1. Introduction
cADPR is a cyclic metabolite of NAD that was first found to release Ca
2+ from intracellular stores in sea urchins by Hon Chong Lee in 1987 [
1] but was later shown to act as Ca
2+-releasing second messenger in a large variety of different cell systems (reviewed in [
2,
3]). Pharmacological data indicated that Ca
2+ release occurs via activation of ryanodine receptors [
4,
5]. Besides releasing Ca
2+ from intracellular stores, cADPR can also evoke Ca
2+ influx (reviewed in [
6]). In the human T-lymphoma cell line Jurkat, microinjection of cADPR leads to an increase in cytosolic Ca
2+ that depends on extracellular Ca
2+ and is not only blocked by the cADPR antagonist 8-NH
2-cADPR but also by extracellular Zn
2+ and the channel blocker SK&F-96365 [
7]. In smooth muscle cells, cADPR and the nonhydrolysable cADPR agonist 3-deaza-cADPR stimulate Ca
2+ entry [
8]. This Ca
2+ entry could either be a result of store depletion and activation of capacitative Ca
2+ entry pathways or reflect activation of a Ca
2+ permeable ion channel by cADPR.
In 2005, Kolisek et al. first reported that TRPM2, a nonselective, Ca
2+-permeable member of the melastatin subfamily of TRP channels, can be activated by cADPR [
9]. Before, TRPM2 activation was considered to be caused mainly by ADPR, a metabolite of NAD or cADPR. The cytosolic C-terminus of TRPM2 exhibits significant homology to NUDT9, an enzyme of the Nudix family that hydrolyses the pyrophosphate bridge of ADPR, which led to the identification of ADPR as the first TRPM2 agonist [
10]. The cADPR concentration needed for TRPM2 activation was clearly supraphysiological (EC
50 of 700 µM, while endogenous concentrations are in the low µM range [
11]), but the authors of the study also demonstrated that cADPR could act synergistically with ADPR at much lower concentrations [
2]. Interestingly, cADPR actions could be specifically blocked by 8-Br-cADPR (a cADPR antagonist), whereas AMP, one of the products of NUDT9, blocked ADPR-mediated activation of TRPM2 (IC
50 of 70 µM), indicating that cADPR acts upon a binding site distinct from the NUDT9 homology domain (NUDT9H) [
9]. Another study put this into question by showing that cADPR-mediated activation of TRPM2 does not only require body temperature (no current evoked by cADPR at 25 °C) but is also dependent on the NUDT9H domain [
12]. In the following years, the question of cADPR-mediated activation of TRPM2 was nearly put to rest by two studies that showed that the effect of cADPR on TRPM2 is due to the contamination of commercial cADPR by ADPR, and that the activating effect of cADPR vanishes when contaminating ADPR is hydrolysed (without affecting cADPR) by nucleotide pyrophosphatase [
13,
14] (see also our recent, more extensive review on the topic [
15]).
Recent developments again raised the question of whether cADPR could be a direct TRPM2 modulator. Seminal work from Kühn and coworkers on the TRPM2 orthologue of
Nematostella vectensis (nvTRPM2) showed that the NUDT9H domain in this channel is not required for activation by ADPR and instead hydrolyses the agonist, which was a first indication that the initial assumption that the NUDT9H domain is solely responsible for gating of TRPM2 is incorrect [
16]. The first cryo-EM structure of TRPM2 from zebrafish (drTRPM2) then revealed a so-far unrecognised nucleotide-binding site in the MHR1/2 domain of the channel [
17]. Interestingly, the ADPR molecule in the MHR1/2 domain assumes a horseshoe-like conformation that resembles the cyclic structure of cADPR (PDB: 6DRJ). This horseshoe-like conformation was confirmed in a later cryo-EM structure of human TRPM2 (PDB: 6PUS, [
18]). The ADPR found in the NUDT9H domain of human TRPM2, in contrast, assumes an elongated conformation. The structure of human TRPM2 was also determined in the complex with the cADPR antagonist 8-Br-cADPR (PDB: 6PUU). Matching the other observations, 8-Br-cADPR was found in the MHR1/2 domain but not in the NUDT9H domain of the channel. While this could point towards a role of the MHR1/2 domain as a cADPR-binding site, another recent study showed effects of purified cADPR on TRPM2 and proposed that cADPR binds to the NUDT9H domain based on molecular dynamics simulations, mutagenesis studies, and surface plasmon resonance measurements (SPR) [
19].
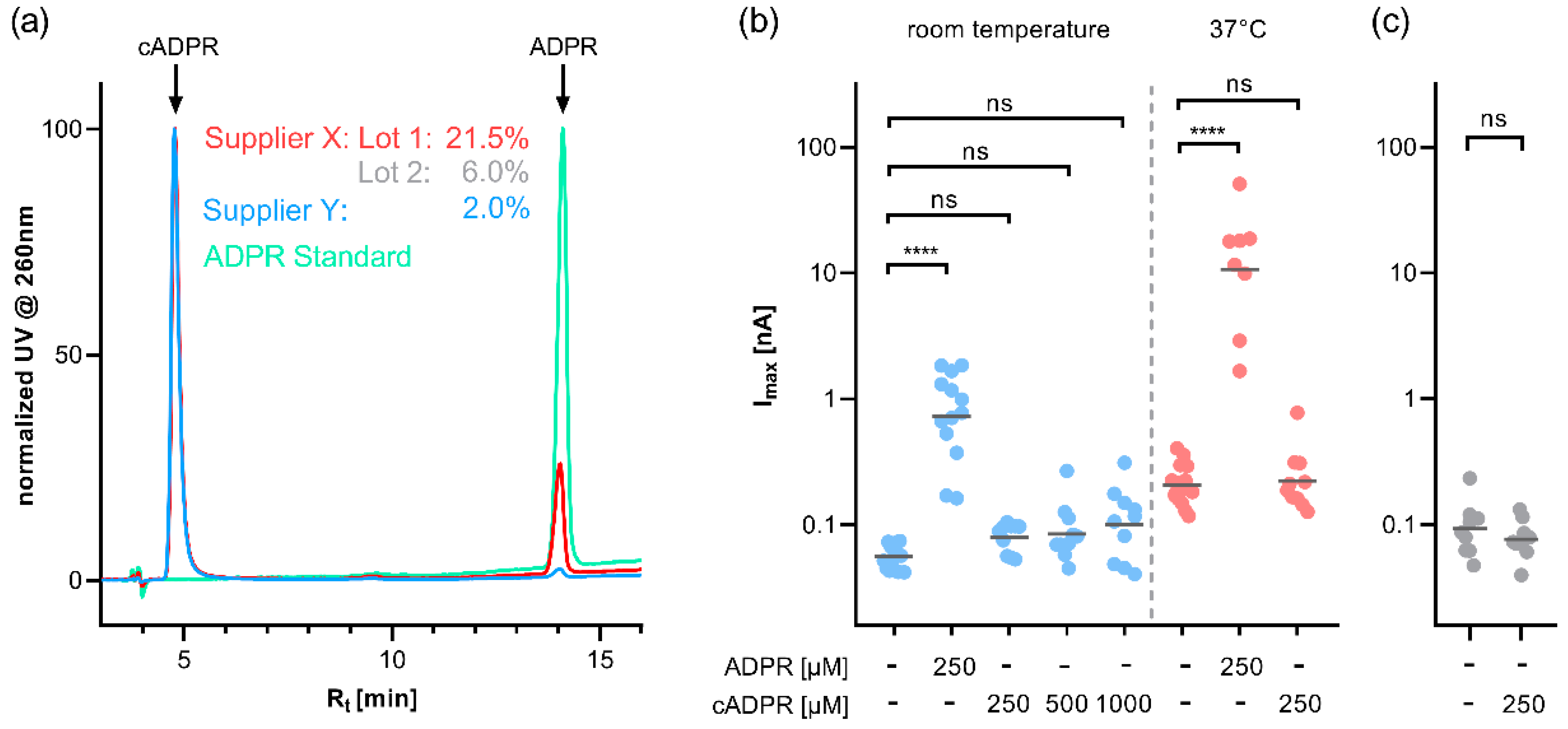
These developments led us to revisit the role of cADPR in TRPM2 activation. We tested cADPR, which we checked for purity by HPLC, for potential activation of TRPM2 under a variety of conditions, introduced mutations into the MHR1/2 and NUDT9H domain to analyse their role in agonist activation, and determined the binding of the MHR1/2 domain of TRPM2 to ADPR and cADPR.
3. Discussion
Our interest in cADPR as a potential TRPM2 agonist was raised by a recent structural study that showed 8-Br-cADPR bound to the N-terminal MHR1/2 domain of TRPM2 (PDB 6PUU, [
18]). In conjunction with the observation that ADPR probably folds into a horseshoe-like conformation when bound to this domain, and the older observation that 8-Br-cADPR selectively inhibits activation of TRPM2 by cADPR [
9], together this may indicate that the MHR1/2 domain is a binding site for cADPR.
Using the purest cADPR preparation available to us, we tested cADPR for activation of human TRPM2 in whole-cell patch clamp experiments but observed no activation of TRPM2 regardless of temperature, Ca
2+ buffering, or extracellular cation composition. We also observed no additive/synergistic effect of cADPR when coapplied with ADPR at a subthreshold concentration under conditions of low Ca
2+ buffering. These findings are in agreement with the previous reports that hydrolysis of contaminating ADPR using a pyrophosphatase abrogates any effects of cADPR on TRPM2 [
13,
14]. Yu et al. recently proposed binding of cADPR to the ADPR-binding pocket in the C-terminal NUDT9H domain based on MD simulations [
19]. To cope with the problem of ADPR contamination, they either synthesised cADPR themselves or purified cADPR by HPLC. Interestingly, the analysis by HPLC/ESI-MS included with the paper shows, besides the molecule ion peak for cADPR (
m/
z 540, the molecular weight of cADPR 541 Da,) also a smaller peak (
m/
z 558) which the authors attribute to a complex of cADPR + water (541 Da + 18 Da = 559 Da), but which in our opinion more likely is ADPR (m.w. 559 Da). The EC
50 value for cADPR in this study was significantly larger than that for ADPR (250 µM for cADPR vs. 40 µM for ADPR), which likely is consistent with ADPR contamination of their cADPR preparation. Our patch clamp experiments under nearly identical buffer conditions (NaCl-based bath solution, intracellular buffer with low Ca
2+ buffering) exhibited no activation of human TRPM2 by cADPR.
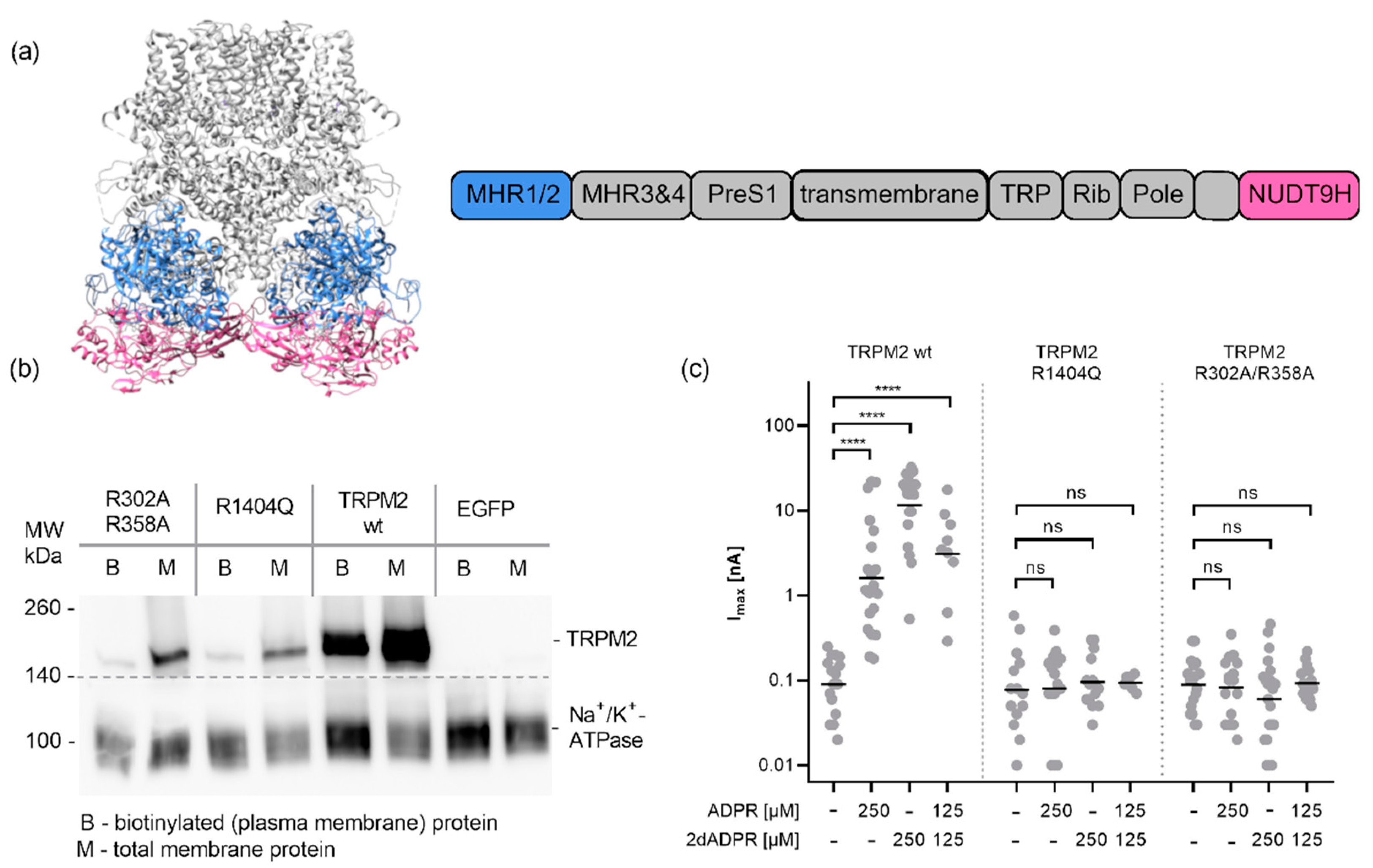
This posed the question of the role of the MHR1/2 domain for the activation of human TRPM2. While the MHR1/2 domain is clearly required for activation of TRPM2 from
Nematostella vectensis and
Danio rerio, there has been some dispute whether this also holds true for the human orthologue of TRPM2. While in their first cryo-EM structure of human TRPM2, Wang et al. did not detect ADPR in the MHR1/2 domain and therefore proposed that the human channel does not require binding of ADPR to this domain [
35], Huang et al. later showed that ADPR binds to the MHR1/2 domain as well as the NUDT9H of human TRPM2. The observation that 8-Br-cADPR was only found in the N-terminal MHR1/2 domain indicates that there might be some differences in selectivity for the ligand and probably also a functional difference between the nucleotide-binding sites. As we proposed recently [
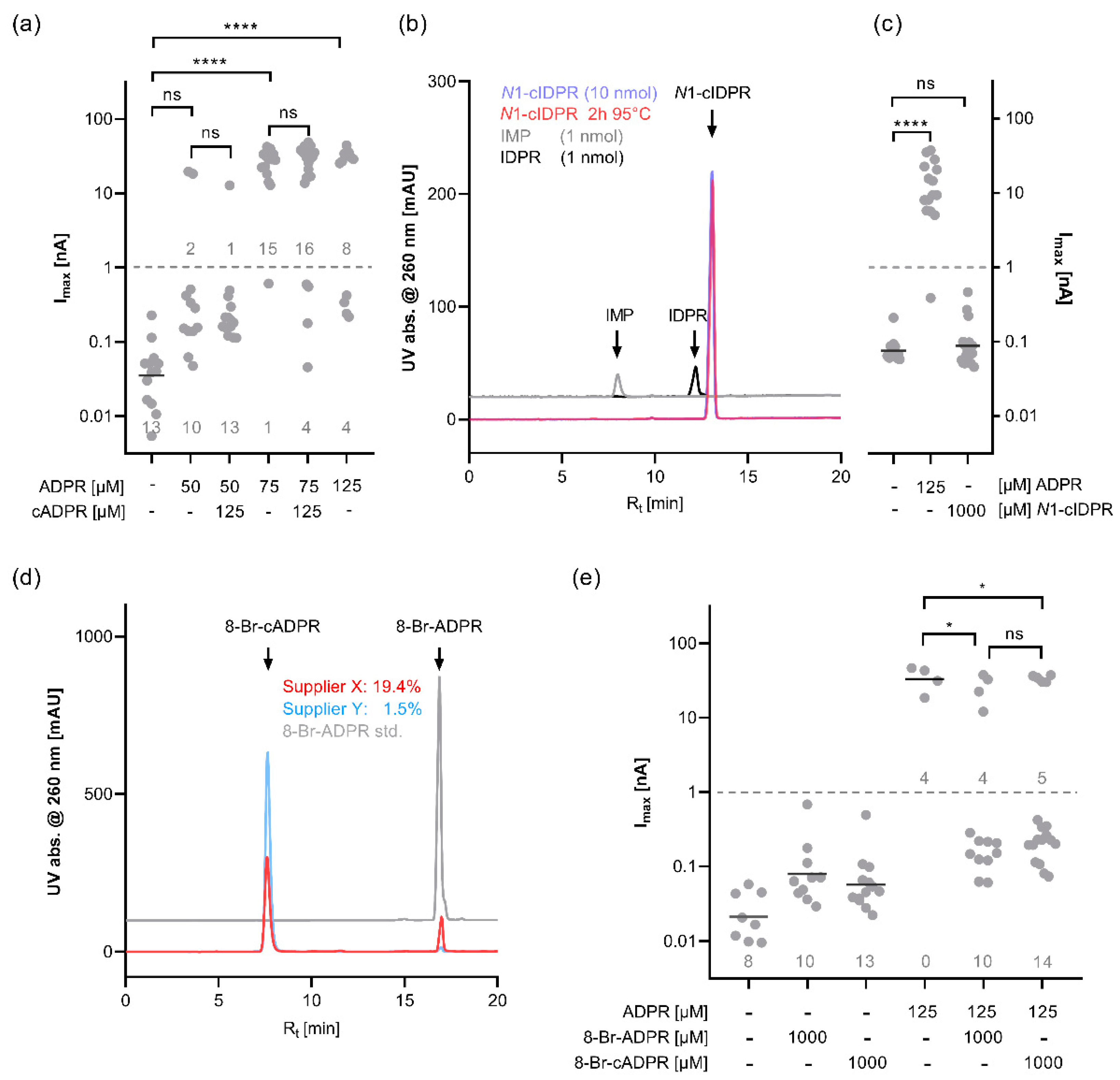
36], this may mean that the sites are required for activation by different agonists. Since we observed no activation by cADPR, we tested variants of human TRPM2 with point mutations in the MHR1/2 and NUDT9H domain for activation by the agonist ADPR and the superagonist 2′-deoxy-ADPR. Our mutagenesis data show that activating human TRPM2 by both agonists requires both domains to be intact.
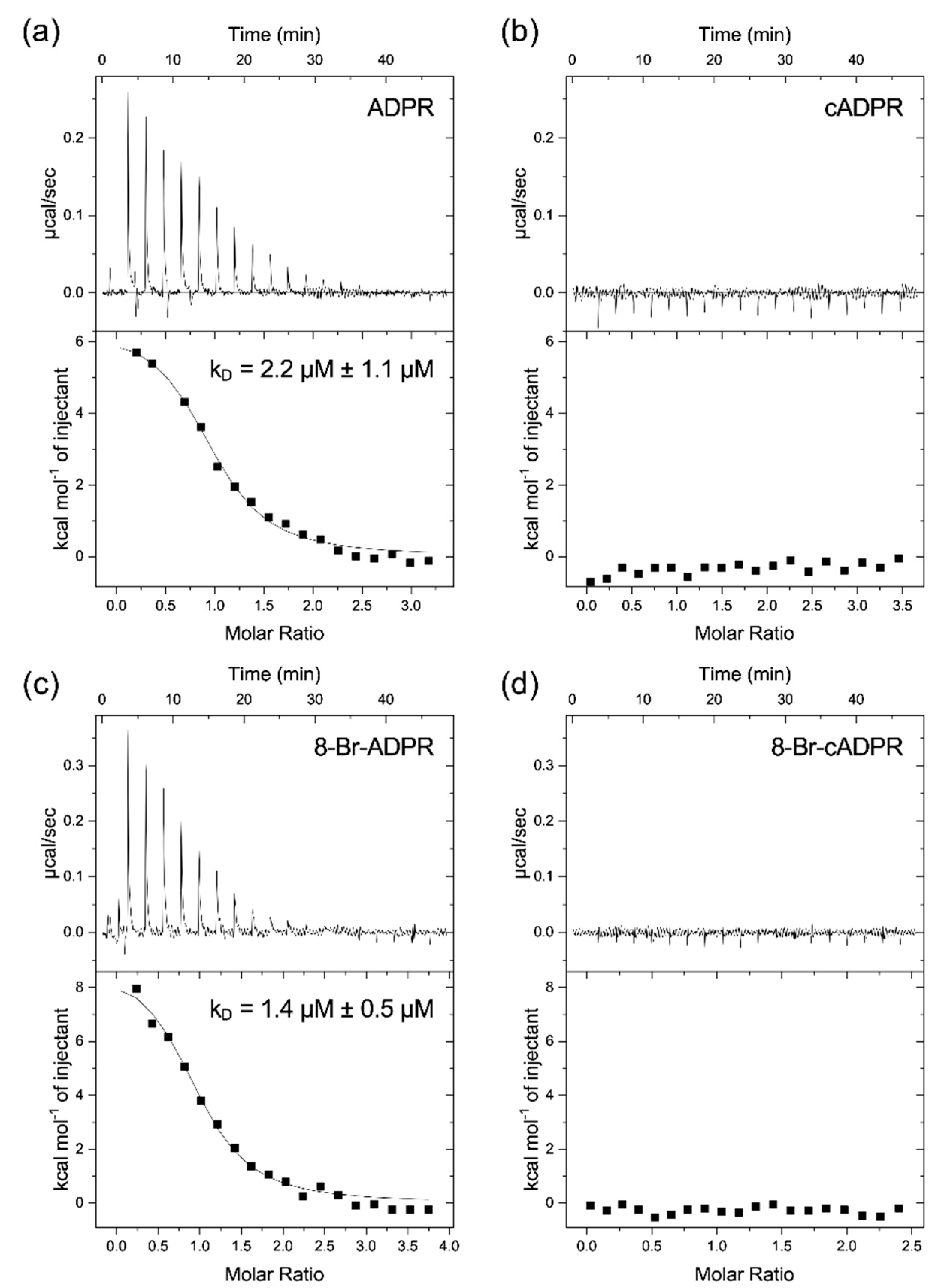
If both domains are required for activation by either agonist, this raises the question about the previous reports on the binding of 8-Br-cADPR to the MHR1/2 domain [
18] and selective inhibition of cADPR-mediated activation of TRPM2 by 8-Br-cADPR [
9]. Our HPLC analysis shows that, similar to cADPR, commercial preparations of 8-Br-cADPR can contain significant amounts of 8-Br-ADPR, a compound that we have previously shown to act as an ADPR antagonist. The local resolution of the cryo-EM structure at the MHR1/2 domain is not that high (PDB: 6PUU) and presumably does not allow to conclusively differentiate between an 8-Br-ADPR molecule in a horseshoe-like conformation and a cyclic 8-Br-cADPR molecule. Interestingly, our patch clamp data indicate that 8-Br-cADPR, similar to the linear analogue, does indeed inhibit activation of the channel by ADPR. However, while 8-Br-ADPR binds to the isolated MHR1/2 domain, 8-Br-cADPR exhibited no binding in our ITC experiments. This may indicate that 8-Br-cADPR is rapidly hydrolysed in the patched cells to the ADPR antagonist 8-Br-ADPR [
29]. This leaves the question of whether cADPR does bind to the MHR1/2 domain, and our ITC data show conclusively that this is not the case.
In conclusion, while both nucleotide-binding sites, the N-terminal MHR1/2 domain as well as the C-terminal NUDT9H domain, are required for activation of human TRPM2 by the nucleotide agonists ADPR and 2′-deoxy-ADPR (2dADPR), we found no evidence for a role of the cyclic nucleotide cADPR in the activation of the human channel. The “cADPR antagonist” 8-Br-cADPR inhibits activation of the channel by ADPR, which might reflect hydrolysis to the ADPR antagonist 8-Br-ADPR, and the isolated MHR1/2 domain of TRPM2 binds with high-affinity binding to the TRPM2 agonist ADPR but does not bind to the cyclic nucleotide cADPR.
4. Materials and Methods
4.1. Cell Culture
Wildtype HEK293 cells were cultivated in DMEM (with 4.5 g/L glucose and without pyruvate, ThermoFisher Scientific, Waltham, MA, USA) with 10% fetal calf serum (Merck, Darmstadt, Germany), 100 units/mL penicillin and 100 µg/mL streptomycin (ThermoFisher Scientific, Waltham, MA, USA) For HEK 293 #24 (stably expressing TRPM2) 400 µg/mL G418 (Merck, Darmstadt, Germany) were added to the medium. The cells were kept at 37 °C and 5% CO2 and tested for mycoplasma contamination on a regular basis (using MycoAlert™ Mycoplasma Detection Kit, Lonza, Basel, Switzerland).
4.2. HPLC Analysis of Nucleotides
Nucleotides were checked by ion pair reverse-phase HPLC (RP-HPLC) as described before [
33]. Samples of 1 nmol or 10 nmol of the nucleotides were analysed on a 1260 Infinity system (Agilent Technologies, Waldbronn, Germany) with a Multohyp BDS-C18 column (250 mm × 4.6 mm; particle size: 5 μm; CS Chromatographie Service, Langerwehe, Germany) protected by a guard cartridge (4 mm × 3 mm) containing a C18 ODS filter element (Phenomenex, Aschaffenburg, Germany). The mobile phase consisted of buffer A (20 mmol/L KH
2PO
4 and 5 mmol/L tetrabutylammonium dihydrogen phosphate, pH 6.0) with increasing amounts of methanol, resulting in the following gradient: 15% from start to 3 min, 31.25% at 11 min, 50% at 25 min, 50% at 27 min, 15% at 29 min, 15% at 38 min. The flow rate was 0.8 mL/min. Nucleotides were detected by their UV absorption at 260 nm and identified and quantified using external standards. Chromatograms were analysed offline using ChemStation Software (Revision C.01.05, Agilent Technologies, Waldbronn, Germany).
4.3. Site-Directed Mutagenesis
Construction of the expression vector for human TRPM2 (pIRES2-EGFP-hTRPM2) has been described before [
15]. The mutation in the NUDT9H domain (R1404Q) was introduced using the QuikChange Site-Directed Mutagenesis Kit (Agilent Technologies, Waldbronn, Germany) using the primers 5′-CGGAAGCTGAAGCAGATCCTCCGGCAG-3′ and 5′-CTGCCGGAGGATCTGCTTCAGCTTCCG-3′. The coding sequence of pIRES2-EGFP-hTRPM2 R1404Q was confirmed by DNA sequencing (Eurofins Genomics, Ebersberg, Germany). The two mutations in the MHR1/2 (R302A/R358A) were introduced by a commercial service (Eurofins Genomics, Ebersberg, Germany).
4.4. Heterologous Expression and Electrophysiological Measurements (Patch Clamp)
Wild type HEK 293 cells were transfected with either pIRES-EGFP-TRPM2-wt (control), pIRES2-EGFP-hTRPM2-R302A/R358A, or pIRES2-EGFP-hTRPM2-R1404Q and 1 µg of vector DNA was incubated with 5 µL of JetPEI solution (polyPlus Transfection, Illkirch, France) in 150 mM NaCl solution (polyPlus Transfection, Illkirch, France) at a total volume of 250 µL for 30 min. The mix was added to Greiner 35 mm culture dishes (ThermoFisher Scientific, Waltham, MA, USA) containing 2.5 × 105 cells in a cell culture medium as described above without antibiotics. Cells were kept at 37 °C and 5% CO2 overnight.
Before the electrophysiological experiments, the culture medium was removed, and cells were washed with the respective bath solution twice. During the experiments, the cells were kept in 1 mL of the bath solution. The standard bath solution was based on NMDG (140 mM N-methyl-D-glucamine (NMDG), 5 mM KCl, 5 mM D-Glucose, 10 mM HEPES, 3.3 mM MgCl2, 1 mM CaCl2, pH 7.4 at room temperature). For experiments at 37 °C, the temperature dependence of the buffer was taken into account and the pH adjusted to pH 7.63 at room temperature to make sure that the solution had a pH of 7.4 at 37 °C. In some experiments, the NMDG in the bath solution was replaced by 140 mM NaCl. 1.10 mm × 1.50 mm × 80 mm capillaries from borosilicate glass were pulled into pipettes with resistance between 1.5 and 3.5 MΩ using a P-97 horizontal puller (Sutter, Novato, CA, USA) and filled with pipette solution (120 mM KCl, 10 mM HEPES, 10 mM EGTA, 8 mM NaCl, 1 mM MgCl2, and 5.6 mM CaCl2, pH 7.2 at room temperature, resulting in a free Ca2+ concentration of 200 nM). For the experiments performed at 37 °C, the buffer contained 5.947 mM CaCl2, and the pH was adjusted to 7.42 at room temperature to make sure the solution had 200 nM free Ca2+ and pH 7.2 at 37 °C. Ca2+ concentrations were calculated using MaxChelator. For some experiments, Ca2+ buffering was reduced. In these cases the pipette solution contained 129.3 mM KCl, 10 mM HEPES, 100 µM EGTA, 8 mM NaCl, and 1 mM MgCl2 at pH 7.2.
Whole-cell patch clamp experiments were performed using an EPC-10 amplifier (HEKA, Reutlingen, Germany) and PatchMaster software (v2x32, HEKA, Reutlingen, Germany). After the opening of the cells, the holding potential was set to −50 mV, and 90 voltage ramps between −85 and +20 mV (over 140 ms) were applied, one every 5 s. Compensation of series resistance was set to 70%. For the measurements that required temperature control, a peristaltic pump (miniplus2, Gilson, Limburg-Offheim, Germany) and a single channel heat controller (Warner Instruments, Holliston, MA, USA) were added to the setup, and the cells were continuously perfused with prewarmed bath solution at a flow rate of 0.5 mL/min. The temperature within the dish was checked using a thermocouple thermometer.
Adenine nucleotides (ADPR (SigmaAldrich, Taufkirchen, Germany), 2′-deoxy-ADPR (Biolog, Bremen, Germany), cADPR (Biolog, Bremen, Germany and SigmaAldrich, Taufkirchen, Germany), 8-Br-ADPR (Biolog, Bremen, Germany), 8-Br-cADPR (Biolog, Bremen, Germany and SigmaAldrich, Taufkirchen, Germany), and cIDPR (Biolog, Bremen, Germany)) were added to the pipette solution at the indicated concentrations.
4.5. Surface Biotinylation of Proteins of the Plasma Membrane
For each condition, one T25 flask of wild type HEK293 cells was prepared at 75% confluency and transfected using the Lipofectamine LTX and plus reagents (ThermoFisher Scientific, Waltham, MA, USA) using the TRPM2 expression vectors described in
Section 4.3.
48 h post-transfection, successful transfection was confirmed by checking EGFP fluorescence by fluorescence microscopy. Then cells were washed three times with D-PBS (with Ca2+ and Mg2+) and treated with 1 mg/mL EZ-Link Sulfo-NHS-LC-Biotin (ThermoFisher Scientific, Waltham, MA, USA) for 30 min. Afterwards, cells were removed from the flask using 2 mM EGTA in PBS and collected by centrifugation (500× g). The cells were washed two times with PBS/EGTA, and membrane proteins were extracted using the ProteoExtract Native Membrane Protein Extraction Kit (Merck, Darmstadt, Germany). Total membrane protein samples were collected and stored at −20 °C; membrane extraction samples were mixed with NeutrAvidin Agarose beads (ThermoFisher Scientific, Waltham, MA, USA) overnight, shaking with 20 rpm at 4 °C, centrifugated (2500× g, 2 min at RT) and stored at −20 °C as well.
Both sample groups were mixed with 10 µL SDS sample buffer per sample, heated to 75 °C for 7 min, and centrifugated (500× g, 1 min at RT) and separated using a precast 4–15 % SDS gel at 200 V for ca. 40 min until the coloured band left the gel. The gel was transferred to a PVDF-Membrane Immobilon-P via Western blot using 25 mM Tris, 192 mM glycine, 20% methanol, 0.025% SDS transfer buffer. The membrane was cut at the 140 kDa marker band (Spectra Multicolor Broad Range Ladder, ThermoFisher Scientific, Waltham, MA, USA). Both parts were washed three times and blocked overnight using 5% milk powder in TBS-T at 4 °C. The upper membrane part was incubated with rabbit a-TRPM2-antibodies (NB 500-241, Novus Biologicals, Wiesbaden Nordenstadt, Germany) 1:50,000 in TBS-T containing 2.5% milk powder for 1 h at RT. Rabbit α-Na+/K+-ATPase-antibodies (#3010, Cell Signalling Technology, Frankfurt am Main, Germany) 1:10,000 were used on the lower membrane part at the same conditions. Membranes were washed in TBS-T three times for 5 min and incubated with HRP-conjugated goat anti-rabbit antibodies (#111-035-045, Dianova, Hamburg, Germany) 1:10,000 at the same conditions as before. Membranes were washed three times for 5 min in TBS-T.
SuperSignal West Dura chemiluminescence substrate (ThermoFisher Scientific, Waltham, MA, USA) was mixed 1:10 with SuperSignal West Pico chemiluminescence substrate (ThermoFisher Scientific, Waltham, MA, USA), put on the membranes for 5 min and carefully dried. The membrane was photographed at one picture per minute.
4.6. Expression and Purification of the Isolated MHR1/2 Domain from drTRPM2
A pnEK-vH vector was used to express the zebrafish TRPM2 MHR1/2 domain (drMHR1/2, residues 1-419 of drTRPM2) with an N-terminal His6-tag. E. coli BL21 Gold (DE3) cells were grown in Terrific Broth medium supplemented with 25 µg/mL kanamycin at 37 °C until a density of 0.8 (measured at 600 nm) was reached. Protein expression was induced by 0.1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) and carried out for 16 h at 20 °C. The cells were harvested by centrifugation, and lysis was carried out using a high-pressure homogenizer (EmulsiFlex-C3, Avestin, Ottawa, Ontario, Canada). The His6-tagged protein was purified from the clarified lysate using immobilised metal affinity chromatography (IMAC) and subsequent size exclusion chromatography on a Superdex S200 increase 10/300 column (Cytiva, Freiburg, Germany) using HEPES buffer (20 mM HEPES pH 7.5, 150 mM NaCl).
4.7. Isothermal Titration Calorimetry
Binding assays by isothermal titration calorimetry (ITC) were carried out at 25 °C on a MicroCal ITC-200 isothermal titration calorimeter (Malvern Panalytical, Malvern, UK). Thermodynamic parameters were analysed with the MicroCal ORIGINTM software (Additive GmbH, Friedrichsdorf, Germany). Adenine nucleotides were dissolved in HEPES buffer to a concentration of 300 µM and placed in the ITC syringe. Nineteen injections of the ligand (2 µL each) were titrated into 20 µM drMHR1/2 in the ITC sample cell. Each individual injection was interspaced by 150 s, and the stirring speed was set to 750 rpm. The ligand was titrated into HEPES buffer to allow for baseline correction. All measurements were performed as triplicates, and binding curves were fitted with a one-site binding model.
4.8. Statistical Analysis
Statistical analysis of the data was performed using GraphPad Prism (v8.0.1, GraphPad Software, San Diego, CA, USA). Data in the text are represented as mean ± SEM if not indicated otherwise. Since the currents obtained from whole-cell recordings were significantly skewed towards positive values, they were log-transformed to achieve normality. Normality of transformed data was confirmed by Kolmogorov–Smirnov normality test. Normal log-transformed data were tested by one-way ANOVA, using Bonferroni correction to adjust p values for multiple testing. The data acquired with decreased Ca2+ buffering in the pipette solution showed a bimodal distribution; since both modes showed the skewed distribution, these data were also log-transformed but tested using a nonparametric Kruskal–Wallis test with Dunn’s correction. In the case of the experiments with 8-Br-ADPR/8-Br-cADPR, a threshold was set between the modes, and the distributions were tested pairwise using Fisher’s exact test. In all cases, test results with p < 0.05 were considered to be significant.