Loss of Microglial Insulin Receptor Leads to Sex-Dependent Metabolic Disorders in Obese Mice
Abstract
:1. Introduction
2. Results
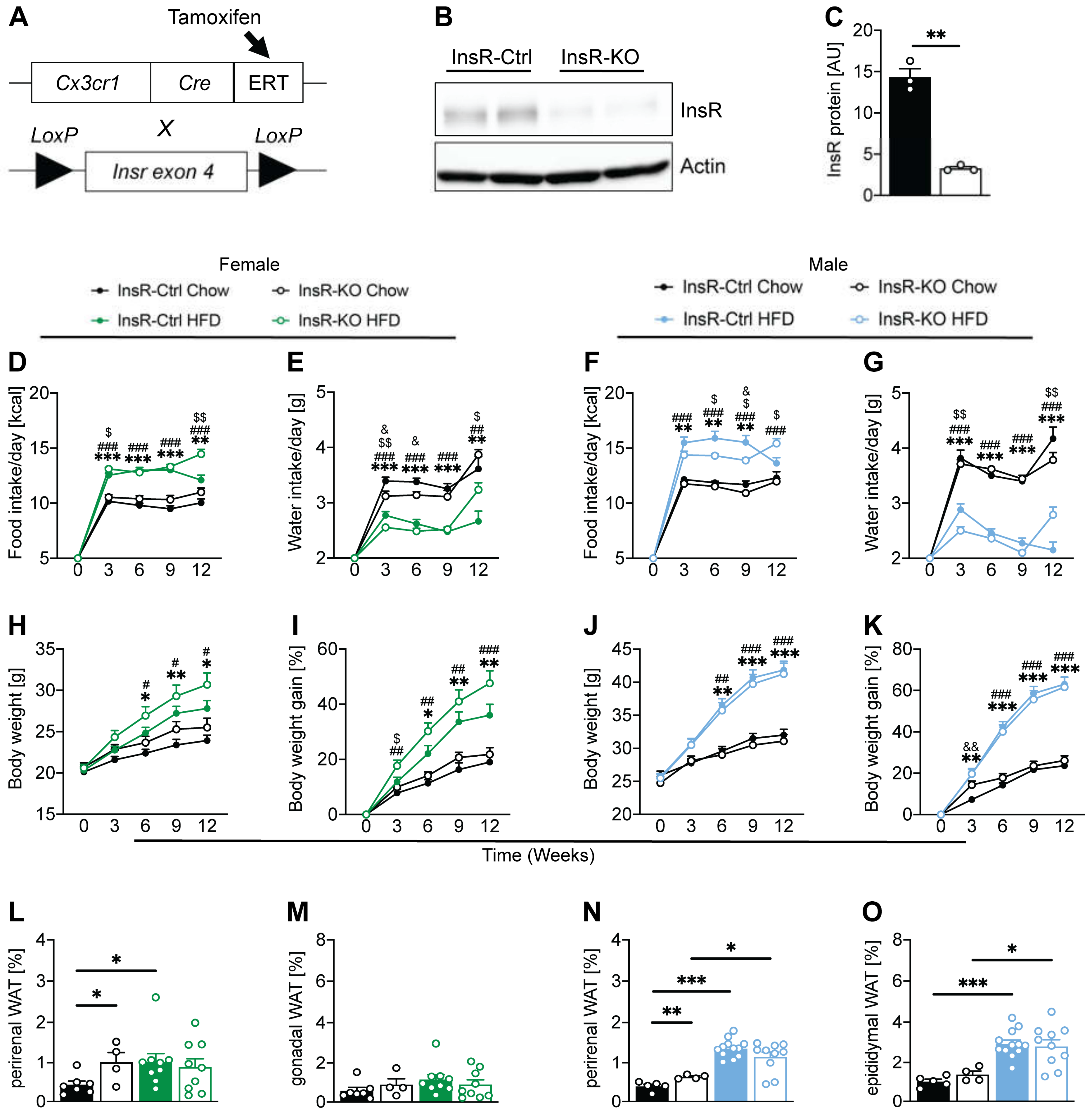
2.1. Microglial InsR Deletion in Mice Affects Food and Water Intake during Dietary-Induced Obesity in a Sex-Dependent Manner
2.2. Microglial Insulin Signaling in Female Mice Plays an Important Role in Maintaining Homeostatic Glucose and Insulin Concentrations
2.3. Microglial Insulin Function Has a Sex-Dependent Effect on Energy Metabolism in Obesity
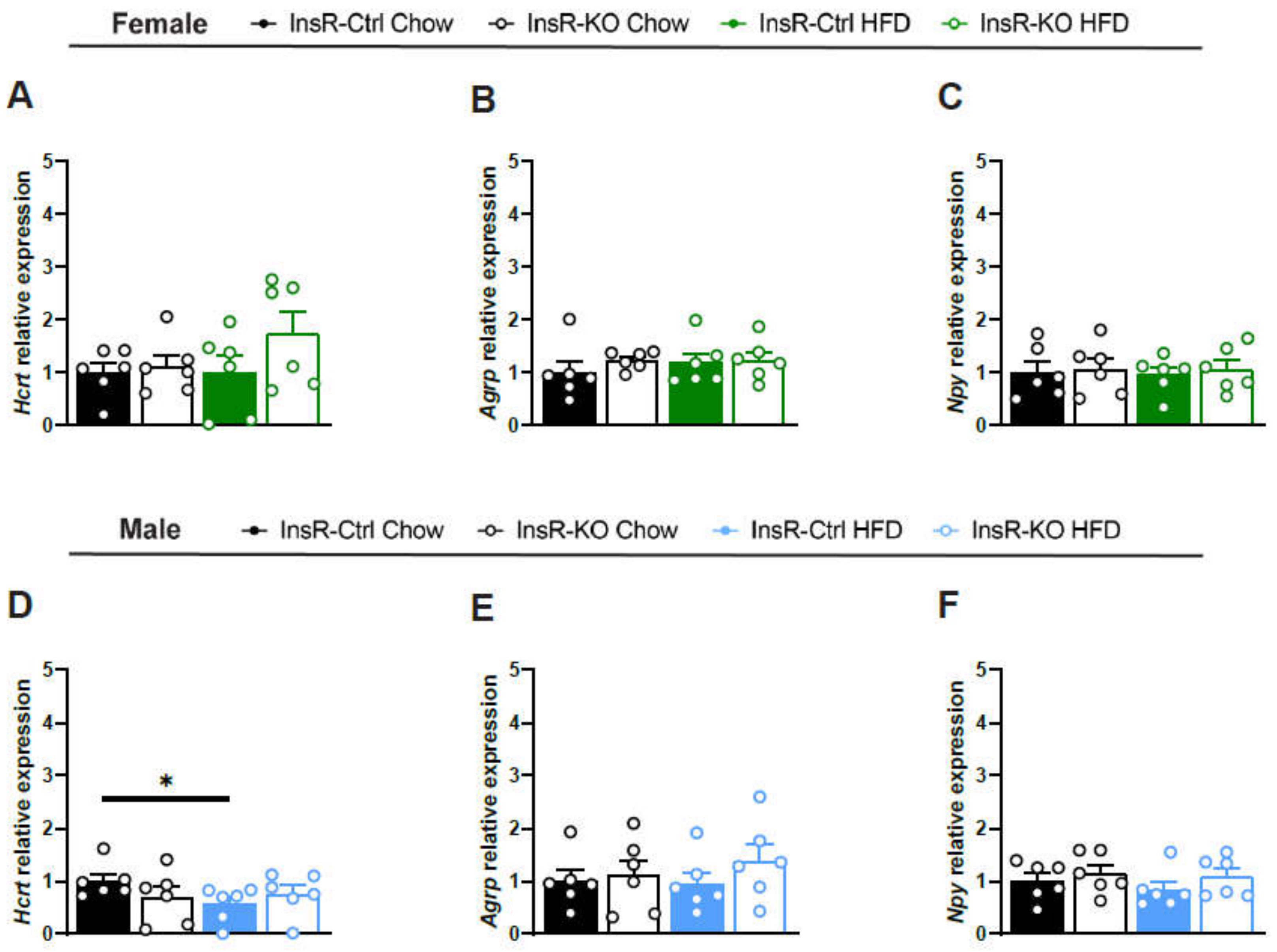
2.4. Lack of Microglial InsR Has No Effect on Satiety-Involved Hypothalamic Neuropeptides in Physiological and Obesogenic Conditions
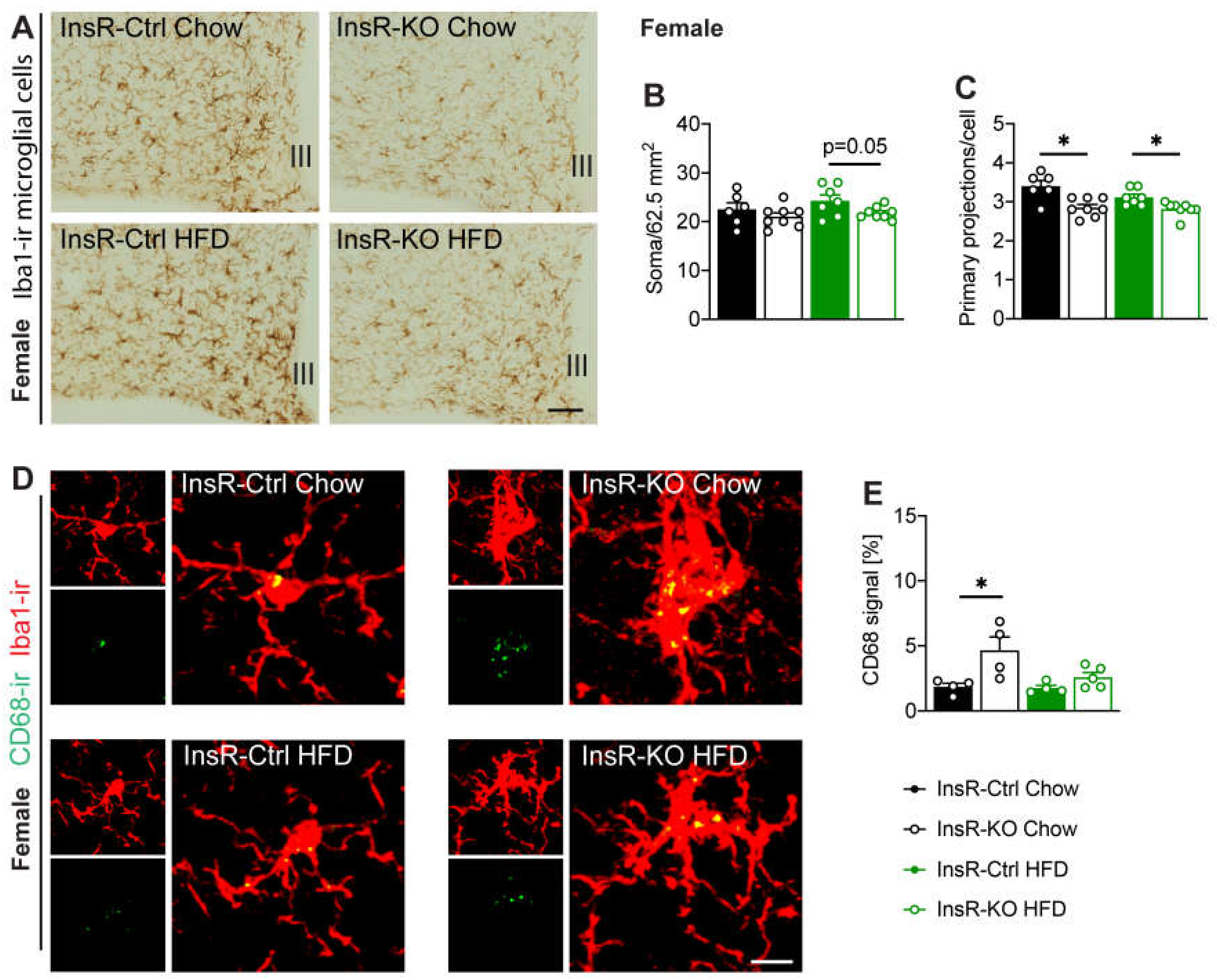
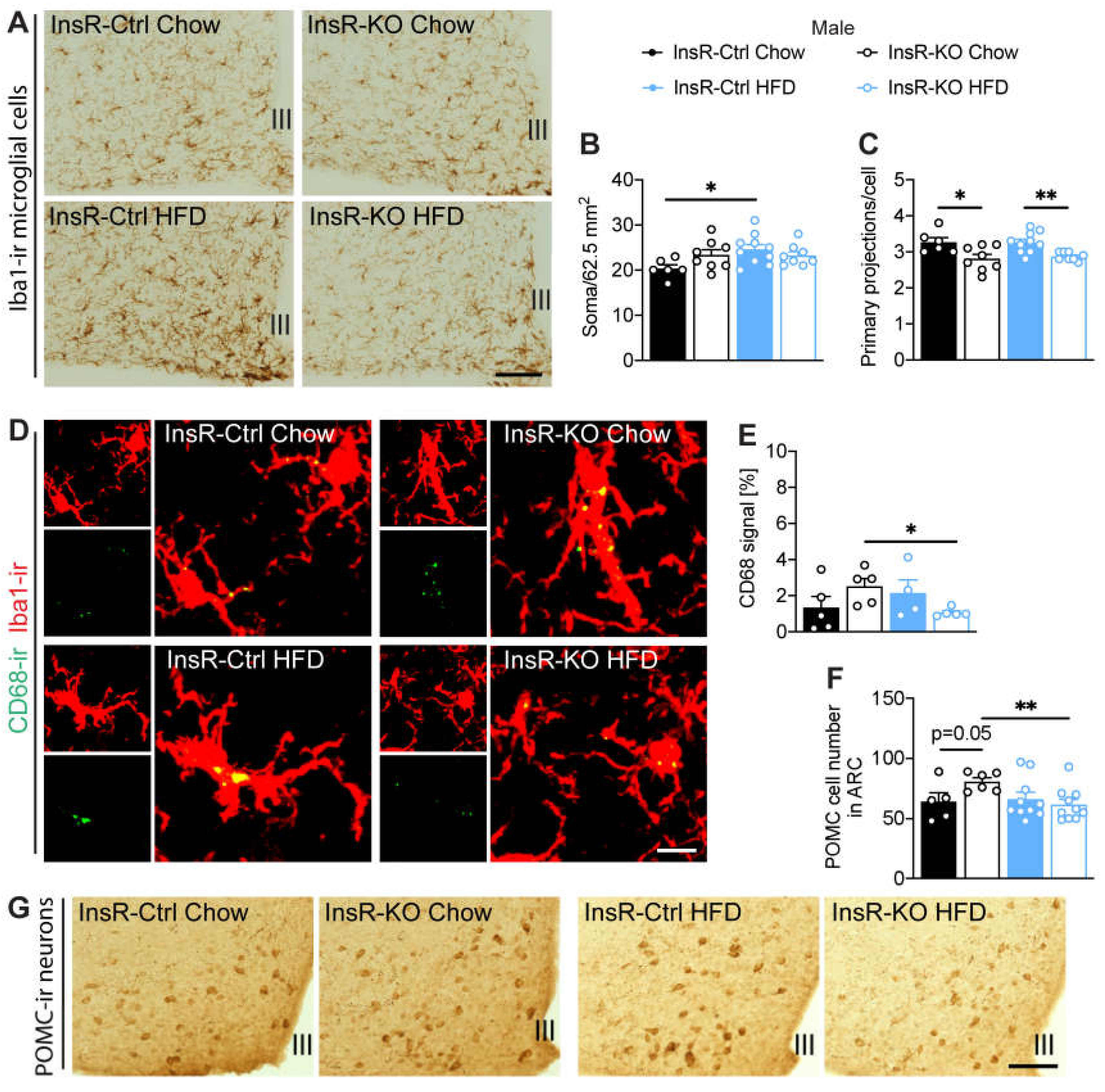
2.5. Hypothalamic Microglial Morphology and Phagocytic Capacity Are Dependent on Insulin Signaling in Male and Female Mice
2.6. InsR Loss in Microglial Cells Leads to a Decrease of POMC Neurons during Obesity in Male Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Primary Microglial Cell Culture
4.3. Western Blot
4.4. Metabolic Phenotyping
4.5. Insulin Tolerance Test
4.6. Insulin ELISA
4.7. PCR
4.8. Immunohistochemical and Immunofluorescent Staining
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Oussaada, S.M.; van Galen, K.A.; Cooiman, M.I.; Kleinendorst, L.; Hazebroek, E.J.; van Haelst, M.M.; ter Horst, K.W.; Serlie, M.J. The pathogenesis of obesity. Metabolism 2019, 92, 26–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central nervous system control of food intake and body weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Coll, A.P.; Farooqi, I.S.; O’Rahilly, S. The Hormonal Control of Food Intake. Cell 2007, 129, 251–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763. [Google Scholar] [CrossRef]
- Varela, L.; Horvath, T.L. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep. 2012, 13, 1079–1086. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Woods, S.C.; Porte, D.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef]
- Havrankova, J.; Roth, J.; Brownstein, M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature 1978, 272, 827–829. [Google Scholar] [CrossRef]
- Brüning, J.C.; Gautam, D.; Burks, D.J.; Gillette, J.; Schubert, M.; Orban, P.C.; Klein, R.; Krone, W.; Müller-Wieland, D.; Kahn, C.R. Role of brain insulin receptor in control of body weight and reproduction. Science 2000, 289, 2122–2125. [Google Scholar] [CrossRef]
- Obici, S.; Feng, Z.; Karkanias, G.; Baskin, D.G.; Rossetti, L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat. Neurosci. 2002, 5, 566–572. [Google Scholar] [CrossRef]
- McGowan, M.K.; Andrews, K.M.; Kelly, J.; Grossman, S.P. Effects of chronic intrahypothalamic infusion of insulin on food intake and diurnal meal patterning in the rat. Behav. Neurosci. 1990, 104, 373–385. [Google Scholar] [CrossRef]
- Benoit, S.C.; Air, E.L.; Coolen, L.M.; Strauss, R.; Jackman, A.; Clegg, D.J.; Seeley, R.J.; Woods, S.C. The catabolic action of insulin in the brain is mediated by melanocortins. J. Neurosci. 2002, 22, 9048–9052. [Google Scholar] [CrossRef]
- Choudhury, A.I.; Heffron, H.; Smith, M.A.; Al-Qassab, H.; Xu, A.W.; Selman, C.; Simmgen, M.; Clements, M.; Claret, M.; MacColl, G.; et al. The role of insulin receptor substrate 2 in hypothalamic and β cell function. J. Clin. Investig. 2005, 115, 940–950. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, M.W.; Sipols, A.J.; Marks, J.L.; Sanacora, G.; White, J.D.; Scheurink, A.; E Kahn, S.; Baskin, D.G.; Woods, S.C.; Figlewicz, D.P. Inhibition of hypothalamic neuropeptide Y gene expression by insulin. Endocrinology 1992, 130, 3608–3616. [Google Scholar] [CrossRef]
- Sipols, A.J.; Baskin, D.G.; Schwartz, M.W. Effect of intracerebroventricular insulin infusion on diabetic hyperphagia and hypothalamic neuropeptide gene expression. Diabetes 1995, 44, 147–151. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Broken Energy Homeostasis and Obesity Pathogenesis: The Surrounding Concepts. J. Clin. Med. 2018, 7, 453. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, A.S.; Belchior, T.; Lira, F.S.; Bishop, N.C.; Wessner, B.; Rosa, J.C.; Festuccia, W.T. Regulation of Metabolic Disease-Associated Inflammation by Nutrient Sensors. Mediat. Inflamm. 2018, 2018, 8261432. [Google Scholar] [CrossRef] [Green Version]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Morari, J.; Anhe, G.F.; Nascimento, L.F.; de Moura, R.F.; Razolli, D.; Solon, C.; Guadagnini, D.; Souza, G.; Mattos, A.H.; Tobar, N.; et al. Fractalkine (CX3CL1) is involved in the early activation of hypothalamic inflammation in experimental obesity. Diabetes 2014, 63, 3770–3784. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Bielohuby, M.; Fleming, T.; Grabner, G.F.; Foppen, E.; Bernhard, W.; Guzmán-Ruiz, M.; Layritz, C.; Legutko, B.; Zinser, E.; et al. Dietary sugars, not lipids, drive hypothalamic inflammation. Mol. Metab. 2017, 6, 897–908. [Google Scholar] [CrossRef]
- Gao, Y.; Ottaway, N.; Schriever, S.C.; Legutko, B.; García-Cáceres, C.; De La Fuente, E.; Mergen, C.; Bour, S.; Thaler, J.P.; Seeley, R.; et al. Hormones and diet, but not body weight, control hypothalamic microglial activity. Glia 2014, 62, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Valdearcos, M.; Robblee, M.M.; Benjamin, D.I.; Nomura, D.K.; Xu, A.W.; Koliwad, S.K. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014, 9, 2124–2138. [Google Scholar] [CrossRef] [Green Version]
- Valdearcos, M.; Douglass, J.D.; Robblee, M.M.; Dorfman, M.D.; Stifler, D.R.; Bennett, M.L.; Gerritse, I.; Fasnacht, R.; Barres, B.A.; Thaler, J.P.; et al. Microglial Inflammatory Signaling Orchestrates the Hypothalamic Immune Response to Dietary Excess and Mediates Obesity Susceptibility. Cell Metab. 2017, 26, 185–197.e3. [Google Scholar] [CrossRef] [Green Version]
- Thaler, J.P.; Yi, C.X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Borst, K.; Schwabenland, M.; Prinz, M. Microglia metabolism in health and disease. Neurochem. Int. 2019, 130, 104331. [Google Scholar] [CrossRef]
- Lindsay, J.S.; Manpreet, B.; Jonathan, P.L.; Douglas, G.W.; Andis, K. Insulin Modulates In Vitro Secretion of Cytokines and Cytotoxins by Human Glial Cells. Curr. Alzheimer Res. 2015, 12, 684–693. [Google Scholar]
- Brabazon, F.; Bermudez, S.; Shaughness, M.; Khayrullina, G.; Byrnes, K.R. The effects of insulin on the inflammatory activity of BV2 microglia. PLoS ONE 2018, 13, e0201878. [Google Scholar] [CrossRef] [Green Version]
- Geer, E.B.; Shen, W. Gender differences in insulin resistance, body composition, and energy balance. Gend. Med. 2009, 6 (Suppl. S1), 60–75. [Google Scholar] [CrossRef] [Green Version]
- Joksimovic, J.; Selakovic, D.; Jovicic, N.; Mitrovic, S.; Mihailovic, V.; Katanic, J.; Milovanovic, D.; Rosic, G. Exercise Attenuates Anabolic Steroids-Induced Anxiety via Hippocampal NPY and MC4 Receptor in Rats. Front. Neurosci. 2019, 13, 172. [Google Scholar] [CrossRef]
- Joksimovic, J.; Selakovic, D.; Matovic, M.; Zaletel, I.; Puskas, N.; Rosic, G. The role of neuropeptide-Y in nandrolone decanoate-induced attenuation of antidepressant effect of exercise. PLoS ONE 2017, 12, e0178922. [Google Scholar] [CrossRef]
- Quarta, C.; Fioramonti, X.; Cota, D. POMC Neurons Dysfunction in Diet-induced Metabolic Disease: Hallmark or Mechanism of Disease? Neuroscience 2020, 447, 3–14. [Google Scholar] [CrossRef] [PubMed]
- García-Cáceres, C.; Quarta, C.; Varela, L.; Gao, Y.; Gruber, T.; Legutko, B.; Jastroch, M.; Johansson, P.; Ninkovic, J.; Yi, C.-X.; et al. Astrocytic Insulin Signaling Couples Brain Glucose Uptake with Nutrient Availability. Cell 2016, 166, 867–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, L.H.; Warden, S.; Lenz, K.M. Sex differences in microglial phagocytosis in the neonatal hippocampus. Brain Behav. Immun. 2017, 64, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, E.; Guzzardi, M.A.; Panetta, D.; Tripodi, M.; De Sena, V.; Quaglierini, M.; Burchielli, S.; Salvadori, P.A.; Iozzo, P. Combined Effect of Fatty Diet and Cognitive Decline on Brain Metabolism, Food Intake, Body Weight, and Counteraction by Intranasal Insulin Therapy in 3×Tg Mice. Front. Cell. Neurosci. 2019, 13, 188. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Ma, D.; Wang, Y.; Jiang, T.; Hu, S.; Zhang, M.; Yu, X.; Gong, C.-X. Intranasal Insulin Ameliorates Tau Hyperphosphorylation in a Rat Model of Type 2 Diabetes. J. Alzheimer’s Dis. 2013, 33, 329–338. [Google Scholar] [CrossRef]
- Ramos-Rodriguez, J.J.; Sanchez-Sotano, D.; Doblas-Marquez, A.; Infante-Garcia, C.; Lubian-Lopez, S.; Garcia-Alloza, M. Intranasal insulin reverts central pathology and cognitive impairment in diabetic mother offspring. Mol. Neurodegener. 2017, 12, 57. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Vidal-Itriago, A.; Kalsbeek, M.J.; Layritz, C.; Garcia-Caceres, C.; Tom, R.Z.; Eichmann, T.O.; Vaz, F.M.; Houtkooper, R.H.; der Wel, N.; et al. Lipoprotein Lipase Maintains Microglial Innate Immunity in Obesity. Cell Rep. 2017, 20, 3034–3042. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-L.; Kooijman, S.; Gao, Y.; Tzeplaeff, L.; Cosquer, B.; Milanova, I.; Wolff, S.E.C.; Korpel, N.; Champy, M.-F.; Petit-Demoulière, B.; et al. Microglia-specific knock-down of Bmal1 improves memory and protects mice from high fat diet-induced obesity. Mol. Psychiatry 2021, 26, 6336–6349. [Google Scholar] [CrossRef]
- Qiu, J.; Bosch, M.A.; Meza, C.; Navarro, U.-V.; Nestor, C.C.; Wagner, E.J.; Rønnekleiv, O.K.; Kelly, M.J. Estradiol Protects Proopiomelanocortin Neurons Against Insulin Resistance. Endocrinology 2017, 159, 647–664. [Google Scholar] [CrossRef]
- Han, J.; Fan, Y.; Zhou, K.; Blomgren, K.; Harris, R.A. Uncovering sex differences of rodent microglia. J. Neuroinflammation 2021, 18, 74. [Google Scholar] [CrossRef]
- Milanova, I.V.; Correa-da-Silva, F.; Kalsbeek, A.; Yi, C.-X. Mapping of Microglial Brain Region, Sex and Age Heterogeneity in Obesity. Int. J. Mol. Sci. 2021, 22, 3141. [Google Scholar] [CrossRef]
- Dorfman, M.D.; Krull, J.E.; Douglass, J.D.; Fasnacht, R.; Lara-Lince, F.; Meek, T.H.; Shi, X.; Damian, V.; Nguyen, H.T.; Matsen, M.E.; et al. Sex differences in microglial CX3CR1 signalling determine obesity susceptibility in mice. Nat. Commun. 2017, 8, 14556. [Google Scholar] [CrossRef] [Green Version]
- Mendez, P.; Wandosell, F.; Garcia-Segura, L.M. Cross-talk between estrogen receptors and insulin-like growth factor-I receptor in the brain: Cellular and molecular mechanisms. Front. Neuroendocrinol. 2006, 27, 391–403. [Google Scholar] [CrossRef] [Green Version]
- Kodama, L.; Gan, L. Do Microglial Sex Differences Contribute to Sex Differences in Neurodegenerative Diseases? Trends Mol. Med. 2019, 25, 741–749. [Google Scholar] [CrossRef]
- Brüning, J.C.; Michael, M.; Winnay, J.N.; Hayashi, T.; Hörsch, D.; Accili, D.; Goodyear, L.J.; Kahn, C. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol. Cell 1998, 2, 559–569. [Google Scholar] [CrossRef]
- Yona, S.; Kim, K.W.; Wolf, Y.; Mildner, A.; Varol, D.; Breker, M.; Strauss-Ayali, D.; Viukov, S.; Guilliams, M.; Misharin, A.; et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013, 38, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milanova, I.V.; Korpel, N.L.; Correa-da-Silva, F.; Berends, E.; Osman, S.; la Fleur, S.E.; Fliers, E.; Kalsbeek, A.; Yi, C.-X. Loss of Microglial Insulin Receptor Leads to Sex-Dependent Metabolic Disorders in Obese Mice. Int. J. Mol. Sci. 2022, 23, 2933. https://doi.org/10.3390/ijms23062933
Milanova IV, Korpel NL, Correa-da-Silva F, Berends E, Osman S, la Fleur SE, Fliers E, Kalsbeek A, Yi C-X. Loss of Microglial Insulin Receptor Leads to Sex-Dependent Metabolic Disorders in Obese Mice. International Journal of Molecular Sciences. 2022; 23(6):2933. https://doi.org/10.3390/ijms23062933
Chicago/Turabian StyleMilanova, Irina V., Nikita L. Korpel, Felipe Correa-da-Silva, Eline Berends, Samar Osman, Susanne E. la Fleur, Eric Fliers, Andries Kalsbeek, and Chun-Xia Yi. 2022. "Loss of Microglial Insulin Receptor Leads to Sex-Dependent Metabolic Disorders in Obese Mice" International Journal of Molecular Sciences 23, no. 6: 2933. https://doi.org/10.3390/ijms23062933
APA StyleMilanova, I. V., Korpel, N. L., Correa-da-Silva, F., Berends, E., Osman, S., la Fleur, S. E., Fliers, E., Kalsbeek, A., & Yi, C.-X. (2022). Loss of Microglial Insulin Receptor Leads to Sex-Dependent Metabolic Disorders in Obese Mice. International Journal of Molecular Sciences, 23(6), 2933. https://doi.org/10.3390/ijms23062933






