The EPH/Ephrin System in Gynecological Cancers: Focusing on the Roots of Carcinogenesis for Better Patient Management
Abstract
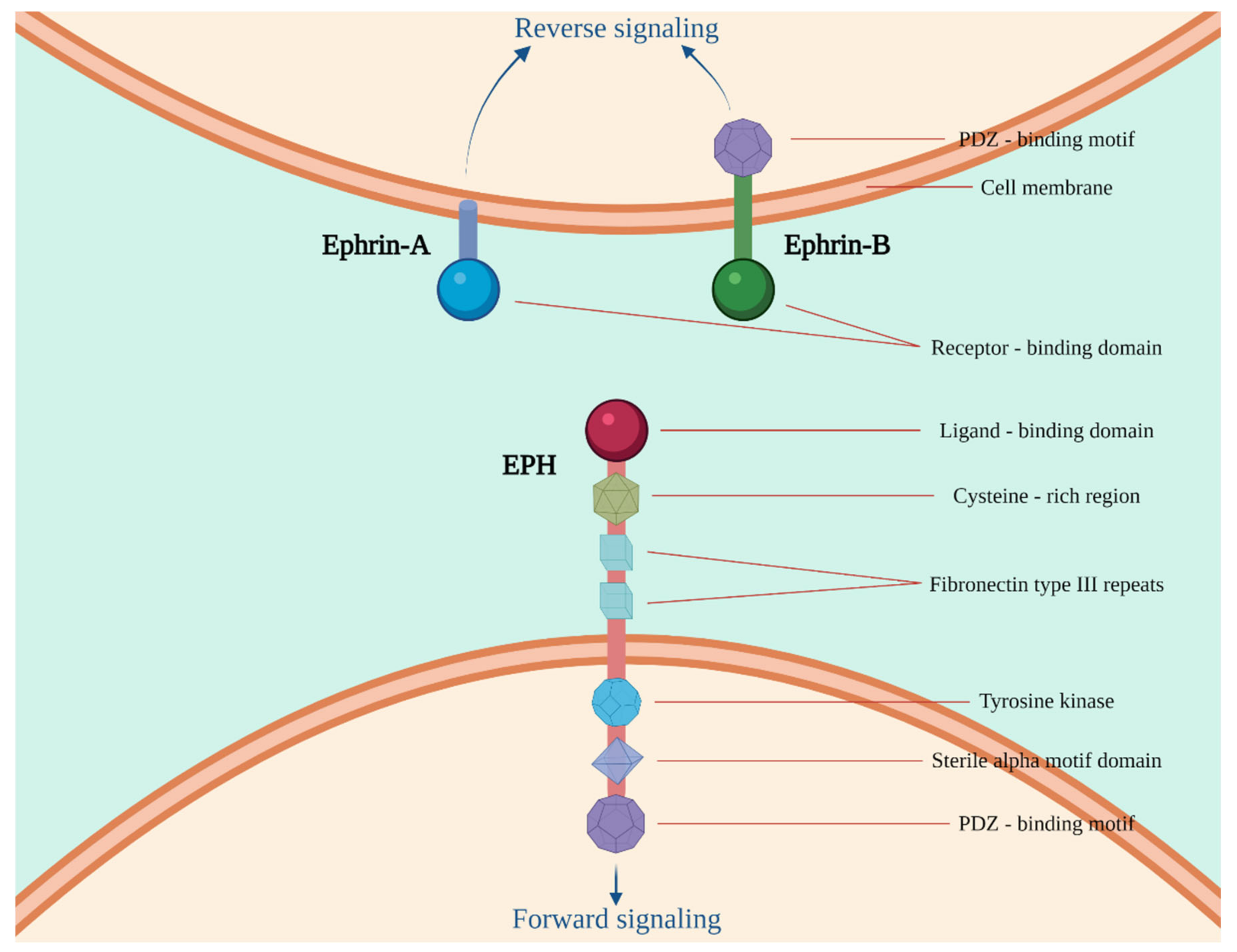
1. Introduction
| EPHs/Ephrins | Molecular Function in Carcinogenesis |
|---|---|
| EPHA1 | High affinity to ephrin-A1. Cell attachment induction to the extracellular matrix. Cell spreading and motility inhibition through integrin-linked protein kinase (ILK) regulation and Ras Homolog Family Member A (RHOA) and Rac Family Small GTPase (RAC) downstream. |
| EPHA2 | Activation by the ligand ephrin-A1. Regulation of cell migration, integrin-mediated adhesion, proliferation and differentiation through Desmoglein-1, and inhibition of the extracellular signal-regulated kinases 1/2 (ERK1/2) signaling pathway. |
| EPHA3 | High affinity to ephrin-A5. Regulation of cell–cell adhesion, cytoskeletal organization and cell migration. |
| EPHA4 | Activation by ephrin-A1 and -B3. Cell morphology modulation and integrin-dependent cell adhesion through regulation of the RAC, Ras-related protein (RAP) and Rhodopsin (Rho) GTPases activity. |
| EPHA5 | High affinity to ephrin-A5. |
| EPHA6 | Contact-dependent bidirectional signaling into neighboring cells. |
| EPHA7 | High affinity to ephrin-A5. |
| EPHA8 | Activation by ephrin-A2, -3, and -5. |
| EPHA10 | Activation by ephrin-A3, -4, and -5. |
| EPHB1 | Activation by ephrin-B1, -2, and -3. Cell migration regulation through activation of the ERK signaling pathway. Cell adhesion regulation through activation of the c-Jun N-terminal kinase (JNK) signaling cascade. |
| EPHB2 | Activation by ephrin-B2. |
| EPHB3 | Activation by ephrin-B2. |
| EPHB4 | Activation by ephrin-B2. Regulation of cell adhesion and migration. Cellular repulsion and segregation control. |
| EPHB6 | High affinity to ephrin-B1, and -2. Cell adhesion and migration modulation, inhibition of JNK activation, T-cell receptor-induced IL-2 secretion, and CD25 expression upon stimulation with ephrin-B2. |
| ephrin-A1 | Induction of RAC1 GTPase activation and vascular endothelial cell migration and assembly. |
| ephrin-A2 | Contact-dependent bidirectional signaling into neighboring cells. |
| ephrin-A3 | Contact-dependent bidirectional signaling into neighboring cells. |
| ephrin-A4 | Contact-dependent bidirectional signaling into neighboring cells. |
| ephrin-A5 | Compartmentalized signaling induction within a caveolae-like membrane microdomain when bound to the extracellular domain of its cognate receptor through the activity of the Fyn tyrosine kinase. Cell-cell adhesion and cytoskeletal organization regulation. |
| ephrin-B1 | Contact-dependent bidirectional signaling into neighboring cells. |
| ephrin-B2 | Cellular repulsion and segregation control. |
| ephrin-B3 | Contact-dependent bidirectional signaling into neighboring cells. |
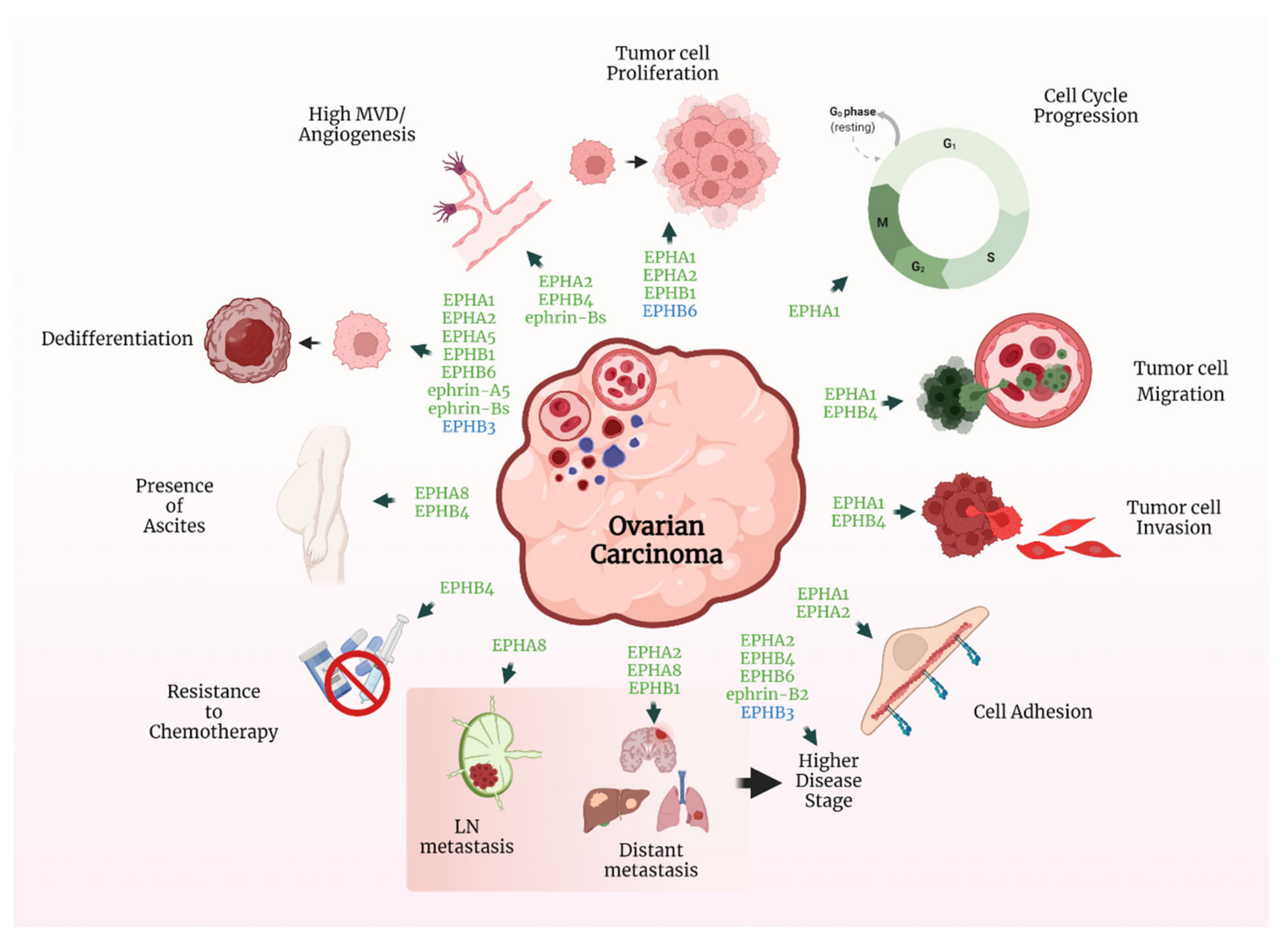
2. Ovarian Cancer
2.1. The EPH/Ephrin System in OC Cell Lines and Human Xenograft Models
2.2. The EPH/Ephrin System in OC Patient Tissue Samples
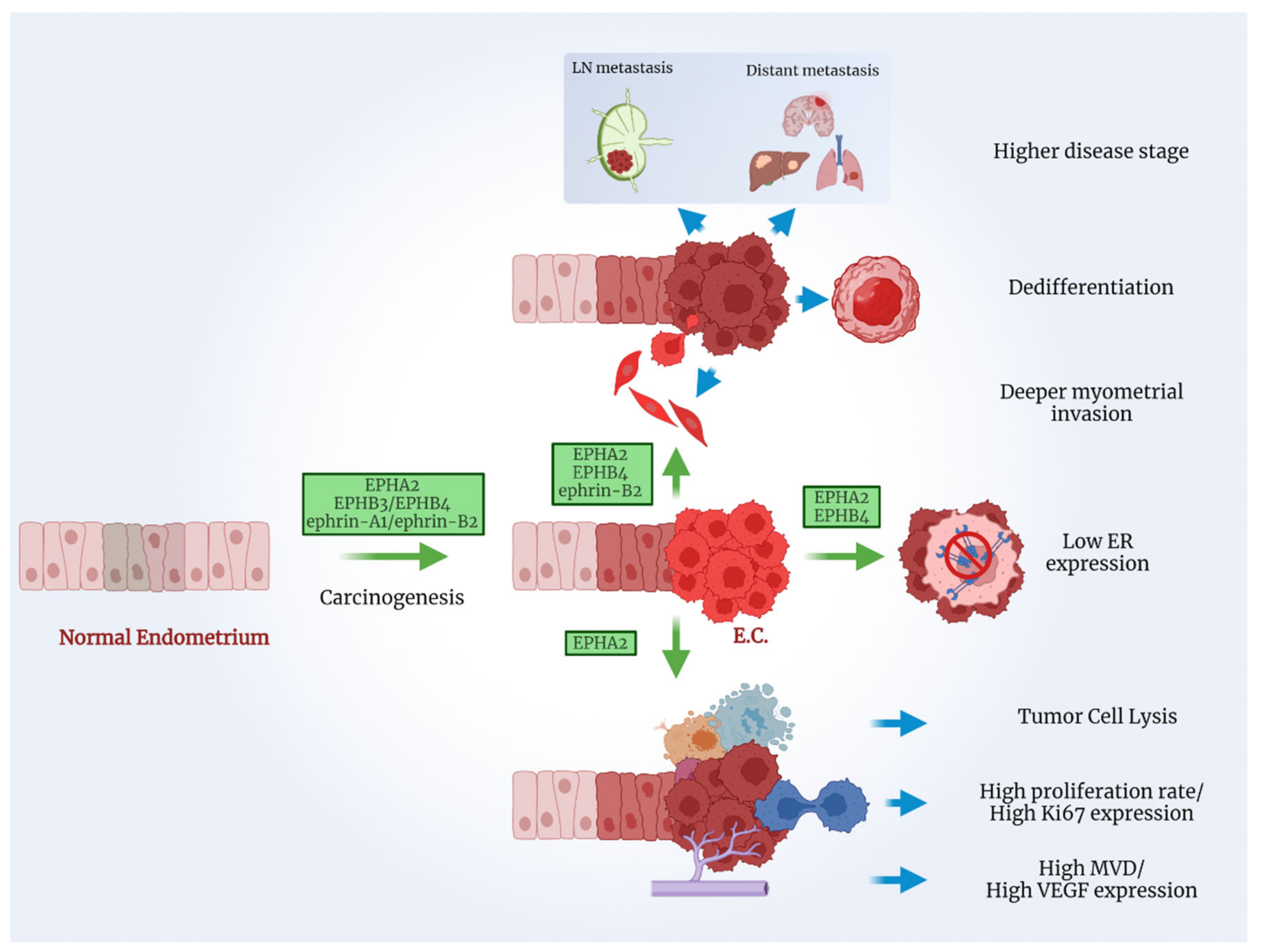
3. Endometrial Cancer
3.1. The EPH/Ephrin System in EC Cell Lines and Human Xenograft Models
3.2. The EPH/Ephrin System in EC Patient Tissue Samples
4. Cervical Cancer
4.1. The EPH/Ephrin System in CC Cell Lines and Human Xenograft Models
4.2. The EPH/Ephrin System in CC Patient Tissue Samples
4.3. The EPH/Ephrin System as a Treatment Target in GC
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eph Nomenclature Committee. Unified nomenclature for Eph family receptors and their ligands, the ephrins. Cell 1997, 90, 403–404. [Google Scholar] [CrossRef]
- Wei, Q.; Liu, J.; Wang, N.; Zhang, X.; Jin, J.; Chin-Sang, I.; Zheng, J.; Jia, Z. Structures of an Eph receptor tyrosine kinase and its potential activation mechanism. Acta Cryst. D Biol. Crystallogr. 2014, 70, 3135–3143. [Google Scholar] [CrossRef] [PubMed]
- Kania, A.; Klein, R. Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat. Rev. Mol. Cell Biol. 2016, 17, 240–256. [Google Scholar] [CrossRef] [PubMed]
- Shiuan, E.; Chen, J. Eph Receptor Tyrosine Kinases in Tumor Immunity. Cancer Res. 2016, 76, 6452–6457. [Google Scholar] [CrossRef] [PubMed]
- Gene. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. 2004. Available online: https://www.ncbi.nlm.nih.gov/gene/ (accessed on 29 December 2021).
- Stelzer, G.; Rosen, R.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Iny Stein, T.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The genecards suite: From gene data mining to disease genome sequence analysis. Curr. Protoc. Bioinform. 2016, 54, 1–30. [Google Scholar] [CrossRef]
- Yamada, T.; Yuasa, M.; Masaoka, T.; Taniyama, T.; Maehara, H.; Torigoe, I.; Yoshii, T.; Shinomiya, K.; Okawa, A.; Sotome, S. After repeated division, bone marrow stromal cells express inhibitory factors with osteogenic capabilities, and EphA5 is a primary candidate. Bone 2013, 57, 343–354. [Google Scholar] [CrossRef]
- Hiramoto-Yamaki, N.; Takeuchi, S.; Ueda, S.; Harada, K.; Fujimoto, S.; Negishi, M.; Katoh, H. Ephexin4 and EphA2 mediate cell migration through a RhoG-dependent mechanism. J. Cell Biol. 2010, 190, 461–477. [Google Scholar] [CrossRef]
- Neill, T.; Buraschi, S.; Goyal, A.; Sharpe, C.; Natkanski, E.; Schaefer, L.; Morrione, A.; Iozzo, R.V. EphA2 is a functional receptor for the growth factor progranulin. J. Cell Biol. 2016, 215, 687–703. [Google Scholar] [CrossRef]
- Liang, L.Y.; Patel, O.; Janes, P.W.; Murphy, J.M.; Lucet, I.S. Eph receptor signalling: From catalytic to non-catalytic functions. Oncogene 2019, 38, 6567–6584. [Google Scholar] [CrossRef]
- Coulthard, M.G.; Duffy, S.; Down, M.; Evans, B.; Power, M.; Smith, F.; Stylianou, C.; Kleikamp, S.; Oates, A.; Lackmann, M.; et al. The role of the Eph-ephrin signalling system in the regulation of developmental patterning. Int. J. Dev. Biol. 2002, 46, 375–384. [Google Scholar]
- Rudno-Rudzińska, J.; Kielan, W.; Frejlich, E.; Kotulski, K.; Hap, W.; Kurnol, K.; Dzierżek, P.; Zawadzki, M.; Hałoń, A. A review on Eph/ephrin, angiogenesis and lymphangiogenesis in gastric, colorectal and pancreatic cancers. Chin. J. Cancer Res. 2017, 29, 303–312. [Google Scholar] [CrossRef] [PubMed]
- GeneCards: The Human Gene Database. Available online: https://www.genecards.org/ (accessed on 11 March 2022).
- Edwards, C.M.; Mundy, G.R. Eph receptors and ephrin signaling pathways: A role in bone homeostasis. Int. J. Med. Sci. 2008, 5, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Pergaris, A.; Danas, E.; Goutas, D.; Sykaras, A.G.; Soranidis, A.; Theocharis, S. The clinical impact of the EPH/Ephrin system in cancer: Unwinding the thread. Int. J. Mol. Sci. 2021, 22, 8412. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Gynecologic Cancer Incidence, United States—2012–2016. USCS Data Brief, no 11; Centers for Disease Control and Prevention, US Department of Health and Human Services: Atlanta, GA, USA, 2019. [Google Scholar]
- American Cancer Society. Key Statistics for Ovarian Cancer; American Cancer Society: Atlanta, GA, USA, 2021. [Google Scholar]
- Prat, J. Ovarian carcinomas: Five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012, 460, 237–249. [Google Scholar] [CrossRef]
- Seebacher, V.; Reinthaller, A.; Koelbl, H.; Concin, N.; Nehoda, R.; Polterauer, S. The Impact of the Duration of Adjuvant Chemotherapy on Survival in Patients with Epithelial Ovarian Cancer—A Retrospective Study. PLoS ONE 2017, 12, e0169272. [Google Scholar] [CrossRef]
- American Cancer Society. Survival Rates for Ovarian Cancer; American Cancer Society: Atlanta, GA, USA, 2021. [Google Scholar]
- Książek, K. Molecular Biology of Ovarian Cancer: From Mechanisms of Intraperitoneal Metastasis to Therapeutic Opportunities. Cancers 2021, 13, 1661. [Google Scholar] [CrossRef]
- Cui, Y.; Wu, B.O.; Flamini, V.; Evans, B.A.J.; Zhou, D.; Jiang, W.G. Knockdown of EPHA1 using CRISPR/CAS9 suppresses aggressive properties of ovarian cancer cells. Anticancer Res. 2017, 37, 4415–4424. [Google Scholar]
- Jin, Y.; Zou, Y.; Wan, L.; Lu, M.; Liu, Y.; Huang, G.; Wang, J.; Xi, Q. Decreased Eph receptor-A1 expression is related to grade in ovarian serous carcinoma. Mol. Med. Rep. 2018, 17, 5409–5415. [Google Scholar] [CrossRef]
- Takasugi, M.; Okada, R.; Takahashi, A.; Virya Chen, D.; Watanabe, S.; Hara, E. Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through EphA2. Nat. Commun. 2017, 8, 15729. [Google Scholar] [CrossRef]
- Thaker, P.H.; Deavers, M.; Celestino, J.; Thornton, A.; Fletcher, M.S.; Landen, C.N.; Kinch, M.S.; Kiener, P.A.; Sood, A.K. EphA2 expression is associated with aggressive features in ovarian carcinoma. Clin. Cancer Res. 2004, 10, 5145–5150. [Google Scholar] [CrossRef]
- Han, L.; Dong, Z.; Qiao, Y.; Kristensen, G.B.; Holm, R.; Nesland, J.M.; Suo, Z. The clinical significance of EphA2 and Ephrin A-1 in epithelial ovarian carcinomas. Gynecol. Oncol. 2005, 99, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Shahzad, M.M.; Wang, H.; Landen, C.N.; Kim, S.W.; Allen, J.; Nick, A.M.; Jennings, N.; Kinch, M.S.; Bar-Eli, M.; et al. EphA2 overexpression promotes ovarian cancer growth. Cancer Biol. Ther. 2008, 7, 1098–1103. [Google Scholar] [CrossRef]
- Wu, Q.; Lind, G.E.; Aasheim, H.C.; Micci, F.; Silins, I.; Tropé, C.G.; Nesland, J.M.; Lothe, R.A.; Suo, Z. The EPH receptor Bs (EPHBs) promoters are unmethylated in colon and ovarian cancers. Epigenetics 2007, 2, 237–243. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Davidson, B.A.; Rubatt, J.M.; Corcoran, D.L.; Teoh, D.K.; Bernardini, M.Q.; Grace, L.A.; Soper, W.J.; Berchuck, A.; Siamakpour-Reihani, S.; Chen, W.; et al. Differential Angiogenic Gene Expression in TP53 Wild-Type and Mutant Ovarian Cancer Cell Lines. Front. Oncol. 2014, 4, 163. [Google Scholar] [CrossRef]
- Kumar, S.R.; Masood, R.; Spannuth, W.A.; Singh, J.; Scehnet, J.; Kleiber, G.; Jennings, N.; Deavers, M.; Krasnoperov, V.; Dubeau, L.; et al. The receptor tyrosine kinase EphB4 is overexpressed in ovarian cancer, provides survival signals and predicts poor outcome. Br. J. Cancer 2007, 96, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Luo, D.; Li, K.; Liu, R.; Liu, Y.; Zhu, T.; Deng, D.; Zhou, J.; Meng, L.; Wang, S.; et al. Suppression of EphB4 improves the inhibitory effect of mTOR shRNA on the biological behaviors of ovarian cancer cells by down-regulating Akt phosphorylation. J. Huazhong Univ. Sci. Technol. Med. Sci. 2012, 32, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Deregowski, V.; Delhalle, S.; Benoit, V.; Bours, V.; Merville, M.P. Identification of cytokine-induced nuclear factor-kappaB target genes in ovarian and breast cancer cells. Biochem. Pharmacol. 2002, 64, 873–881. [Google Scholar] [CrossRef]
- Jukonen, J.; Moyano-Galceran, L.; Höpfner, K.; Pietilä, E.A.; Lehtinen, L.; Huhtinen, K.; Gucciardo, E.; Hynninen, J.; Hietanen, S.; Grénman, S.; et al. Aggressive and recurrent ovarian cancers upregulate ephrinA5, a non-canonical effector of EphA2 signaling duality. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Wong, Y.L.; Dali, A.Z.; Mohamed Rose, I.; Jamal, R.; Mokhtar, N.M. Potential molecular signatures in epithelial ovarian cancer by genome wide expression profiling. Asia Pac. J. Clin. Oncol. 2016, 12, e259–e268. [Google Scholar] [CrossRef]
- Herath, N.I.; Spanevello, M.D.; Sabesan, S.; Newton, T.; Cummings, M.; Duffy, S.; Lincoln, D.; Boyle, G.; Parsons, P.G.; Boyd, A.W. Over-expression of Eph and ephrin genes in advanced ovarian cancer: Ephrin gene expression correlates with shortened survival. BMC Cancer 2006, 6, 144. [Google Scholar] [CrossRef]
- Reinartz, S.; Finkernagel, F.; Adhikary, T.; Rohnalter, V.; Schumann, T.; Schober, Y.; Nockher, W.A.; Nist, A.; Stiewe, T.; Jansen, J.M.; et al. A transcriptome-based global map of signaling pathways in the ovarian cancer microenvironment associated with clinical outcome. Genome Biol. 2016, 17, 1–22. [Google Scholar] [CrossRef]
- Lin, Y.G.; Han, L.Y.; Kamat, A.A.; Merritt, W.M.; Landen, C.N.; Deavers, M.T.; Fletcher, M.S.; Urbauer, D.L.; Kinch, M.S.; Sood, A.K. EphA2 overexpression is associated with angiogenesis in ovarian cancer. Cancer 2007, 109, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Hamasaki, M.; Aoki, M.; Koga, K.; Koshikawa, N.; Miyamoto, S.; Nabeshima, K. Activated EphA2 processing by MT1-MMP is involved in malignant transformation of ovarian tumours in vivo. Anticancer Res. 2018, 38, 4257–4266. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, X.; Wei, X.; Wang, J. EphA5 protein, a potential marker for distinguishing histological grade and prognosis in 7 ovarian serous carcinoma. J. Ovarian Res. 2016, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, Y.; Jin, Q.; Wang, W.; Zhang, S.; Wang, X.; Zhang, Y.; Xu, X.; Huang, J. EphA8 is a prognostic marker for epithelial ovarian cancer. Oncotarget 2016, 7, 20801–20809. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wen, J.; Wang, H.; Guo, Q.; Shi, S.; Shi, Q.; Zhou, X.; Liu, Q.; Lu, G.; Wang, J. Loss of expression of EphB1 protein in serous carcinoma of ovary associated with metastasis and poor survival. Int. J. Clin. Exp. Pathol. 2013, 7, 313–321. [Google Scholar]
- Wu, Q.; Suo, Z.; Kristensen, G.B.; Baekelandt, M.; Nesland, J.M. The prognostic impact of EphB2/B4 expression on patients with advanced ovarian carcinoma. Gynecol. Oncol. 2006, 102, 15–21. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Q.; Wang, Y.; Wang, J.; Zhang, S. EphB3 protein is associated with histological grade and FIGO stage in ovarian serous carcinomas. APMIS 2017, 125, 122–127. [Google Scholar] [CrossRef]
- Alam, S.M.; Fujimoto, J.; Jahan, I.; Sato, E.; Tamaya, T. Coexpression of EphB4 and ephrinB2 in tumour advancement of ovarian cancers. Br. J. Cancer 2008, 98, 845–851. [Google Scholar] [CrossRef]
- Gu, Y.; Li, F.; Qian, N.; Chen, X.; Wang, H.; Wang, J. Expression of EphB6 in ovarian serous carcinoma is associated with grade, TNM stage and survival. J. Clin. Pathol. 2016, 69, 448–453. [Google Scholar] [CrossRef]
- Sabesan, S.S.; Wyld, D.; Herath, N.; Boyd, A. Analysis of expression of Eph/Ephrin tyrosine kinases in advanced ovarian cancer. J. Clin. Oncol. 2005, 23, 5105. [Google Scholar] [CrossRef]
- Castellvi, J.; Garcia, A.; de la Torre, J.; Hernandez, J.; Gil, A.; Xercavins, J.; Ramón y Cajal, S. Ephrin B expression in epithelial ovarian neoplasms correlates with tumor differentiation and angiogenesis. Hum. Pathol. 2006, 37, 883–889. [Google Scholar] [CrossRef]
- Schaner, M.E.; Ross, D.T.; Ciaravino, G.; Sorlie, T.; Troyanskaya, O.; Diehn, M.; Wang, Y.C.; Duran, G.E.; Sikic, T.L.; Caldeira, S.; et al. Gene expression patterns in ovarian carcinomas. Mol. Biol. Cell 2003, 14, 4376–4386. [Google Scholar] [CrossRef]
- Castellano, G.; Reid, J.F.; Alberti, P.; Carcangiu, M.L.; Tomassetti, A.; Canevari, S. New potential ligand-receptor signaling loops in ovarian cancer identified in multiple gene expression studies. Cancer Res. 2006, 66, 10709–10719. [Google Scholar] [CrossRef]
- American Cancer Society. Facts & Figures 2021; American Cancer Society: Atlanta, GA, USA, 2021. [Google Scholar]
- Sherman, M.E.; Bur, M.E.; Kurman, R.J. P53 in endometrial cancer and its putative precursors: Evidence for diverse pathways of tumorigenesis. Hum. Pathol. 1995, 26, 1268–1274. [Google Scholar] [CrossRef]
- Ueda, T.; Takai, N.; Nishida, M.; Nasu, K.; Narahara, H. Apicidin, a novel histone deacetylase inhibitor, has profound anti-growth activity in human endometrial and ovarian cancer cells. Int. J. Mol. Med. 2007, 19, 301–308. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Survival Rates for Endometrial Cancer; American Cancer Society: Atlanta, GA, USA, 2021. [Google Scholar]
- Wang, Y.; Yang, D.; Cogdell, D.; Hu, L.; Xue, F.; Broaddus, R.; Zhang, W. Genomic characterization of gene copy-number aberrations in endometrial carcinoma cell lines derived from endometrioid-type endometrial adenocarcinoma. Technol. Cancer Res. Treat. 2010, 9, 179–189. [Google Scholar] [CrossRef]
- Hudecek, R.; Kohlova, B.; Siskova, I.; Piskacek, M.; Knight, A. Blocking of EphA2 on Endometrial Tumor Cells Reduces Susceptibility to Vδ1 Gamma-Delta T-Cell-Mediated Killing. Front. Immunol. 2021, 12, 752646. [Google Scholar] [CrossRef]
- Fujii, H.; Fujiwara, H.; Horie, A.; Suginami, K.; Sato, Y.; Konishi, I. EphrinA1 stimulates cell attachment and inhibits cell aggregation through the EphA receptor pathway in human endometrial carcinoma-derived Ishikawa cells. Hum. Reprod. 2011, 26, 1163–1170. [Google Scholar] [CrossRef]
- Fujii, H.; Fujiwara, H.; Horie, A.; Sato, Y.; Konishi, I. Ephrin A1 induces intercellular dissociation in Ishikawa cells: Possible implication of the Eph-ephrin A system in human embryo implantation. Hum. Reprod. 2011, 26, 299–306. [Google Scholar] [CrossRef]
- Merritt, W.M.; Kamat, A.A.; Hwang, J.Y.; Bottsford-Miller, J.; Lu, C.; Lin, Y.G.; Coffey, D.; Spannuth, W.A.; Nugent, E.; Han, L.Y.; et al. Clinical and biological impact of EphA2 overexpression and angiogenesis in endometrial cancer. Cancer Biol. Ther. 2010, 10, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Kamat, A.A.; Coffey, D.; Merritt, W.M.; Nugent, E.; Urbauer, D.; Lin, Y.G.; Edwards, C.; Broaddus, R.; Coleman, R.L.; Sood, A.K. EphA2 overexpression is associated with lack of hormone receptor expression and poor outcome in endometrial cancer. Cancer 2009, 115, 2684–2692. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.M.; Fujimoto, J.; Jahan, I.; Sato, E.; Tamaya, T. Overexpression of ephrinB2 and EphB4 in tumor advancement of uterine endometrial cancers. Ann. Oncol. 2007, 18, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Berclaz, G.; Karamitopoulou, E.; Mazzucchelli, L.; Rohrbach, V.; Dreher, E.; Ziemiecki, A.; Andres, A.C. Activation of the receptor protein tyrosine kinase EphB4 in endometrial hyperplasia and endometrial carcinoma. Ann. Oncol. 2003, 14, 220–226. [Google Scholar] [CrossRef]
- Dong, L.D.; Cheng, X.L.; Zhou, L.; Huang, Q.; Li, J.C.; Yi, C.J. Overexpression of erythropoietin-producing hepatocyte receptor B4 and ephrin-B2 is associated with estrogen receptor expression in endometrial adenocarcinoma. Oncol. Lett. 2017, 13, 2109–2114. [Google Scholar] [CrossRef]
- Takai, N.; Miyazaki, T.; Fujisawa, K.; Nasu, K.; Miyakawa, I. Expression of receptor tyrosine kinase EphB4 and its ligand ephrin-B2 is associated with malignant potential in endometrial cancer. Oncol. Rep. 2001, 8, 567–573. [Google Scholar] [CrossRef]
- Takai, N.; Ueda, T.; Nishida, M.; Nasu, K.; Miyakawa, I. The relationship between oncogene expression and clinical outcome in endometrial carcinoma. Curr. Cancer Drug Targets 2004, 4, 511–520. [Google Scholar] [CrossRef]
- American Cancer Society. Key Statistics for Cervical Cancer; American Cancer Society: Atlanta, GA, USA, 2021. [Google Scholar]
- Fang, J.; Zhang, H.; Jin, S. Epigenetics and cervical cancer: From pathogenesis to therapy. Tumour Biol. 2014, 35, 5083–5093. [Google Scholar] [CrossRef]
- American Cancer Society. Treatment Options for Cervical Cancer, by Stage; American Cancer Society: Atlanta, GA, USA, 2021. [Google Scholar]
- American Cancer Society. Survival Rates for Cervical Cancer; American Cancer Society: Atlanta, GA, USA, 2021. [Google Scholar]
- Huang, C.; Chen, Z.; He, Y.; He, Z.; Ban, Z.; Zhu, Y.; Ding, L.; Yang, C.; Jeong, J.H.; Yuan, W.; et al. EphA2 promotes tumorigenicity of cervical cancer by up-regulating CDK6. J. Cell Mol. Med. 2021, 25, 2967–2975. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, W.; Cai, J.; Li, M.; Gao, Y.; Lin, W.; Li, Z. EphB2 promotes cervical cancer progression by inducing epithelial-mesenchymal transition. Hum. Pathol. 2014, 45, 372–381. [Google Scholar] [CrossRef]
- Duan, S.; Wu, A.; Chen, Z.; Yang, Y.; Liu, L.; Shu, Q. miR-204 Regulates Cell Proliferation and Invasion by Targeting EphB2 in Human Cervical Cancer. Oncol. Res. 2018, 26, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Holm, R.; de Putte, G.V.; Suo, Z.; Lie, A.K.; Kristensen, G.B. Expressions of EphA2 and EphrinA-1 in early squamous cell cervical carcinomas and their relation to prognosis. Int. J. Med. Sci. 2008, 5, 121–126. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, D.; Suo, Z.; Kristensen, G.B.; Li, S.; Troen, G.; Holm, R.; Nesland, J.M. Prognostic value of EphA2 and EphrinA-1 in squamous cell cervical carcinoma. Gynecol. Oncol. 2004, 94, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Phan, N.N.; Liu, S.; Wang, C.Y.; Hsu, H.P.; Lai, M.D.; Li, C.Y.; Chen, C.F.; Chiao, C.C.; Yen, M.C.; Sun, Z.; et al. Overexpressed gene signature of EPH receptor A/B family in cancer patients-comprehensive analyses from the public high-throughput database. Int. J. Clin. Exp. Pathol. 2020, 13, 1220–1242. [Google Scholar] [PubMed]
- Alam, S.M.; Fujimoto, J.; Jahan, I.; Sato, E.; Tamaya, T. Coexpression of EphB4 and ephrinB2 in tumor advancement of uterine cervical cancers. Gynecol. Oncol. 2009, 114, 84–88. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, T.; Liang, M. Expression of Eph B4 and Ephrin B2 in cervical cancer tissues and angiogenesis. Int. J. Gynaecol. Obstet. 2007, 96, 46–47. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, W.; Zou, S.; Shen, Q.; Zhu, X. A Five-Genes-Based Prognostic Signature for Cervical Cancer Overall Survival Prediction. Int. J. Genom. 2020, 2020, 8347639. [Google Scholar] [CrossRef]
- Nishimura, M.; Jung, E.J.; Shah, M.Y.; Lu, C.; Spizzo, R.; Shimizu, M.; Han, H.D.; Ivan, C.; Rossi, S.; Zhang, X.; et al. Therapeutic synergy between microRNA and siRNA in ovarian cancer treatment. Cancer Discov. 2013, 3, 1302–1315. [Google Scholar] [CrossRef]
- Landen, C.N.; Chavez-Reyes, A.; Bucana, C.; Schmandt, R.; Deavers, M.T.; Lopez-Berestein, G.; Sood, A.K. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005, 65, 6910–6918. [Google Scholar] [CrossRef]
- Moyano-Galceran, L.; Pietilä, E.A.; Turunen, S.P.; Corvigno, S.; Hjerpe, E.; Bulanova, D.; Joneborg, U.; Alkasalias, T.; Miki, Y.; Yashiro, M.; et al. Adaptive RSK-EphA2-GPRC5A signaling switch triggers chemotherapy resistance in ovarian cancer. EMBO Mol. Med. 2020, 12, e11177. [Google Scholar] [CrossRef]
- Xiong, C.; Wen, Y.; Zhao, J.; Yin, D.; Xu, L.; Chelariu-Raicu, A.; Yao, C.; Leng, X.; Liu, J.; Chaudhari, R.R.; et al. Targeting Forward and Reverse EphB4/EFNB2 Signaling by a Peptide with Dual Functions. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Werner, T.L.; Wade, M.L.; Agarwal, N.; Boucher, K.; Patel, J.; Luebke, A.; Sharma, S. A pilot study of JI-101, an inhibitor of VEGFR-2, PDGFR-β, and EphB4 receptors, in combination with everolimus and as a single agent in an ovarian cancer expansion cohort. Investig. New Drugs 2015, 33, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Damelin, M.; Bankovich, A.; Park, A.; Aguilar, J.; Anderson, W.; Santaguida, M.; Aujay, M.; Fong, S.; Khandke, K.; Pulito, V.; et al. Anti-EFNA4 Calicheamicin Conjugates Effectively Target Triple-Negative Breast and Ovarian Tumor-Initiating Cells to Result in Sustained Tumor Regressions. Clin. Cancer Res. 2015, 21, 4165–4173. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Laguna, I.; Krop, I.; Burris, H.A.; Hamilton, E., 3rd; Braiteh, F.; Weise, A.M.; Abu-Khalaf, M.; Werner, T.L.; Pirie-Shepherd, S.; Zopf, C.J.; et al. First-in-human, phase I study of PF-06647263, an anti-EFNA4 calicheamicin antibody-drug conjugate, in patients with advanced solid tumors. Int. J. Cancer 2019, 145, 1798–1808. [Google Scholar] [CrossRef]
- Lee, J.W.; Stone, R.L.; Lee, S.J.; Nam, E.J.; Roh, J.W.; Nick, A.M.; Han, H.D.; Shahzad, M.M.; Kim, H.S.; Mangala, L.S.; et al. EphA2 targeted chemotherapy using an antibody drug conjugate in endometrial carcinoma. Clin. Cancer Res. 2010, 16, 2562–2570. [Google Scholar] [CrossRef]
- Expasy-Cellosaurus. Available online: https://web.expasy.org/cellosaurus/ (accessed on 11 March 2022).
| EPHs/Ephrins | Cell Lines | Methods | Main Results | Refs. |
|---|---|---|---|---|
| EPHAs | ||||
| EPHA1 | SKOV3, COV504 | RT-PCR, Western blot, cell cycle analysis, cell matrix adhesion/wound healing/invasion/ migration/motility assays | Knockdown suppresses cell cycle arrest, cell adhesion migration, proliferation, and invasion. | [22] |
| HO8910, A2780 | Cell viability assay, flow cytometry | Low expression levels were reported only in A2780 OC cells. Transfection with EPHA1 plasmid resulted in a significant reduction in the proliferation rate of OC cells. | [23] | |
| EPHA2 | OVK-18 | Immunoblotting, ELISA, immunoprecipitation | Growth promotion. | [24] |
| HIO-180, EG, 222, SKOV3, A2780-PAR | Western blot, immunoprecipitation | Overexpression in EG, 222, and SKOV3 OC cell lines. Low to absent expression in A2780-PAR and HIO-180. | [25] | |
| OVCAR3, SKOV3 | Semiquantitative RT-PCR, Western blot | Strong EPHA2 and ephrin-A1 mRNA expression. | [26] | |
| A2780 | Western blot, immunoprecipitation, cell viability/attachment assay, murine tumor xenograft model | Increased expression resulted in the reduction of cell–cell contact, promotion of cell–extracellular matrix attachment, and an increase in anchorage-independent cell growth. Overexpression promoted tumorigenesis, angiogenesis, and metastasis in OC xenografts. | [27] | |
| EPHBs | ||||
| EPHB2 | ES-2, OVCAR-3, OV-90, SKOV-3 | Semiquantitative RT-PCR, Northern blot | Similarities in RNA expression across OC cell lines and clinical samples. No association between promoter hypermethylation of EPHB2, EPHB3, EPHB4, and OC. | [28] |
| A2780wtTP53, A2780mTP53 | Affymetrix U133A array analysis | Upregulation in wild-type TP53 OC cell lines. | [29] | |
| EPHB4 | A2780wtTP53, A2780mTP53 | Affymetrix U133A array analysis | Upregulation in the larger pool of mutant TP53 lines. | [29] |
| ML5, ML10, MCV 50, HOC-7, OVCAR-3 | Western blot, cell cycle analysis, wound healing/ migration/viability/ apoptosis assays, murine tumor xenograft model | Upregulation in OC cell lines correlated with apoptosis inhibition, tumor cell migration and invasion. Progesterone treatment resulted in a dose-dependent reduction in EPHB4 expression, thus promoting apoptosis via activation of the death receptor caspase pathway. Knockdown induced apoptosis and reduced vascularization in murine OC xenografts. | [30] | |
| A2780, SKOV3 | RT-PCR, Western blot, MTT/apoptosis/migration/invasion assays | Downregulation of EPHB4 led to cell growth inhibition, apoptosis induction, and reduced invasive ability in OC cells. | [31] | |
| Ephrins | ||||
| ephrin-A1 | OVCAR-3 | real-time RT-qPCR, Western blot | NFκB induced ephrin-A1 expression after stimulation with TNF-α and IL-1β. | [32] |
| ephrin-A3 | A2780wtTP53, A2780mTP53 | Affymetrix U133A array analysis | Upregulation in hypoxia treated A2780mTP53 cells. | [29] |
| ephrin-A5 | OVCAR3, OVCAR4, OVCAR8 | Treatment with dimeric and monomeric recombinant ephrins | Endogenous ephrin-A5 inefficiently activates EPHA2–pY588 signaling and receptor internalization. | [33] |
| ephrin-B2 | A2780wtTP53, A2780mTP53 | Affymetrix U133A array analysis | Upregulation in the A2780mTP53 cells. | [29] |
| EPHs/Ephrins | Tissue Samples | Methods | Main Results | Refs. |
|---|---|---|---|---|
| EPHAs | ||||
| EPHA1 | 8 OC samples, 8 benign ovarian samples | IHC | Upregulation in OC | [34] |
| 24 OC samples, 4 benign ovarian samples | real-time RT-qPCR | Greater than 10-fold overexpression in OC | [35] | |
| Ascites of 28 patients with high-grade serous OC, 1 patient with serous borderline tumor | RT-qPCR, survival-associated gene expression analysis | Adverse clinical association | [36] | |
| EPHA2 | 31 OC stroma tissue samples, 8 normal ovarian stroma samples | Microarray data analysis | Upregulation in the stroma of OC | [24] |
| 5 benign ovarian masses, 10 ovarian tumors of low malignant potential, 79 invasive OC samples | IHC | Overexpression in OC relates to higher tumor grade, advanced stage of disease, and significantly shorter median survival. | [25] | |
| 118 advanced epithelial OC samples | Semiquantitative RT-PCR, IHC | Higher levels of protein expression correlated with a shorter disease-specific survival in OC. | [26] | |
| 24 OC samples, 4 benign ovarian samples | real-time RT-qPCR | Overexpression in OC | [35] | |
| 77 invasive epithelial OC samples | IHC | Overexpression in OC is associated with increased MVD, invasion, high-grade histology, advanced FIGO stage and overexpression of stromal and epithelial MMP-9, epithelial MMP-2, and epithelial MT1-MMP. | [37] | |
| 107 OC samples, 54 ovarian borderline tumors, 45 adenomas | IHC, Western blot, in situ proximity ligation assay | C-EPHA2 was expressed diffusely throughout the tumor in most OC. In OCs, fewer signals of MT1-MMP and N-EPHA2 were observed compared with MT1-MMP and C- EPHA2. No significant difference between MT1-MMP and C/N-EPHA2 interaction was detected in adenomas. | [38] | |
| EPHA4 | Ascites of 28 patients with high-grade serous OC, 1 patient with serous borderline tumor | RT-qPCR, survival-associated gene expression analysis | Adverse clinical association | [36] |
| EPHA5 | 61 OC samples, 24 benign ovarian serous tumors, 42 serous borderline tumors, 20 normal fallopian tube samples | IHC | Loss of expression was associated with tumor grade, FIGO stage, and poor outcome. | [39] |
| EPHA8 | 18 normal ovarian tissue samples, 20 normal fallopian tube tissue samples, 30 benign ovarian tumors, 30 borderline ovarian tumors, 125 OC samples | RT-qPCR, TMA-IHC | High protein level was associated with older age at diagnosis, higher FIGO stage, positive LNs, presence of metastasis, positive ascitic fluid, and higher serum CA-125 level. | [40] |
| EPHBs | ||||
| EPHB1 | 74 OC samples, 12 normal ovarian epithelial tissue samples | IHC | Loss of expression was associated with higher tumor grade, metastasis, high proliferative index, Ki67 expression, and significantly worse OS. | [41] |
| EPHB2 | 115 OC samples | RT-PCR, IHC | OC patients older than 60 years of age exhibited higher expression than younger ones. High levels correlated with poorer OS. | [42] |
| EPHB3 | 19 normal fallopian tube samples, 17 serous borderline tumor samples, 50 OC specimens | IHC | Expression is significantly reduced in OC compared with normal fallopian tubes and borderline tumors, and is negatively associated with histological grade and FIGO stage of OC. | [43] |
| EPHB4 | 7 normal ovarian specimens, 85 invasive OC samples | IHC, Western Blot | Upregulation in invasive OC | [30] |
| Ascites of 28 patients with high-grade serous OC, 1 patient with serous borderline tumor | RT-qPCR, survival-associated gene expression analysis | Adverse clinical association | [36] | |
| 115 OC samples | RT-PCR, IHC | High levels correlated with poorer OS and poorer response to chemotherapy. | [42] | |
| 72 OC samples | Real-time RT-PCR, IHC | Upregulation in OC, increased with clinical stage and correlated with poor survival. | [44] | |
| EPHB6 | 55 OC samples, 24 benign ovarian serous tumors, 37 serous borderline tumors, 20 normal fallopian tube samples | IHC | High expression was observed in 100% of normal fallopian tube samples, 100% of benign epithelial ovarian tumors, 78% of ovarian serous borderline tumors, and 18% of OC. The expression was significantly associated with grade, TNM stage, and poorer OS, and inversely associated with Ki-67. | [45] |
| Ephrins | ||||
| ephrin-A1 | 24 OC samples, 4 benign ovarian samples | real-time RT-qPCR | Expression correlated with poor survival. | [42] |
| ephrin-A5 | High-grade OC samples | IHC, tissue microarrays | Overexpression in the most aggressive high-grade OC and upregulation in the high-grade OC cells upon disease progression. High expression was most strongly associated with poor OS. | [33] |
| 24 OC samples, 4 benign ovarian samples | real-time RT-qPCR | Expression correlated with poor survival. | [35] | |
| 25 OC specimens, 2 normal ovarian tissue samples, 2 benign ovarian tumors | real-time RT-qPCR | Expression was associated with poorer progression-free survival. | [46] | |
| ephrin-B | 112 OC samples | IHC, Western blot | High-grade OC showed greatest expression. A correlation was found between ephrin-B expression and MVD. Expression was associated with higher rates of disease recurrence and a decrease in OS. | [47] |
| ephrin-B1 | 162 OC samples | IHC | Upregulation in OC cell lines. | [48] |
| ephrin-B2 | 72 OC samples | Real-time RT-PCR, IHC | Upregulation in OC, increase with clinical stage, and correlation with poor survival. | [44] |
| EPHs/Ephrins | Cell Lines | Methods | Main Results | Refs. |
|---|---|---|---|---|
| EPHAs | ||||
| EPHA2 | AN3CA, ECC-1, Ishikawa, HEC1A, HEC1B | Array CGH | Amplification in two of the EC cell lines | [54] |
| Ishikawa | Real-time PCR, flow cytometric cytotoxicity assay | Blocking of expression resulted in significant inhibition of EC killing, mediated by Vδ1 γδ T-cells. | [55] | |
| EPHBs | ||||
| EPHB3 | AN3CA, ECC-1, Ishikawa, HEC1A, HEC1B | Array CGH | Amplification in four of five EC cell lines | [54] |
| EPHB4 | AN3CA, ECC-1, Ishikawa, HEC1A, HEC1B | Array CGH | Amplification in four of five EC cell lines | [54] |
| Ephrins | ||||
| ephrin-A1 | Ishikawa | RT-PCR, Western blot, cell aggregation assay | Stimulation of cell attachment and inhibition of cell aggregation through the EPHA receptor pathway | [56] |
| Ishikawa | RT-PCR, Western blot, permeability assay | Intercellular dissociation induction | [57] | |
| EPHs/Ephrins | Tissue Samples | Methods | Main Results | Refs. |
|---|---|---|---|---|
| EPHAs | ||||
| EPHA2 | 85 EC samples | IHC | Overexpression in 47% of tumors. Significant correlation with angiogenesis induction and poorer disease-specific survival. | [58] |
| 139 EC samples, 10 benign endometrial samples | IHC | High expression in 48% of EEC samples. Significant correlation with high stage and grade, increased myometrial invasion depth, low hormone receptor levels, high Ki-67 expression, and poorer disease-specific survival. | [59] | |
| EPHBs | ||||
| EPHB4 | 68 EC samples, 16 normal endometrium tissue samples | Real-time RT-PCR, IHC | Significant association with clinical stages, dedifferentiation, myometrial invasion depth, and patient survival rates. | [60] |
| 26 normal endometrium specimens, 15 hyperplasias, 102 EC samples | IHC | Protein-expressing glandular epithelial cell proliferation in hyperplasias and ECs. Highly significant positive correlation with postmenopausal stage. | [61] | |
| 12 control endometrial samples, 20 atypical EH tissue samples, 34 EC samples | IHC | Overexpression in atypical hyperplasia and hormone positive EC. Positive correlation with ER expression in EC. | [62] | |
| 20 EC samples, 20 normal endometrial samples | IHC | Significant correlation with histological grade and certain clinical stages of EC. | [63] | |
| EC samples (number not specified) | Significant association with PCNA-labeling index, clinical stage, histopathological grade, myometrium invasion depth, and clinical outcome. | [64] | ||
| Ephrins | ||||
| ephrin-B2 | 68 EC samples, 16 normal endometrium tissue samples | Real-time RT-PCR, IHC | Significant correlation with clinical stage, histopathological grade, myometrial invasion depth, and survival rates in EC. | [60] |
| 12 control endometrial samples, 20 atypical EH tissue samples, 34 EC samples | IHC | Overexpression in atypical hyperplasia and hormone-positive EC. Positive correlation with ER expression in EC. | [62] | |
| 20 EC samples, 20 normal endometrial samples | IHC | Significant correlation with myometrium invasion depth. | [63] | |
| EC samples (number not specified) | Significant association with PCNA labeling index, clinical stage, histopathological grade, myometrium invasion depth, and clinical outcome. | [64] | ||
| EPHs/Ephrins | Cell Lines | Methods | Main Results | Refs. |
|---|---|---|---|---|
| EPHAs | ||||
| EPHA2 | SiHa, HeLa, C4-i | real-time qPCR, IHC, Western blot, cell proliferation/wound healing/tumor sphere formation/transwell migration assay, murine tumor xenograft model | Knockdown decreased CC tumorigenicity in vitro and vivo. Overexpression promotes CC tumorigenicity in vitro and in vivo. EPHA2 conferred CC cell chemotherapy resistance by upregulating CDK6 expression. Expression levels were correlated with CDK6 levels. | [69] |
| EPHBs | ||||
| EPHB2 | HeLa, C33A | Real-time PCR, Western blot, immunoprecipitation, tumor xenotransplantation | Upregulation in CC and high expression in metastatic CC cell lines. Stem cell state induction. EPHB2/R-Ras signaling is activated in CC. | [70] |
| EPHs/Ephrins | Tissue Samples | Methods | Main Results | Refs. |
|---|---|---|---|---|
| EPHAs | ||||
| EPHA2 | 158 CC samples, 103 CIN samples, 58 cervicitis samples | IHC | Knockdown decreased CC tumorigenicity in vitro and vivo. Overexpression promotes CC tumorigenicity in vitro and in vivo. EPHA2 conferred CC cell chemotherapy resistance by upregulating CDK6 expression. Expression levels were correlated with CDK6 levels. | [69] |
| 217 CC samples | IHC | Upregulation in CC | [72] | |
| 206 CC samples | RT-PCR, IHC | High level was significantly associated with OS. | [73] | |
| EPHBs | ||||
| EPHB2 | CC samples | Oncomine Database, Human Protein Atlas | Upregulation in CC | [74] |
| EPHB4 | 62 CC samples | Real-time RT-PCR, IHC | High expression correlated with adverse clinical disease stage, larger tumor size, LN metastasis, and poor survival. | [75] |
| 90 CC samples, 15 CINs, 15 normal cervix samples | IHC | Overexpression in CC or CIN compared to in normal cervices. Strong expression was correlated with both clinical stage and tumor diameter. Strong expression correlated with MVD in patients with CC. | [76] | |
| Ephrins | ||||
| ephrin-A1 | 217 CC samples | IHC | High expression was associated with poor disease-free and disease-specific survival. | [72] |
| 206 CC samples | RT-PCR, IHC | Moderate to high level was significantly associated with OS. | [73] | |
| 378 CC samples, 45 normal cervical tissue samples | Gene expression data and clinical information of CC patients and health controls from the Cancer Genome Atlas and from three datasets of the Gene Expression Omnibus database | Upregulation in CC | [77] | |
| ephrin-B2 | 62 CC samples | Real-time RT-PCR, IHC | High expression correlated with adverse clinical disease stage, larger tumor size, LN metastasis, and poor survival. | [75] |
| 90 CC samples, 15 CINs, 15 normal cervix samples | IHC | The expression was higher in CC or CIN than in normal cervices. Strong expression was correlated with tumor diameter. Strong expression correlated with MVD in patients with CC. | [76] | |
| EPHs/Ephrins | Cell Lines | Tissue Samples | Methods | Main Results | Refs. |
|---|---|---|---|---|---|
| EPHAs | |||||
| EPHA2 | HeyA8, SKOV3ip1, ES2 | 91 OC tissue samples | In vivo treatment with miR520d-3p-DOPC and si-EphA2-1-DOPC | Synergistic therapeutic efficacy of dual inhibition in vivo using DOPC nano-liposomes loaded with miR-520d-3p and EPHA2-siRNA in OC, due to miR-520d-3p targeting EPHB2. | [78] |
| HeyA8, SKOV3ip1 | In vivo treatment with EphA2-targeting siRNA-DOPC | Tumor growth inhibition after EPHA2-targeting siRNA therapy. Significant tumor growth reduction after combination of EPHA2-targeting siRNA-DOPC with paclitaxel. | [79] | ||
| OVCAR3, OVCAR4, OVCAR8 | Abdominal ascites fluid | Pharmacological inhibition or knockdown of RSK1/2 | RSK1/2 inhibition or knockdown suppressed oncogenic EPHA2-S897 phosphorylation and EPHA2-GPRC5A coregulation, and promoted canonical tumor-suppressive tyrosine phosphorylation and downregulation of EPHA2. The combination of RSK inhibitors with platinum induced GPRC5Ahigh cell apoptosis. | [80] | |
| 85 EC tissue samples | In vitro/vivo treatment with EA5 | EA5 suppressed expression and phosphorylation in vitro. The combination of EA5 and docetaxel resulted in a significant reduction in MVD counts, percent proliferation, and cell death. | [58] | ||
| Ishikawa, Hec-1A, KLE | In vitro/vivo treatment with MEDI-547 | In vitro treatment with MEDI-547 decreased viability by inducing apoptosis in EC cells. In vivo treatment with MEDI-547 decreased EC growth and distant metastasis, and induced apoptosis in EC tumor cells. | [85] | ||
| EPHBs | |||||
| EPHB4 | HeyA8, A2780Cp20 | In vivo treatment with the bi-directional ephrin agonist peptide | BIDEN-AP inhibited invasion, epithelial–mesenchymal transition, endothelial migration, and tube formation in OC cells In vivo treatment with (CCPM-)BIDEN-AP led to significant OC tumor growth inhibition, as well as downregulation of epithelial–mesenchymal transition and angiogenic pathways. | [81] | |
| 4 patients in the pharmacokinetic cohort, 11 patients in the pharmacodynamic cohort | Combined JI-101/everolimus treatment in an OC expansion cohort | No serious adverse events and good toleration of JI-101, alone or in combination with everolimus. Possible drug–drug interaction due to increased exposure of everolimus when combined with JI-101. No response per RECIST criteria. | [82] | ||
| Ephrins | |||||
| ephrin-A4 | OC tissue samples | In vivo treatment with anti-EFNA4 calicheamicin conjugates | Anti-ephrin-A4 calicheamicin conjugates resulted in sustained tumor regression. | [83] | |
| 16 OC patients | Treatment with PF-06647263 in patients with OC | PF-06647263 treatment response was not associated with expression levels. | [84] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psilopatis, I.; Pergaris, A.; Vrettou, K.; Tsourouflis, G.; Theocharis, S. The EPH/Ephrin System in Gynecological Cancers: Focusing on the Roots of Carcinogenesis for Better Patient Management. Int. J. Mol. Sci. 2022, 23, 3249. https://doi.org/10.3390/ijms23063249
Psilopatis I, Pergaris A, Vrettou K, Tsourouflis G, Theocharis S. The EPH/Ephrin System in Gynecological Cancers: Focusing on the Roots of Carcinogenesis for Better Patient Management. International Journal of Molecular Sciences. 2022; 23(6):3249. https://doi.org/10.3390/ijms23063249
Chicago/Turabian StylePsilopatis, Iason, Alexandros Pergaris, Kleio Vrettou, Gerasimos Tsourouflis, and Stamatios Theocharis. 2022. "The EPH/Ephrin System in Gynecological Cancers: Focusing on the Roots of Carcinogenesis for Better Patient Management" International Journal of Molecular Sciences 23, no. 6: 3249. https://doi.org/10.3390/ijms23063249
APA StylePsilopatis, I., Pergaris, A., Vrettou, K., Tsourouflis, G., & Theocharis, S. (2022). The EPH/Ephrin System in Gynecological Cancers: Focusing on the Roots of Carcinogenesis for Better Patient Management. International Journal of Molecular Sciences, 23(6), 3249. https://doi.org/10.3390/ijms23063249








