The Fight against Cancer by Microgravity: The Multicellular Spheroid as a Metastasis Model
Abstract
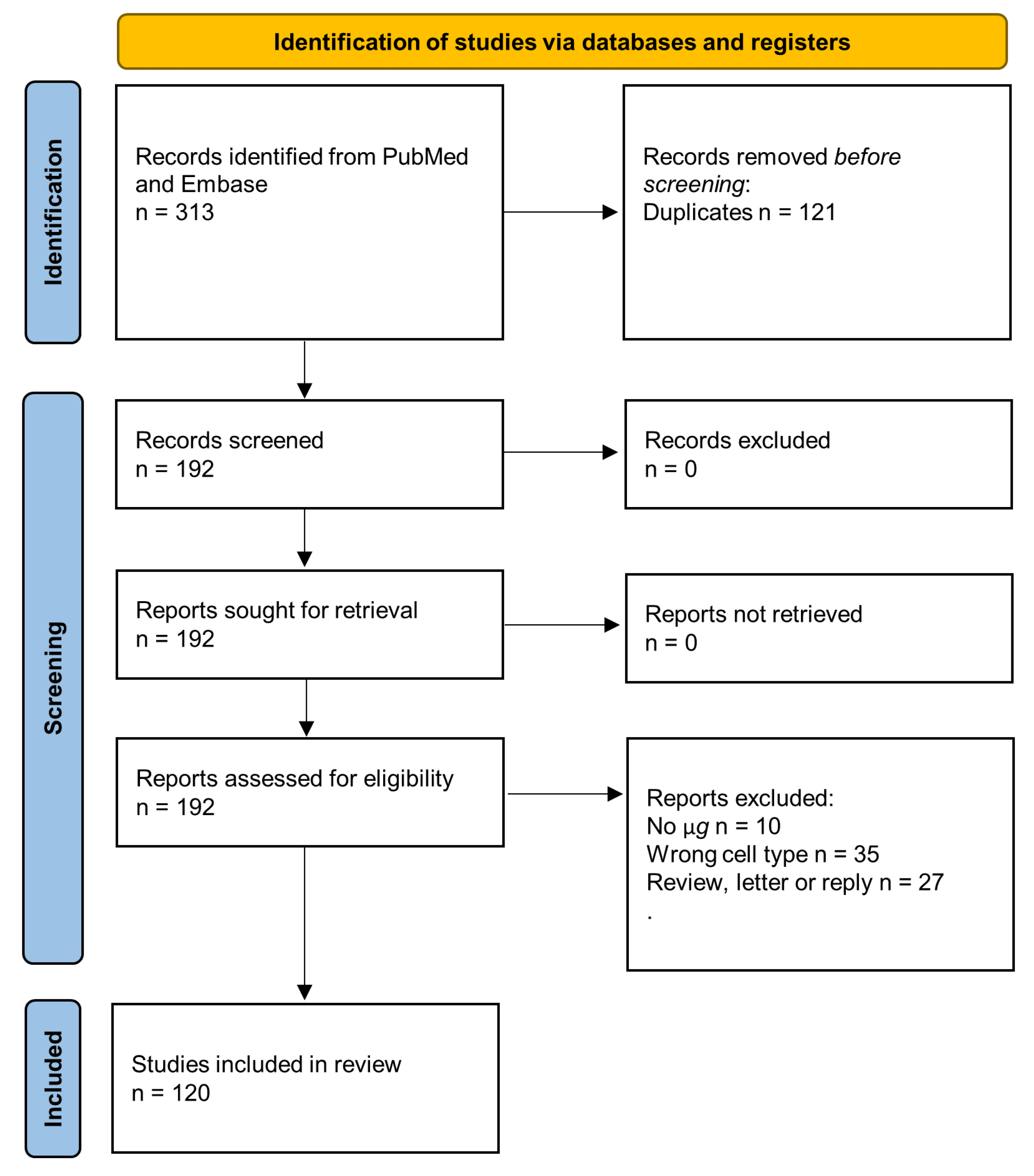
:1. Introduction
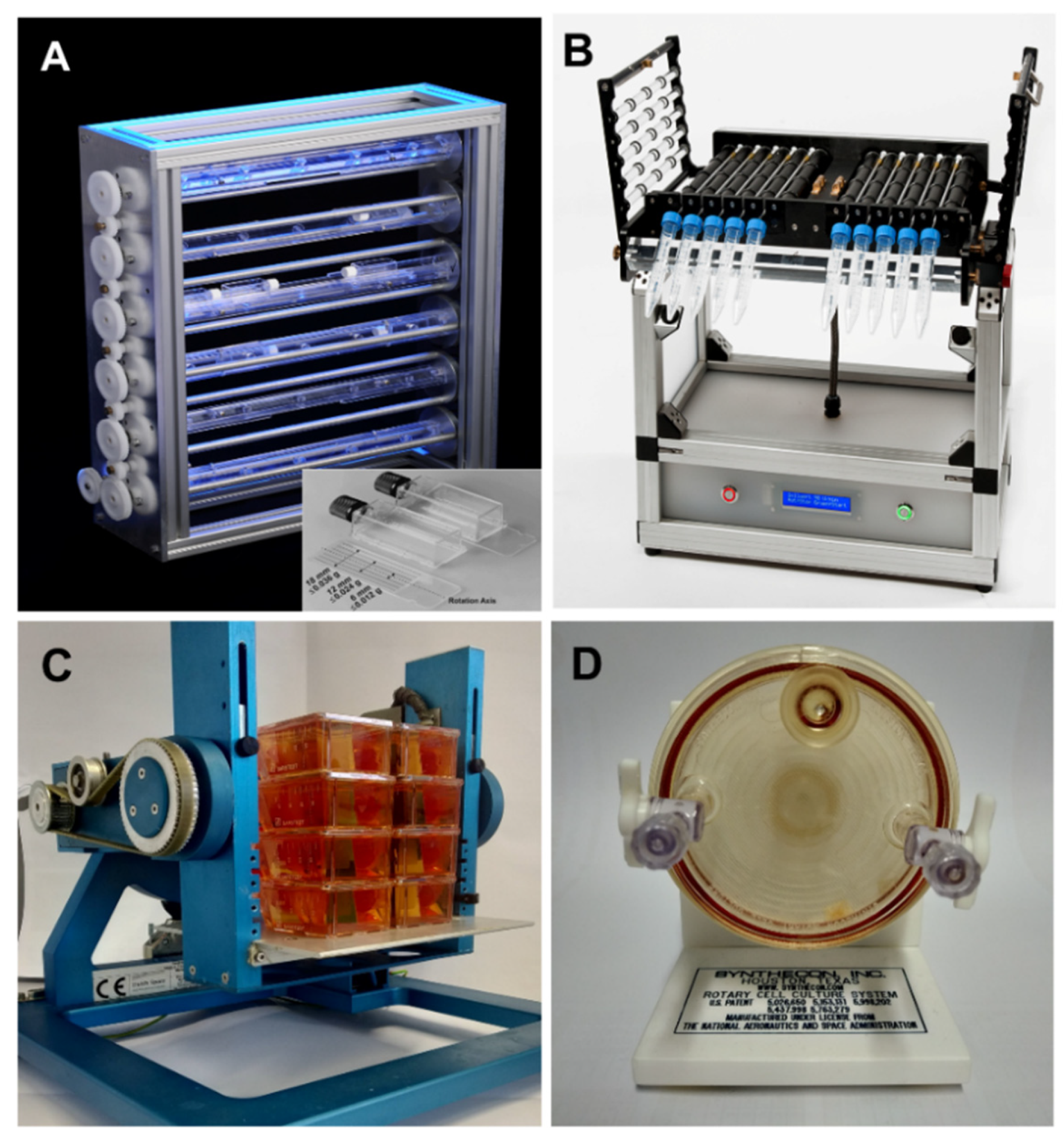
2. Platforms for Microgravity-Based Research
2.1. Ground-Based Facilities: Simulators of Weightlessness on Earth
2.2. Real Microgravity Research Platforms
2.2.1. Drop Tower
2.2.2. Parabolic Flight Maneuvers
2.2.3. Sounding Rockets
2.2.4. International Space Station
3. Definition of Cancer and Cancer Stem Cells
3.1. Definition of Cancer
3.2. Cancer Stem Cells
4. Cancer Research in Microgravity
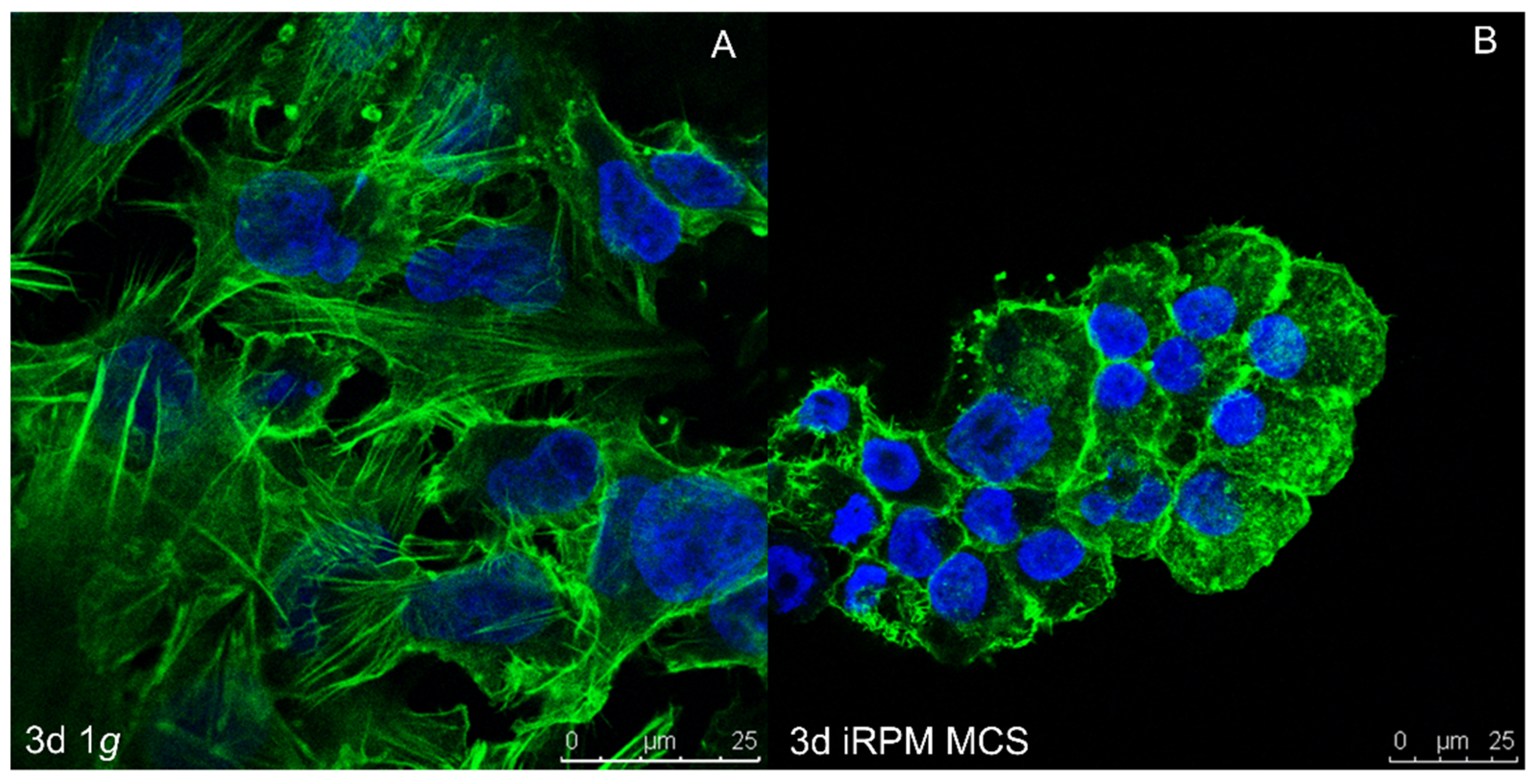
4.1. CSC Exposed to Microgravity
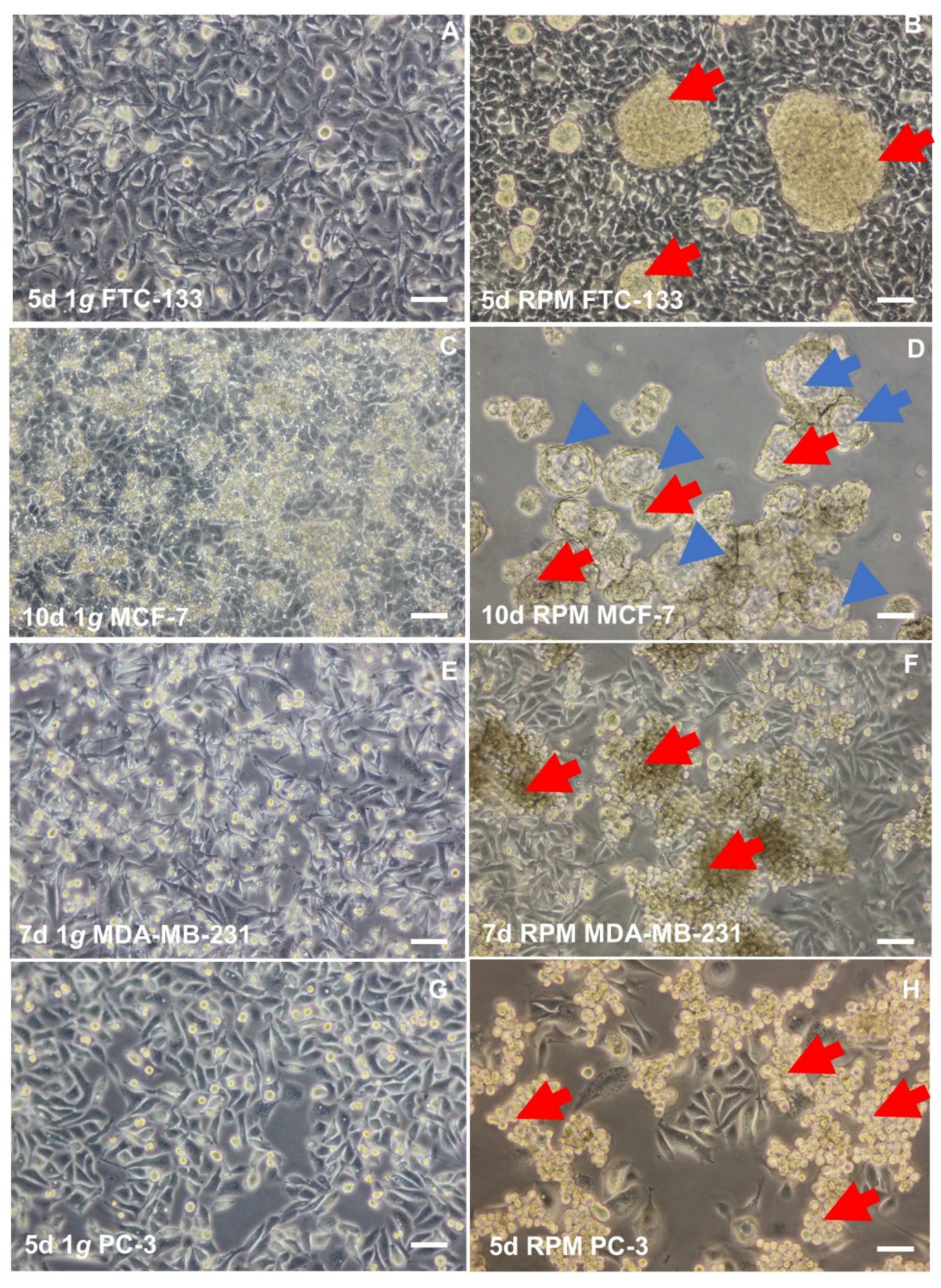
4.2. Thyroid Cancer
4.2.1. Thyroid Cells and Thyroid Cancer Cells Exposed to Real Short-Term Microgravity
4.2.2. Thyroid Cancer Cells Cultured for a Longer Time in Space
4.2.3. Thyroid Cancer Cells and Simulated Microgravity
4.3. Breast Cancer
4.3.1. Breast Cancer Cells Exposed to Real Microgravity
4.3.2. Breast Cancer Cells and Simulated Microgravity
4.4. Prostate Cancer
4.5. Cancers of the Gastrointestinal System
4.5.1. Colorectal Cancer
4.5.2. Hepatocellular Carcinoma Exposed to Simulated Microgravity
4.5.3. Studies Using Gastric and Pancreatic Cancer Cells
4.6. Lung Cancer
4.7. Skin Cancer
5. Extracellular Vesicles and Microgravity
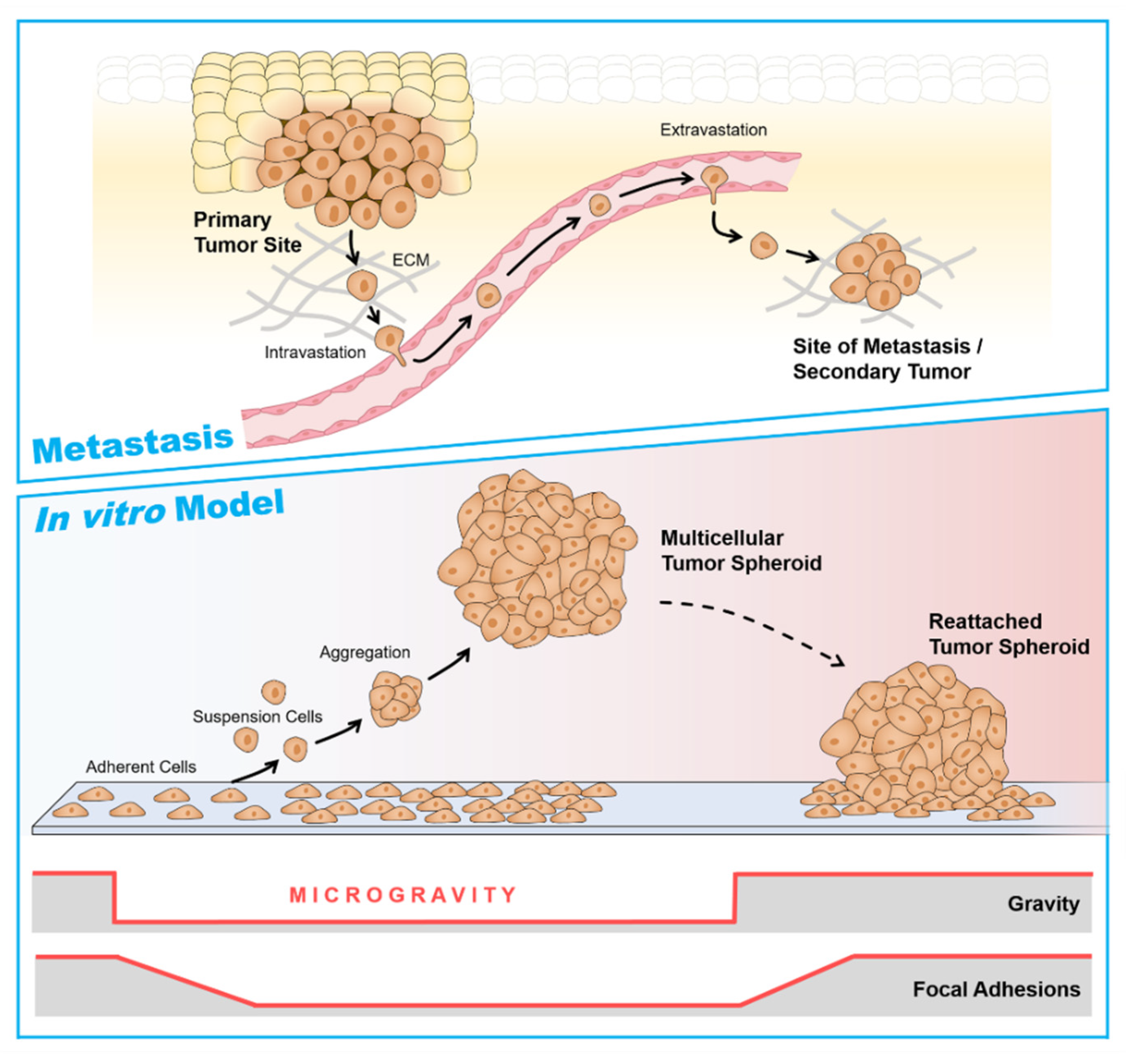
6. Multicellular Tumor Spheroids as a Metastasis Model
7. Current Knowledge about Proteins as Candidates for Future Targeted Tumor Therapy
8. Multi-Omics Analyses, a New Perspective for Microgravity-Related Cancer Research
9. Methods
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- White, R.J.; Averner, M. Humans in space. Nature 2001, 409, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Farkas, Á.; Farkas, G. Effects of Spaceflight on Human Skin. Ski. Pharm. Physiol. 2021, 34, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Genah, S.; Monici, M.; Morbidelli, L. The Effect of Space Travel on Bone Metabolism: Considerations on Today’s Major Challenges and Advances in Pharmacology. Int. J. Mol. Sci. 2021, 22, 4585. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Vaquer, S.; Mazzolai, L.; Roberts, L.N.; Pavela, J.; Watanabe, M.; Weerts, G.; Green, D.A. The effect of microgravity on the human venous system and blood coagulation: A systematic review. Exp. Physiol. 2021, 106, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Horie, K.; Hinoi, E.; Hiraiwa, M.; Kato, A.; Maekawa, Y.; Takahashi, A.; Furukawa, S. How does spaceflight affect the acquired immune system? npj Microgravity 2020, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.R. The biomedical challenges of space flight. Annu. Rev. Med. 2003, 54, 245–256. [Google Scholar] [CrossRef]
- Bateman, G.A.; Bateman, A.R. A perspective on spaceflight associated neuro-ocular syndrome causation secondary to elevated venous sinus pressure. npj Microgravity 2022, 8, 3. [Google Scholar] [CrossRef]
- Lee, P.H.U.; Chung, M.; Ren, Z.; Mair, D.B.; Kim, D.H. Factors mediating spaceflight-induced skeletal muscle atrophy. Am. J. Physiol. Cell. Physiol. 2022. [Google Scholar] [CrossRef]
- Hammond, T.G.; Stodieck, L.; Birdsall, H.H.; Becker, J.L.; Koenig, P.; Hammond, J.S.; Gunter, M.A.; Allen, P.L. Effects of microgravity on the virulence of Listeria monocytogenes, Enterococcus faecalis, Candida albicans, and methicillin-resistant Staphylococcus aureus. Astrobiology 2013, 13, 1081–1090. [Google Scholar] [CrossRef]
- Wu, X.T.; Yang, X.; Tian, R.; Li, Y.H.; Wang, C.Y.; Fan, Y.B.; Sun, L.W. Cells respond to space microgravity through cytoskeleton reorganization. FASEB J. 2022, 36, e22114. [Google Scholar] [CrossRef]
- Gardiner, J. Cytoskeletal Tensegrity in Microgravity. Life 2021, 11, 1091. [Google Scholar] [CrossRef] [PubMed]
- Freed, L.E.; Hollander, A.P.; Martin, I.; Barry, J.R.; Langer, R.; Vunjak-Novakovic, G. Chondrogenesis in a cell-polymer-bioreactor system. Exp. Cell Res. 1998, 240, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Ohyabu, Y.; Kida, N.; Kojima, H.; Taguchi, T.; Tanaka, J.; Uemura, T. Cartilaginous tissue formation from bone marrow cells using rotating wall vessel (RWV) bioreactor. Biotechnol. Bioeng. 2006, 95, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Yang, Z.; Liu, T.; Zhi, W.; Li, X.; Deng, L.; Cui, Z.; Ma, X. Fabrication and detection of tissue-engineered bones with bio-derived scaffolds in a rotating bioreactor. Biotechnol. Appl. Biochem. 2006, 45, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Chiu, B.; Wan, J.Z.; Abley, D.; Akabutu, J. Induction of vascular endothelial phenotype and cellular proliferation from human cord blood stem cells cultured in simulated microgravity. Acta Astronaut. 2005, 56, 918–922. [Google Scholar] [CrossRef]
- Grimm, D.; Infanger, M.; Westphal, K.; Ulbrich, C.; Pietsch, J.; Kossmehl, P.; Vadrucci, S.; Baatout, S.; Flick, B.; Paul, M.; et al. A delayed type of three-dimensional growth of human endothelial cells under simulated weightlessness. Tissue Eng. Part A 2009, 15, 2267–2275. [Google Scholar] [CrossRef] [PubMed]
- Freed, L.E.; Langer, R.; Martin, I.; Pellis, N.R.; Vunjak-Novakovic, G. Tissue engineering of cartilage in space. Proc. Natl. Acad. Sci. USA 1997, 94, 13885–13890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietsch, J.; Gass, S.; Nebuloni, S.; Echegoyen, D.; Riwaldt, S.; Baake, C.; Bauer, J.; Corydon, T.J.; Egli, M.; Infanger, M.; et al. Three-dimensional growth of human endothelial cells in an automated cell culture experiment container during the SpaceX CRS-8 ISS space mission—The SPHEROIDS project. Biomaterials 2017, 124, 126–156. [Google Scholar] [CrossRef] [PubMed]
- Hargens, A.R.; Vico, L. Long-duration bed rest as an analog to microgravity. J. Appl. Physiol. 2016, 120, 891–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morey-Holton, E.R.; Globus, R.K. Hindlimb unloading of growing rats: A model for predicting skeletal changes during space flight. Bone 1998, 22, 83s–88s. [Google Scholar] [CrossRef]
- Häder, D.-P.; Braun, M.; Grimm, D.; Hemmersbach, R. Gravireceptors in eukaryotes—A comparison of case studies on the cellular level. npj Microgravity 2017, 3, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemmersbach, R.; Simon, A.; Waßer, K.; Hauslage, J.; Christianen, P.C.; Albers, P.W.; Lebert, M.; Richter, P.; Alt, W.; Anken, R. Impact of a high magnetic field on the orientation of gravitactic unicellular organisms—A critical consideration about the application of magnetic fields to mimic functional weightlessness. Astrobiology 2014, 14, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Brungs, S.; Egli, M.; Wuest, S.L.; Christianen, P.C.M.; van Loon, J.J.W.A.; Ngo Anh, T.J.; Hemmersbach, R. Facilities for Simulation of Microgravity in the ESA Ground-Based Facility Programme. Microgravity Sci. Technol. 2016, 28, 191–203. [Google Scholar] [CrossRef]
- Herranz, R.; Anken, R.; Boonstra, J.; Braun, M.; Christianen, P.C.; de Geest, M.; Hauslage, J.; Hilbig, R.; Hill, R.J.; Lebert, M.; et al. Ground-based facilities for simulation of microgravity: Organism-specific recommendations for their use, and recommended terminology. Astrobiology 2013, 13, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Ferranti, F.; Del Bianco, M.; Pacelli, C. Advantages and Limitations of Current Microgravity Platforms for Space Biology Research. Appl. Sci. 2021, 11, 68. [Google Scholar] [CrossRef]
- Briegleb, W. Some qualitative and quantitative aspects of the fast-rotating clinostat as a research tool. ASGSB Bull. 1992, 5, 23–30. [Google Scholar]
- Eiermann, P.; Kopp, S.; Hauslage, J.; Hemmersbach, R.; Gerzer, R.; Ivanova, K. Adaptation of a 2-D Clinostat for Simulated Microgravity Experiments with Adherent Cells. Microgravity Sci. Technol. 2013, 25, 153–159. [Google Scholar] [CrossRef]
- Borst, A.G.; van Loon, J.J.W.A. Technology and Developments for the Random Positioning Machine, RPM. Microgravity Sci. Technol. 2008, 21, 287. [Google Scholar] [CrossRef]
- Wuest, S.L.; Richard, S.; Kopp, S.; Grimm, D.; Egli, M. Simulated Microgravity: Critical Review on the Use of Random Positioning Machines for Mammalian Cell Culture. BioMed Res. Int. 2015, 2015, 971474. [Google Scholar] [CrossRef] [Green Version]
- Hauslage, J.; Cevik, V.; Hemmersbach, R. Pyrocystis noctiluca represents an excellent bioassay for shear forces induced in ground-based microgravity simulators (clinostat and random positioning machine). npj Microgravity 2017, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Svejgaard, B.; Wehland, M.; Ma, X.; Kopp, S.; Sahana, J.; Warnke, E.; Aleshcheva, G.; Hemmersbach, R.; Hauslage, J.; Grosse, J.; et al. Common Effects on Cancer Cells Exerted by a Random Positioning Machine and a 2D Clinostat. PLoS ONE 2015, 10, e0135157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warnke, E.; Pietsch, J.; Wehland, M.; Bauer, J.; Infanger, M.; Görög, M.; Hemmersbach, R.; Braun, M.; Ma, X.; Sahana, J.; et al. Spheroid formation of human thyroid cancer cells under simulated microgravity: A possible role of CTGF and CAV1. Cell Commun. Signal. 2014, 12, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammond, T.G.; Allen, P.L.; Gunter, M.A.; Chiang, J.; Giaever, G.; Nislow, C.; Birdsall, H.H. Physical Forces Modulate Oxidative Status and Stress Defense Meditated Metabolic Adaptation of Yeast Colonies: Spaceflight and Microgravity Simulations. Microgravity Sci. Technol. 2018, 30, 195–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Loon, J.J.W.A. Mechanomics and Physicomics in Gravisensing. Microgravity Sci. Technol. 2008, 21, 159. [Google Scholar] [CrossRef] [Green Version]
- Kohn, F.; Hauslage, J.; Hanke, W. Membrane Fluidity Changes, A Basic Mechanism of Interaction of Gravity with Cells? Microgravity Sci. Technol. 2017, 29, 337–342. [Google Scholar] [CrossRef]
- Phelan, M.; Lelkes, P.; Swaroop, A. Customized rotating wall vessel bioreactors produce improved retinal organoids with reduced operational costs and less frequent experimental failure. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3316. [Google Scholar]
- Von Kampen, P.; Kaczmarczik, U.; Rath, H.J. The new Drop Tower catapult system. Acta Astronaut. 2006, 59, 278–283. [Google Scholar] [CrossRef]
- National Research, C. Zero-G Devices and Weightlessness Simulators; The National Academies Press: Washington, DC, USA, 1961. [Google Scholar] [CrossRef]
- Aleshcheva, G.; Wehland, M.; Sahana, J.; Bauer, J.; Corydon, T.J.; Hemmersbach, R.; Frett, T.; Egli, M.; Infanger, M.; Grosse, J.; et al. Moderate alterations of the cytoskeleton in human chondrocytes after short-term microgravity produced by parabolic flight maneuvers could be prevented by up-regulation of BMP-2 and SOX-9. FASEB J. 2015, 29, 2303–2314. [Google Scholar] [CrossRef]
- Wehland, M.; Ma, X.; Braun, M.; Hauslage, J.; Hemmersbach, R.; Bauer, J.; Grosse, J.; Infanger, M.; Grimm, D. The impact of altered gravity and vibration on endothelial cells during a parabolic flight. Cell. Physiol. Biochem. 2013, 31, 432–451. [Google Scholar] [CrossRef] [PubMed]
- Caprace, D.-G.; Gontier, C.; Iranmanesh, M.; Scoubeau, M.; Pletser, V. Experimental Characterization of Weightlessness During Glider Parabolic Flights. Microgravity Sci. Technol. 2020, 32, 1121–1132. [Google Scholar] [CrossRef]
- Studer, M.; Bradacs, G.; Hilliger, A.; Hürlimann, E.; Engeli, S.; Thiel, C.S.; Zeitner, P.; Denier, B.; Binggeli, M.; Syburra, T.; et al. Parabolic maneuvers of the Swiss Air Force fighter jet F-5E as a research platform for cell culture experiments in microgravity. Acta Astronaut. 2011, 68, 1729–1741. [Google Scholar] [CrossRef]
- Brinckmann, E. New Facilities and Instruments for Developmental Biology Research in Space. In Advances in Space Biology and Medicine; Elsevier: Amsterdam, The Netherlands, 2003; Volume 9, pp. 253–280. [Google Scholar]
- Trichopoulos, D.; Adami, H.O. Introduction: Progress and enigmas in cancer epidemiology. Semin. Cancer Biol. 1998, 8, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Noren, H.; Jove, R.; Beljanski, V.; Grinnemo, K.-H. Differences and similarities between cancer and somatic stem cells: Therapeutic implications. Stem Cell Res. Ther. 2020, 11, 489. [Google Scholar] [CrossRef] [PubMed]
- Walcher, L.; Kistenmacher, A.K.; Suo, H.; Kitte, R.; Dluczek, S.; Strauß, A.; Blaudszun, A.R.; Yevsa, T.; Fricke, S.; Kossatz-Boehlert, U. Cancer Stem Cells-Origins and Biomarkers: Perspectives for Targeted Personalized Therapies. Front. Immunol. 2020, 11, 1280. [Google Scholar] [CrossRef] [PubMed]
- Kopinski, P.K.; Singh, L.N.; Zhang, S.; Lott, M.T.; Wallace, D.C. Mitochondrial DNA variation and cancer. Nat. Rev. Cancer 2021, 21, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Sadikovic, B.; Al-Romaih, K.; Squire, J.A.; Zielenska, M. Cause and consequences of genetic and epigenetic alterations in human cancer. Curr. Genom. 2008, 9, 394–408. [Google Scholar] [CrossRef] [Green Version]
- Elliott, K.; Larsson, E. Non-coding driver mutations in human cancer. Nat. Rev. Cancer 2021, 21, 500–509. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atashzar, M.R.; Baharlou, R.; Karami, J.; Abdollahi, H.; Rezaei, R.; Pourramezan, F.; Zoljalali Moghaddam, S.H. Cancer stem cells: A review from origin to therapeutic implications. J. Cell. Physiol. 2020, 235, 790–803. [Google Scholar] [CrossRef]
- Papaccio, F.; Paino, F.; Regad, T.; Papaccio, G.; Desiderio, V.; Tirino, V. Concise Review: Cancer Cells, Cancer Stem Cells, and Mesenchymal Stem Cells: Influence in Cancer Development. Stem Cells Transl. Med. 2017, 6, 2115–2125. [Google Scholar] [CrossRef]
- Kleinsmith, L.J.; Pierce, G.B., Jr. Multipotentiality of Single Embryonal Carcinoma Cells. Cancer Res. 1964, 24, 1544–1551. [Google Scholar]
- Spillane, J.B.; Henderson, M.A. Cancer stem cells: A review. ANZ J. Surg. 2007, 77, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Pattabiraman, D.R.; Weinberg, R.A. Tackling the cancer stem cells—What challenges do they pose? Nat. Rev. Drug Discov. 2014, 13, 497–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, D.G. Understanding cancer stem cell heterogeneity and plasticity. Cell. Res. 2012, 22, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.B.; Pastushenko, I.; Skibinski, A.; Blanpain, C.; Kuperwasser, C. Phenotypic Plasticity: Driver of Cancer Initiation, Progression, and Therapy Resistance. Cell Stem Cell 2019, 24, 65–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polyak, M.E.; Zaklyazminskaya, E.V. New genetic variant in the SERPINC1 gene: Hereditary Antithrombin deficiency case report, familial thrombosis and considerations on genetic counseling. BMC Med. Genet. 2020, 21, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topal, U.; Zamur, C. Microgravity, Stem Cells, and Cancer: A New Hope for Cancer Treatment. Stem Cells Int. 2021, 2021, 5566872. [Google Scholar] [CrossRef] [PubMed]
- Kopp, S.; Warnke, E.; Wehland, M.; Aleshcheva, G.; Magnusson, N.E.; Hemmersbach, R.; Corydon, T.J.; Bauer, J.; Infanger, M.; Grimm, D. Mechanisms of three-dimensional growth of thyroid cells during long-term simulated microgravity. Sci. Rep. 2015, 5, 16691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopp, S.; Slumstrup, L.; Corydon, T.J.; Sahana, J.; Aleshcheva, G.; Islam, T.; Magnusson, N.E.; Wehland, M.; Bauer, J.; Infanger, M.; et al. Identifications of novel mechanisms in breast cancer cells involving duct-like multicellular spheroid formation after exposure to the Random Positioning Machine. Sci. Rep. 2016, 6, 26887. [Google Scholar] [CrossRef] [PubMed]
- Hybel, T.E.; Dietrichs, D.; Sahana, J.; Corydon, T.J.; Nassef, M.Z.; Wehland, M.; Krüger, M.; Magnusson, N.E.; Bauer, J.; Utpatel, K.; et al. Simulated Microgravity Influences VEGF, MAPK, and PAM Signaling in Prostate Cancer Cells. Int. J. Mol. Sci. 2020, 21, 1263. [Google Scholar] [CrossRef] [Green Version]
- Masiello, M.G.; Cucina, A.; Proietti, S.; Palombo, A.; Coluccia, P.; D’Anselmi, F.; Dinicola, S.; Pasqualato, A.; Morini, V.; Bizzarri, M. Phenotypic switch induced by simulated microgravity on MDA-MB-231 breast cancer cells. BioMed Res. Int. 2014, 2014, 652434. [Google Scholar] [CrossRef]
- Melnik, D.; Krüger, M.; Schulz, H.; Kopp, S.; Wehland, M.; Bauer, J.; Baselet, B.; Vermeesen, R.; Baatout, S.; Corydon, T.J.; et al. The CellBox-2 Mission to the International Space Station: Thyroid Cancer Cells in Space. Int. J. Mol. Sci. 2021, 22, 8777. [Google Scholar] [CrossRef]
- Sahana, J.; Corydon, T.J.; Wehland, M.; Krüger, M.; Kopp, S.; Melnik, D.; Kahlert, S.; Relja, B.; Infanger, M.; Grimm, D. Alterations of Growth and Focal Adhesion Molecules in Human Breast Cancer Cells Exposed to the Random Positioning Machine. Front. Cell Dev. Biol. 2021, 9, 672098. [Google Scholar] [CrossRef] [PubMed]
- Lelkes, P.I.; Galvan, D.L.; Hayman, G.T.; Goodwin, T.J.; Chatman, D.Y.; Cherian, S.; Garcia, R.M.; Unsworth, B.R. Simulated microgravity conditions enhance differentiation of cultured PC12 cells towards the neuroendocrine phenotype. In Vitro Cell. Dev. Biol. Anim. 1998, 34, 316–325. [Google Scholar] [CrossRef]
- Kelly, S.E.; Di Benedetto, A.; Greco, A.; Howard, C.M.; Sollars, V.E.; Primerano, D.A.; Valluri, J.V.; Claudio, P.P. Rapid selection and proliferation of CD133+ cells from cancer cell lines: Chemotherapeutic implications. PLoS ONE 2010, 5, e10035. [Google Scholar] [CrossRef] [Green Version]
- Pisanu, M.E.; Noto, A.; De Vitis, C.; Masiello, M.G.; Coluccia, P.; Proietti, S.; Giovagnoli, M.R.; Ricci, A.; Giarnieri, E.; Cucina, A.; et al. Lung cancer stem cell lose their stemness default state after exposure to microgravity. BioMed Res. Int. 2014, 2014, 470253. [Google Scholar] [CrossRef] [PubMed]
- Mancini, R.; Giarnieri, E.; De Vitis, C.; Malanga, D.; Roscilli, G.; Noto, A.; Marra, E.; Laudanna, C.; Zoppoli, P.; De Luca, P.; et al. Spheres derived from lung adenocarcinoma pleural effusions: Molecular characterization and tumor engraftment. PLoS ONE 2011, 6, e21320. [Google Scholar] [CrossRef] [PubMed]
- Arun, R.P.; Sivanesan, D.; Patra, B.; Varadaraj, S.; Verma, R.S. Simulated microgravity increases polyploid giant cancer cells and nuclear localization of YAP. Sci. Rep. 2019, 9, 10684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Society, T.A.C. Key Statistics for Thyroid Cancer. How Common Is Thyroid Cancer? Available online: https://www.cancer.org/cancer/thyroid-cancer/about/key-statistics.html (accessed on 24 January 2022).
- Grimm, D.; Bauer, J.; Wise, P.; Krüger, M.; Simonsen, U.; Wehland, M.; Infanger, M.; Corydon, T.J. The role of SOX family members in solid tumours and metastasis. Semin. Cancer Biol. 2020, 67, 122–153. [Google Scholar] [CrossRef]
- Ancker, O.V.; Krüger, M.; Wehland, M.; Infanger, M.; Grimm, D. Multikinase Inhibitor Treatment in Thyroid Cancer. Int. J. Mol. Sci. 2019, 21, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, K.N.; Shaha, A.R. Poorly differentiated thyroid cancer. Curr. Opin. Otolaryngol. Head Neck Surg. 2014, 22, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Leboulleux, S. Current practice in patients with differentiated thyroid cancer. Nat. Rev. Endocrinol. 2021, 17, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Albi, E.; Ambesi-Impiombato, F.S.; Peverini, M.; Damaskopoulou, E.; Fontanini, E.; Lazzarini, R.; Curcio, F.; Perrella, G. Thyrotropin receptor and membrane interactions in FRTL-5 thyroid cell strain in microgravity. Astrobiology 2011, 11, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Bauer, J.; Kossmehl, P.; Shakibaei, M.; Schöberger, J.; Pickenhahn, H.; Schulze-Tanzil, G.; Vetter, R.; Eilles, C.; Paul, M.; et al. Simulated microgravity alters differentiation and increases apoptosis in human follicular thyroid carcinoma cells. FASEB J. 2002, 16, 604–606. [Google Scholar] [CrossRef]
- Warnke, E.; Pietsch, J.; Kopp, S.; Bauer, J.; Sahana, J.; Wehland, M.; Krüger, M.; Hemmersbach, R.; Infanger, M.; Lützenberg, R.; et al. Cytokine Release and Focal Adhesion Proteins in Normal Thyroid Cells Cultured on the Random Positioning Machine. Cell. Physiol. Biochem. 2017, 43, 257–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grosse, J.; Wehland, M.; Pietsch, J.; Schulz, H.; Saar, K.; Hübner, N.; Eilles, C.; Bauer, J.; Abou-El-Ardat, K.; Baatout, S.; et al. Gravity-sensitive signaling drives 3-dimensional formation of multicellular thyroid cancer spheroids. FASEB J. 2012, 26, 5124–5140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulbrich, C.; Pietsch, J.; Grosse, J.; Wehland, M.; Schulz, H.; Saar, K.; Hübner, N.; Hauslage, J.; Hemmersbach, R.; Braun, M.; et al. Differential gene regulation under altered gravity conditions in follicular thyroid cancer cells: Relationship between the extracellular matrix and the cytoskeleton. Cell. Physiol. Biochem. 2011, 28, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Kopp, S.; Krüger, M.; Feldmann, S.; Oltmann, H.; Schütte, A.; Schmitz, B.; Bauer, J.; Schulz, H.; Saar, K.; Huebner, N.; et al. Thyroid cancer cells in space during the TEXUS-53 sounding rocket mission—The THYROID Project. Sci. Rep. 2018, 8, 10355. [Google Scholar] [CrossRef] [Green Version]
- Kopp, S.; Krüger, M.; Bauer, J.; Wehland, M.; Corydon, T.J.; Sahana, J.; Nassef, M.Z.; Melnik, D.; Bauer, T.J.; Schulz, H.; et al. Microgravity Affects Thyroid Cancer Cells during the TEXUS-53 Mission Stronger than Hypergravity. Int. J. Mol. Sci. 2018, 19, 4001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corydon, T.J.; Kopp, S.; Wehland, M.; Braun, M.; Schütte, A.; Mayer, T.; Hülsing, T.; Oltmann, H.; Schmitz, B.; Hemmersbach, R.; et al. Alterations of the cytoskeleton in human cells in space proved by life-cell imaging. Sci. Rep. 2016, 6, 20043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, C.; Kohn, F.P.; Bauer, J. Preparing normal tissue cells for space flight experiments. Prep. Biochem. Biotechnol. 2016, 46, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, J.; Ma, X.; Wehland, M.; Aleshcheva, G.; Schwarzwälder, A.; Segerer, J.; Birlem, M.; Horn, A.; Bauer, J.; Infanger, M.; et al. Spheroid formation of human thyroid cancer cells in an automated culturing system during the Shenzhou-8 Space mission. Biomaterials 2013, 34, 7694–7705. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Pietsch, J.; Wehland, M.; Schulz, H.; Saar, K.; Hübner, N.; Bauer, J.; Braun, M.; Schwarzwälder, A.; Segerer, J.; et al. Differential gene expression profile and altered cytokine secretion of thyroid cancer cells in space. FASEB J. 2014, 28, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Riwaldt, S.; Bauer, J.; Pietsch, J.; Braun, M.; Segerer, J.; Schwarzwälder, A.; Corydon, T.J.; Infanger, M.; Grimm, D. The Importance of Caveolin-1 as Key-Regulator of Three-Dimensional Growth in Thyroid Cancer Cells Cultured under Real and Simulated Microgravity Conditions. Int. J. Mol. Sci. 2015, 16, 28296–28310. [Google Scholar] [CrossRef] [PubMed]
- Riwaldt, S.; Pietsch, J.; Sickmann, A.; Bauer, J.; Braun, M.; Segerer, J.; Schwarzwälder, A.; Aleshcheva, G.; Corydon, T.J.; Infanger, M.; et al. Identification of proteins involved in inhibition of spheroid formation under microgravity. Proteomics 2015, 15, 2945–2952. [Google Scholar] [CrossRef]
- Grimm, D.; Kossmehl, P.; Shakibaei, M.; Schulze-Tanzil, G.; Pickenhahn, H.; Bauer, J.; Paul, M.; Cogoli, A. Effects of simulated microgravity on thyroid carcinoma cells. J. Gravit. Physiol. 2002, 9, P253–P256. [Google Scholar] [PubMed]
- Kossmehl, P.; Shakibaei, M.; Cogoli, A.; Infanger, M.; Curcio, F.; Schönberger, J.; Eilles, C.; Bauer, J.; Pickenhahn, H.; Schulze-Tanzil, G.; et al. Weightlessness induced apoptosis in normal thyroid cells and papillary thyroid carcinoma cells via extrinsic and intrinsic pathways. Endocrinology 2003, 144, 4172–4179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Infanger, M.; Kossmehl, P.; Shakibaei, M.; Schulze-Tanzil, G.; Cogoli, A.; Faramarzi, S.; Bauer, J.; Curcio, F.; Paul, M.; Grimm, D. Longterm conditions of mimicked weightlessness influences the cytoskeleton in thyroid cells. J. Gravit. Physiol. 2004, 11, P169–P172. [Google Scholar]
- Infanger, M.; Kossmehl, P.; Shakibaei, M.; Bauer, J.; Kossmehl-Zorn, S.; Cogoli, A.; Curcio, F.; Oksche, A.; Wehland, M.; Kreutz, R.; et al. Simulated weightlessness changes the cytoskeleton and extracellular matrix proteins in papillary thyroid carcinoma cells. Cell Tissue Res. 2006, 324, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Riwaldt, S.; Bauer, J.; Wehland, M.; Slumstrup, L.; Kopp, S.; Warnke, E.; Dittrich, A.; Magnusson, N.E.; Pietsch, J.; Corydon, T.J.; et al. Pathways Regulating Spheroid Formation of Human Follicular Thyroid Cancer Cells under Simulated Microgravity Conditions: A Genetic Approach. Int. J. Mol. Sci. 2016, 17, 528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melnik, D.; Sahana, J.; Corydon, T.J.; Kopp, S.; Nassef, M.Z.; Wehland, M.; Infanger, M.; Grimm, D.; Krüger, M. Dexamethasone Inhibits Spheroid Formation of Thyroid Cancer Cells Exposed to Simulated Microgravity. Cells 2020, 9, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, J.; Kopp, S.; Schlagberger, E.M.; Grosse, J.; Sahana, J.; Riwaldt, S.; Wehland, M.; Luetzenberg, R.; Infanger, M.; Grimm, D. Proteome Analysis of Human Follicular Thyroid Cancer Cells Exposed to the Random Positioning Machine. Int. J. Mol. Sci. 2017, 18, 546. [Google Scholar] [CrossRef] [Green Version]
- Bauer, J.; Grimm, D.; Gombocz, E. Semantic analysis of thyroid cancer cell proteins obtained from rare research opportunities. J. Biomed. Inf. 2017, 76, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Wehland, M.; Infanger, M.; Grimm, D.; Gombocz, E. Semantic Analysis of Posttranslational Modification of Proteins Accumulated in Thyroid Cancer Cells Exposed to Simulated Microgravity. Int. J. Mol. Sci. 2018, 19, 2257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, A.; Zhou, A.; Gordon, R.E.; Henderson, S.C.; Schwartz, A.E.; Schwartz, A.E.; Friedman, E.W.; Davies, T.F. Thyroid organoid formation in simulated microgravity: Influence of keratinocyte growth factor. Thyroid 2000, 10, 481–487. [Google Scholar] [CrossRef]
- Tan, X.; Xu, A.; Zhao, T.; Zhao, Q.; Zhang, J.; Fan, C.; Deng, Y.; Freywald, A.; Genth, H.; Xiang, J. Simulated microgravity inhibits cell focal adhesions leading to reduced melanoma cell proliferation and metastasis via FAK/RhoA-regulated mTORC1 and AMPK pathways. Sci. Rep. 2018, 8, 3769. [Google Scholar] [CrossRef] [Green Version]
- Thiel, C.S.; Tauber, S.; Seebacher, C.; Schropp, M.; Uhl, R.; Lauber, B.; Polzer, J.; Neelam, S.; Zhang, Y.; Ullrich, O. Real-Time 3D High-Resolution Microscopy of Human Cells on the International Space Station. Int. J. Mol. Sci. 2019, 20, 2033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietsch, J.; Kussian, R.; Sickmann, A.; Bauer, J.; Weber, G.; Nissum, M.; Westphal, K.; Egli, M.; Grosse, J.; Schönberger, J.; et al. Application of free-flow IEF to identify protein candidates changing under microgravity conditions. Proteomics 2010, 10, 904–913. [Google Scholar] [CrossRef]
- Melnik, D.; Krüger, M.; Kopp, S.; Wehland, M.; Bauer, J.; Infanger, M.; Grimm, D. Microgravity-based Modulation of VEGF Expression in Human Thyroid Carcinoma Cells. In Proceedings of the 39th ISGP Meeting & ESA Life Sciences Meeting, Noordwijk, The Netherlands, 18–22 June 2018. [Google Scholar]
- Wise, P.M.; Neviani, P.; Riwaldt, S.; Corydon, T.J.; Wehland, M.; Braun, M.; Krüger, M.; Infanger, M.; Grimm, D. Changes in Exosome Release in Thyroid Cancer Cells after Prolonged Exposure to Real Microgravity in Space. Int. J. Mol. Sci. 2021, 22, 2132. [Google Scholar] [CrossRef] [PubMed]
- Wise, P.M.; Neviani, P.; Riwaldt, S.; Corydon, T.J.; Wehland, M.; Braun, M.; Krüger, M.; Infanger, M.; Grimm, D. Changes in Exosomal miRNA Composition in Thyroid Cancer Cells after Prolonged Exposure to Real Microgravity in Space. Int. J. Mol. Sci. 2021, 22, 12841. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Leo, C.P.; Leo, C.; Szucs, T.D. Breast cancer drug approvals by the US FDA from 1949 to 2018. Nat. Rev. Drug Discov. 2020, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Qian, A.; Zhang, W.; Xie, L.; Weng, Y.; Yang, P.; Wang, Z.; Hu, L.; Xu, H.; Tian, Z.; Shang, P. Simulated weightlessness alters biological characteristics of human breast cancer cell line MCF-7. Acta Astronaut. 2008, 63, 947–958. [Google Scholar] [CrossRef]
- Nassef, M.Z.; Kopp, S.; Wehland, M.; Melnik, D.; Sahana, J.; Krüger, M.; Corydon, T.J.; Oltmann, H.; Schmitz, B.; Schütte, A.; et al. Real Microgravity Influences the Cytoskeleton and Focal Adhesions in Human Breast Cancer Cells. Int. J. Mol. Sci. 2019, 20, 3156. [Google Scholar] [CrossRef] [Green Version]
- Nassef, M.Z.; Kopp, S.; Melnik, D.; Corydon, T.J.; Sahana, J.; Krüger, M.; Wehland, M.; Bauer, T.J.; Liemersdorf, C.; Hemmersbach, R.; et al. Short-Term Microgravity Influences Cell Adhesion in Human Breast Cancer Cells. Int. J. Mol. Sci. 2019, 20, 5730. [Google Scholar] [CrossRef] [Green Version]
- Vassy, J.; Portet, S.; Beil, M.; Millot, G.; Fauvel-Lafève, F.; Gasset, G.; Schoevaert, D. Weightlessness acts on human breast cancer cell line MCF-7. Adv. Space Res. 2003, 32, 1595–1603. [Google Scholar] [CrossRef]
- Kopp, S.; Sahana, J.; Islam, T.; Petersen, A.G.; Bauer, J.; Corydon, T.J.; Schulz, H.; Saar, K.; Huebner, N.; Slumstrup, L.; et al. The role of NFκB in spheroid formation of human breast cancer cells cultured on the Random Positioning Machine. Sci. Rep. 2018, 8, 921. [Google Scholar] [CrossRef]
- Sahana, J.; Nassef, M.Z.; Wehland, M.; Kopp, S.; Krüger, M.; Corydon, T.J.; Infanger, M.; Bauer, J.; Grimm, D. Decreased E-Cadherin in MCF7 Human Breast Cancer Cells Forming Multicellular Spheroids Exposed to Simulated Microgravity. Proteomics 2018, 18, e1800015. [Google Scholar] [CrossRef]
- Strube, F.; Infanger, M.; Dietz, C.; Romswinkel, A.; Kraus, A. Short-term effects of simulated microgravity on morphology and gene expression in human breast cancer cells. Physiol. Int. 2019, 106, 311–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strube, F.; Infanger, M.; Wehland, M.; Delvinioti, X.; Romswinkel, A.; Dietz, C.; Kraus, A. Alteration of Cytoskeleton Morphology and Gene Expression in Human Breast Cancer Cells under Simulated Microgravity. Cell J. 2020, 22, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Coinu, R.; Chiaviello, A.; Galleri, G.; Franconi, F.; Crescenzi, E.; Palumbo, G. Exposure to modeled microgravity induces metabolic idleness in malignant human MCF-7 and normal murine VSMC cells. FEBS Lett. 2006, 580, 2465–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietrichs, D.; Grimm, D.; Sahana, J.; Melnik, D.; Corydon, T.J.; Wehland, M.; Krüger, M.; Vermeesen, R.; Baselet, B.; Baatout, S.; et al. Three-Dimensional Growth of Prostate Cancer. Cells Exposed to Simulated Microgravity. Front. Cell Dev. Biol. 2022, 10, 841017. [Google Scholar] [CrossRef] [PubMed]
- Monti, N.; Masiello, M.G.; Proietti, S.; Catizone, A.; Ricci, G.; Harrath, A.H.; Alwasel, S.H.; Cucina, A.; Bizzarri, M. Survival Pathways Are Differently Affected by Microgravity in Normal and Cancerous Breast Cells. Int. J. Mol. Sci. 2021, 22, 862. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.J.; Gombocz, E.; Wehland, M.; Bauer, J.; Infanger, M.; Grimm, D. Insight in Adhesion Protein Sialylation and Microgravity Dependent Cell Adhesion—An Omics Network Approach. Int. J. Mol. Sci. 2020, 21, 1749. [Google Scholar] [CrossRef] [Green Version]
- Grimm, D.; Wehland, M.; Corydon, T.J.; Richter, P.; Prasad, B.; Bauer, J.; Egli, M.; Kopp, S.; Lebert, M.; Krüger, M. The effects of microgravity on differentiation and cell growth in stem cells and cancer stem cells. Stem Cells Transl. Med. 2020, 9, 882–894. [Google Scholar] [CrossRef]
- Vamvakidou, A.P.; Mondrinos, M.J.; Petushi, S.P.; Garcia, F.U.; Lelkes, P.I.; Tozeren, A. Heterogeneous breast tumoroids: An in vitro assay for investigating cellular heterogeneity and drug delivery. J. Biomol. Screen 2007, 12, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Li, Q.; Cao, Q.; Diao, Y.; Zhang, Y.; Yue, L.; Wei, L. EMT Transcription Factors Are Involved in the Altered Cell Adhesion under Simulated Microgravity Effect or Overloading by Regulation of E-cadherin. Int. J. Mol. Sci. 2020, 21, 1349. [Google Scholar] [CrossRef] [Green Version]
- Jiang, N.; Chen, Z.-Y.; Li, B.-B.; Guo, S.; Li, A.; Zhang, T.; Fu, X.-Y.; Si, S.-y.; Cui, Y. Effects of rotary cell culture system-simulated microgravity on the ultrastructure and biological behavior of human MDA MB 231 breast cancer cells. Precis. Radiat. Oncol. 2019, 3, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Yang, X.; Cui, X.; Jiang, M.; Gui, Y.; Zhang, Y.; Luo, X. Adrenomedullin is a key Protein Mediating Rotary Cell Culture System that Induces the Effects of Simulated Microgravity on Human Breast Cancer Cells. Microgravity Sci. Technol. 2015, 27, 417–426. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, F.; Russo, A.; Wan, Y. Proteomic Analysis of Extracellular Vesicles Derived from MDA-MB-231 Cells in Microgravity. Protein J. 2021, 40, 108–118. [Google Scholar] [CrossRef]
- Vassy, J.; Portet, S.; Beil, M.; Millot, G.; Fauvel-Lafève, F.; Karniguian, A.; Gasset, G.; Irinopoulou, T.; Calvo, F.; Rigaut, J.P.; et al. The effect of weightlessness on cytoskeleton architecture and proliferation of human breast cancer cell line MCF-7. FASEB J. 2001, 15, 1104–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandaglia, G.; Abdollah, F.; Schiffmann, J.; Trudeau, V.; Shariat, S.F.; Kim, S.P.; Perrotte, P.; Montorsi, F.; Briganti, A.; Trinh, Q.D.; et al. Distribution of metastatic sites in patients with prostate cancer: A population-based analysis. Prostate 2014, 74, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, R.P.; Goodwin, T.J.; Wolf, D.A. Cell culture for three-dimensional modeling in rotating-wall vessels: An application of simulated microgravity. J. Tissue Cult. Methods 1992, 14, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Clejan, S.; O’Connor, K.C.; Cowger, N.L.; Cheles, M.K.; Haque, S.; Primavera, A.C. Effects of simulated microgravity on DU 145 human prostate carcinoma cells. Biotechnol. Bioeng. 1996, 50, 587–597. [Google Scholar] [CrossRef]
- O’Connor, K.C.; Enmon, R.M.; Dotson, R.S.; Primavera, A.C.; Clejan, S. Characterization of Autocrine Growth Factors, Their Receptors and Extracellular Matrix Present in Three-Dimensional Cultures of DU 145 Human Prostate Carcinoma Cells Grown in Simulated Microgravity. Tissue Eng. 1997, 3, 161–171. [Google Scholar] [CrossRef]
- Clejan, S.; O’Connor, K.; Rosensweig, N. Tri-dimensional prostate cell cultures in simulated microgravity and induced changes in lipid second messengers and signal transduction. J. Cell. Mol. Med. 2001, 5, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Zhau, H.E.; Goodwin, T.J.; Chang, S.M.; Baker, T.L.; Chung, L.W. Establishment of a three-dimensional human prostate organoid coculture under microgravity-simulated conditions: Evaluation of androgen-induced growth and PSA expression. In Vitro Cell. Dev. Biol. Anim. 1997, 33, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Twombly, R. Prostate modeling experiment success becomes part of legacy of shuttle astronauts. J. Natl. Cancer Inst. 2003, 95, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Ingram, M.; Techy, G.B.; Saroufeem, R.; Yazan, O.; Narayan, K.S.; Goodwin, T.J.; Spaulding, G.F. Three-dimensional growth patterns of various human tumor cell lines in simulated microgravity of a NASA bioreactor. In Vitro Cell. Dev. Biol. Anim. 1997, 33, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodwin, T.J.; Jessup, J.M.; Wolf, D.A. Morphologic differentiation of colon carcinoma cell lines HT-29 and HT-29KM in rotating-wall vessels. In Vitro Cell. Dev. Biol. 1992, 28a, 47–60. [Google Scholar] [CrossRef]
- Jessup, J.M.; Brown, K.; Ishii, S.; Ford, R.; Goodwin, T.J.; Spaulding, G. Simulated microgravity does not alter epithelial cell adhesion to matrix and other molecules. Adv. Space Res. 1994, 14, 71–76. [Google Scholar] [CrossRef]
- Jessup, J.M.; Brown, D.; Fitzgerald, W.; Ford, R.D.; Nachman, A.; Goodwin, T.J.; Spaulding, G. Induction of carcinoembryonic antigen expression in a three-dimensional culture system. In Vitro Cell. Dev. Biol. Anim. 1997, 33, 352–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessup, J.M.; Frantz, M.; Sonmez-Alpan, E.; Locker, J.; Skena, K.; Waller, H.; Battle, P.; Nachman, A.; Bhatti; Weber, M.E.; et al. Microgravity culture reduces apoptosis and increases the differentiation of a human colorectal carcinoma cell line. In Vitro Cell. Dev. Biol. Anim. 2000, 36, 367–373. [Google Scholar] [CrossRef]
- Skardal, A.; Devarasetty, M.; Rodman, C.; Atala, A.; Soker, S. Liver-Tumor Hybrid Organoids for Modeling Tumor Growth and Drug Response In Vitro. Ann. Biomed. Eng. 2015, 43, 2361–2373. [Google Scholar] [CrossRef] [Green Version]
- Devarasetty, M.; Wang, E.; Soker, S.; Skardal, A. Mesenchymal stem cells support growth and organization of host-liver colorectal-tumor organoids and possibly resistance to chemotherapy. Biofabrication 2017, 9, 021002. [Google Scholar] [CrossRef] [PubMed]
- Arun, R.P.; Sivanesan, D.; Vidyasekar, P.; Verma, R.S. PTEN/FOXO3/AKT pathway regulates cell death and mediates morphogenetic differentiation of Colorectal Cancer Cells under Simulated Microgravity. Sci. Rep. 2017, 7, 5952. [Google Scholar] [CrossRef]
- La Barbera, G.; Capriotti, A.L.; Michelini, E.; Piovesana, S.; Calabretta, M.M.; Zenezini Chiozzi, R.; Roda, A.; Laganà, A. Proteomic analysis and bioluminescent reporter gene assays to investigate effects of simulated microgravity on Caco-2 cells. Proteomics 2017, 17, 1700081. [Google Scholar] [CrossRef]
- Gouws, C.; Smit, T.; Willers, C.; Svitina, H.; Calitz, C.; Wrzesinski, K. Anticancer Potential of Sutherlandia frutescens and Xysmalobium undulatum in LS180 Colorectal Cancer Mini-Tumors. Molecules 2021, 26, 605. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ramadori, P.; Pfister, D.; Seehawer, M.; Zender, L.; Heikenwalder, M. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat. Rev. Cancer 2021, 21, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Khaoustov, V.I.; Risin, D.; Pellis, N.R.; Yoffe, B. Microarray analysis of genes differentially expressed in HepG2 cells cultured in simulated microgravity: Preliminary report. In Vitro Cell. Dev. Biol. Anim. 2001, 37, 84–88. [Google Scholar] [CrossRef]
- Clement, J.Q.; Lacy, S.M.; Wilson, B.L. Genome-wide gene expression profiling of microgravity effect on human liver cells. J. Gravit. Physiol. 2007, 14, P121–P122. [Google Scholar] [PubMed]
- Chang, T.T.; Hughes-Fulford, M. Monolayer and spheroid culture of human liver hepatocellular carcinoma cell line cells demonstrate distinct global gene expression patterns and functional phenotypes. Tissue Eng. Part A 2009, 15, 559–567. [Google Scholar] [CrossRef]
- Tang, J.; Cui, J.; Chen, R.; Guo, K.; Kang, X.; Li, Y.; Gao, D.; Sun, L.; Xu, C.; Chen, J.; et al. A three-dimensional cell biology model of human hepatocellular carcinoma in vitro. Tumour Biol. 2011, 32, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.X.; Song, H.Y.; Dong, Y.Y.; Hu, C.; Zheng, Q.D.; Xue, T.C.; Liu, X.H.; Zhang, Y.; Chen, J.; Ren, Z.G.; et al. Dynamic expression patterns of differential proteins during early invasion of hepatocellular carcinoma. PLoS ONE 2014, 9, e88543. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Dong, Y.; Xie, X.; Chen, J.; Gao, D.; Liu, Y.; Ren, Z.; Cui, J. Screening candidate metastasis-associated genes in three-dimensional HCC spheroids with different metastasis potential. Int. J. Clin. Exp. Pathol. 2014, 7, 2527–2535. [Google Scholar] [PubMed]
- Fukazawa, T.; Tanimoto, K.; Shrestha, L.; Imura, T.; Takahashi, S.; Sueda, T.; Hirohashi, N.; Hiyama, E.; Yuge, L. Simulated microgravity enhances CDDP-induced apoptosis signal via p53-independent mechanisms in cancer cells. PLoS ONE 2019, 14, e0219363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Štampar, M.; Sedighi Frandsen, H.; Rogowska-Wrzesinska, A.; Wrzesinski, K.; Filipič, M.; Žegura, B. Hepatocellular carcinoma (HepG2/C3A) cell-based 3D model for genotoxicity testing of chemicals. Sci. Total Environ. 2021, 755, 143255. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Jiang, N.; Guo, S.; Li, B.B.; Yang, J.Q.; Chai, S.B.; Yan, H.F.; Sun, P.M.; Zhang, T.; Sun, H.W.; et al. Effect of simulated microgravity on metabolism of HGC-27 gastric cancer cells. Oncol. Lett. 2020, 19, 3439–3450. [Google Scholar] [CrossRef]
- Nakamura, K.; Kuga, H.; Morisaki, T.; Baba, E.; Sato, N.; Mizumoto, K.; Sueishi, K.; Tanaka, M.; Katano, M. Simulated microgravity culture system for a 3-D carcinoma tissue model. Biotechniques 2002, 33, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, J.S.; Huang, H.Y.; Shi, J.F.; Li, N.; Zhang, Y.; Dai, M. International trends in lung cancer incidence from 1973 to 2007. Cancer Med. 2018, 7, 1479–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, A.K.; Gupta, U.; Jain, S. Epidemiology of lung cancer and approaches for its prediction: A systematic review and analysis. Chin. J. Cancer 2016, 35, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratz, J.R.; Yagui-Beltrán, A.; Jablons, D.M. Cancer stem cells in lung tumorigenesis. Ann. Thorac. Surg. 2010, 89, S2090–S2095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borthwick, D.W.; Shahbazian, M.; Krantz, Q.T.; Dorin, J.R.; Randell, S.H. Evidence for stem-cell niches in the tracheal epithelium. Am. J. Respir. Cell. Mol. Biol. 2001, 24, 662–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, J.C.; Teisanu, R.M.; Stripp, B.R. Endogenous lung stem cells and contribution to disease. J. Pathol. 2009, 217, 254–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, K.U.; Reynolds, S.D.; Watkins, S.; Fuchs, E.; Stripp, B.R. In vivo differentiation potential of tracheal basal cells: Evidence for multipotent and unipotent subpopulations. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L643–L649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoch, K.G.; Lori, A.; Burns, K.A.; Eldred, T.; Olsen, J.C.; Randell, S.H. A subset of mouse tracheal epithelial basal cells generates large colonies in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L631–L642. [Google Scholar] [CrossRef] [Green Version]
- Hong, K.U.; Reynolds, S.D.; Watkins, S.; Fuchs, E.; Stripp, B.R. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am. J. Pathol. 2004, 164, 577–588. [Google Scholar] [CrossRef] [Green Version]
- Evans, M.J.; Cabral-Anderson, L.J.; Freeman, G. Role of the Clara cell in renewal of the bronchiolar epithelium. Lab. Investig. 1978, 38, 648–653. [Google Scholar]
- Teisanu, R.M.; Lagasse, E.; Whitesides, J.F.; Stripp, B.R. Prospective isolation of bronchiolar stem cells based upon immunophenotypic and autofluorescence characteristics. Stem Cells 2009, 27, 612–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, S.D.; Giangreco, A.; Power, J.H.; Stripp, B.R. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am. J. Pathol. 2000, 156, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, S.D.; Hong, K.U.; Giangreco, A.; Mango, G.W.; Guron, C.; Morimoto, Y.; Stripp, B.R. Conditional clara cell ablation reveals a self-renewing progenitor function of pulmonary neuroendocrine cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 278, L1256–L1263. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.U.; Reynolds, S.D.; Giangreco, A.; Hurley, C.M.; Stripp, B.R. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am. J. Respir. Cell. Mol. Biol. 2001, 24, 671–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giangreco, A.; Groot, K.R.; Janes, S.M. Lung cancer and lung stem cells: Strange bedfellows? Am. J. Respir. Crit. Care Med. 2007, 175, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Gutova, M.; Najbauer, J.; Gevorgyan, A.; Metz, M.Z.; Weng, Y.; Shih, C.C.; Aboody, K.S. Identification of uPAR-positive chemoresistant cells in small cell lung cancer. PLoS ONE 2007, 2, e243. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.Y.; Peyrollier, K.; Xia, W.; Gilad, E. Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J. Biol. Chem. 2008, 283, 17635–17651. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.F.; Jackson, E.L.; Woolfenden, A.E.; Lawrence, S.; Babar, I.; Vogel, S.; Crowley, D.; Bronson, R.T.; Jacks, T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005, 121, 823–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eramo, A.; Lotti, F.; Sette, G.; Pilozzi, E.; Biffoni, M.; Di Virgilio, A.; Conticello, C.; Ruco, L.; Peschle, C.; De Maria, R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008, 15, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, G.; Roz, L.; Perego, P.; Tortoreto, M.; Fontanella, E.; Gatti, L.; Pratesi, G.; Fabbri, A.; Andriani, F.; Tinelli, S.; et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc. Natl. Acad. Sci. USA 2009, 106, 16281–16286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, J.H.; Ahn, C.B.; Son, K.H.; Yi, E.; Son, H.S.; Kim, H.S.; Lee, S.H. Simulated Microgravity Effects on Nonsmall Cell Lung Cancer Cell Proliferation and Migration. Aerosp. Med. Hum. Perform. 2017, 88, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Dietz, C.; Infanger, M.; Romswinkel, A.; Strube, F.; Kraus, A. Apoptosis Induction and Alteration of Cell Adherence in Human Lung Cancer Cells under Simulated Microgravity. Int. J. Mol. Sci. 2019, 20, 3601. [Google Scholar] [CrossRef] [Green Version]
- Ahn, C.B.; Lee, J.-H.; Han, D.G.; Kang, H.-W.; Lee, S.-H.; Lee, J.-I.; Son, K.H.; Lee, J.W. Simulated microgravity with floating environment promotes migration of non-small cell lung cancers. Sci. Rep. 2019, 9, 14553. [Google Scholar] [CrossRef]
- Chang, D.; Xu, H.; Guo, Y.; Jiang, X.; Liu, Y.; Li, K.; Pan, C.; Yuan, M.; Wang, J.; Li, T.; et al. Simulated microgravity alters the metastatic potential of a human lung adenocarcinoma cell line. In Vitro Cell. Dev. Biol. Anim. 2013, 49, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Degan, P.; Cortese, K.; Pulliero, A.; Bruno, S.; Gagliani, M.C.; Congiu, M.; Izzotti, A. Simulated Microgravity Effects on Human Adenocarcinoma Alveolar Epithelial Cells: Characterization of Morphological, Functional, and Epigenetic Parameters. Int. J. Mol. Sci. 2021, 22, 6951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, L.; Chen, J.; Wang, J. Behavior of stem cells under outer-space microgravity and ground-based microgravity simulation. Cell Biol. Int. 2015, 39, 647–656. [Google Scholar] [CrossRef]
- Shi, Y.; Fu, X.; Hua, Y.; Han, Y.; Lu, Y.; Wang, J. The side population in human lung cancer cell line NCI-H460 is enriched in stem-like cancer cells. PLoS ONE 2012, 7, e33358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NIH. National Cancer Institute. Cancer Stat Facts: Melanoma of the Skin. Available online: https://seer.cancer.gov/statfacts/html/melan.html (accessed on 14 February 2022).
- Taga, M.; Yamauchi, K.; Odle, J.; Furian, L.; Sundaresan, A.; Ramesh, G.T.; Pellis, N.R.; Andrassy, R.J.; Kulkarni, A.D. Melanoma growth and tumorigenicity in models of microgravity. Aviat. Space Environ. Med. 2006, 77, 1113–1116. [Google Scholar]
- Zhao, T.; Tang, X.; Umeshappa, C.S.; Ma, H.; Gao, H.; Deng, Y.; Freywald, A.; Xiang, J. Simulated Microgravity Promotes Cell Apoptosis Through Suppressing Uev1A/TICAM/TRAF/NF-κB-Regulated Anti-Apoptosis and p53/PCNA- and ATM/ATR-Chk1/2-Controlled DNA-Damage Response Pathways. J. Cell. Biochem. 2016, 117, 2138–2148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, R.; Tan, X.; Zhang, J.; Fan, C.; Zhao, Q.; Deng, Y.; Xu, A.; Lukong, K.E.; Genth, H.; et al. Simulated Microgravity Reduces Focal Adhesions and Alters Cytoskeleton and Nuclear Positioning Leading to Enhanced Apoptosis via Suppressing FAK/RhoA-Mediated mTORC1/NF-κB and ERK1/2 Pathways. Int. J. Mol. Sci. 2018, 19, 1994. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, K.; Eiermann, P.; Tsiockas, W.; Hauslage, J.; Hemmersbach, R.; Gerzer, R. Natriuretic peptide-sensitive guanylyl cyclase expression is down-regulated in human melanoma cells at simulated weightlessness. Acta Astronaut. 2011, 68, 652–655. [Google Scholar] [CrossRef]
- Ivanova, K.; Eiermann, P.; Tsiockas, W.; Hemmersbach, R.; Gerzer, R. Differential Regulation of cGMP Signaling in Human Melanoma Cells at Altered Gravity: Simulated Microgravity Down-Regulates Cancer-Related Gene Expression and Motility. Microgravity Sci. Technol. 2018, 30, 457–467. [Google Scholar] [CrossRef] [Green Version]
- Licato, L.L.; Prieto, V.G.; Grimm, E.A. A novel preclinical model of human malignant melanoma utilizing bioreactor rotating-wall vessels. In Vitro Cell. Dev. Biol. Anim. 2001, 37, 121–126. [Google Scholar] [CrossRef]
- Marrero, B.; Messina, J.L.; Heller, R. Generation of a tumor spheroid in a microgravity environment as a 3D model of melanoma. In Vitro Cell. Dev. Biol. Anim. 2009, 45, 523–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Przystupski, D.; Górska, A.; Michel, O.; Podwin, A.; Śniadek, P.; Łapczyński, R.; Saczko, J.; Kulbacka, J. Testing Lab-on-a-Chip Technology for Culturing Human Melanoma Cells under Simulated Microgravity. Cancers 2021, 13, 402. [Google Scholar] [CrossRef]
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu Rev. Cell. Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, G.M. The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev. Cell. Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- Orozco, A.F.; Lewis, D.E. Flow cytometric analysis of circulating microparticles in plasma. Cytom. Part A 2010, 77A, 502–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzales, P.A.; Pisitkun, T.; Hoffert, J.D.; Tchapyjnikov, D.; Star, R.A.; Kleta, R.; Wang, N.S.; Knepper, M.A. Large-scale proteomics and phosphoproteomics of urinary exosomes. J. Am. Soc. Nephrol. 2009, 20, 363–379. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Gillespie, B.M.; Palanisamy, V.; Gimzewski, J.K. Quantitative nanostructural and single-molecule force spectroscopy biomolecular analysis of human-saliva-derived exosomes. Langmuir 2011, 27, 14394–14400. [Google Scholar] [CrossRef] [Green Version]
- Kang, C.Y.; Zou, L.; Yuan, M.; Wang, Y.; Li, T.Z.; Zhang, Y.; Wang, J.F.; Li, Y.; Deng, X.W.; Liu, C.T. Impact of simulated microgravity on microvascular endothelial cell apoptosis. Eur. J. Appl. Physiol. 2011, 111, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Corydon, T.J.; Mann, V.; Slumstrup, L.; Kopp, S.; Sahana, J.; Askou, A.L.; Magnusson, N.E.; Echegoyen, D.; Bek, T.; Sundaresan, A.; et al. Reduced Expression of Cytoskeletal and Extracellular Matrix Genes in Human Adult Retinal Pigment Epithelium Cells Exposed to Simulated Microgravity. Cell. Physiol. Biochem. 2016, 40, 1–17. [Google Scholar] [CrossRef]
- Li, N.; Wang, C.; Sun, S.; Zhang, C.; Lü, D.; Chen, Q.; Long, M. Microgravity-Induced Alterations of Inflammation-Related Mechanotransduction in Endothelial Cells on Board SJ-10 Satellite. Front. Physiol. 2018, 9, 1025. [Google Scholar] [CrossRef] [Green Version]
- Prasad, B.; Grimm, D.; Strauch, S.M.; Erzinger, G.S.; Corydon, T.J.; Lebert, M.; Magnusson, N.E.; Infanger, M.; Richter, P.; Krüger, M. Influence of Microgravity on Apoptosis in Cells, Tissues, and Other Systems In Vivo and In Vitro. Int. J. Mol. Sci. 2020, 21, 9373. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, J.; Bauer, J.; Egli, M.; Infanger, M.; Wise, P.; Ulbrich, C.; Grimm, D. The effects of weightlessness on the human organism and mammalian cells. Curr. Mol. Med. 2011, 11, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Zanello, S.B.; Tadigotla, V.; Hurley, J.; Skog, J.; Stevens, B.; Calvillo, E.; Bershad, E. Inflammatory gene expression signatures in idiopathic intracranial hypertension: Possible implications in microgravity-induced ICP elevation. npj Microgravity 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Chen, J.; Wang, H.; Lu, X.; Li, K.; Yang, C.; Wu, F.; Xu, Z.; Nie, H.; Ding, B.; et al. Serum Metabolomics Associating with Circulating MicroRNA Profiles Reveal the Role of miR-383-5p in Rat Hippocampus Under Simulated Microgravity. Front. Physiol. 2020, 11, 939. [Google Scholar] [CrossRef] [PubMed]
- Bezdan, D.; Grigorev, K.; Meydan, C.; Pelissier Vatter, F.A.; Cioffi, M.; Rao, V.; MacKay, M.; Nakahira, K.; Burnham, P.; Afshinnekoo, E.; et al. Cell-free DNA (cfDNA) and Exosome Profiling from a Year-Long Human Spaceflight Reveals Circulating Biomarkers. iScience 2020, 23, 101844. [Google Scholar] [CrossRef] [PubMed]
- Nassef, M.Z.; Melnik, D.; Kopp, S.; Sahana, J.; Infanger, M.; Lützenberg, R.; Relja, B.; Wehland, M.; Grimm, D.; Krüger, M. Breast Cancer Cells in Microgravity: New Aspects for Cancer Research. Int. J. Mol. Sci. 2020, 21, 7345. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.L.; Moriarity, D.M.; Campbell, P.S. Use of microgravity bioreactors for development of an in vitro rat salivary gland cell culture model. J. Cell. Biochem. 1993, 51, 265–273. [Google Scholar] [CrossRef]
- Bauer, J.; Wehland, M.; Pietsch, J.; Sickmann, A.; Weber, G.; Grimm, D. Annotated Gene and Proteome Data Support Recognition of Interconnections Between the Results of Different Experiments in Space Research. Microgravity Sci. Technol. 2016, 28, 357–365. [Google Scholar] [CrossRef]
- Desai, S.D.; Reed, R.E.; Burks, J.; Wood, L.M.; Pullikuth, A.K.; Haas, A.L.; Liu, L.F.; Breslin, J.W.; Meiners, S.; Sankar, S. ISG15 disrupts cytoskeletal architecture and promotes motility in human breast cancer cells. Exp. Biol Med. 2012, 237, 38–49. [Google Scholar] [CrossRef]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFκB-signaling pathway in cancer. OncoTargets Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietsch, J.; Sickmann, A.; Weber, G.; Bauer, J.; Egli, M.; Wildgruber, R.; Infanger, M.; Grimm, D. A proteomic approach to analysing spheroid formation of two human thyroid cell lines cultured on a random positioning machine. Proteomics 2011, 11, 2095–2104. [Google Scholar] [CrossRef]
- Krüger, M.; Pietsch, J.; Bauer, J.; Kopp, S.; Carvalho, D.T.O.; Baatout, S.; Moreels, M.; Melnik, D.; Wehland, M.; Egli, M.; et al. Growth of Endothelial Cells in Space and in Simulated Microgravity—A Comparison on the Secretory Level. Cell. Physiol. Biochem. 2019, 52, 1039–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvard University Press. Aristotle. Met. 7.1041b. In Aristotle in 23 Volumes; Harvard University Press: Cambridge, MA, USA, 1933; Volume 17. [Google Scholar]
- Nam, A.S.; Chaligne, R.; Landau, D.A. Integrating genetic and non-genetic determinants of cancer evolution by single-cell multi-omics. Nat. Rev. Genet. 2021, 22, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Vasaikar, S.V.; Straub, P.; Wang, J.; Zhang, B. LinkedOmics: Analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018, 46, D956–D963. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Hosen, M.I.; Ahmed, M.; Shekhar, H.U. Onco-Multi-OMICS Approach: A New Frontier in Cancer Research. BioMed Res. Int. 2018, 2018, 9836256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albaradei, S.; Thafar, M.; Alsaedi, A.; Van Neste, C.; Gojobori, T.; Essack, M.; Gao, X. Machine learning and deep learning methods that use omics data for metastasis prediction. Comput. Struct. Biotechnol. J. 2021, 19, 5008–5018. [Google Scholar] [CrossRef]
- Sone, K.; Toyohara, Y.; Taguchi, A.; Miyamoto, Y.; Tanikawa, M.; Uchino-Mori, M.; Iriyama, T.; Tsuruga, T.; Osuga, Y. Application of artificial intelligence in gynecologic malignancies: A review. J. Obs. Gynaecol. Res. 2021, 47, 2577–2585. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.A.; Kondrashova, O.; Bradley, A.; Williams, E.D.; Pearson, J.V.; Waddell, N. Deep learning in cancer diagnosis, prognosis and treatment selection. Genome Med. 2021, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, I.; Furukawa, T.; Morise, M. The current issues and future perspective of artificial intelligence for developing new treatment strategy in non-small cell lung cancer: Harmonization of molecular cancer biology and artificial intelligence. Cancer Cell. Int. 2021, 21, 454. [Google Scholar] [CrossRef] [PubMed]
- Rutter, L.; Barker, R.; Bezdan, D.; Cope, H.; Costes, S.V.; Degoricija, L.; Fisch, K.M.; Gabitto, M.I.; Gebre, S.; Giacomello, S.; et al. A New Era for Space Life Science: International Standards for Space Omics Processing. Patterns 2020, 1, 100148. [Google Scholar] [CrossRef]
- Fujita, S.-I.; Rutter, L.; Ong, Q.; Muratani, M. Integrated RNA-seq Analysis Indicates Asynchrony in Clock Genes between Tissues under Spaceflight. Life 2020, 10, 196. [Google Scholar] [CrossRef]
- Guo, J.-H.; Qu, W.-M.; Chen, S.-G.; Chen, X.-P.; Lv, K.; Huang, Z.-L.; Wu, Y.-L. Keeping the right time in space: Importance of circadian clock and sleep for physiology and performance of astronauts. Mil. Med. Res. 2014, 1, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haus, E.L.; Smolensky, M.H. Shift work and cancer risk: Potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med. Rev. 2013, 17, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Sauer, L.A.; Dauchy, R.T.; Blask, D.E. Polyunsaturated fatty acids, melatonin, and cancer prevention. Biochem. Pharm. 2001, 61, 1455–1462. [Google Scholar] [CrossRef]
- Underhill, H.R.; Kitzman, J.O.; Hellwig, S.; Welker, N.C.; Daza, R.; Baker, D.N.; Gligorich, K.M.; Rostomily, R.C.; Bronner, M.P.; Shendure, J. Fragment Length of Circulating Tumor DNA. PLoS Genet. 2016, 12, e1006162. [Google Scholar] [CrossRef]
- Shen, S.Y.; Singhania, R.; Fehringer, G.; Chakravarthy, A.; Roehrl, M.H.A.; Chadwick, D.; Zuzarte, P.C.; Borgida, A.; Wang, T.T.; Li, T.; et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature 2018, 563, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Sixt, S.U.; Dahlmann, B. Extracellular, circulating proteasomes and ubiquitin—Incidence and relevance. Biochim. Biophys. Acta 2008, 1782, 817–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, J.L.; Souza, G.R. Using space-based investigations to inform cancer research on Earth. Nat. Rev. Cancer 2013, 13, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Chua, H.L.; Bhat-Nakshatri, P.; Clare, S.E.; Morimiya, A.; Badve, S.; Nakshatri, H. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: Potential involvement of ZEB-1 and ZEB-2. Oncogene 2007, 26, 711–724. [Google Scholar] [CrossRef] [Green Version]
- Cristobal, A.; van den Toorn, H.W.P.; van de Wetering, M.; Clevers, H.; Heck, A.J.R.; Mohammed, S. Personalized Proteome Profiles of Healthy and Tumor Human Colon Organoids Reveal Both Individual Diversity and Basic Features of Colorectal Cancer. Cell Rep. 2017, 18, 263–274. [Google Scholar] [CrossRef] [Green Version]


| Cell Line | Biological Process | Genes/Proteins/Pathways Major Results | Microgravity | Reference |
|---|---|---|---|---|
| FTC-133 | Adhesion | VCAM1 | Space–CellBox-1 | [89,90] |
| VCL, PXN, ICAM1 | Space–CellBox-2 | [65] | ||
| FTC-133 | Angiogenesis | VEGFA, VEGFD, FLK1 | Space–SimBox | [88] |
| VEGF-A | Space–CellBox-1 | [89] | ||
| Angiopoetin-2 | Space–CellBox-2 | [65] | ||
| VEGFA, VEGF-A | Space–CellBox-2 | [104] | ||
| FTC-133 | Caveolae | CAV1 | Space–CellBox-1 | [89,90] |
| CAV1 | Space–CellBox-2 | [65] | ||
| FTC-133 | Extracellular Matrix | SPP1, MMP2, MMP3, TIMP1 | Space–SimBox, RPM | [88] |
| TIMP1, MMP3 | Space–CellBox-1 | [89] | ||
| COL1A1, ITGB1 | Space–CellBox-2 | [65] | ||
| FTC-133 | Cytokines | IL6, CXCL8, IL15 | Space–SimBox, RPM | [88] |
| IL6, IL8, IL7, IL18, MCP1, MIP-1 beta | Space–CellBox-1 | [89] | ||
| IL6 | Space–CellBox-2 | [65] | ||
| ML1, RO82-W1 | IL6, MCP1 | RPM, Clinostat | [31] | |
| IL6, IL8 | 1g Liquid-overlay | |||
| FTC-133 | Cell Signaling | ERK1/2, RELA | Space–CellBox-2 | [65] |
| FTC-133 | Protein Kinases | PRKAA, PRKACA | Space–SimBox, RPM | [88] |
| FTC-133 | Growth Factors | EGF, CTGF, FGF17 | Space–SimBox, RPM | [87,88] |
| EGFR | Space–CellBox-2 | [65] | ||
| FTC-133 | Cytoskeleton | ACTB, TUBB, F-actin | Space–TX52 | [85] |
| ML1 | ACTB, KRT80 | PFC | [82] | |
| Cytokeratin, vimentin, tubulin | PFC | [82] | ||
| FTC-133 | Exosomes, Exosomal miRNA | CD9, CD63, CD81 | Space-CellBox-1 | [105] |
| Array scan of a total of 754 miRNA targets revealed more than 100 differentially expressed miRNAs: miR-199 family | Space-CellBox-1 | [106] |
| Cell Line | Biolog. Process | Genes/Proteins/Pathways Major Result | Microgravity | Ref. |
|---|---|---|---|---|
| MCF-7 | Cytoskeleton | Upregulation of KRT8, RDX, TIMP1, CXCL8 mRNAs, downregulation of VCL, reduced E-cadherin protein and rearrangement of F-actin and tubulin | r-μg/TEXUS SR & PFC | [110] |
| MDA-MB231 | Cell adhesion | Upregulation of ICAM1, VCAM1, CD44 and down-regulation of NFκB-p65 and annexin-2 protein. | r-μg/PFC | [111] |
| MCF-7 | ECM, Cell cycle, Proliferation | Loosely organized perinuclear cytokeratin network, arrested cell cycle and decreased proliferation | r-μg/spaceflight | [112]. |
| MCF-7 | Cytoskeleton, Mitosis | Altered microtubule structure, prolonged cell cycle | r-μg/spaceflight | [127] |
| MCF-7 | MCS Cytoskeleton | ACTB, TUBB, EZR, RDX, FN1, VEGFA, FLK1, CASP9, CASP3, PRKCA mRNAs were downregulated in 5 d-MCS, duct-like and compact MCS | s-μg/RPM | [62] |
| CRL-2351 | Cell reparation and adhesion, MCS | BRCA1 increased, KRAS decreased in AD cells; VCAM1 upregulated, VIM downregulated in µg | s-μg/iRPM | [115] |
| CRL-2351 | Morphology and gene expression, MCS | Upregulated RHOA gene and over expressed MAPK1 gene and protein | s-μg/RPM | [116] |
| MCF- 7 | MCS formation and adhesion | Decreased E-cadherin in MCS, PP2 prevented MCS formation | s-μg/RPM | [114] |
| MCF-7 | MCS formation, apoptosis | Upregulation of ANXA1, ANXA2, CTGF, CAV2, ICAM1, FAS, CASP8, BAX, TP53, CYC1, and PARP1 in MCS, upregulated apoptosis related protein p53, CYC1, PARP1, FAS, CASP8, and ANXA1 | s-μg/RPM | [83] |
| MDA-MB231 | Phenotypic switch | G2/M inhibited and cyclin D1 decreased | s-μg/RPM | [64] |
| MCF-7, MDA-MB231 | MCS formation | Vinculin and β-catenin are critical to form MCS | s-μg/RPM | [66] |
| MCF-10A, MCF-7 | Apoptosis | increased AKT and ERK pathway activity, decreased apoptosis | s-μg/RPM | [119] |
| MDA-MB231 | Cell cycle apoptosis | Increased lysosomal vesicles, cyclin D3, decreased Bcl-2 and MMP9 proteins. | s-μg/RCSS | [124] |
| MCF7 | Metastatis ability | Cell invasion and migration decreased | s-μg/MG-6C clinostat system | [109] |
| MDA-MB 231 | dysregulation extracellular vesicle | Proteomics show significant correlation with GTPases and proliferation | s-μg/Gravite | [126] |
| Cell Line | Biological Process | Genes/Proteins | Microgravity | Reference |
|---|---|---|---|---|
| DU145 | Cytoskeleton | Cytokeratins-8 and -18, actin, vimentin | s-µg: high aspect ratio vessel (HARV) | [130] |
| DU145 | Regulatory and matrix proteins | EGF, EGF receptor, TGF-β1, TGF-β receptor, collagen IV and laminin | s-µg: (HARV) | [131] |
| DU145 | Transduction-second messenger | DAG, ceramide, PA, PEt, choline, AA and cAMP | s-µg: (HARV) | [132] |
| LNCaP | Prostate specific peptidase | PSA | s-µg: (HARV) | [133] |
| PC-3 | Cell adhesion molecules | CD44 and E-cadherin | s-µg: (HARV) | [135] |
| PC-3 | Epithelial marker | cytokeratin VIII | s-µg: (HARV) | [135] |
| PC-3 | Collagen deposition | collagen IV | s-µg: (HARV) | [135] |
| PC-3 | VEGF signaling | VEGFA, FLK1, RAF1, SRC1, AKT1, MTOR, MAP2K1, ERK2, LCN2, protein supernatant: NGAL, VEGF | s-µg: RPM | [63] |
| PC-3 | Collagen deposition | LAMA3, LAMB2, FN1 | s-µg: RPM | [63] |
| PC-3 | Focal adhesion | CDH1 | s-µg: RPM | [63] |
| PC-3 | Cytokines | IL-1α, IL-1β, IL-6 and IL-8 | s-µg: RPM | [118] |
| Cell Line | Biological Process | Genes/Proteins/Pathways Major Results | Microgravity | Reference |
|---|---|---|---|---|
| Colorectal Cancer Cells | ||||
| HT-29, HT-29KM, Co-culture with normal human colonic fibroblasts | Differentiation | proliferation at an accelerated rate, organizing themselves into 3D MCS (1.0–1.5 cm), signs of a well-differentiated colon tissue | RWV | [137] |
| HT-29KM CCL 188 KM-12c and MIP-101 | Cell adhesion | µg does not alter epithelial cell adhesion | RWV | [138] |
| MIP-101 | Proliferation, differentiation | The petri and RWV cultures continued to proliferate the full 14 d. Induced expression of CEA | RWV with 5 mg/mL Cytodex 3 microcarrier beads | [139] |
| MIP-101 | Differentiation, Apoptosis, Proliferation | Rotation appears to increase apoptosis and decrease proliferation, whereas static 3D cultures in either unit or microgravity have less apoptosis, and reduced rotation in microgravity increases CEA expression | on Teflon-coated non-adherent surfaces (static 3D) or RWV either in r-µg low-earth orbit or in unit gravity on the ground (3D 1g) | [140] |
| HCT-116 | 3D liver metastasis model with CRC cells Drug testing with 5-FU | In 2D they displayed an epithelial phenotype, and only after transition to the organoids did the cells present with a mesenchymal phenotype. WNT pathway might be involved in the phenotypic changes In vitro 3D liver-tumor organoid model for metastasis growth and suitable for drug testing | RWV | [141] |
| HCT116 | 3D spheroids Metastasis model for drug testing-5-FU | Host-liver CRC- spheroids composed of primary human hepatocytes, MSC and HCT116 cells The presence of MSC appeared to drive self-organization and formation of a stroma-like tissue surrounding the tumor foci and hepatocytes. | RWV | [142] |
| DLD1, HCT116 SW620 | Apoptosis 3D aggregates (clumps) | Apoptosis under s-µg Upregulation of the tumor suppressors PTEN and FOXO3 mRNAs leading to AKT downregulation and apoptosis induction Clumps formed in µg showed elevated hypoxia and mitochondrial membrane potential | RCCS-HARV | [143] |
| HCT 116 | Stemness regulators, differentiation | upregulation of markers like CD133/CD44, YAP nuclear localization and increase the number of polyploid giant cancer cells, Yamanaka factor upregulation | RCCS-HARV | [71] |
| Caco-2 cells | Proteomics | 38 and 26 proteins differently regulated by simulated microgravity after 48 and 72 h lower NF-kB basal activation in s-µg conditions | 2D clinostat | [144] |
| LS180 | Tissue engineering, phytomedicine testing | 3D LS180 cell mini-tumors, suitable for drug testing | 2D Clinostat | [145] |
| Hepatocellular Carcinoma Cells | ||||
| HepG2 | 3D formation Gene expression | Early stage of 3D assembly: changes in the expression of 95 genes (overexpression of 85 and downregulation in 10) | RCCS | [148] |
| HepG2 | Gene expression | 139 genes significantly altered in s-µg | RCCS | [149] |
| HepG2 | MCS formation, cytoskeleton, Gene expression | MCS up to 100 µm in diameter within 72 h and up to 1 mm with long-term culture. MCS: cortical actin organization RWV MCS: upregulation of metabolic and synthetic genes liver-specific functions of cytochrome P450 activity and albumin production are higher in the MCS | RWV | [150] |
| MHCC97H | MCS formation, Morphology Nude mice model | MCS: mirrored clinical pathological features of HCC in vivo: morphology, ultrastructure, protein production and secretion, glucose metabolism, tissue-specific gene expression, and apoptosis. Xenografts into livers of nude mice resulted in tumorigenesis and distant metastasis | RWV | [151] |
| MHCC97H | Co-culture of CRC cells and liver fragments Invasion simulation | time-course analysis showed dynamic gene alterations: MMP2, MMP7, MMP9, CD44, SPP1, CXCR4, CXCL12, and CDH1. Increase in vitronectin, Met, clusterin, ICAM1, GSN proteins | RWV | [152] |
| MHCC97H, Hep3B | Metastasis—low and high potential, gene expression | Differences between two HCC MCS types in gene expression patterns of adhesion molecules, matrix secretion, invasion etc. | RWV | [153] |
| HepG2 | Apoptosis, cis-diamminedi-chloroplatinum (CDDP) | µg altered CDDP sensitivity through activation of caspase-3 by p53-independent mechanism | Gravite (3D clinostat) | [154] |
| HepG2/C3A | 3D model for genotoxicity testing of chemicals | 21-day old MCS: higher basal expression of genes encoding metabolic enzymes compared to monolayer culture. Sensitive and promising in vitro model for genotoxicity and environmental studies. | dynamic clinostat bioreactor system (CelVivo BAM/bioreactor) | [155] |
| Gastric and Pancreatic Cancer Cells | ||||
| HGC-27 | Metabolomics | A total of 67 differentially regulated metabolites were identified, including upregulated and downregulated metabolites. Phosphatidyl ethanolamine, phosphatidyl choline, arachidonic acid and sphinganine were significantly upregulated in s-µg. sphingomyelin, phosphatidyl serine, phosphatidic acid, L-proline, creatine, pantothenic acid, oxidized glutathione, adenosine diphosphate and adenosine triphosphate were significantly downregulated | RCCS | [156] |
| NOR-P1 | 3D tissues, apoptosis | s-µg: NOR-P1 cells showed greater numbers of mitotic, cycling (Ki-67-positive), nuclear factor-kappa B-activating cells, and a lower number of apoptotic cells compared to 1g | RCCS-4D | [157] |
| Pharmacological Agent and Drugs | Target Protein | References |
|---|---|---|
| PP2 (4-amino-5-(4-chlorophenyl)-7-(dimethylethyl) pyrazolo [3,4-d] pyrimidine) | Proto-oncogene tyrosine-protein kinase Src | [114] |
| Daidzein | Caveolin-1 | [89,97] |
| Camptothecin | Ubiquitin-like protein ISG15 | [212] |
| SP600125 | Mitogen-activated protein kinase 8/JNK1 | [97] |
| Dexamethasone, BAY 11-7082 | NFκB p65 | [96,97,113] |
| GSK2256098, MPAP | Focal adhesion kinase 1 | [97] |
| MT189 | Paxillin | [97] |
| Cetuximab, Panitumumab, Sym004 | EGF receptor | [65] |
| Interleukin-6 Inhibitor (Siltuximab), Tocilizumab | Interleukin 6, IL-6 receptor | [65,81,88] |
| HuMax-IL8 (BMS-986253) antibody, CXCL8-IP10 (Analogue), Reparixin | CXCL8, CXCL8 receptor | [65,81,88] |
| AKT Inhibitor, Ipatasertib | AKT | [63,119] |
| mTOR inhibitors | mTOR | [63] |
| Curcumin | HMOX-1 | [113] |
| TM5441 | Plasminogen activator inhibitor 1 | [113] |
| UK370106 | Stromelysin, (MMP3) | [95] |
| Monoclonal antibody | Integrin-ß1, Fibronektin, CD44, E-cadherin, ICAM-1, VEGF | [63,81,88,113,114,215] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grimm, D.; Schulz, H.; Krüger, M.; Cortés-Sánchez, J.L.; Egli, M.; Kraus, A.; Sahana, J.; Corydon, T.J.; Hemmersbach, R.; Wise, P.M.; et al. The Fight against Cancer by Microgravity: The Multicellular Spheroid as a Metastasis Model. Int. J. Mol. Sci. 2022, 23, 3073. https://doi.org/10.3390/ijms23063073
Grimm D, Schulz H, Krüger M, Cortés-Sánchez JL, Egli M, Kraus A, Sahana J, Corydon TJ, Hemmersbach R, Wise PM, et al. The Fight against Cancer by Microgravity: The Multicellular Spheroid as a Metastasis Model. International Journal of Molecular Sciences. 2022; 23(6):3073. https://doi.org/10.3390/ijms23063073
Chicago/Turabian StyleGrimm, Daniela, Herbert Schulz, Marcus Krüger, José Luis Cortés-Sánchez, Marcel Egli, Armin Kraus, Jayashree Sahana, Thomas J. Corydon, Ruth Hemmersbach, Petra M. Wise, and et al. 2022. "The Fight against Cancer by Microgravity: The Multicellular Spheroid as a Metastasis Model" International Journal of Molecular Sciences 23, no. 6: 3073. https://doi.org/10.3390/ijms23063073
APA StyleGrimm, D., Schulz, H., Krüger, M., Cortés-Sánchez, J. L., Egli, M., Kraus, A., Sahana, J., Corydon, T. J., Hemmersbach, R., Wise, P. M., Infanger, M., & Wehland, M. (2022). The Fight against Cancer by Microgravity: The Multicellular Spheroid as a Metastasis Model. International Journal of Molecular Sciences, 23(6), 3073. https://doi.org/10.3390/ijms23063073











