Clinical Application of Adipose Derived Stem Cells for the Treatment of Aseptic Non-Unions: Current Stage and Future Perspectives—Systematic Review
Abstract
1. Introduction
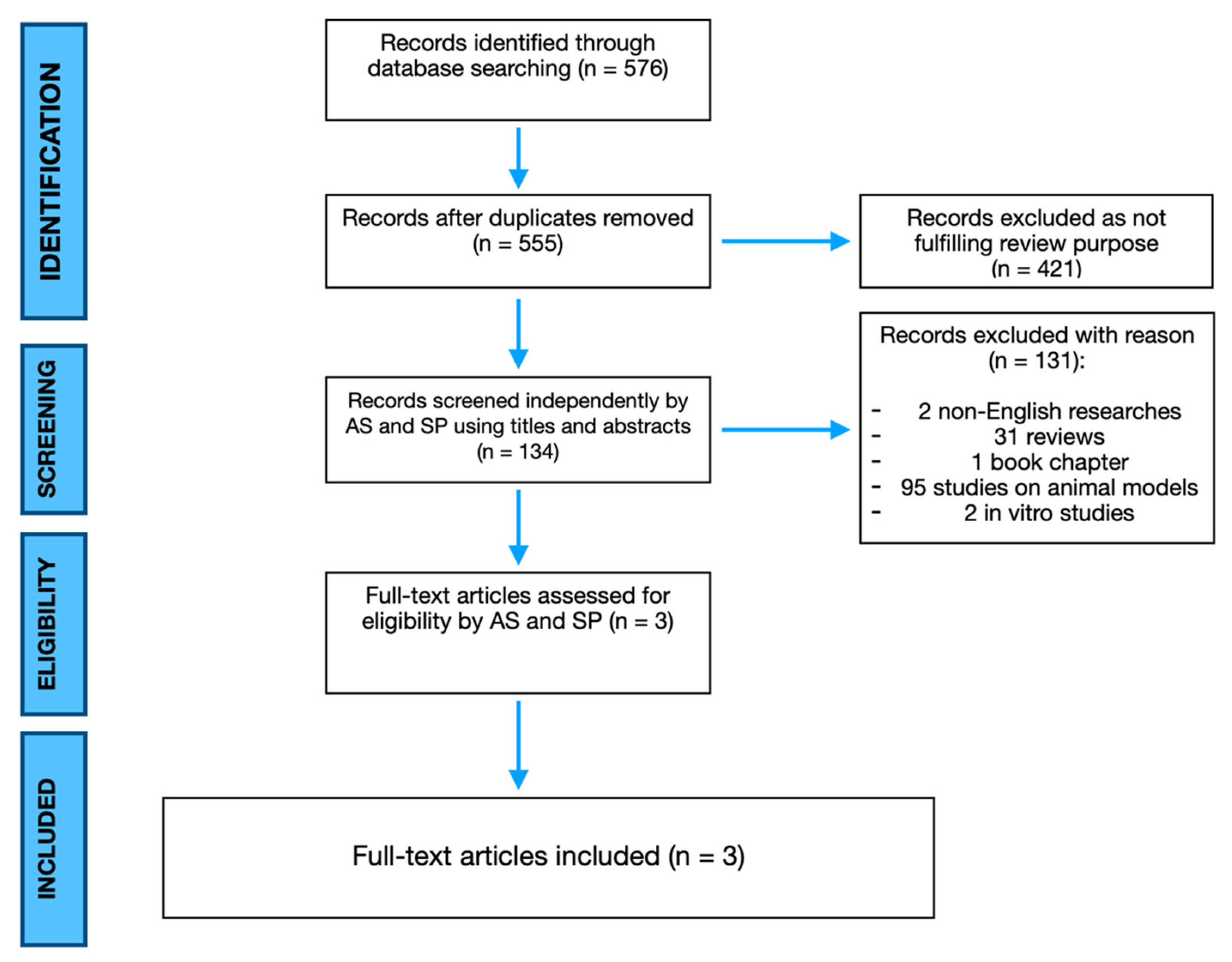
2. Materials and Methods
2.1. Study Design and Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Analysis
3. Results
4. Discussion
4.1. Orthopaedic Overview on the Issue
4.2. Current Stage of ADSCs Human Application in Non-Unions
4.3. The Role of Bone Grafts
4.4. What’s New from Basic Science? Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nauth, A.; Lee, M.; Gardner, M.J.; Brinker, M.R.; Warner, S.J.; Tornetta, P., III; Leucht, P. Principles of Nonunion Management: State of the Art. J. Orthop. Trauma 2018, 32, S52–S57. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, H.C.; Giannoudis, P.V.; Vrahas, M.S.; Smith, R.M.; Moran, C.; Pape, H.C.; Krettek, C.; Jupiter, J.B. The role of stem cells in fracture healing and nonunion. Int. Orthop. 2011, 35, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Tay, W.H.; de Steiger, R.; Richardson, M.; Gruen, R.; Balogh, Z.J. Health outcomes of delayed union and nonunion of femoral and tibial shaft fractures. Injury 2014, 45, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Igoe, E.; Kleftouris, G.; Papachristos, I.V.; Papakostidis, C.; Giannoudis, P.V. Physical Health and Psychological Outcomes in Adult Patients with Long-bone Fracture Non-unions: Evidence Today. J. Clin. Med. 2019, 8, 1998. [Google Scholar] [CrossRef] [PubMed]
- Hak, D.J.; Fitzpatrick, D.; Bishop, J.A.; Marsh, J.L.; Tilp, S.; Schnettler, R.; Simpson, H.; Alt, V. Delayed union and nonunions: Epidemiology, clinical issues, and financial aspects. Injury 2014, 45, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Gálvez-Sirvent, E.; Ibarzábal-Gil, A.; Rodríguez-Merchán, E.C. Treatment options for aseptic tibial diaphyseal nonunion: A review of selected studies. EFORT Open Rev. 2020, 5, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [PubMed]
- Cicione, C.; Di Taranto, G.; Barba, M.; Isgrò, M.A.; D’Alessio, A.; Cervelli, D.; Sciarretta, F.V.; Pelo, S.; Michetti, F.; Lattanzi, W. In Vitro Validation of a Closed Device Enabling the Purification of the Fluid Portion of Liposuction Aspirates. Plast. Reconstr. Surg. 2016, 137, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Di Taranto, G.; Cicione, C.; Visconti, G.; Isgrò, M.A.; Barba, M.; Di Stasio, E.; Stigliano, E.; Bernardini, C.; Michetti, F.; Salgarello, M.; et al. Qualitative and quantitative differences of adipose-derived stromal cells from superficial and deep subcutaneous lipoaspirates: A matter of fat. Cytotherapy 2015, 17, 1076–1089. [Google Scholar] [CrossRef] [PubMed]
- Mocini, F.; Monteleone, A.S.; Piazza, P.; Cardona, V.; Vismara, V.; Messinese, P.; Campana, V.; Sircana, G.; Maccauro, G.; Saccomanno, M.F. The role of adipose derived stem cells in the treatment of rotator cuff tears: From basic science to clinical application. Orthop. Rev. 2020, 12, 8682. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Perdisa, F.; Roffi, A.; Marcacci, M.; Kon, E. Stem cells in articular cartilage regeneration. J. Orthop. Surg. Res. 2016, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, M.A.; Rovere, G.; Fulchignoni, C.; De Santis, V.; Ziranu, A.; Maccauro, G.; Pataia, E. Autologous fat transplantation for the treatment of trapeziometacarpal joint osteoarthritis. Orthop. Rev. 2020, 12, 8666. [Google Scholar]
- Yoon, E.; Dhar, S.; Chun, D.E.; Gharibjanian, N.A.; Evans, G.R. In Vivo osteogenic potential of human adipose-derived stem cells/poly lactide-co-glycolic acid constructs for bone regeneration in a rat critical-sized calvarial defect model. Tissue Eng. 2007, 13, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Tajima, S.; Tobita, M.; Mizuno, H. Bone regeneration with a combination of adipose-derived stem cells and platelet-rich plasma. In Adipose-Derived Stem Cells; Springer: Totowa, NJ, USA, 2018; pp. 261–272. [Google Scholar]
- Saulnier, N.; Lattanzi, W.; Puglisi, M.A.; Pani, G.; Barba, M.; Piscaglia, A.C.; Giachelia, M.; Alfieri, S.; Neri, G.; Gasbarrini, G.; et al. Mesenchymal stromal cells multipotency and plasticity: Induction toward the hepatic lineage. Eur. Rev. Med. Pharmacol. Sci. 2009, 13, 71–78. [Google Scholar] [PubMed]
- Fetoni, A.R.; Lattanzi, W.; Eramo, S.L.; Barba, M.; Paciello, F.; Moriconi, C.; Rolesi, R.; Michetti, F.; Troiani, D.; Paludetti, G. Grafting and early expression of growth factors from adipose-derived stem cells transplanted into the cochlea, in a Guinea pig model of acoustic trauma. Front. Cell Neurosci. 2014, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H. Adipose-derived stem cells for tissue repair and regeneration: Ten years of research and a literature review. J. Nippon. Med. Sch. 2009, 76, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Vériter, S.; André, W.; Aouassar, N.; Poirel, H.A.; Lafosse, A.; Docquier, P.L.; Dufrane, D. Human Adipose-Derived Mesenchymal Stem Cells in Cell Therapy: Safety and Feasibility in Different “Hospital Exemption” Clinical Applications. PLoS ONE 2015, 10, e0139566. [Google Scholar] [CrossRef] [PubMed]
- Dufrane, D.; Docquier, P.L.; Delloye, C.; Poirel, H.A.; André, W.; Aouassar, N. Scaffold-free Three-dimensional Graft from Autologous Adipose-derived Stem Cells for Large Bone Defect Reconstruction: Clinical Proof of Concept. Medicine 2015, 94, e2220. [Google Scholar] [CrossRef] [PubMed]
- Khalpey, Z.; Marsh, K.M.; Ferng, A.; Riaz, I.B.; Hemphill, C.; Johnson, K.; Oliva, I.; Friedman, M. First in Man: Sternal Reconstruction with Autologous Stem Cells. Asaio J. 2015, 61, e31–e32. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2007, 38, S3–S6. [Google Scholar] [CrossRef]
- Barba, M.; Di Taranto, G.; Lattanzi, W. Adipose-derived stem cell therapies for bone regeneration. Expert. Opin. Biol. Ther. 2017, 17, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Bajek, A.; Gurtowska, N.; Olkowska, J.; Kazmierski, L.; Maj, M.; Drewa, T. Adipose-Derived Stem Cells as a Tool in Cell-Based Therapies. Arch. Immunol. Ther. Exp. 2016, 64, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Barba, M.; Cicione, C.; Bernardini, C.; Michetti, F.; Lattanzi, W. Adipose-derived mesenchymal cells for bone regereneration: State of the art. Biomed. Res. Int. 2013, 2013, 416391. [Google Scholar] [CrossRef] [PubMed]
- Meza-Zepeda, L.A.; Noer, A.; Dahl, J.A.; Micci, F.; Myklebost, O.; Collas, P. High-resolution analysis of genetic stability of human adipose tissue stem cells cultured to senescence. J. Cell Mol. Med. 2008, 12, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rey, E.; Gonzalez, M.A.; Varela, N.; O’Valle, F.; Hernandez-Cortes, P.; Rico, L.; Büscher, D.; Delgado, M. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann. Rheum. Dis. 2010, 69, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Chen, Y.; Yuan, L.; Liu, H.; Wang, J.; Liu, Q.; Zhang, Y. Adipose-Derived Stem Cells: Current Applications and Future Directions in the Regeneration of Multiple Tissues. Stem. Cells Int. 2020, 2020, 8810813. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, C.; Saulnier, N.; Bernardini, C.; Patti, R.; Tartaglione, T.; Fetoni, A.R.; Pola, E.; Paludetti, G.; Michetti, F.; Lattanzi, W. Undifferentiated human adipose tissue-derived stromal cells induce mandibular bone healing in rats. Arch. Otolaryngol. Head Neck Surg. 2011, 137, 463–470. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zura, R.; Xiong, Z.; Einhorn, T.; Watson, J.T.; Ostrum, R.F.; Prayson, M.J.; Della Rocca, G.J.; Mehta, S.; McKinley, T.; Wang, Z.; et al. Epidemiology of Fracture Nonunion in 18 Human Bones. JAMA Surg. 2016, 151, e162775. [Google Scholar] [CrossRef] [PubMed]
- Schmal, H.; Brix, M.; Bue, M.; Ekman, A.; Ferreira, N.; Gottlieb, H.; Kold, S.; Taylor, A.; Toft Tengberg, P.; Ban, I. Nonunion—Consensus from the 4th annual meeting of the Danish Orthopaedic Trauma Society. EFORT Open Rev. 2020, 5, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.W.; Muschler, G.F. Bone graft materials. An overview of the basic science. Clin. Orthop. Relat. Res. 2000, 371, 10–27. [Google Scholar]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, R.A.; Rozental, T.D. Bone graft substitutes. Hand Clin. 2012, 28, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, A.S.; Boden, S.D.; Golsdberg, V.M.; Khan, Y.; Laurencin, C.T.; Rosier, R.N. Bone-graft substitutes: Facts, fictions, and applications. JBJS 2001, 83, S98–S103. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, E.; D’Agostino, A.; Iaquinta, M.R.; Bononi, I.; Trevisiol, L.; Rotondo, J.C.; Patergnani, S.; Giorgi, C.; Gunson, M.J.; Arnett, G.W.; et al. Hydroxylapatite-collagen hybrid scaffold induces human adipose-derived mesenchymal stem cells to osteogenic differentiation in vitro and bone regrowth in patients. Stem. Cells Transl. Med. 2020, 9, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Saçak, B.; Certel, F.; Akdeniz, Z.D.; Karademir, B.; Ercan, F.; Özkan, N.; Akpinar, İ.N.; Çelebiler, Ö. Repair of critical size defects using bioactive glass seeded with adipose-derived mesenchymal stem cells. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1002–1008. [Google Scholar] [PubMed]
- Romero, R.; Chubb, L.; Travers, J.K.; Gonzales, T.R.; Ehrhart, N.P.; Kipper, M.J. Coating cortical bone allografts with periosteum-mimetic scaffolds made of chitosan, trimethyl chitosan, and heparin. Carbohydr. Polym. 2015, 122, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dai, K.; Tang, T.; Zhang, X.; Yan, M.; Lou, J. Bone regeneration by implantation of adipose-derived stromal cells expressing BMP. Biochem. Biophys. Res. Commun. 2007, 356, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kim, Y.; Kweon, O.K.; Kang, B.J. Use of stem-cell sheets expressing bone morphogenetic protein-7 in the management of a nonunion radial fracture in a Toy Poodle. J. Vet. Sci. 2017, 18, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, O.A.; Campbell, B.; Poche, J.N.; Ma, M.; Rogers, E.; Gaupp, D.; Harrison, M.A.A.; Bunnell, B.A.; Hayes, D.J.; Gimble, J.M. Decellularized Adipose Tissue Hydrogel Promotes Bone Regeneration in Critical-Sized Mouse Femoral Defect Model. Front. Bioeng. Biotechnol. 2019, 7, 211. [Google Scholar] [CrossRef] [PubMed]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef] [PubMed]
- Lendeckel, S.; Jödicke, A.; Christophis, P.; Heidinger, K.; Wolff, J.; Fraser, J.K.; Hedrick, M.H.; Berthold, L.; Howaldt, H.P. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: Case report. J. Craniomaxillofac. Surg. 2004, 32, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.; Pigeot, S.; Müller, J.; Schaefer, D.J.; Martin, I.; Scherberich, A. Fractionated human adipose tissue as a native biomaterial for the generation of a bone organ by endochondral ossification. Acta Biomater. 2018, 77, 142–154. [Google Scholar] [CrossRef] [PubMed]
| N° of Patients | Age | Sex | Type of Lesion | Surgical Treatment | ADSCs Application | Outcomes ** | Follow-Up | Complications | |
|---|---|---|---|---|---|---|---|---|---|
| Khalpey Z. Et al. 2015 | 1 | 65 | M | Sternal non-union with bone loss | Open reduction, augmentation and plate fixation | Injection of autologous SVF cells | Good | 6 months | - |
| Veriter S. Et al. * 2015 | 11 | 18 | 8M, 3F | 6 bone tumors, 4 congenital and 1 iatrogenic non-union | Wide oncological resection with growing prosthesis implantation or bone grafting | Scaffold-free osteogenic 3D adipose-derived graft | Good | 28.7 months | Delayed wound healing Intercalary allograft infection Subcutaneous collection Cellulite |
| Dufrane D. Et al. * 2015 | 6 | 9.7 | 5M, 1F | 3 bone tumors, 3 congenital pseudoarthrosis | Wide oncological resection with growing prosthesis implantation or bone grafting | Scaffold-free osteogenic 3D adipose-derived graft | Good | 39 months | Intercalary allograft infection Screw and plate infection |
| Osteo-Conduction | Osteo-Induction | Osteo-Genesis | Osteo-Integration | Structural Support | Disadvantages | ||
|---|---|---|---|---|---|---|---|
| Autologous Bone Graft | Cancellous bone | +++ | +++ | +++ | +++ | − | Limited availability, donor site morbidity, blood loss |
| Cortical Bone | + | + | + | + | ++++ | Limited availability, donor site morbidity, blood loss | |
| Allogeneic Bone Graft | Cancellous bone | + | + | − | ++ | − | Risk of disease transmission and rejection |
| Cortical Bone | + | − | − | + | +++ | Risk of disease transmission and rejection | |
| Demineralized Bone Matrix | + | ++ | − | ++ | − | Variable osteo-conduction | |
| Synthetic Bone Substitutes | Calcium solfate | + | − | − | ++ | + | Rapid resorption, osteo-conduction only |
| Hydroxyapatite | + | − | − | − | ++ | Slow resorption, osteo-conduction only | |
| Calcium Phosphate Ceramic | + | − | − | + | ++ | Osteo-conduction only | |
| Calcium Phosphate Cement | + | − | − | + | + | Osteo-conduction only | |
| Bioactive Glass | + | − | − | − | Bioactive osteo-conduction only | ||
| Poly (methyl-methacrylate) | − | − | − | − | +++ | Inert, exothermic, monomer-mediate toxic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smakaj, A.; De Mauro, D.; Rovere, G.; Pietramala, S.; Maccauro, G.; Parolini, O.; Lattanzi, W.; Liuzza, F. Clinical Application of Adipose Derived Stem Cells for the Treatment of Aseptic Non-Unions: Current Stage and Future Perspectives—Systematic Review. Int. J. Mol. Sci. 2022, 23, 3057. https://doi.org/10.3390/ijms23063057
Smakaj A, De Mauro D, Rovere G, Pietramala S, Maccauro G, Parolini O, Lattanzi W, Liuzza F. Clinical Application of Adipose Derived Stem Cells for the Treatment of Aseptic Non-Unions: Current Stage and Future Perspectives—Systematic Review. International Journal of Molecular Sciences. 2022; 23(6):3057. https://doi.org/10.3390/ijms23063057
Chicago/Turabian StyleSmakaj, Amarildo, Domenico De Mauro, Giuseppe Rovere, Silvia Pietramala, Giulio Maccauro, Ornella Parolini, Wanda Lattanzi, and Francesco Liuzza. 2022. "Clinical Application of Adipose Derived Stem Cells for the Treatment of Aseptic Non-Unions: Current Stage and Future Perspectives—Systematic Review" International Journal of Molecular Sciences 23, no. 6: 3057. https://doi.org/10.3390/ijms23063057
APA StyleSmakaj, A., De Mauro, D., Rovere, G., Pietramala, S., Maccauro, G., Parolini, O., Lattanzi, W., & Liuzza, F. (2022). Clinical Application of Adipose Derived Stem Cells for the Treatment of Aseptic Non-Unions: Current Stage and Future Perspectives—Systematic Review. International Journal of Molecular Sciences, 23(6), 3057. https://doi.org/10.3390/ijms23063057







