Adhesion of Oral Bacteria to Commercial d-PTFE Membranes: Polymer Microstructure Makes a Difference
Abstract
:1. Introduction
2. Results
2.1. PTFE Microstructure
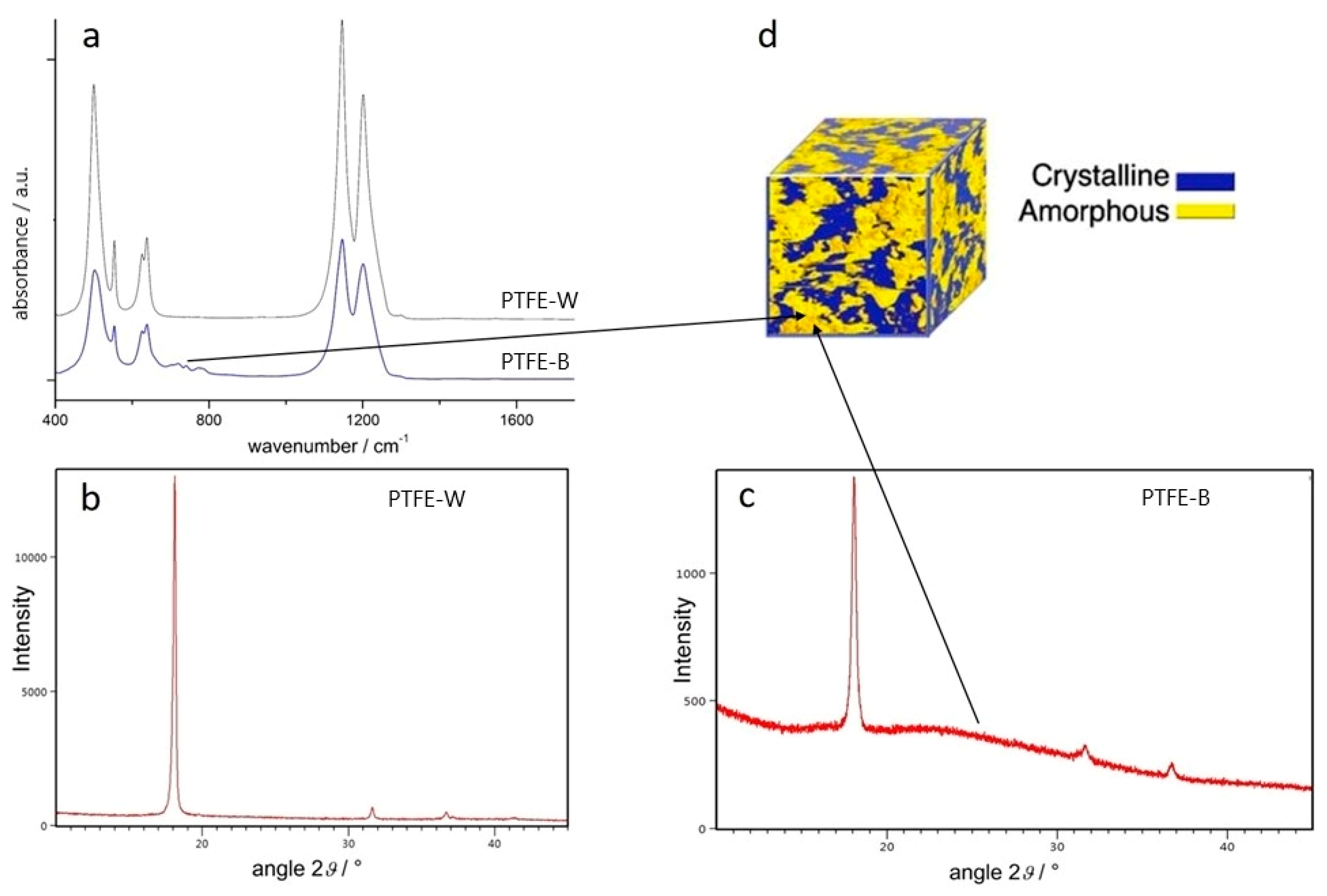
2.1.1. ATR-FTIR Spectroscopy
2.1.2. X-ray Diffraction
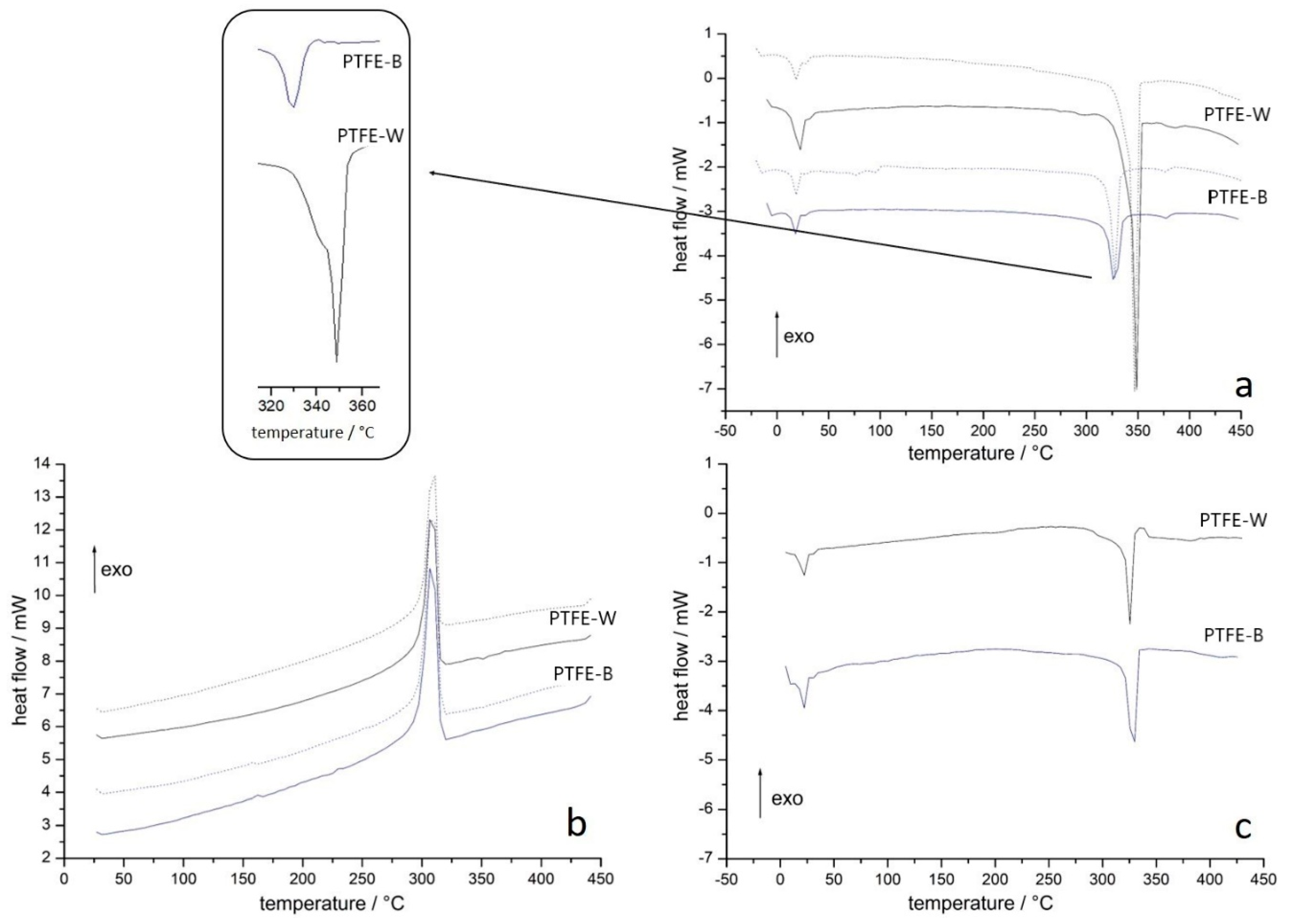
2.1.3. Differential Scanning Calorimetry
2.2. Surface Roughness and Topography
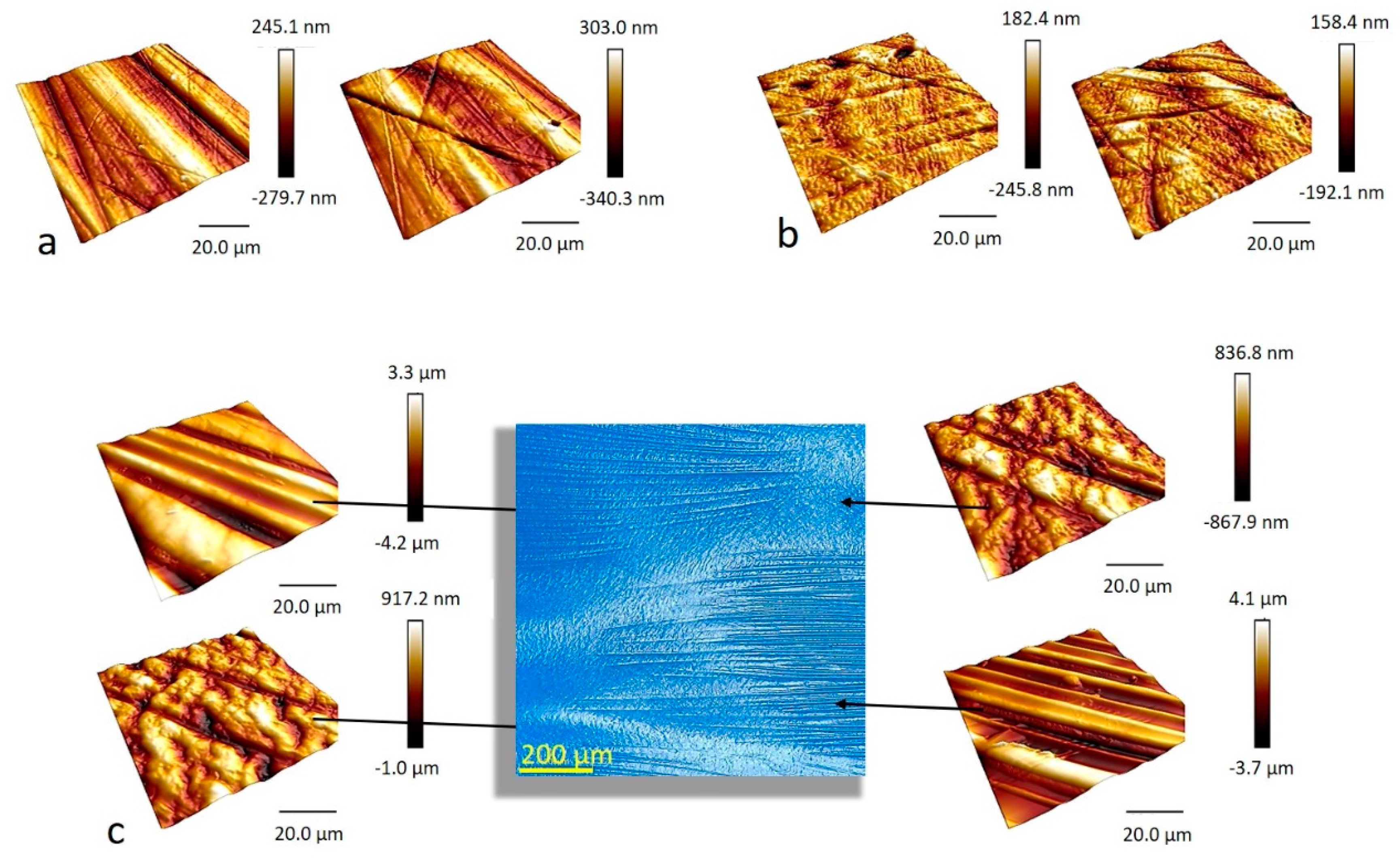
2.2.1. Microscopic Surface Roughness by Atomic Force Microscopy
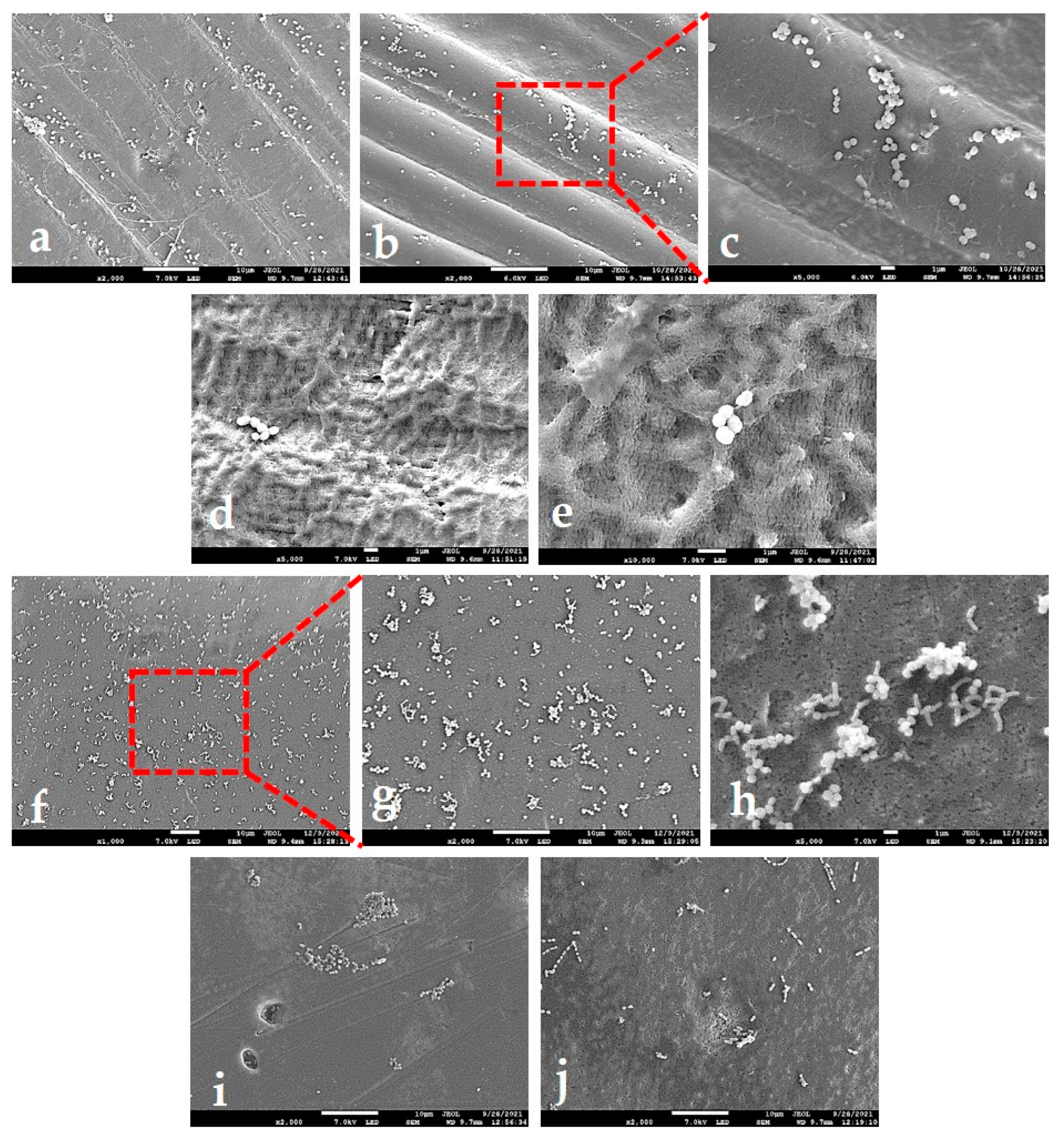
2.2.2. SEM Analysis
2.3. Contact Angles and Surface Free Energy Parameters
2.4. Microbial Adhesion
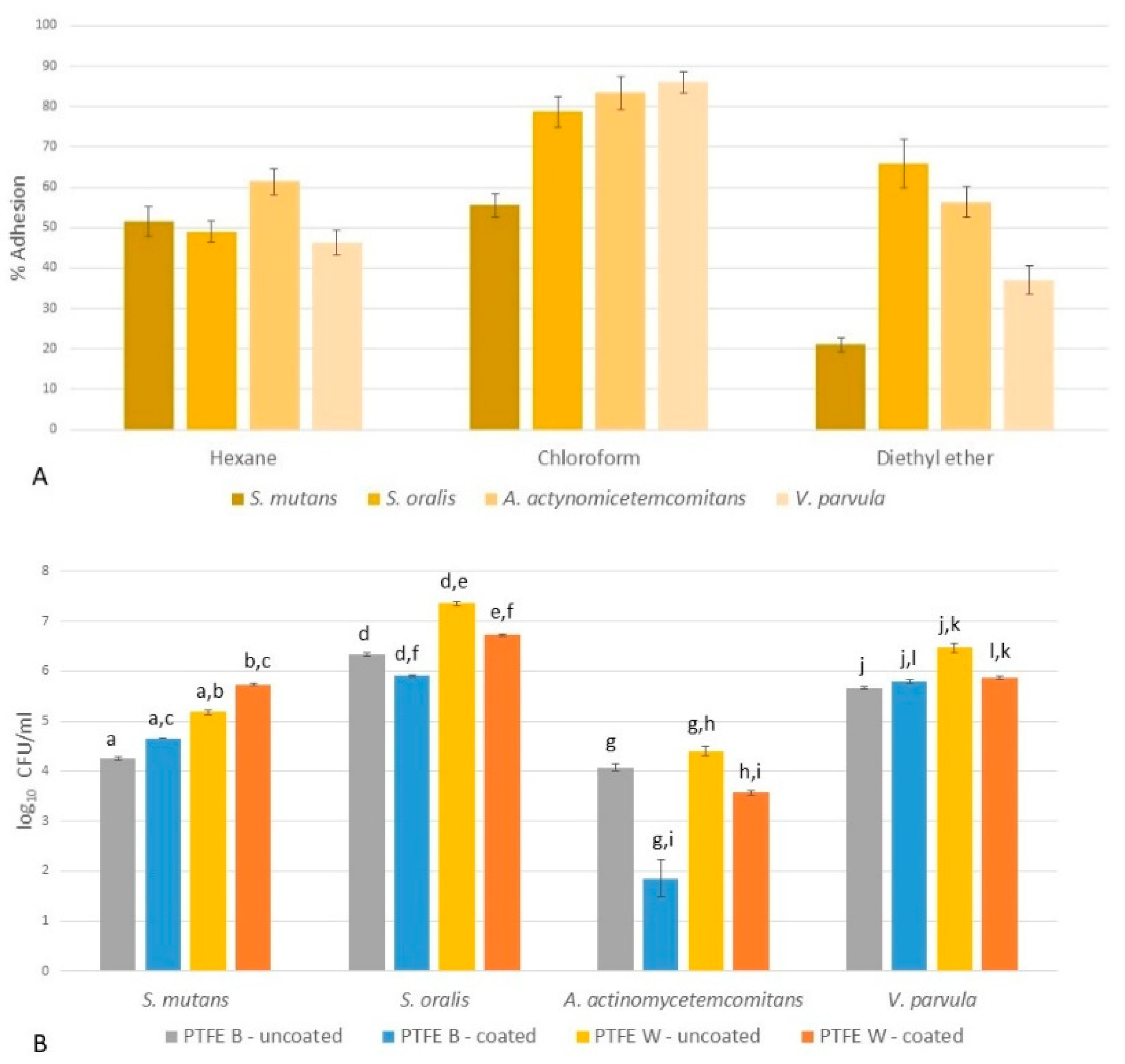
2.4.1. Microbial Adhesion to Solvents (MATS)
2.4.2. Microbial Adhesion to Membranes
2.4.3. SEM Analysis of Adhered Bacteria
3. Discussion
4. Materials and Methods
4.1. PTFE Membranes
4.2. Bacterial Strains and Inoculum Preparation
4.3. Bacterial Adhesion to PTFE Membranes
4.4. Attenuated Total Reflection-Fourier Transform Infrared (ATR-FTIR) Spectroscopy
4.5. Differential Scanning Calorimetry (DSC)
4.6. X-ray Diffraction (XRD)
4.7. Scanning Electron Microscopy (SEM) Analysis of Adherent Bacteria on PTFE Membranes
4.8. Atomic Force Microscopy (AFM)
4.9. Digital Microscopy
4.10. Microbial Adhesion to Solvents (MATS)
4.11. Surface Free Energy (SFE) Determination
4.11.1. PTFE Membranes
4.11.2. Bacterial Cells SFE
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morra, M.; Cassinelli, C. Bacterial Adhesion to Polymer Surfaces: A Critical Review of Surface Thermodynamic Approaches. J. Biomater. Sci. Polym. Ed. 1998, 9, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of Material Characteristics and/or Surface Topography on Biofilm Development. Clin. Oral Implants Res. 2006, 17, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Sterzenbach, T.; Helbig, R.; Hannig, C.; Hannig, M. Bioadhesion in the Oral Cavity and Approaches for Biofilm Management by Surface Modifications. Clin. Oral Investig. 2020, 24, 4237–4260. [Google Scholar] [CrossRef] [PubMed]
- Lorenzetti, M.; Dogša, I.; Stošicki, T.; Stopar, D.; Kalin, M.; Kobe, S.; Novak, S. The Influence of Surface Modification on Bacterial Adhesion to Titanium-Based Substrates. ACS Appl. Mater. Interfaces 2015, 7, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef] [PubMed]
- Asha, A.B.; Chen, Y.; Zhang, H.; Ghaemi, S.; Ishihara, K.; Liu, Y.; Narain, R. Rapid Mussel-Inspired Surface Zwitteration for Enhanced Antifouling and Antibacterial Properties. Langmuir 2019, 35, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Preedy, E.; Perni, S.; Nipiĉ, D.; Bohinc, K.; Prokopovich, P. Surface Roughness Mediated Adhesion Forces between Borosilicate Glass and Gram-Positive Bacteria. Langmuir 2014, 30, 9466–9476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreve, S.; Reis, A.C.D. Bacterial Adhesion to Biomaterials: What Regulates This Attachment? A Review. Jpn. Dent. Sci. Rev. 2021, 57, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.; Gupta, R.; Lele, T.P.; Lele, P.P. A Skeptic’s Guide to Bacterial Mechanosensing. J. Mol. Biol. 2020, 432, 523–533. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Chaudhury, M.K.; Good, R.J. Interfacial Lifshitz-van Der Waals and Polar Interactions in Macroscopic Systems. Chem. Rev. 1988, 88, 927–941. [Google Scholar] [CrossRef]
- Bos, R. Physico-Chemistry of Initial Microbial Adhesive Interactions—Its Mechanisms and Methods for Study. FEMS Microbiol. Rev. 1999, 23, 179–229. [Google Scholar] [CrossRef]
- Cavitt, T.B.; Pathak, N. Modeling Bacterial Attachment Mechanisms on Superhydrophobic and Superhydrophilic Substrates. Pharmaceuticals 2021, 14, 977. [Google Scholar] [CrossRef]
- Krasowska, A.; Sigler, K. How Microorganisms Use Hydrophobicity and What Does This Mean for Human Needs? Front. Cell. Infect. Microbiol. 2014, 4, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, Q.; Yan, T.; Jiang, Z.; Zhang, X.; Zuo, Y.Y. Quantitatively Predicting Bacterial Adhesion Using Surface Free Energy Determined with a Spectrophotometric Method. Environ. Sci. Technol. 2015, 49, 6164–6171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trobos, M.; Juhlin, A.; Shah, F.A.; Hoffman, M.; Sahlin, H.; Dahlin, C. In Vitro Evaluation of Barrier Function against Oral Bacteria of Dense and Expanded Polytetrafluoroethylene (PTFE) Membranes for Guided Bone Regeneration. Clin. Implant Dent. Relat. Res. 2018, 20, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided Bone Regeneration: Materials and Biological Mechanisms Revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological Principle and Therapeutic Applications: Guided Bone Regeneration. Clin. Oral Implants Res. 2010, 21, 567–576. [Google Scholar] [CrossRef]
- Carbonell, J.M.; Martín, I.S.; Santos, A.; Pujol, A.; Sanz-Moliner, J.D.; Nart, J. High-Density Polytetrafluoroethylene Membranes in Guided Bone and Tissue Regeneration Procedures: A Literature Review. Int. J. Oral Maxillofac. Surg. 2014, 43, 75–84. [Google Scholar] [CrossRef]
- Mazzucchi, G.; Lollobrigida, M.; Laurito, D.; Di Nardo, D.; Berlutti, F.; Passariello, C.; Serafini, G.; Testarelli, L.; De Biase, A. Microbiological and FE-SEM Assessment of d-PTFE Membrane Exposed to Oral Environment after Alveolar Socket Preservation Managed with Granular Nc-HA. J. Contemp. Dent. Pract. 2020, 21, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Machtei, E.E.; Cho, M.I.; Dunford, R.; Norderyd, J.; Zambon, J.J.; Genco, R.J. Clinical, Microbiological, and Histological Factors Which Influence the Success of Regenerative Periodontal Therapy. J. Periodontol. 1994, 65, 154–161. [Google Scholar] [CrossRef]
- Yoshinari, N.; Tohya, T.; Mori, A.; Koide, M.; Kawase, H.; Takada, T.; Inagaki, K.; Noguchi, T. Inflammatory Cell Population and Bacterial Contamination of Membranes Used for Guided Tissue Regenerative Procedures. J. Periodontol. 1998, 69, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Yaghobee, S.; Samadi, N.; Khorsand, A.; Ghahroudi, A.A.R.; Kadkhodazadeh, M. Comparison of the Penetration and Passage of Streptococcus Mutans and Aggregatibacter Actinomycetemcomitans through Membranes Loaded with Tetracycline, Amoxicillin, and Chlorhexidine: An In Vitro Study. J. Basic Clin. Physiol. Pharmacol. 2014, 25, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical Applications of Polymer-Composite Materials: A Review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Crombez, M.; Chevallier, P.; -Gaudreault, R.C.; Petitclerc, E.; Mantovani, D.; Laroche, G. Improving Arterial Prosthesis Neo-Endothelialization: Application of a Proactive VEGF Construct onto PTFE Surfaces. Biomaterials 2005, 26, 7402–7409. [Google Scholar] [CrossRef] [PubMed]
- Onder, S.; Kazmanli, K.; Kok, F.N. Alteration of PTFE Surface to Increase Its Blood Compatibility. J. Biomater. Sci. Polym. Ed. 2011, 22, 1443–1457. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Franke, R.; Gohs, U.; Lehmann, D.; Heinrich, G. Friction and Wear Behaviour of Electron Beam Modified PTFE Filled EPDM Compounds. Wear 2009, 266, 175–183. [Google Scholar] [CrossRef]
- Dalvi, V.H.; Rossky, P.J. Molecular Origins of Fluorocarbon Hydrophobicity. Proc. Natl. Acad. Sci. USA 2010, 107, 13603–13607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glaris, P.; Coulon, J.-F.; Dorget, M.; Poncin-Epaillard, F. Thermal Annealing as a New Simple Method for PTFE Texturing. Polymer 2013, 54, 5858–5864. [Google Scholar] [CrossRef]
- Collaud Coen, M.; Lehmann, R.; Groening, P.; Schlapbach, L. Modification of the Micro- and Nanotopography of Several Polymers by Plasma Treatments. Appl. Surf. Sci. 2003, 207, 276–286. [Google Scholar] [CrossRef]
- Venkataraman, M.; Mishra, R.; Yang, K.; Militky, J.; Kremenakova, D.; Zhu, G.; Yao, J. Preparation of Electrosprayed Microporous Membranes. IOP Conf. Ser. Mater. Sci. Eng. 2018, 460, 012017. [Google Scholar] [CrossRef]
- Papi, P.; Di Murro, B.; Tromba, M.; Passarelli, P.C.; D’Addona, A.; Pompa, G. The Use of a Non-Absorbable Membrane as an Occlusive Barrier for Alveolar Ridge Preservation: A One Year Follow-Up Prospective Cohort Study. Antibiotics 2020, 9, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puckett, S.D.; Taylor, E.; Raimondo, T.; Webster, T.J. The Relationship between the Nanostructure of Titanium Surfaces and Bacterial Attachment. Biomaterials 2010, 31, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Li, M.; Gregory, R.L. Bacterial Interactions in Dental Biofilm. Virulence 2011, 2, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, G.; He, Y. Structure and Properties of Polytetrafluoroethylene (PTFE) Fibers. e-Polymers 2017, 17, 215–220. [Google Scholar] [CrossRef]
- Moynihan, R.E. The Molecular Structure of Perfluorocarbon Polymers. Infrared Studies on Polytetrafluoroethylene. J. Am. Chem. Soc. 1959, 81, 1045–1050. [Google Scholar] [CrossRef]
- Saidi-Amroun, N.; Mouaci, S.; Mezouar, A.; Saidi, M.; Griseri, V.; Teyssedre, G. Analysis of the Gamma Irradiation Effect on PTFE Films by FTIR and DSC. In Proceedings of the 2018 IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Cancun, Mexico, 21–24 October 2018; pp. 430–433. [Google Scholar]
- Chen, J.; Asano, M.; Yamaki, T.; Yoshida, M. Preparation of Sulfonated Crosslinked PTFE--Poly(Alkyl Vinyl Ether) Membranes for Polymer Electrolyte Membrane Fuel Cells by Radiation Processing. J. Membr. Sci. 2005, 256, 38–45. [Google Scholar] [CrossRef]
- Brown, E.N.; Rae, P.J.; Dattelbaum, D.M.; Clausen, B.; Brown, D.W. In-Situ Measurement of Crystalline Lattice Strains in Polytetrafluoroethylene. Exp. Mech. 2008, 48, 119–131. [Google Scholar] [CrossRef] [Green Version]
- Sadiq, M.; Ahmad, S.; Shafiq, M.; Zakaullah, M. Enhanced Crystallinity of PTFE by Ion Irradiation in a Dense Plasma Focus. Plasma Process. Polym. 2007, 4, 186–191. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A.J.C. Scherrer after Sixty Years: A Survey and Some New Results in the Determination of Crystallite Size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Clark, E.S. The Molecular Conformations of Polytetrafluoroethylene: Forms II and IV. Polymer 1999, 40, 4659–4665. [Google Scholar] [CrossRef]
- Luo, C.; Pei, J.; Zhuo, W.; Niu, Y.; Li, G. Phase Transition Behavior and Deformation Mechanism of Polytetrafluoroethylene under Stretching. RSC Adv. 2021, 11, 39813–39820. [Google Scholar] [CrossRef]
- Ranjbarzadeh-Dibazar, A.; Barzin, J.; Shokrollahi, P. Microstructure Crystalline Domains Disorder Critically Controls Formation of Nano-Porous/Long Fibrillar Morphology of EPTFE Membranes. Polymer 2017, 121, 75–87. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, Z.; Wang, W.; Liu, W.; Xue, Q. Various Curing Conditions for Controlling PTFE Micro/Nano-Fiber Texture of a Bionic Superhydrophobic Coating Surface. Mater. Chem. Phys. 2010, 119, 40–47. [Google Scholar] [CrossRef]
- Sciuti, V.F.; Melo, C.C.; Canto, L.B.; Canto, R.B. Influence of Surface Crystalline Structures on DSC Analysis of PTFE. Mater. Res. 2017, 20, 1350–1359. [Google Scholar] [CrossRef] [Green Version]
- Ozbay, S.; Erdogan, N.; Erden, F.; Ekmekcioglu, M.; Ozdemir, M.; Aygun, G.; Ozyuzer, L. Surface Free Energy Analysis of ITO/Au/ITO Multilayer Thin Films on Polycarbonate Substrate by Apparent Contact Angle Measurements. Appl. Surf. Sci. 2020, 529, 147111. [Google Scholar] [CrossRef]
- Kwok, D.Y. The Usefulness of the Lifshitz–van Der Waals/Acid–Base Approach for Surface Tension Components and Interfacial Tensions. Colloids Surf. Physicochem. Eng. Asp. 1999, 156, 191–200. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Neumann, A.W. Contact Angle Measurement and Contact Angle Interpretation. Adv. Colloid Interface Sci. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- Costanzo, P.M.; Giese, R.F.; Van Oss, C.J. Determination of the Acid-Base Characteristics of Clay Mineral Surfaces by Contact Angle Measurements-Implications for the Adsorption of Organic Solutes from Aqueous Media. J. Adhes. Sci. Technol. 1990, 4, 267–275. [Google Scholar] [CrossRef]
- Erbil, H.Y. The Debate on the Dependence of Apparent Contact Angles on Drop Contact Area or Three-Phase Contact Line: A Review. Surf. Sci. Rep. 2014, 69, 325–365. [Google Scholar] [CrossRef]
- Meiron, T.S.; Marmur, A.; Saguy, I.S. Contact Angle Measurement on Rough Surfaces. J. Colloid Interface Sci. 2004, 274, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D.; Veeramasuneni, S.; Drelich, J.; Yalamanchili, M.R.; Yamauchi, G. Effect of Roughness as Determined by Atomic Force Microscopy on the Wetting Properties of PTFE Thin Films. Polym. Eng. Sci. 1996, 36, 1849–1855. [Google Scholar] [CrossRef]
- Youngblood, J.P.; McCarthy, T.J. Ultrahydrophobic Polymer Surfaces Prepared by Simultaneous Ablation of Polypropylene and Sputtering of Poly(Tetrafluoroethylene) Using Radio Frequency Plasma. Macromolecules 1999, 32, 6800–6806. [Google Scholar] [CrossRef]
- Öner, D.; McCarthy, T.J. Ultrahydrophobic Surfaces. Effects of Topography Length Scales on Wettability. Langmuir 2000, 16, 7777–7782. [Google Scholar] [CrossRef]
- Singh, J.; Ching, Y.; Liu, D.; Ching, K.; Razali, S.; Gan, S. Effects of PTFE Micro-Particles on the Fiber-Matrix Interface of Polyoxymethylene/Glass Fiber/Polytetrafluoroethylene Composites. Materials 2018, 11, 2164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Wu, S. Calculation of Interfacial Tension in Polymer Systems. J. Polym. Sci. Part C Polym. Symp. 1971, 34, 19–30. [Google Scholar] [CrossRef]
- Chen, W.; Fadeev, A.Y.; Hsieh, M.C.; Öner, D.; Youngblood, J.; McCarthy, T.J. Ultrahydrophobic and Ultralyophobic Surfaces: Some Comments and Examples. Langmuir 1999, 15, 3395–3399. [Google Scholar] [CrossRef]
- Włoch, J.; Terzyk, A.P.; Wiśniewski, M.; Kowalczyk, P. Nanoscale Water Contact Angle on Polytetrafluoroethylene Surfaces Characterized by Molecular Dynamics–Atomic Force Microscopy Imaging. Langmuir 2018, 34, 4526–4534. [Google Scholar] [CrossRef] [PubMed]
- Fox, H.W.; Zisman, W.A. The Spreading of Liquids on Low Energy Surfaces. I. Polytetrafluoroethylene. J. Colloid Sci. 1950, 5, 514–531. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Han, Y. Superhydrophobic PTFE Surfaces by Extension. Macromol. Rapid Commun. 2004, 25, 1105–1108. [Google Scholar] [CrossRef]
- Veeramasuneni, S.; Drelich, J.; Miller, J.D.; Yamauchi, G. Hydrophobicity of Ion-Plated PTFE Coatings. Prog. Org. Coat. 1997, 31, 265–270. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Lee, H.-K.; Kwon, Y.-N. Review on Blueprint of Designing Anti-Wetting Polymeric Membrane Surfaces for Enhanced Membrane Distillation Performance. Polymers 2019, 12, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowkes, F.M. Attractive Forces at Interfaces. Ind. Eng. Chem. 1964, 56, 40–52. [Google Scholar] [CrossRef]
- Zielecka, M.; Kozakiewicz, J.; Przybylski, J.; Sobczak, J. Studies on the Surface Properties of Silicone-Containing Hybrid Systems. Surf. Coat. Int. Part B Coat. Trans. 2002, 85, 215–220. [Google Scholar] [CrossRef]
- Mańko, D.; Zdziennicka, A.; Jańczuk, B. Surface Tension of Polytetrafluoroethylene and Its Wetting by Aqueous Solution of Some Surfactants and Their Mixtures. Appl. Surf. Sci. 2017, 392, 117–125. [Google Scholar] [CrossRef]
- Jaṅczuk, B.; Zdziennicka, A.; Wȯjcik, W. RELATIONSHIP BETWEEN WETTING OF TEFLON BY CETYLTRIMETHYLAMMONIUM BROMIDE SOLUTION AND ADSORPTION. Eur. Polym. J. 1997, 33, 1093–1098. [Google Scholar] [CrossRef]
- Lazzini, G.; Lutey, A.H.A.; Romoli, L.; Fuso, F. Molecular Dynamics Model for the Antibactericity of Textured Surfaces. Colloids Surf. B Biointerfaces 2021, 199, 111504. [Google Scholar] [CrossRef] [PubMed]
- Kolewe, K.W.; Peyton, S.R.; Schiffman, J.D. Fewer Bacteria Adhere to Softer Hydrogels. ACS Appl. Mater. Interfaces 2015, 7, 19562–19569. [Google Scholar] [CrossRef] [PubMed]
- Guégan, C.; Garderes, J.; Le Pennec, G.; Gaillard, F.; Fay, F.; Linossier, I.; Herry, J.-M.; Fontaine, M.-N.B.; Réhel, K.V. Alteration of Bacterial Adhesion Induced by the Substrate Stiffness. Colloids Surf. B Biointerfaces 2014, 114, 193–200. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Yan, T.; Jiang, Z.; Zhang, X.; Zuo, Y.Y. Surface Free Energy Activated High-Throughput Cell Sorting. Anal. Chem. 2014, 86, 9350–9355. [Google Scholar] [CrossRef] [Green Version]
- Fine, D.H.; Patil, A.G.; Velusamy, S.K. Aggregatibacter Actinomycetemcomitans (Aa) Under the Radar: Myths and Misunderstandings of Aa and Its Role in Aggressive Periodontitis. Front. Immunol. 2019, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- Inouye, T.; Ohta, H.; Kokeguchi, S.; Fukui, K.; Kato, K. Colonial Variation and Fimbriation of Actinobacillus Actinomycetemcomitans. FEMS Microbiol. Lett. 1990, 69, 13–17. [Google Scholar] [CrossRef]
- Roger, V.; Tenovuo, J.; Lenander-Lumikari, M.; Söderling, E.; Vilja, P. Lysozyme and Lactoperoxidase Inhibit the Adherence of Streptococcus Mutans NCTC 10449 (Serotype c) to Saliva-Treated Hydroxyapatite in Vitro. Caries Res. 1994, 28, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Cross, B.W.; Ruhl, S. Glycan Recognition at the Saliva—Oral Microbiome Interface. Cell. Immunol. 2018, 333, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Badihi Hauslich, L.; Sela, M.N.; Steinberg, D.; Rosen, G.; Kohavi, D. The Adhesion of Oral Bacteria to Modified Titanium Surfaces: Role of Plasma Proteins and Electrostatic Forces. Clin. Oral Implants Res. 2013, 24, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.C.; Llama-Palacios, A.; Blanc, V.; León, R.; Herrera, D.; Sanz, M. Structure, Viability and Bacterial Kinetics of an in Vitro Biofilm Model Using Six Bacteria from the Subgingival Microbiota. J. Periodontal Res. 2011, 46, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Pratten, J.; Smith, A.W.; Wilson, M. Response of Single Species Biofilms and Microcosm Dental Plaques to Pulsing with Chlorhexidine. J. Antimicrob. Chemother. 1998, 42, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Fai Lau, S.; Suzuki, H.; Wunderlich, B. The Thermodynamic Properties of Polytetrafluoroethylene. J. Polym. Sci. Polym. Phys. Ed. 1984, 22, 379–405. [Google Scholar] [CrossRef]
- Bellon-Fontaine, M.-N.; Rault, J.; van Oss, C.J. Microbial Adhesion to Solvents: A Novel Method to Determine the Electron-Donor/Electron-Acceptor or Lewis Acid-Base Properties of Microbial Cells. Colloids Surf. B Biointerfaces 1996, 7, 47–53. [Google Scholar] [CrossRef]


| RMS/nm | Average RMS/nm | |
|---|---|---|
| PTFE-W side1a | 90.3 | 83.0 |
| PTFE-W side1b | 75.6 | |
| PTFE-W side2a | 50.8 | 49.2 |
| PTFE-W side2b | 47.5 | |
| PTFE-B side1a | 281 | 693.5 |
| PTFE-B side1b | 1106 | |
| PTFE-B side2a | 252 | 735.0 |
| PTFE-B side2b | 1218 |
| PTFE-W | PTFE-B | |||
|---|---|---|---|---|
| SIDE 1 | SIDE 2 | SIDE 1 | SIDE 2 | |
| water | 127.1 ± 2.6 | 120.9 ± 2.7 | 127.8 ± 3.7 | 122.5 ± 4.9 |
| formamide | 106.5 ± 2.8 | 109.3 ± 2.6 | 113.4 ± 3.8 | 110.1 ± 2.2 |
| diiodomethane | 95.7 ± 5.0 | 95.8 ± 1.5 | 99.3 ± 2.8 | 88.3 ± 4.1 |
| (a) Dispersive | Polar | (b) Lifshitz–van der Waals | Acid | Base | Total SFE | |
|---|---|---|---|---|---|---|
| water | 21.8 | 51.0 | 21.8 | 25.5 | 25.5 | 72.8 |
| formamide | 39.0 | 19.0 | 39.0 | 2.28 | 39.6 | 58.0 |
| diiodomethane | 50.8 | 0.00 | 50.8 | 0.00 | 0.00 | 50.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begić, G.; Petković Didović, M.; Lučić Blagojević, S.; Jelovica Badovinac, I.; Žigon, J.; Perčić, M.; Cvijanović Peloza, O.; Gobin, I. Adhesion of Oral Bacteria to Commercial d-PTFE Membranes: Polymer Microstructure Makes a Difference. Int. J. Mol. Sci. 2022, 23, 2983. https://doi.org/10.3390/ijms23062983
Begić G, Petković Didović M, Lučić Blagojević S, Jelovica Badovinac I, Žigon J, Perčić M, Cvijanović Peloza O, Gobin I. Adhesion of Oral Bacteria to Commercial d-PTFE Membranes: Polymer Microstructure Makes a Difference. International Journal of Molecular Sciences. 2022; 23(6):2983. https://doi.org/10.3390/ijms23062983
Chicago/Turabian StyleBegić, Gabrijela, Mirna Petković Didović, Sanja Lučić Blagojević, Ivana Jelovica Badovinac, Jure Žigon, Marko Perčić, Olga Cvijanović Peloza, and Ivana Gobin. 2022. "Adhesion of Oral Bacteria to Commercial d-PTFE Membranes: Polymer Microstructure Makes a Difference" International Journal of Molecular Sciences 23, no. 6: 2983. https://doi.org/10.3390/ijms23062983
APA StyleBegić, G., Petković Didović, M., Lučić Blagojević, S., Jelovica Badovinac, I., Žigon, J., Perčić, M., Cvijanović Peloza, O., & Gobin, I. (2022). Adhesion of Oral Bacteria to Commercial d-PTFE Membranes: Polymer Microstructure Makes a Difference. International Journal of Molecular Sciences, 23(6), 2983. https://doi.org/10.3390/ijms23062983










