model of Uveitis. Ophthalmic Res. 2008, 40, 151–153. [Google Scholar] [CrossRef] [Green Version]
- Jones, N. The Manchester Uveitis Clinic: The First 3000 Patients, 2: Uveitis Manifestations, Complications, Medical and Surgical Management. Ocul. Immunol. Inflamm. 2015, 23, 127–134. [Google Scholar] [CrossRef]
- Srivastava, A.; Rajappa, M.; Kaur, J. Uveitis: Mechanisms and Recent Advances in Therapy. Clin. Chim. Acta 2010, 411, 1165–1171. [Google Scholar] [CrossRef]
- Willermain, F.; Rosenbaum, J.T.; Bodaghi, B.; Rosenzweig, H.L.; Childers, S.; Behrend, T.; Wildner, G.; Dick, A.D. Interplay between Innate and Adaptive Immunity in the Development of Non-Infectious Uveitis. Prog. Retin. Eye Res. 2012, 31, 182–194. [Google Scholar] [CrossRef] [Green Version]
- Crabtree, E.; Gilger, B.C.; Hirsch, M.; Bower, J.; Smith, S.; Salmon, B.; Song, L. Prevention of Experimental Autoimmune Uveitis by Intravitreal AAV-EqIL-10. Investig. Ophthalmol. Vis. Sci. 2021, 62, 994. [Google Scholar]
- Chu, C.; Barker, S.; Dick, A.; Ali, R. Gene Therapy for Noninfectious Uveitis. Ocul. Immunol. Inflamm. 2012, 20, 394–405. [Google Scholar] [CrossRef]
- Ildefonso, C.J.; Jaime, H.; Biswal, M.R.; Boye, S.E.; Li, Q.; Hauswirth, W.W.; Lewin, A.S. Gene Therapy with the Caspase Activation and Recruitment Domain Reduces the Ocular Inflammatory Response. Mol. Ther. 2015, 23, 875–884. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Tao, L.; Zheng, S.; Lin, R.; Fu, X.; Chen, Z.; Lei, C.; Wang, J.; Li, H.; Li, Q. AAV8-Mediated Angiotensin-Converting Enzyme 2 Gene Delivery Prevents Experimental Autoimmune Uveitis by Regulating MAPK, NF-ΚB and STAT3 Pathways. Sci. Rep. 2016, 6, 31912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, R.K.; Silver, P.B.; Caspi, R.R. Rodent Models of Experimental Autoimmune Uveitis. In Autoimmunity; Perl, A., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 900, pp. 443–469. ISBN 978-1-60761-719-8. [Google Scholar]
- Caspi, R.R. Animal Models of Autoimmune and Immune-Mediated Uveitis. Drug Discov. Today Dis. Models 2006, 3, 3–9. [Google Scholar] [CrossRef]
- Cukras, C.; Wiley, H.E.; Jeffrey, B.G.; Sen, H.N.; Turriff, A.; Zeng, Y.; Vijayasarathy, C.; Marangoni, D.; Ziccardi, L.; Kjellstrom, S. Retinal AAV8-RS1 Gene Therapy for X-Linked Retinoschisis: Initial Findings from a Phase I/IIa Trial by Intravitreal Delivery. Mol. Ther. 2018, 26, 2282–2294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandler, L.C.; McClements, M.E.; Yusuf, I.H.; de la Camara, C.M.-F.; MacLaren, R.E.; Xue, K. Characterizing the Cellular Immune Response to Subretinal AAV Gene Therapy in the Murine Retina. Mol. Ther.-Methods Clin. Dev. 2021, 22, 52–65. [Google Scholar] [CrossRef]
- Tummala, G.; Crain, A.; Rowlan, J.; Pepple, K.L. Characterization of Gene Therapy Associated Uveitis Following Intravitreal Adeno-Associated Virus Injection in Mice. Investig. Ophthalmol. Vis. Sci. 2021, 62, 41. [Google Scholar] [CrossRef]
- Chung, S.H.; Mollhoff, I.N.; Mishra, A.; Sin, T.-N.; Ngo, T.; Ciulla, T.; Sieving, P.; Thomasy, S.M.; Yiu, G. Host Immune Responses after Suprachoroidal Delivery of AAV8 in Nonhuman Primate Eyes. Hum. Gene Ther. 2021, 32, 682–693. [Google Scholar] [CrossRef]
- Xiong, W.; Wu, D.M.; Xue, Y.; Wang, S.K.; Chung, M.J.; Ji, X.; Rana, P.; Zhao, S.R.; Mai, S.; Cepko, C.L. AAV Cis-Regulatory Sequences Are Correlated with Ocular Toxicity. Proc. Natl. Acad. Sci. USA 2019, 116, 5785–5794. [Google Scholar] [CrossRef] [Green Version]

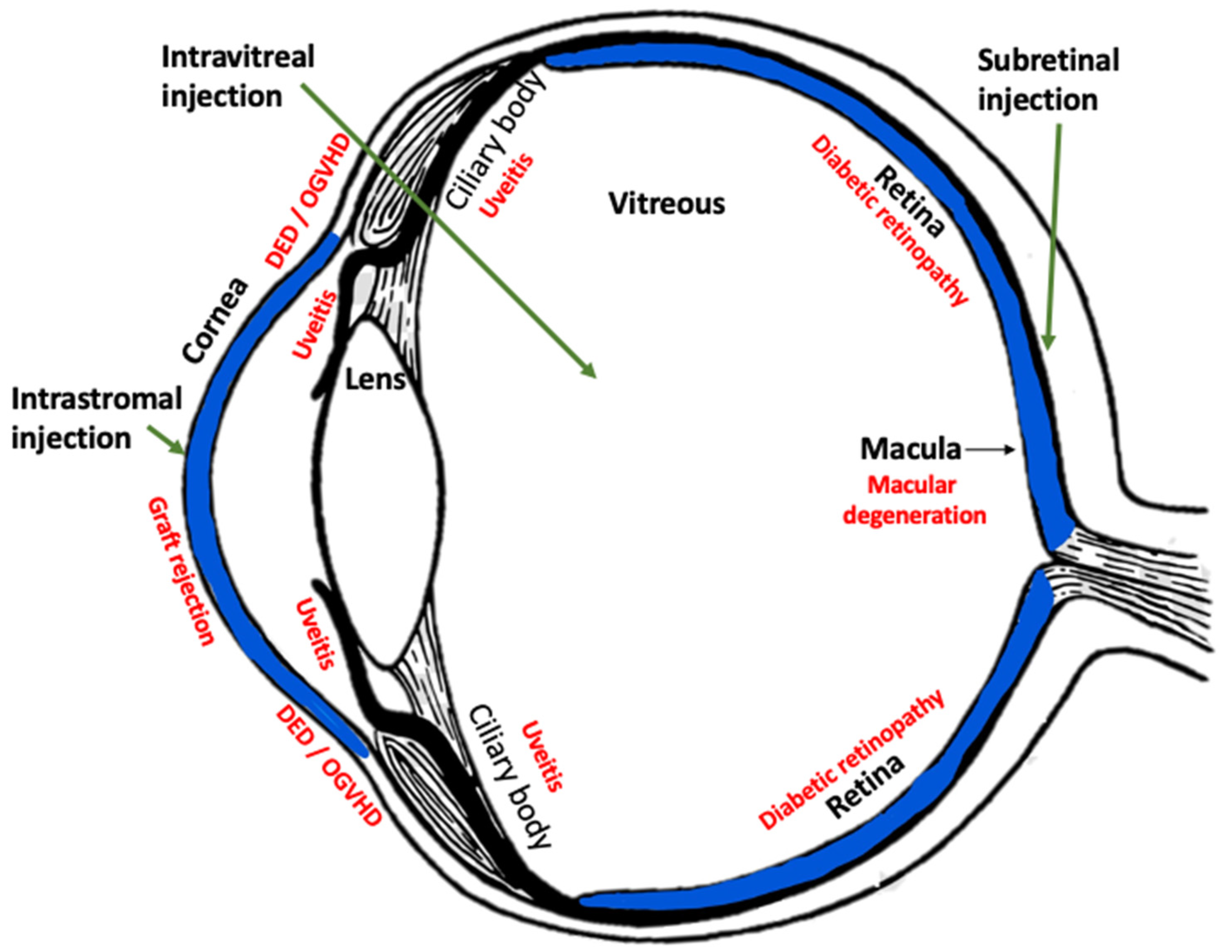
| Target Tissue | Disease | Route of Therapy | Reference |
|---|---|---|---|
| Cornea | Injury/inflammation/neovascularization/fibrosis | Intrastromal injection | Hirsch et al., 2017 [13] |
| Cornea | Transplant rejection | Ex vivo graft incubation | Bastola et al., 2020 [7] Gilger et al., 2018 [14] |
| Conjunctiva | Ocular graft vs. host disease/dry eye | Subconjunctival injection | Nilles et al., 2021 [15] |
| Uvea | Non-infectious uveitis | Intravitreal injection | Crabtree et al., 2019 [16] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilger, B.C.; Hirsch, M.L. Therapeutic Applications of Adeno-Associated Virus (AAV) Gene Transfer of HLA-G in the Eye. Int. J. Mol. Sci. 2022, 23, 3465. https://doi.org/10.3390/ijms23073465
Gilger BC, Hirsch ML. Therapeutic Applications of Adeno-Associated Virus (AAV) Gene Transfer of HLA-G in the Eye. International Journal of Molecular Sciences. 2022; 23(7):3465. https://doi.org/10.3390/ijms23073465
Chicago/Turabian StyleGilger, Brian C., and Matthew L. Hirsch. 2022. "Therapeutic Applications of Adeno-Associated Virus (AAV) Gene Transfer of HLA-G in the Eye" International Journal of Molecular Sciences 23, no. 7: 3465. https://doi.org/10.3390/ijms23073465
APA StyleGilger, B. C., & Hirsch, M. L. (2022). Therapeutic Applications of Adeno-Associated Virus (AAV) Gene Transfer of HLA-G in the Eye. International Journal of Molecular Sciences, 23(7), 3465. https://doi.org/10.3390/ijms23073465






