Bioinspired Polydopamine Coatings Facilitate Attachment of Antimicrobial Peptidomimetics with Broad-Spectrum Antibacterial Activity
Abstract
:1. Introduction
2. Results
2.1. Biofilm Activity of Peptidomimetics
2.2. X-ray Photoelectron Spectroscopy (XPS)
2.3. Water Contact Angle
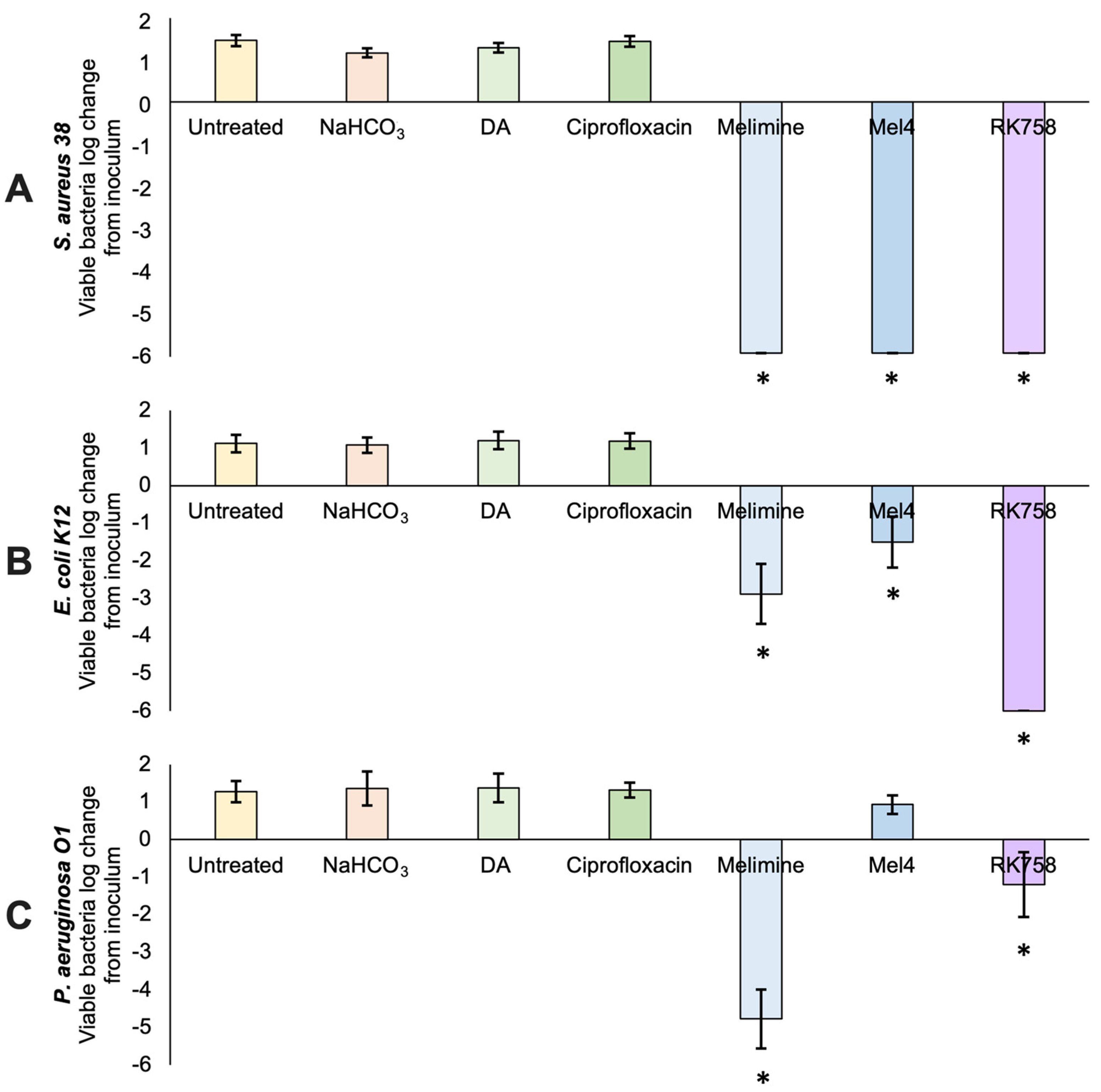
2.4. Antibacterial Activity of Surfaces
2.5. Scanning Electron Microscopy (SEM)
2.6. Haemolysis Assay for Coated Surfaces
2.7. Leaching of Peptidomimetics from Surfaces
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Bacterial Strains and Culture
4.3. Peptidomimetic Design and Synthesis
4.4. Inhibition of Biofilm Formation
4.5. Disruption of Pre-Formed Biofilms
4.6. Polydopamine Attachment to Surfaces
4.7. X-ray Photoelectron Spectroscopy (XPS)
4.8. Water Contact Angle
4.9. Antibacterial Activity of Surfaces
4.10. Scanning Electron Microscopy (SEM)
4.11. Haemolysis Assay for Coated Surfaces
4.12. Leaching of Peptidomimetics from Surfaces
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gebreyohannes, G.; Nyerere, A.; Bii, C.; Sbhatu, D.B. Challenges of Intervention, Treatment, and Antibiotic Resistance of Biofilm-Forming Microorganisms. Heliyon 2019, 5, e02192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufour, D.; Leung, V.; Lévesque, C.M. Bacterial Biofilm: Structure, Function, and Antimicrobial Resistance: Bacterial Biofilm. Endod. Top. 2010, 22, 2–16. [Google Scholar] [CrossRef]
- Allison, D.G.; Gilbert, P. Bacterial Biofilms. Sci. Prog. 1992, 76, 305–321. [Google Scholar]
- Vor, L.; Rooijakkers, S.H.M.; Strijp, J.A.G. Staphylococci Evade the Innate Immune Response by Disarming Neutrophils and Forming Biofilms. FEBS Lett. 2020, 594, 2556–2569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madsen, J.S.; Burmølle, M.; Hansen, L.H.; Sørensen, S.J. The Interconnection between Biofilm Formation and Horizontal Gene Transfer. FEMS Immunol. Med. Microbiol. 2012, 65, 183–195. [Google Scholar] [CrossRef] [Green Version]
- Khoury, A.E.; Lam, K.; Ellis, B.; Costerton, J.W. Prevention and Control of Bacterial Infections Associated with Medical Devices. ASAIO J. 1992, 38, M174–M178. [Google Scholar] [CrossRef]
- Wu, H.; Moser, C.; Wang, H.-Z.; Høiby, N.; Song, Z.-J. Strategies for Combating Bacterial Biofilm Infections. Int. J. Oral Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Parvizi, J.; Aggarwal, V.; Rasouli, M. Periprosthetic Joint Infection: Current Concept. Indian J. Orthop. 2013, 47, 10. [Google Scholar] [CrossRef]
- Wolcott, R.D.; Rhoads, D.D.; Bennett, M.E.; Wolcott, B.M.; Gogokhia, L.; Costerton, J.W.; Dowd, S.E. Chronic Wounds and the Medical Biofilm Paradigm. J. Wound Care 2010, 19, 45–53. [Google Scholar] [CrossRef]
- Klevens, R.M.; Edwards, J.R.; Richards, C.L.; Horan, T.C.; Gaynes, R.P.; Pollock, D.A.; Cardo, D.M. Estimating Health Care-Associated Infections and Deaths in U.S. Hospitals, 2002. Public Health Rep. 2007, 122, 160–166. [Google Scholar] [CrossRef]
- Prokuski, L. Prophylactic Antibiotics in Orthopaedic Surgery. J. Am. Acad. Orthop. Surg. 2008, 16, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Browne, K. Brought to Light: How Ultraviolet Disinfection Can Prevent the Nosocomial Transmission of COVID-19 and Other Infectious Diseases. Appl. Microbiol. 2021, 1, 537–556. [Google Scholar] [CrossRef]
- Huemer, M.; Mairpady Shambat, S.; Bergada-Pijuan, J.; Söderholm, S.; Boumasmoud, M.; Vulin, C.; Gómez-Mejia, A.; Antelo Varela, M.; Tripathi, V.; Götschi, S.; et al. Molecular Reprogramming and Phenotype Switching in Staphylococcus Aureus Lead to High Antibiotic Persistence and Affect Therapy Success. Proc. Natl. Acad. Sci. USA 2021, 118, e2014920118. [Google Scholar] [CrossRef] [PubMed]
- Bistolfi, A.; Massazza, G.; Verné, E.; Massè, A.; Deledda, D.; Ferraris, S.; Miola, M.; Galetto, F.; Crova, M. Antibiotic-Loaded Cement in Orthopedic Surgery: A Review. ISRN Orthop. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.M.; McLaren, A.C.; Sculco, T.P.; Brause, B.; Bostrom, M.; Kates, S.L.; Parvizi, J.; Alt, V.; Arnold, W.V.; Carli, A.; et al. Adjuvant Antibiotic-loaded Bone Cement: Concerns with Current Use and Research to Make It Work. J. Orthop. Res. 2020, 39, 227–239. [Google Scholar] [CrossRef] [Green Version]
- Anagnostakos, K.; Wilmes, P.; Schmitt, E.; Kelm, J. Elution of Gentamicin and Vancomycin from Polymethylmethacrylate Beads and Hip Spacers in Vivo. Acta Orthop. 2009, 80, 193–197. [Google Scholar] [CrossRef] [Green Version]
- Stewart, P.S. Mechanisms of Antibiotic Resistance in Bacterial Biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef]
- Kelm, J.; Regitz, T.; Schmitt, E.; Jung, W.; Anagnostakos, K. In Vivo and In Vitro Studies of Antibiotic Release from and Bacterial Growth Inhibition by Antibiotic-Impregnated Polymethylmethacrylate Hip Spacers. Antimicrob. Agents Chemother. 2006, 50, 332–335. [Google Scholar] [CrossRef] [Green Version]
- Mariconda, M.; Ascione, T.; Balato, G.; Rotondo, R.; Smeraglia, F.; Costa, G.G.; Conte, M. Sonication of Antibiotic-Loaded Cement Spacers in a Two-Stage Revision Protocol for Infected Joint Arthroplasty. BMC Musculoskelet. Disord. 2013, 14, 193. [Google Scholar] [CrossRef] [Green Version]
- Browne, K.; Chakraborty, S.; Chen, R.; Willcox, M.D.; Black, D.S.; Walsh, W.R.; Kumar, N. A New Era of Antibiotics: The Clinical Potential of Antimicrobial Peptides. Int. J. Mol. Sci. 2020, 21, 7047. [Google Scholar] [CrossRef] [PubMed]
- Yazici, H.; O’Neill, M.B.; Kacar, T.; Wilson, B.R.; Oren, E.E.; Sarikaya, M.; Tamerler, C. Engineered Chimeric Peptides as Antimicrobial Surface Coating Agents toward Infection-Free Implants. ACS Appl. Mater. Interfaces 2016, 8, 5070–5081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boix-Lemonche, G.; Guillem-Marti, J.; D’Este, F.; Manero, J.M.; Skerlavaj, B. Covalent Grafting of Titanium with a Cathelicidin Peptide Produces an Osteoblast Compatible Surface with Antistaphylococcal Activity. Colloids Surf. B Biointerfaces 2020, 185, 110586. [Google Scholar] [CrossRef] [PubMed]
- Salvagni, E.; García, C.; Manresa, À.; Müller-Sánchez, C.; Reina, M.; Rodríguez-Abreu, C.; García-Celma, M.J.; Esquena, J. Short and Ultrashort Antimicrobial Peptides Anchored onto Soft Commercial Contact Lenses Inhibit Bacterial Adhesion. Colloids Surf. B Biointerfaces 2020, 196, 111283. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.C.-L.; Harris, J.L.; Khanna, K.K.; Hong, J.-H. A Comprehensive Review on Current Advances in Peptide Drug Development and Design. Int. J. Mol. Sci. 2019, 20, 2383. [Google Scholar] [CrossRef] [Green Version]
- Otvos, L.; Wade, J.D. Current Challenges in Peptide-Based Drug Discovery. Front. Chem. 2014, 2, 62. [Google Scholar] [CrossRef]
- Mueller, L.K.; Baumruck, A.C.; Zhdanova, H.; Tietze, A.A. Challenges and Perspectives in Chemical Synthesis of Highly Hydrophobic Peptides. Front. Bioeng. Biotechnol. 2020, 8, 162. [Google Scholar] [CrossRef] [Green Version]
- Vagner, J.; Qu, H.; Hruby, V.J. Peptidomimetics, a Synthetic Tool of Drug Discovery. Curr. Opin. Chem. Biol. 2008, 12, 292–296. [Google Scholar] [CrossRef] [Green Version]
- Kharb, R.; Rana, M.; Sharma, P.; Shahar Yar, M. Therapeutic Importance of Peptidomimetics in Medicinal Chemistry. J. Chem. Pharm. Res. 2011, 3, 173–186. [Google Scholar]
- Kuppusamy, R.; Yasir, M.; Berry, T.; Cranfield, C.G.; Nizalapur, S.; Yee, E.; Kimyon, O.; Taunk, A.; Ho, K.K.K.; Cornell, B.; et al. Design and Synthesis of Short Amphiphilic Cationic Peptidomimetics Based on Biphenyl Backbone as Antibacterial Agents. Eur. J. Med. Chem. 2018, 143, 1702–1722. [Google Scholar] [CrossRef]
- Kuppusamy, R.; Yasir, M.; Yee, E.; Willcox, M.; Black, D.C.; Kumar, N. Guanidine Functionalized Anthranilamides as Effective Antibacterials with Biofilm Disruption Activity. Org. Biomol. Chem. 2018, 16, 5871–5888. [Google Scholar] [CrossRef] [PubMed]
- Sabir, S.; Yu, T.T.; Kuppusamy, R.; Almohaywi, B.; Iskander, G.; Das, T.; Willcox, M.D.P.; Black, D.S.; Kumar, N. Novel Seleno- and Thio-Urea Containing Dihydropyrrol-2-One Analogues as Antibacterial Agents. Antibiotics 2021, 10, 321. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.T.; Kuppusamy, R.; Yasir, M.; Hassan, M.M.; Alghalayini, A.; Gadde, S.; Deplazes, E.; Cranfield, C.; Willcox, M.D.P.; Black, D.S.; et al. Design, Synthesis and Biological Evaluation of Biphenylglyoxamide-Based Small Molecular Antimicrobial Peptide Mimics as Antibacterial Agents. Int. J. Mol. Sci. 2020, 21, 6789. [Google Scholar] [CrossRef]
- Huang, S.; Liang, N.; Hu, Y.; Zhou, X.; Abidi, N. Polydopamine-Assisted Surface Modification for Bone Biosubstitutes. BioMed Res. Int. 2016, 2016, 2389895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.H.; Floren, M.; Tan, W. Mussel-Inspired Polydopamine for Bio-Surface Functionalization. Biosurf. Biotribol. 2016, 2, 121–136. [Google Scholar] [CrossRef]
- Trzcińska, Z.; Bruggeman, M.; Ijakipour, H.; Hodges, N.J.; Bowen, J.; Stamboulis, A. Polydopamine Linking Substrate for AMPs: Characterisation and Stability on Ti6Al4V. Materials 2020, 13, 3714. [Google Scholar] [CrossRef]
- Su, L.; Yu, Y.; Zhao, Y.; Liang, F.; Zhang, X. Strong Antibacterial Polydopamine Coatings Prepared by a Shaking-Assisted Method. Sci. Rep. 2016, 6, 24420. [Google Scholar] [CrossRef]
- Lim, K.; Chua, R.R.Y.; Ho, B.; Tambyah, P.A.; Hadinoto, K.; Leong, S.S.J. Development of a Catheter Functionalized by a Polydopamine Peptide Coating with Antimicrobial and Antibiofilm Properties. Acta Biomater. 2015, 15, 127–138. [Google Scholar] [CrossRef]
- Dutta, D.; Zhao, T.; Cheah, K.B.; Holmlund, L.; Willcox, M.D.P. Activity of a Melimine Derived Peptide Mel4 against Stenotrophomonas, Delftia, Elizabethkingia, Burkholderia and Biocompatibility as a Contact Lens Coating. Contact Lens Anterior Eye 2017, 40, 175–183. [Google Scholar] [CrossRef]
- Yasir, M.; Dutta, D.; Willcox, M.D.P. Comparative Mode of Action of the Antimicrobial Peptide Melimine and Its Derivative Mel4 against Pseudomonas Aeruginosa. Sci. Rep. 2019, 9, 7063. [Google Scholar] [CrossRef] [Green Version]
- Dhand, C.; Ong, C.Y.; Dwivedi, N.; Varadarajan, J.; Halleluyah Periayah, M.; Jianyang Lim, E.; Mayandi, V.; Goh, E.T.L.; Najjar, R.P.; Chan, L.W.; et al. Mussel-Inspired Durable Antimicrobial Contact Lenses: The Role of Covalent and Noncovalent Attachment of Antimicrobials. ACS Biomater. Sci. Eng. 2020, 6, 3162–3173. [Google Scholar] [CrossRef]
- Yu, K.; Alzahrani, A.; Khoddami, S.; Cheng, J.T.J.; Mei, Y.; Gill, A.; Luo, H.D.; Haney, E.F.; Hilpert, K.; Hancock, R.E.W.; et al. Rapid Assembly of Infection-Resistant Coatings: Screening and Identification of Antimicrobial Peptides Works in Cooperation with an Antifouling Background. ACS Appl. Mater. Interfaces 2021, 13, 36784–36799. [Google Scholar] [CrossRef]
- Yasir, M.; Dutta, D.; Willcox, M.D.P. Enhancement of Antibiofilm Activity of Ciprofloxacin against Staphylococcus Aureus by Administration of Antimicrobial Peptides. Antibiotics 2021, 10, 1159. [Google Scholar] [CrossRef] [PubMed]
- Menzies, K.L.; Jones, L. The Impact of Contact Angle on the Biocompatibility of Biomaterials. Optom. Vis. Sci. 2010, 87, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, M.; Nejib, M.; Naceur, M. Cell Adhesion to Biomaterials: Concept of Biocompatibility. In Advances in Biomaterials Science and Biomedical Applications; Pignatello, R., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-1051-4. [Google Scholar]
- Shalev, T.; Gopin, A.; Bauer, M.; Stark, R.W.; Rahimipour, S. Non-Leaching Antimicrobial Surfaces through Polydopamine Bio-Inspired Coating of Quaternary Ammonium Salts or an Ultrashort Antimicrobial Lipopeptide. J. Mater. Chem. 2012, 22, 2026–2032. [Google Scholar] [CrossRef] [Green Version]
- Yasir, M.; Dutta, D.; Hossain, K.R.; Chen, R.; Ho, K.K.K.; Kuppusamy, R.; Clarke, R.J.; Kumar, N.; Willcox, M.D.P. Mechanism of Action of Surface Immobilized Antimicrobial Peptides Against Pseudomonas Aeruginosa. Front. Microbiol. 2020, 10, 3053. [Google Scholar] [CrossRef]
- Chen, R.; Willcox, M.D.P.; Ho, K.K.K.; Smyth, D.; Kumar, N. Antimicrobial Peptide Melimine Coating for Titanium and Its in Vivo Antibacterial Activity in Rodent Subcutaneous Infection Models. Biomaterials 2016, 85, 142–151. [Google Scholar] [CrossRef]
- Rasul, R. Novel Antimicrobial Biomaterials. Ph.D. Thesis, University of New South Wales, Sydney, Australia, 2010. [Google Scholar]
- Hasan, A.; Lee, K.; Tewari, K.; Pandey, L.M.; Messersmith, P.B.; Faulds, K.; Maclean, M.; Lau, K.H.A. Surface Design for Immobilization of an Antimicrobial Peptide Mimic for Efficient Anti-Biofouling. Chemistry 2020, 26, 5789–5793. [Google Scholar] [CrossRef]
- Hartmann, M.; Berditsch, M.; Hawecker, J.; Ardakani, M.F.; Gerthsen, D.; Ulrich, A.S. Damage of the Bacterial Cell Envelope by Antimicrobial Peptides Gramicidin S and PGLa as Revealed by Transmission and Scanning Electron Microscopy. Antimicrob. Agents Chemother. 2010, 54, 3132–3142. [Google Scholar] [CrossRef] [Green Version]
- Kondejewski, L.H.; Jelokhani-Niaraki, M.; Farmer, S.W.; Lix, B.; Kay, C.M.; Sykes, B.D.; Hancock, R.E.W.; Hodges, R.S. Dissociation of Antimicrobial and Hemolytic Activities in Cyclic Peptide Diastereomers by Systematic Alterations in Amphipathicity. J. Biol. Chem. 1999, 274, 13181–13192. [Google Scholar] [CrossRef] [Green Version]
- Hwang, P.M.; Vogel, H.J. Structure-Function Relationships of Antimicrobial Peptides. Biochem. Cell Biol. 1998, 76, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Ozkan, J.; Willcox, M.D.P. Biocompatibility of Antimicrobial Melimine Lenses: Rabbit and Human Studies. Optom. Vis. Sci. 2014, 91, 570–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willcox, M.D.P.; Hume, E.B.H.; Aliwarga, Y.; Kumar, N.; Cole, N. A Novel Cationic-Peptide Coating for the Prevention of Microbial Colonization on Contact Lenses. J. Appl. Microbiol. 2008, 105, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Lemos, A.S.O.; Campos, L.M.; Melo, L.; Guedes, M.C.M.R.; Oliveira, L.G.; Silva, T.P.; Melo, R.C.N.; Rocha, V.N.; Aguiar, J.A.K.; Apolônio, A.C.M.; et al. Antibacterial and Antibiofilm Activities of Psychorubrin, a Pyranonaphthoquinone Isolated From Mitracarpus Frigidus (Rubiaceae). Front. Microbiol. 2018, 9, 724. [Google Scholar] [CrossRef] [Green Version]
- Ojkic, N.; Lilja, E.; Direito, S.; Dawson, A.; Allen, R.J.; Waclaw, B. A Roadblock-and-Kill Mechanism of Action Model for the DNA-Targeting Antibiotic Ciprofloxacin. Antimicrob. Agents Chemother. 2020, 64, e02487-19. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, C.; Liang, G.; Zhang, M.; Zheng, J. Engineering Antimicrobial Peptides with Improved Antimicrobial and Hemolytic Activities. J. Chem. Inf. Model. 2013, 53, 3280–3296. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of Minimum Inhibitory Concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [Green Version]




| Treatment Group | Elemental Composition (%) | ||||||
|---|---|---|---|---|---|---|---|
| C | N | O | Na | Si | Br | Other | |
| Untreated | 17.3 | 0.3 | 54.3 | 3.5 | 14.7 | 0 | 9.9 |
| NaHCO3 buffer | 17.0 | 1.5 | 56.3 | 2.2 | 15.1 | 0 | 7.9 |
| Polydopamine | 43.0 | 4.7 | 38.2 | 1.3 | 8.6 | 0 | 4.2 |
| +Ciprofloxacin | 37.8 | 3.8 | 40.9 | 2.5 | 10.6 | 0 | 4.4 |
| +Melimine | 60.7 | 22.2 | 17.1 | 0 | 0 | 0 | 0 |
| +Mel4 | 58.2 | 20.2 | 20.3 | 0 | 0.9 | 0 | 0.4 |
| +RK758 | 70.8 | 15.4 | 12.3 | 0.2 | 0.5 | 0.9 | 0 |
| NC | PC | DA | Buffer | Melimine | Mel4 | RK758 | |
|---|---|---|---|---|---|---|---|
| OD540 | 0.124 | 1.785 | 0.137 | 0.108 | 0.118 | 0.130 | 0.105 |
| SD | 0.011 | 0.027 | 0.045 | 0.001 | 0.010 | 0.009 | 0.003 |
| % haemolysis | 0.728 | −0.924 | −0.336 | 0.308 | −1.064 | ||
| p = | 0.0002 | 0.732 | 0.454 | 0.273 | 0.352 | 0.069 |
| Treatment | Untreated | Buffer | DA | Ciprofloxacin | Melimine | Mel4 | RK758 |
|---|---|---|---|---|---|---|---|
| 10 mM NaHCO3 | X | X | X | X | X | X | |
| DA 0.25 mg*mL−1 | X | X | X | X | X | ||
| Ciprofloxacin 4 mg*mL−1 | X | ||||||
| Melimine 4 mg*mL−1 | X | ||||||
| Mel4 4 mg*mL−1 | X | ||||||
| RK758 4 mg*mL−1 | X |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Browne, K.; Kuppusamy, R.; Chen, R.; Willcox, M.D.P.; Walsh, W.R.; Black, D.S.; Kumar, N. Bioinspired Polydopamine Coatings Facilitate Attachment of Antimicrobial Peptidomimetics with Broad-Spectrum Antibacterial Activity. Int. J. Mol. Sci. 2022, 23, 2952. https://doi.org/10.3390/ijms23062952
Browne K, Kuppusamy R, Chen R, Willcox MDP, Walsh WR, Black DS, Kumar N. Bioinspired Polydopamine Coatings Facilitate Attachment of Antimicrobial Peptidomimetics with Broad-Spectrum Antibacterial Activity. International Journal of Molecular Sciences. 2022; 23(6):2952. https://doi.org/10.3390/ijms23062952
Chicago/Turabian StyleBrowne, Katrina, Rajesh Kuppusamy, Renxun Chen, Mark D. P. Willcox, William R. Walsh, David StC. Black, and Naresh Kumar. 2022. "Bioinspired Polydopamine Coatings Facilitate Attachment of Antimicrobial Peptidomimetics with Broad-Spectrum Antibacterial Activity" International Journal of Molecular Sciences 23, no. 6: 2952. https://doi.org/10.3390/ijms23062952
APA StyleBrowne, K., Kuppusamy, R., Chen, R., Willcox, M. D. P., Walsh, W. R., Black, D. S., & Kumar, N. (2022). Bioinspired Polydopamine Coatings Facilitate Attachment of Antimicrobial Peptidomimetics with Broad-Spectrum Antibacterial Activity. International Journal of Molecular Sciences, 23(6), 2952. https://doi.org/10.3390/ijms23062952










