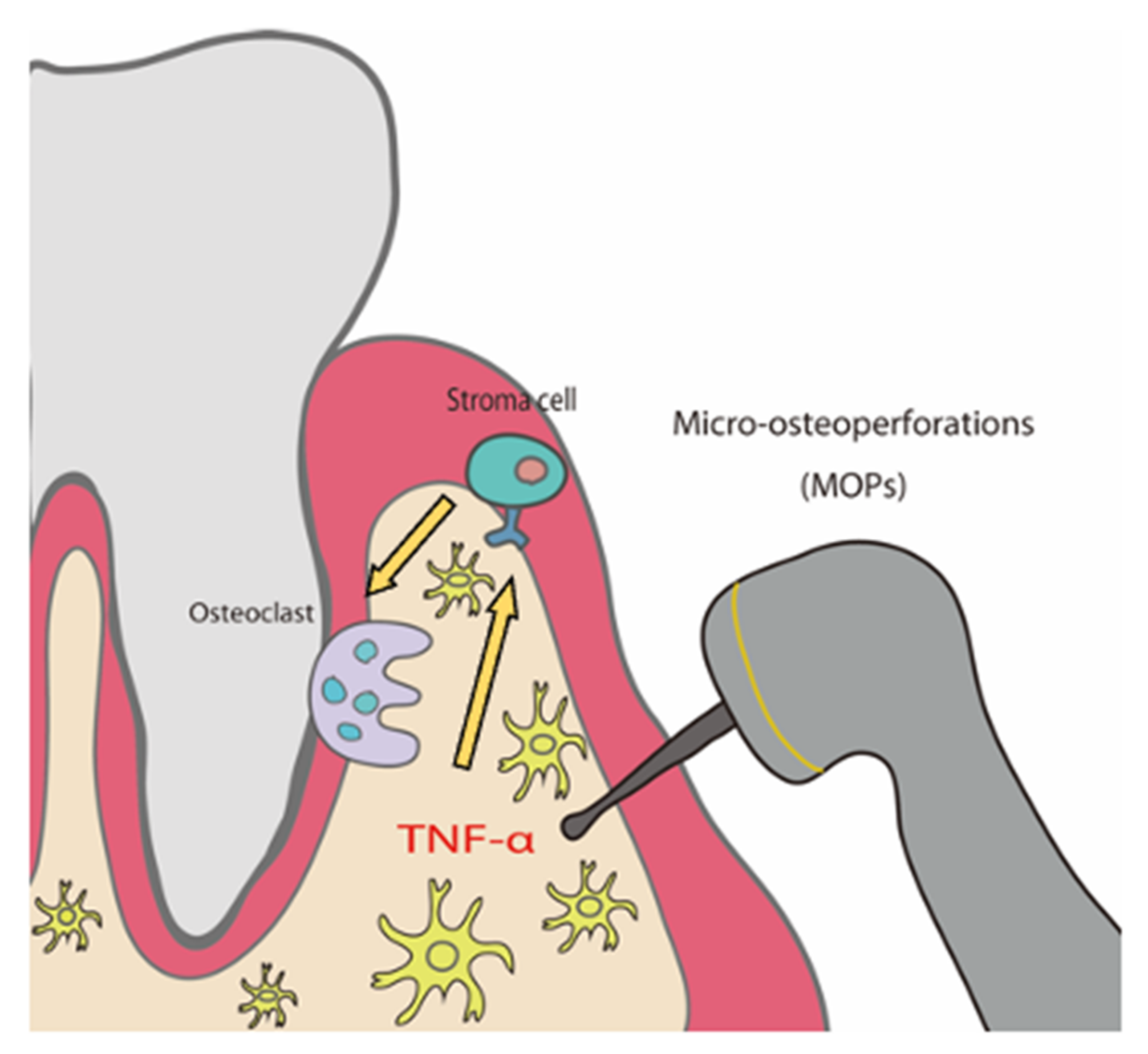
Micro-Osteoperforations Induce TNF-α Expression and Accelerate Orthodontic Tooth Movement via TNF-α-Responsive Stromal Cells
Abstract
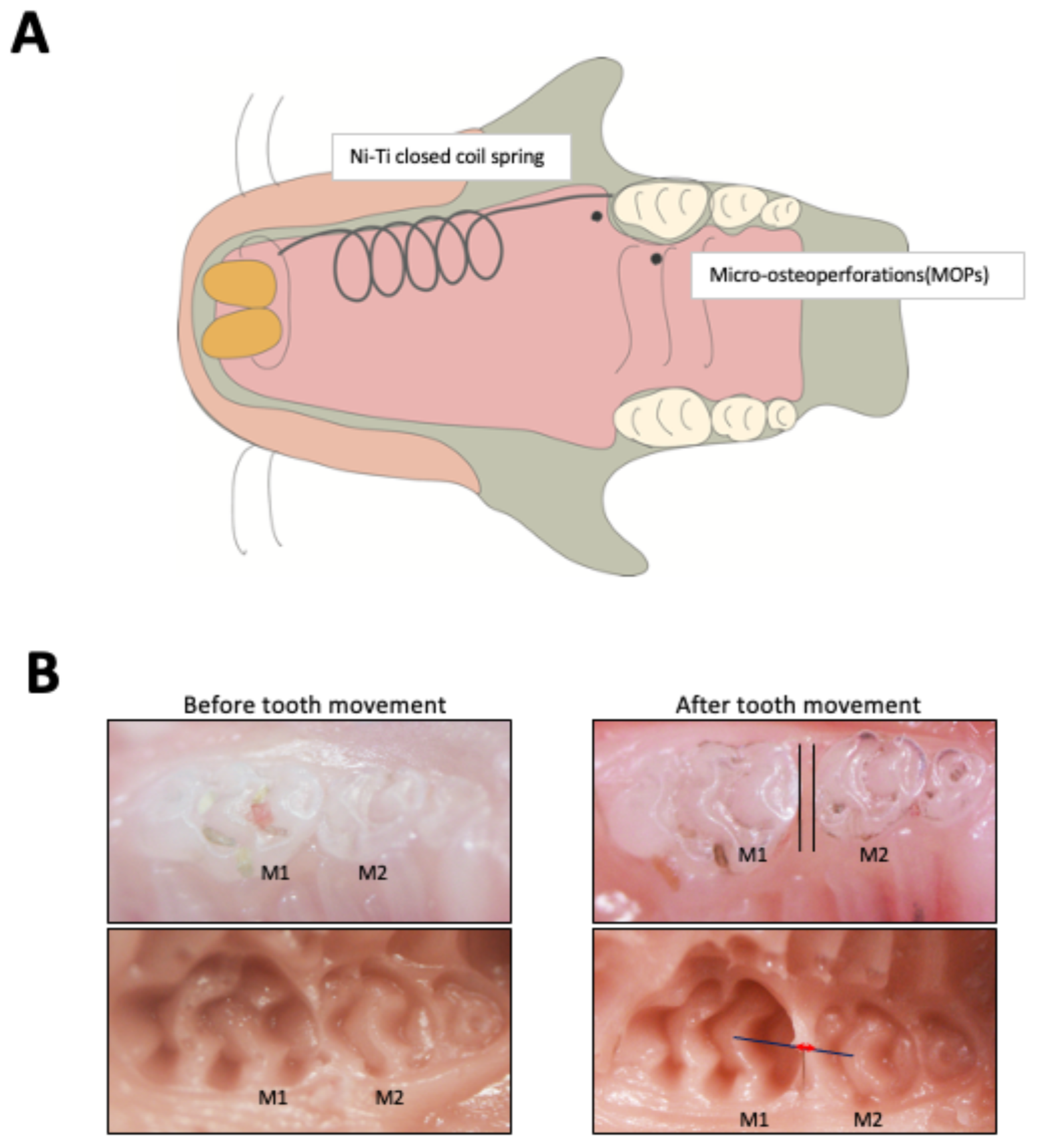
:1. Introduction
2. Results
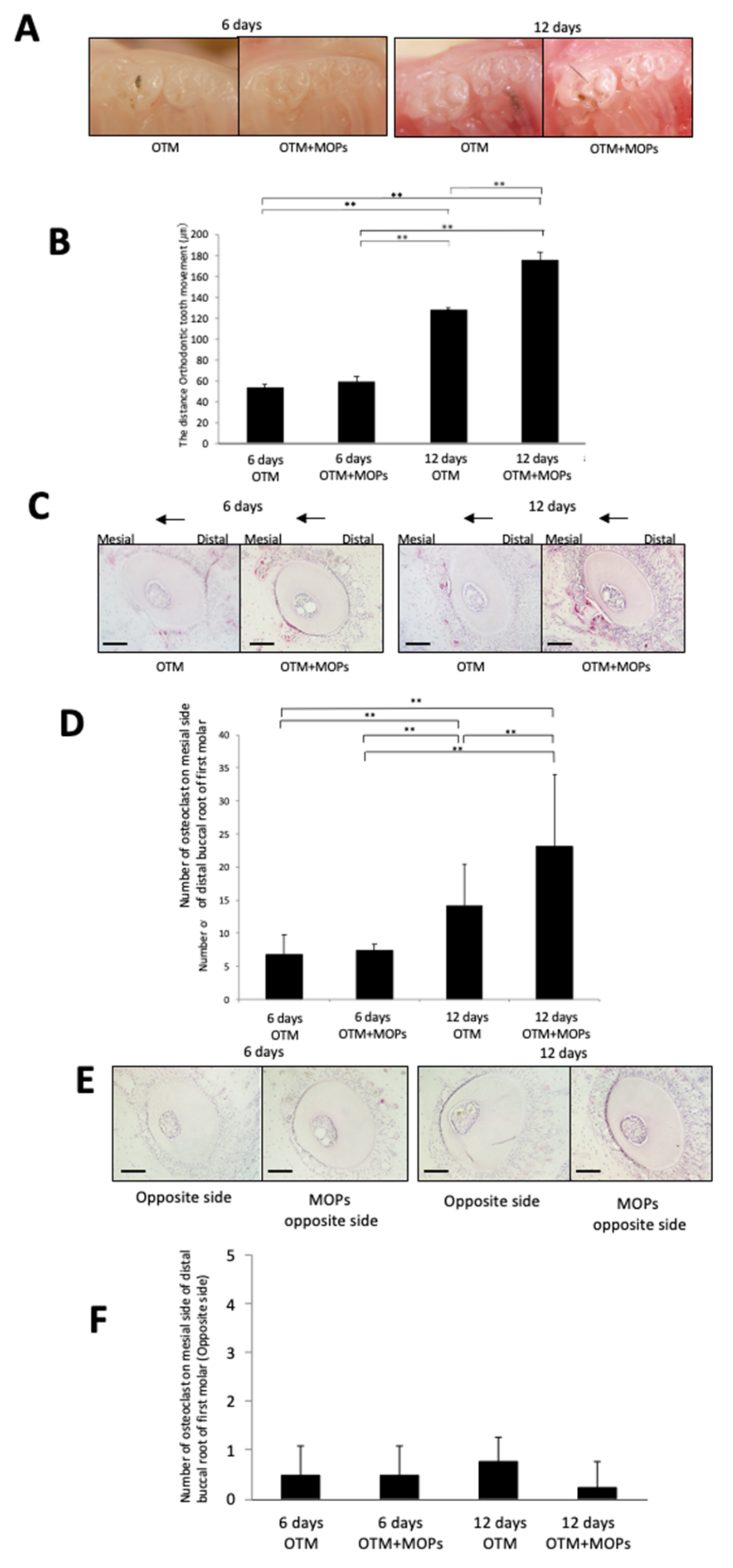
2.1. MOPs Increase Tooth Movement and Osteoclast Formation
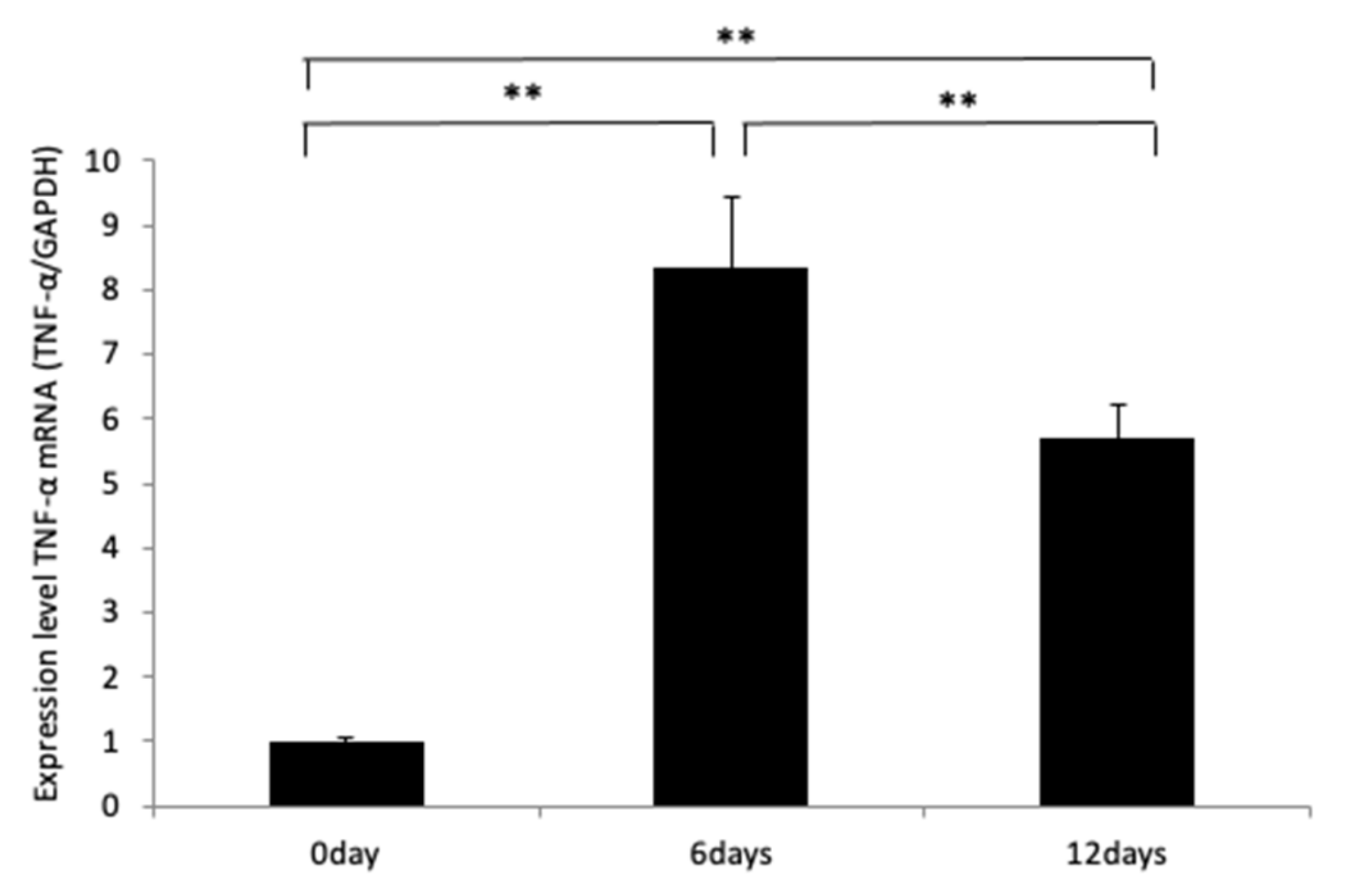
2.2. MOPs Induce TNF-α Expression
2.3. MOPs Induce Tooth Movement and Osteoclast Formation Is Dependent on TNF-α
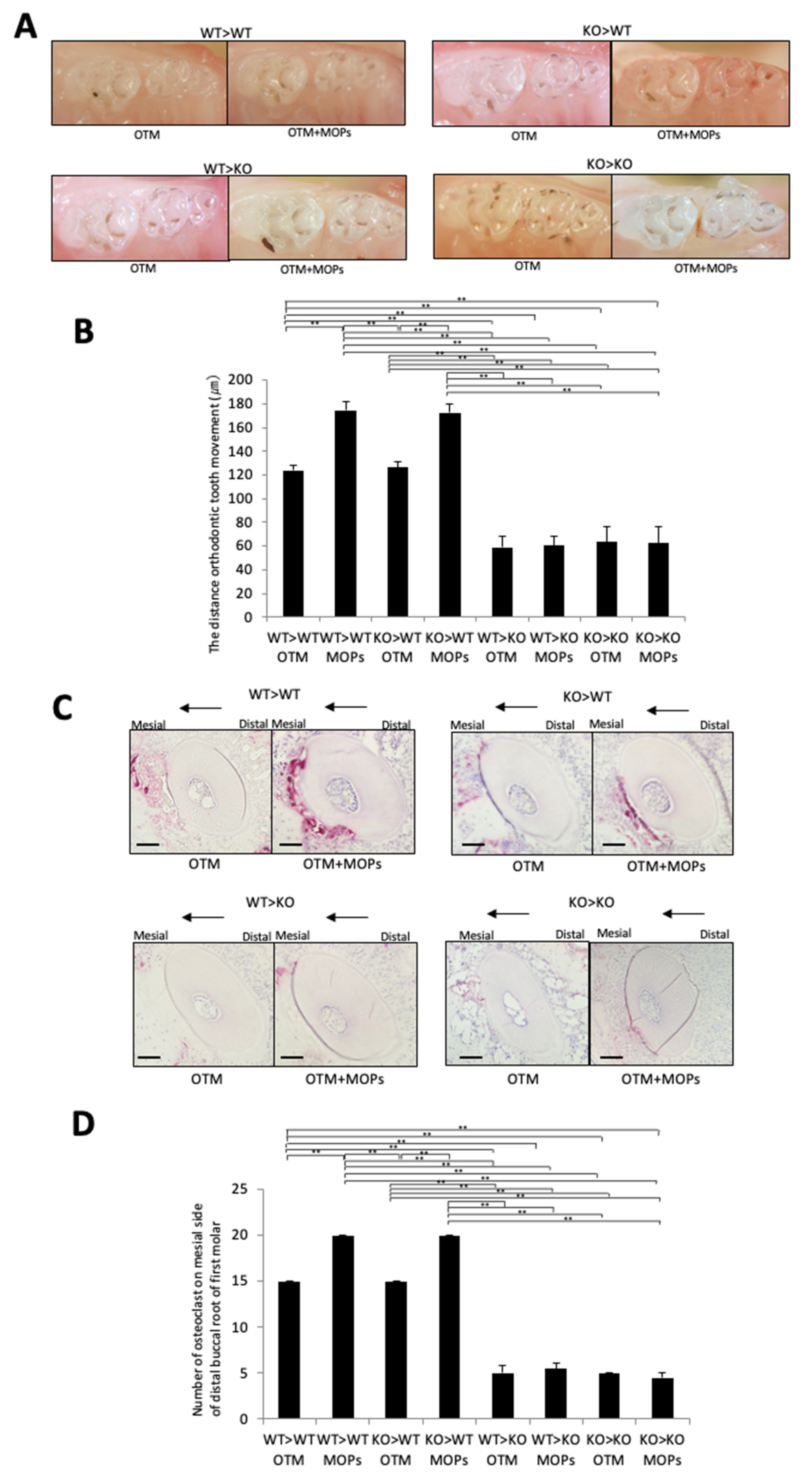
2.4. MOPs Increased Osteoclast Formation and Tooth Movement In Vivo via TNF-α-Responsive Stromal Cells
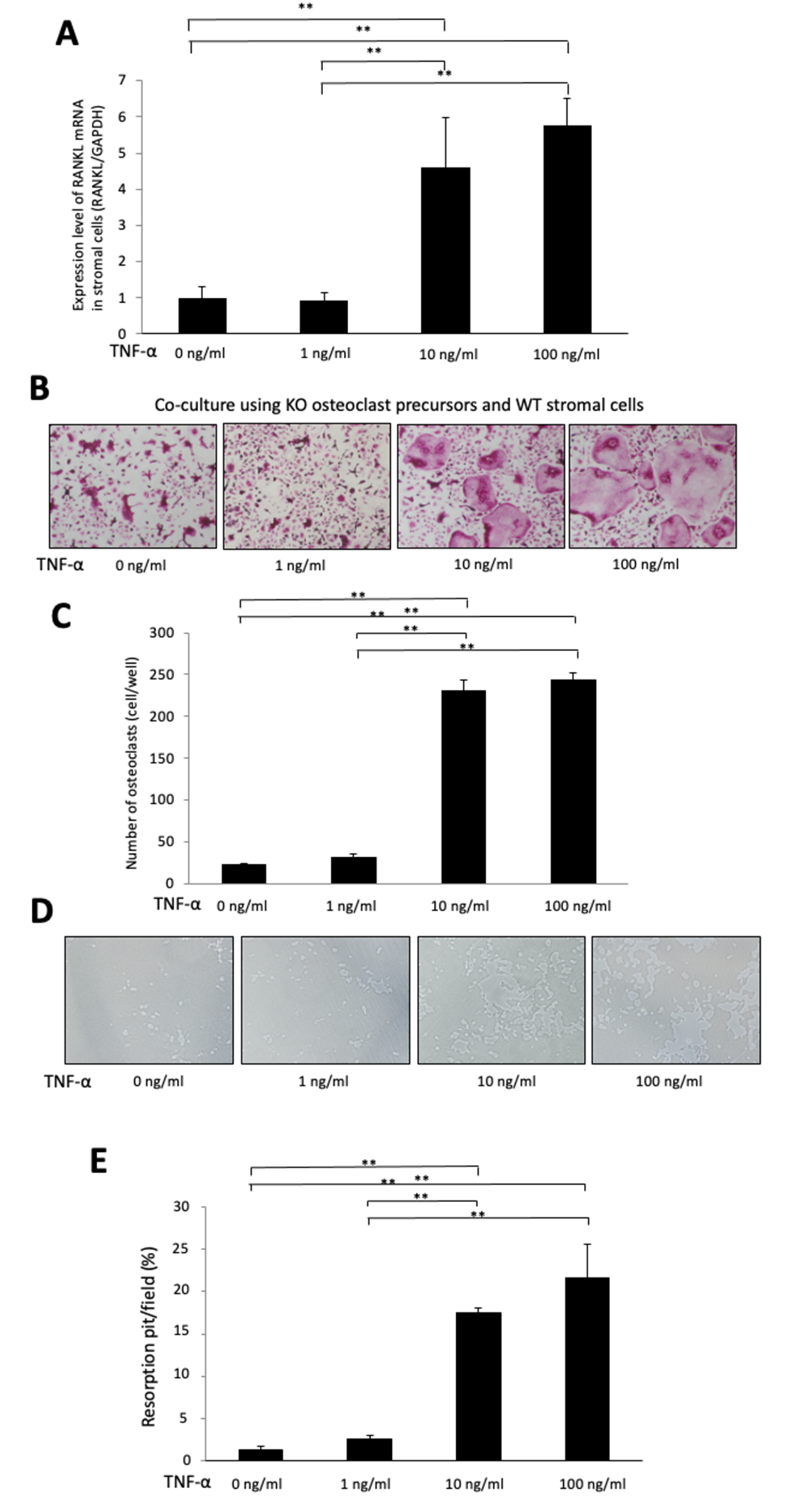
2.5. TNF-α Induces RANKL Expression in Stromal Cells in a Dose-Dependent Manner
2.6. TNF-α Enhances Osteoclast Formation in a Chimeric Co-Culture System, Which Used KO Osteoclast Precursors and WT Stromal Cells
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. RNA Preparation and Real-Time PCR Analysis
4.3. Procedure for Bone Marrow Transplantation
4.4. Sample Preparation for Histological Examination
4.5. Procedure for Bone Marrow Stromal Cells
4.6. Preparation of Osteoclast Precursors and Co-Culturing of Osteoclast Precursors from KO Mice and Stromal Cells from WT
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EDTA | ethylenediaminetetraacetic acid |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| IL | interleukin |
| KO | TNF receptor 1- and TNF receptor 2-deficient |
| M-CSF | macrophage colony-stimulating factor |
| MOPs | micro-osteoperforations |
| Ni-Ti | nickel-titanium |
| OTM | orthodontic tooth movement |
| RANKL | receptor activator of nuclear factor κB ligand |
| RAP | regional acceleratory phenomenon |
| TNF- | tumor necrosis factor- |
| TRAP | tartrate-resistant acid phosphatase |
| WT | wild-type |
References
- Hoogeveen, E.J.; Jansma, J.; Ren, Y. Surgically facilitated orthodontic treatment: A systematic review. Am. J. Orthod. Dentofac. Orthop. 2014, 145, S51–S64. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. The regional acceleratory phenomenon: A review. Henry Ford Hosp. Med. J. 1983, 31, 3–9. [Google Scholar]
- Kole, H. Surgical operations on the alveolar ridge to correct occlusal abnormalities. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 1959, 12, 277–288. [Google Scholar] [CrossRef]
- Wilcko, M.T.; Wilcko, W.M.; Pulver, J.J.; Bissada, N.F.; Bouquot, J.E. Accelerated osteogenic orthodontics technique: A 1-stage surgically facilitated rapid orthodontic technique with alveolar augmentation. J. Oral Maxillofac. Surg. 2009, 67, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, Y.G.; Kang, S.G. Effects of Corticision on paradental remodeling in orthodontic tooth movement. Angle Orthod. 2009, 79, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Dibart, S.; Sebaoun, J.; Surmenian, J. Piezocision: A minimally invasive, periodontally accelerated orthodontic tooth movement procedure. Compend. Contin. Educ. Dent. 2009, 30, 348–350. [Google Scholar]
- Alfawal, A.M.H.; Hajeer, M.Y.; Ajaj, M.A.; Hamadah, O.; Brad, B. Evaluation of piezocision and laser-assisted flapless corticotomy in the acceleration of canine retraction: A randomized controlled trial. Head Face Med. 2018, 14, 4. [Google Scholar] [CrossRef]
- Seifi, M.; Younessian, F.; Ameli, N. The Innovated Laser Assisted Flapless Corticotomy to Enhance Orthodontic Tooth Movement. J. Lasers Med. Sci. 2012, 3, 20–25. [Google Scholar]
- Yilanci, H.; Kara, B.; Ramoglu, S. Comparison of laser and piezo incisions to accelerate orthodontic tooth movement—A pilot rat model study. Ann. Med. Res. 2021, 28, 602–607. [Google Scholar] [CrossRef]
- Alikhani, M.; Raptis, M.; Zoldan, B.; Sangsuwon, C.; Lee, Y.B.; Alyami, B.; Corpodian, C.; Barrera, L.M.; Alansari, S.; Khoo, E.; et al. Effect of micro-osteoperforations on the rate of tooth movement. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 639–648. [Google Scholar] [CrossRef]
- Teixeira, C.C.; Khoo, E.; Tran, J.; Chartres, I.; Liu, Y.; Thant, L.M.; Khabensky, I.; Gart, L.P.; Cisneros, G.; Alikhani, M. Cytokine expression and accelerated tooth movement. J. Dent. Res. 2010, 89, 1135–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attri, S.; Mittal, R.; Batra, P.; Sonar, S.; Sharma, K.; Raghavan, S.; Rai, K.S. Comparison of rate of tooth movement and pain perception during accelerated tooth movement associated with conventional fixed appliances with micro-osteoperforations—A randomised controlled trial. J. Orthod. 2018, 45, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Gulduren, K.; Tumer, H.; Oz, U. Effects of micro-osteoperforations on intraoral miniscrew anchored maxillary molar distalization: A randomized clinical trial. J. Orofac. Orthop. 2020, 81, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kook, Y.A.; Bayome, M.; Park, J.H.; Lee, W.; Choi, H.; Abbas, N.H. Comparison of tooth movement and biological response in corticotomy and micro-osteoperforation in rabbits. Korean J. Orthod. 2019, 49, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Lu, H.P.; Yu, Y.F.; Ding, X.; Zhang, Z.Z.; Zhang, J.N. Comparison of tooth movement and biological response resulting from different force magnitudes combined with osteoperforation in rabbits. J. Appl. Oral Sci. 2021, 29, e20200734. [Google Scholar] [CrossRef]
- Sugimori, T.; Yamaguchi, M.; Shimizu, M.; Kikuta, J.; Hikida, T.; Hikida, M.; Murakami, Y.; Suemitsu, M.; Kuyama, K.; Kasai, K. Micro-osteoperforations accelerate orthodontic tooth movement by stimulating periodontal ligament cell cycles. Am. J. Orthod. Dentofac. Orthop. 2018, 154, 788–796. [Google Scholar] [CrossRef]
- Cheung, T.; Park, J.; Lee, D.; Kim, C.; Olson, J.; Javadi, S.; Lawson, G.; McCabe, J.; Moon, W.; Ting, K.; et al. Ability of mini-implant-facilitated micro-osteoperforations to accelerate tooth movement in rats. Am. J. Orthod. Dentofac. Orthop. 2016, 150, 958–967. [Google Scholar] [CrossRef] [Green Version]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Osteoclasts: What do they do and how do they do it? Am. J. Pathol. 2007, 170, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Takahashi, N.; Jimi, E.; Udagawa, N.; Takami, M.; Kotake, S.; Nakagawa, N.; Kinosaki, M.; Yamaguchi, K.; Shima, N.; et al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J. Exp. Med. 2000, 191, 275–286. [Google Scholar] [CrossRef]
- Azuma, Y.; Kaji, K.; Katogi, R.; Takeshita, S.; Kudo, A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J. Biol. Chem. 2000, 275, 4858–4864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitaura, H.; Sands, M.S.; Aya, K.; Zhou, P.; Hirayama, T.; Uthgenannt, B.; Wei, S.; Takeshita, S.; Novack, D.V.; Silva, M.J.; et al. Marrow stromal cells and osteoclast precursors differentially contribute to TNF-alpha-induced osteoclastogenesis in vivo. J. Immunol. 2004, 173, 4838–4846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitaura, H.; Zhou, P.; Kim, H.J.; Novack, D.V.; Ross, F.P.; Teitelbaum, S.L. M-CSF mediates TNF-induced inflammatory osteolysis. J. Clin. Investig. 2005, 115, 3418–3427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teitelbaum, S.L. Osteoclasts; culprits in inflammatory osteolysis. Arthritis Res. Ther. 2006, 8, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitaura, H.; Kimura, K.; Ishida, M.; Sugisawa, H.; Kohara, H.; Yoshimatsu, M.; Takano-Yamamoto, T. Effect of cytokines on osteoclast formation and bone resorption during mechanical force loading of the periodontal membrane. Sci. World J. 2014, 2014, 617032. [Google Scholar] [CrossRef]
- Sato, T.; Miyazawa, K.; Suzuki, Y.; Mizutani, Y.; Uchibori, S.; Asaoka, R.; Arai, M.; Togari, A.; Goto, S. Selective beta2-adrenergic Antagonist Butoxamine Reduces Orthodontic Tooth Movement. J. Dent. Res. 2014, 93, 807–812. [Google Scholar] [CrossRef] [Green Version]
- Andrade, I., Jr.; Silva, T.A.; Silva, G.A.; Teixeira, A.L.; Teixeira, M.M. The role of tumor necrosis factor receptor type 1 in orthodontic tooth movement. J. Dent. Res. 2007, 86, 1089–1094. [Google Scholar] [CrossRef]
- Basaran, G.; Ozer, T.; Kaya, F.A.; Kaplan, A.; Hamamci, O. Interleukine-1beta and tumor necrosis factor-alpha levels in the human gingival sulcus during orthodontic treatment. Angle Orthod. 2006, 76, 830–836. [Google Scholar] [CrossRef]
- Garlet, T.P.; Coelho, U.; Silva, J.S.; Garlet, G.P. Cytokine expression pattern in compression and tension sides of the periodontal ligament during orthodontic tooth movement in humans. Eur. J. Oral Sci. 2007, 115, 355–362. [Google Scholar] [CrossRef]
- Lowney, J.J.; Norton, L.A.; Shafer, D.M.; Rossomando, E.F. Orthodontic forces increase tumor necrosis factor alpha in the human gingival sulcus. Am. J. Orthod. Dentofac. Orthop. 1995, 108, 519–524. [Google Scholar] [CrossRef]
- Ren, Y.; Hazemeijer, H.; de Haan, B.; Qu, N.; de Vos, P. Cytokine profiles in crevicular fluid during orthodontic tooth movement of short and long durations. J. Periodontol. 2007, 78, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, S.; Mogi, M.; Deguchi, T. Interleukin (IL)-1 beta, IL-6, tumor necrosis factor-alpha, epidermal growth factor, and beta 2-microglobulin levels are elevated in gingival crevicular fluid during human orthodontic tooth movement. J. Dent. Res. 1996, 75, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Benjakul, S.; Unat, B.; Thammanichanon, P.; Leethanakul, C. Vibration synergistically enhances IL-1beta and TNF-alpha in compressed human periodontal ligament cells in the frequency-dependent manner. J. Oral Biol. Craniofac. Res. 2020, 10, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, P.K.; Basavanna, J.M.; Grewal, H.; Modi, P.; Sapawat, P.; Bohara, P.D. Elevated levels of Interleukin (IL)-1beta, IL-6, tumor necrosis factor-alpha, epidermal growth factor, and beta2-microglobulin levels in gingival crevicular fluid during human Orthodontic tooth movement (OTM). J. Fam. Med. Prim. Care 2019, 8, 1602–1606. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, H.; Yoshimatsu, M.; Fujimura, Y.; Eguchi, T.; Kohara, H.; Yamaguchi, A.; Yoshida, N. An anti-c-Fms antibody inhibits orthodontic tooth movement. J. Dent. Res. 2008, 87, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, M.; Shibata, Y.; Kitaura, H.; Chang, X.; Moriishi, T.; Hashimoto, F.; Yoshida, N.; Yamaguchi, A. Experimental model of tooth movement by orthodontic force in mice and its application to tumor necrosis factor receptor-deficient mice. J. Bone Miner. Metab. 2006, 24, 20–27. [Google Scholar] [CrossRef]
- Ogawa, S.; Kitaura, H.; Kishikawa, A.; Qi, J.; Shen, W.R.; Ohori, F.; Noguchi, T.; Marahleh, A.; Nara, Y.; Ochi, Y.; et al. TNF-α is responsible for the contribution of stromal cells to osteoclast and odontoclast formation during orthodontic tooth movement. PLoS ONE 2019, 14, e0223989. [Google Scholar] [CrossRef]
- Zhu, W.; Tan, Y.; Qiu, Q.; Li, X.; Huang, Z.; Fu, Y.; Liang, M. Comparison of the properties of human CD146+ and CD146- periodontal ligament cells in response to stimulation with tumour necrosis factor alpha. Arch. Oral Biol. 2013, 58, 1791–1803. [Google Scholar] [CrossRef]
- Luo, H.; Zhu, W.; Mo, W.; Liang, M. High-glucose concentration aggravates TNF-alpha-induced cell viability reduction in human CD146-positive periodontal ligament cells via TNFR-1 gene demethylation. Cell Biol. Int. 2020, 44, 2383–2394. [Google Scholar] [CrossRef]
- Chen, J.; Yu, M.; Li, X.; Sun, Q.F.; Yang, C.Z.; Yang, P.S. Progranulin promotes osteogenic differentiation of human periodontal ligament stem cells via tumor necrosis factor receptors to inhibit TNF-alpha sensitized NF-kB and activate ERK/JNK signaling. J. Periodontal. Res. 2020, 55, 363–373. [Google Scholar] [CrossRef]
- de Vries, T.J.; Yousovich, J.; Schoenmaker, T.; Scheres, N.; Everts, V. Tumor necrosis factor-alpha antagonist infliximab inhibits osteoclast formation of peripheral blood mononuclear cells but does not affect periodontal ligament fibroblast-mediated osteoclast formation. J. Periodontal. Res. 2016, 51, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Marahleh, A.; Kitaura, H.; Ohori, F.; Kishikawa, A.; Ogawa, S.; Shen, W.R.; Qi, J.; Noguchi, T.; Nara, Y.; Mizoguchi, I. TNF-alpha Directly Enhances Osteocyte RANKL Expression and Promotes Osteoclast Formation. Front. Immunol. 2019, 10, 2925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Amer, Y.; Abbas, S.; Hirayama, T. TNF receptor type 1 regulates RANK ligand expression by stromal cells and modulates osteoclastogenesis. J. Cell. Biochem. 2004, 93, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Kwan Tat, S.; Padrines, M.; Theoleyre, S.; Heymann, D.; Fortun, Y. IL-6, RANKL, TNF-alpha/IL-1: Interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004, 15, 49–60. [Google Scholar] [CrossRef]
- Yang, B.; Sun, H.; Xu, X.; Zhong, H.; Wu, Y.; Wang, J. YAP1 inhibits the induction of TNF-alpha-stimulated bone-resorbing mediators by suppressing the NF-kappaB signaling pathway in MC3T3-E1 cells. J. Cell. Physiol. 2020, 235, 4698–4708. [Google Scholar] [CrossRef] [PubMed]
- Kirschneck, C.; Fanghanel, J.; Wahlmann, U.; Wolf, M.; Roldan, J.C.; Proff, P. Interactive effects of periodontitis and orthodontic tooth movement on dental root resorption, tooth movement velocity and alveolar bone loss in a rat model. Ann. Anat.-Anat. Anz. 2017, 210, 32–43. [Google Scholar] [CrossRef]
- Okamoto, A.; Ohnishi, T.; Bandow, K.; Kakimoto, K.; Chiba, N.; Maeda, A.; Fukunaga, T.; Miyawaki, S.; Matsuguchi, T. Reduction of orthodontic tooth movement by experimentally induced periodontal inflammation in mice. Eur. J. Oral Sci. 2009, 117, 238–247. [Google Scholar] [CrossRef]
- Noguchi, T.; Kitaura, H.; Ogawa, S.; Qi, J.; Shen, W.R.; Ohori, F.; Marahleh, A.; Nara, Y.; Pramusita, A.; Mizoguchi, I. TNF-alpha stimulates the expression of RANK during orthodontic tooth movement. Arch. Oral Biol. 2020, 117, 104796. [Google Scholar] [CrossRef]
- Kishikawa, A.; Kitaura, H.; Kimura, K.; Ogawa, S.; Qi, J.; Shen, W.R.; Ohori, F.; Noguchi, T.; Marahleh, A.; Nara, Y.; et al. Docosahexaenoic Acid Inhibits Inflammation-Induced Osteoclast Formation and Bone Resorption In Vivo through GPR120 by Inhibiting TNF-alpha Production in Macrophages and Directly Inhibiting Osteoclast Formation. Front. Endocrinol. 2019, 10, 157. [Google Scholar] [CrossRef]
- Ishida, M.; Shen, W.R.; Kimura, K.; Kishikawa, A.; Shima, K.; Ogawa, S.; Qi, J.; Ohori, F.; Noguchi, T.; Marahleh, A.; et al. DPP-4 inhibitor impedes lipopolysaccharide-induced osteoclast formation and bone resorption in vivo. Biomed. Pharmacother. 2019, 109, 242–253. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinjo, R.; Kitaura, H.; Ogawa, S.; Ohori, F.; Noguchi, T.; Marahleh, A.; Nara, Y.; Pramusita, A.; Ma, J.; Kanou, K.; et al. Micro-Osteoperforations Induce TNF-α Expression and Accelerate Orthodontic Tooth Movement via TNF-α-Responsive Stromal Cells. Int. J. Mol. Sci. 2022, 23, 2968. https://doi.org/10.3390/ijms23062968
Kinjo R, Kitaura H, Ogawa S, Ohori F, Noguchi T, Marahleh A, Nara Y, Pramusita A, Ma J, Kanou K, et al. Micro-Osteoperforations Induce TNF-α Expression and Accelerate Orthodontic Tooth Movement via TNF-α-Responsive Stromal Cells. International Journal of Molecular Sciences. 2022; 23(6):2968. https://doi.org/10.3390/ijms23062968
Chicago/Turabian StyleKinjo, Ria, Hideki Kitaura, Saika Ogawa, Fumitoshi Ohori, Takahiro Noguchi, Aseel Marahleh, Yasuhiko Nara, Adya Pramusita, Jinghan Ma, Kayoko Kanou, and et al. 2022. "Micro-Osteoperforations Induce TNF-α Expression and Accelerate Orthodontic Tooth Movement via TNF-α-Responsive Stromal Cells" International Journal of Molecular Sciences 23, no. 6: 2968. https://doi.org/10.3390/ijms23062968
APA StyleKinjo, R., Kitaura, H., Ogawa, S., Ohori, F., Noguchi, T., Marahleh, A., Nara, Y., Pramusita, A., Ma, J., Kanou, K., & Mizoguchi, I. (2022). Micro-Osteoperforations Induce TNF-α Expression and Accelerate Orthodontic Tooth Movement via TNF-α-Responsive Stromal Cells. International Journal of Molecular Sciences, 23(6), 2968. https://doi.org/10.3390/ijms23062968





