Targeting Endothelial Connexin37 Reduces Angiogenesis and Decreases Tumor Growth
Abstract
1. Introduction
2. Results
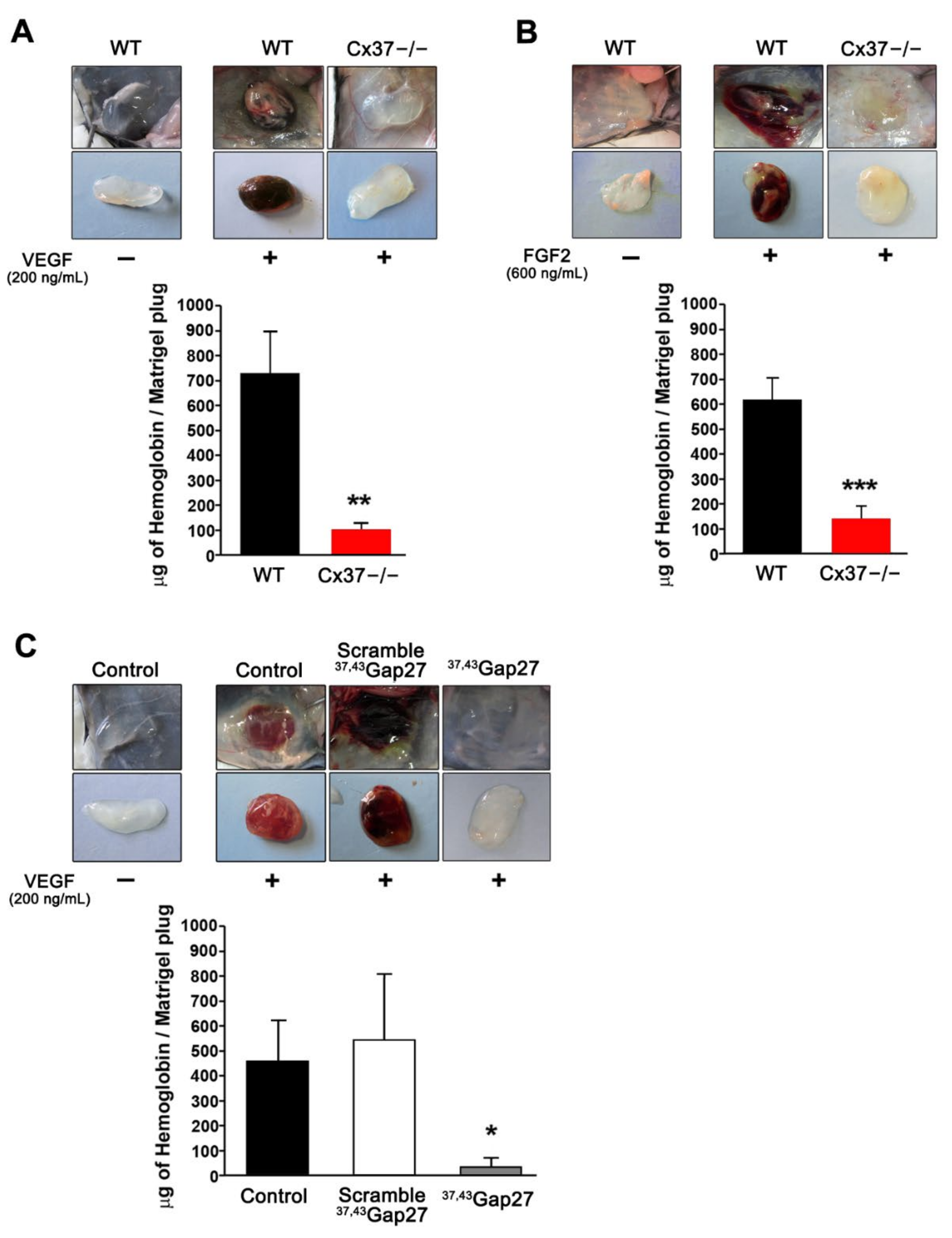
2.1. Loss of Cx37 Decreases Angiogenesis
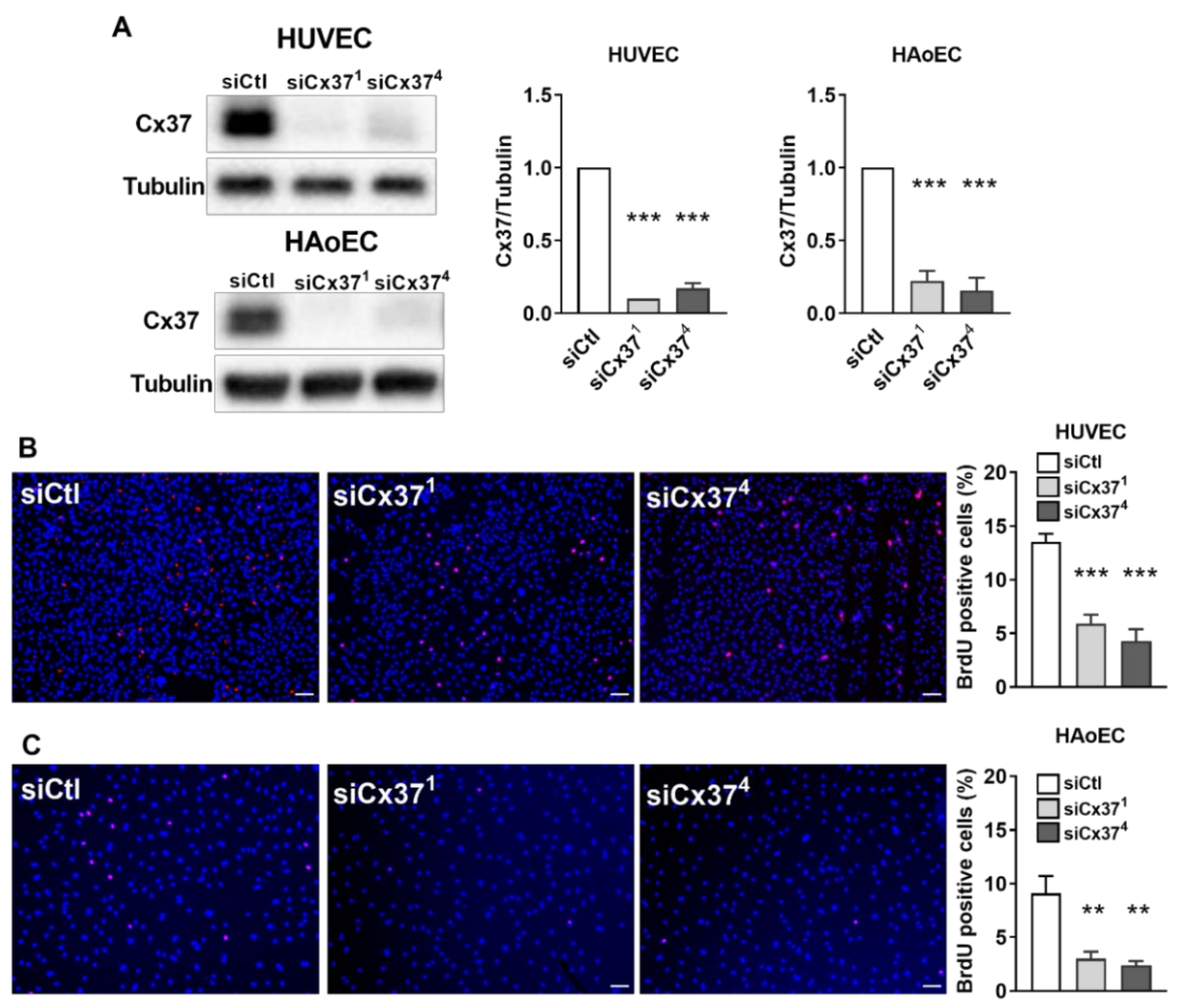
2.2. Silencing Cx37 Reduces EC Proliferation
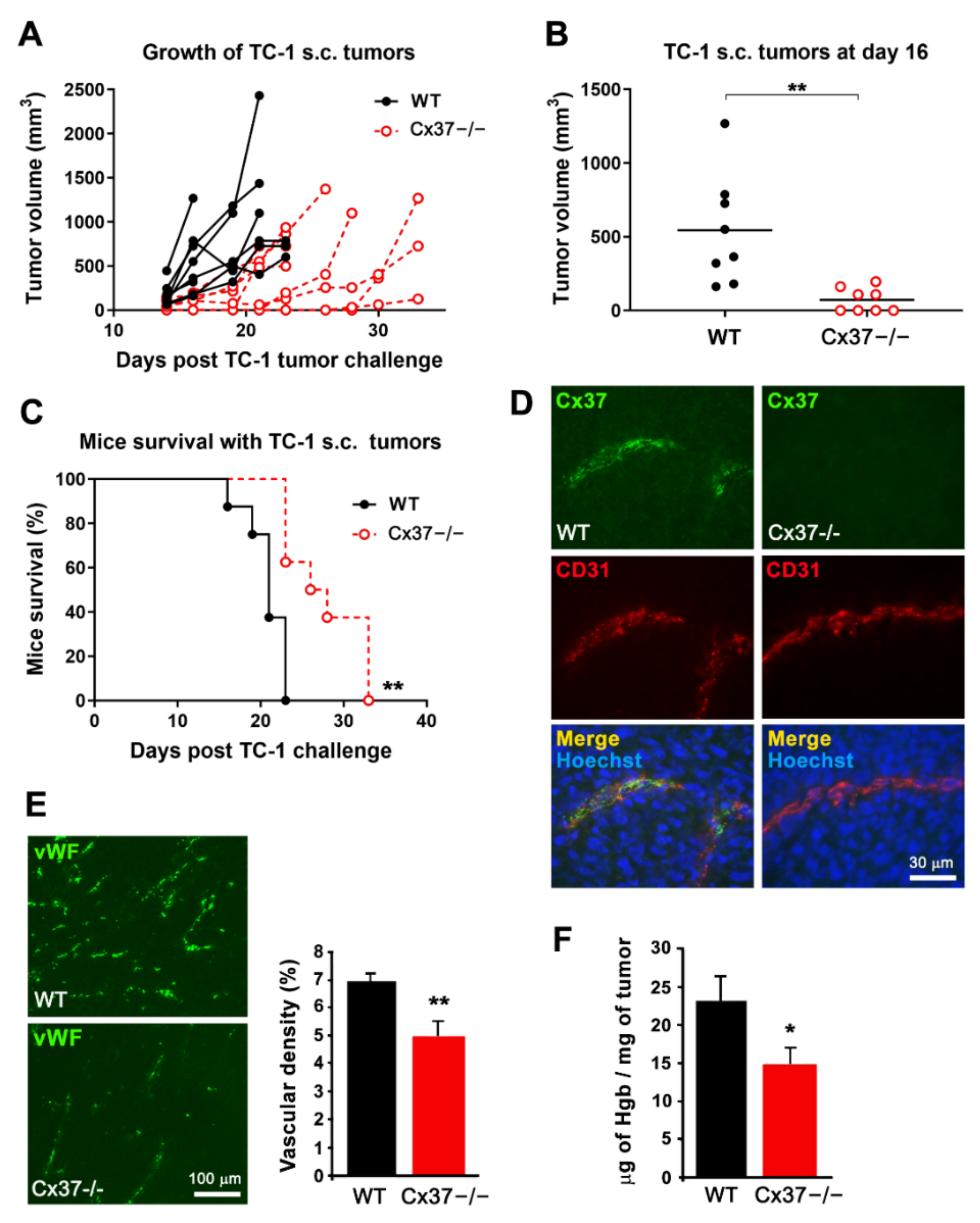
2.3. Loss of Cx37 Decreases the Growth and the Vascularization of Tumors
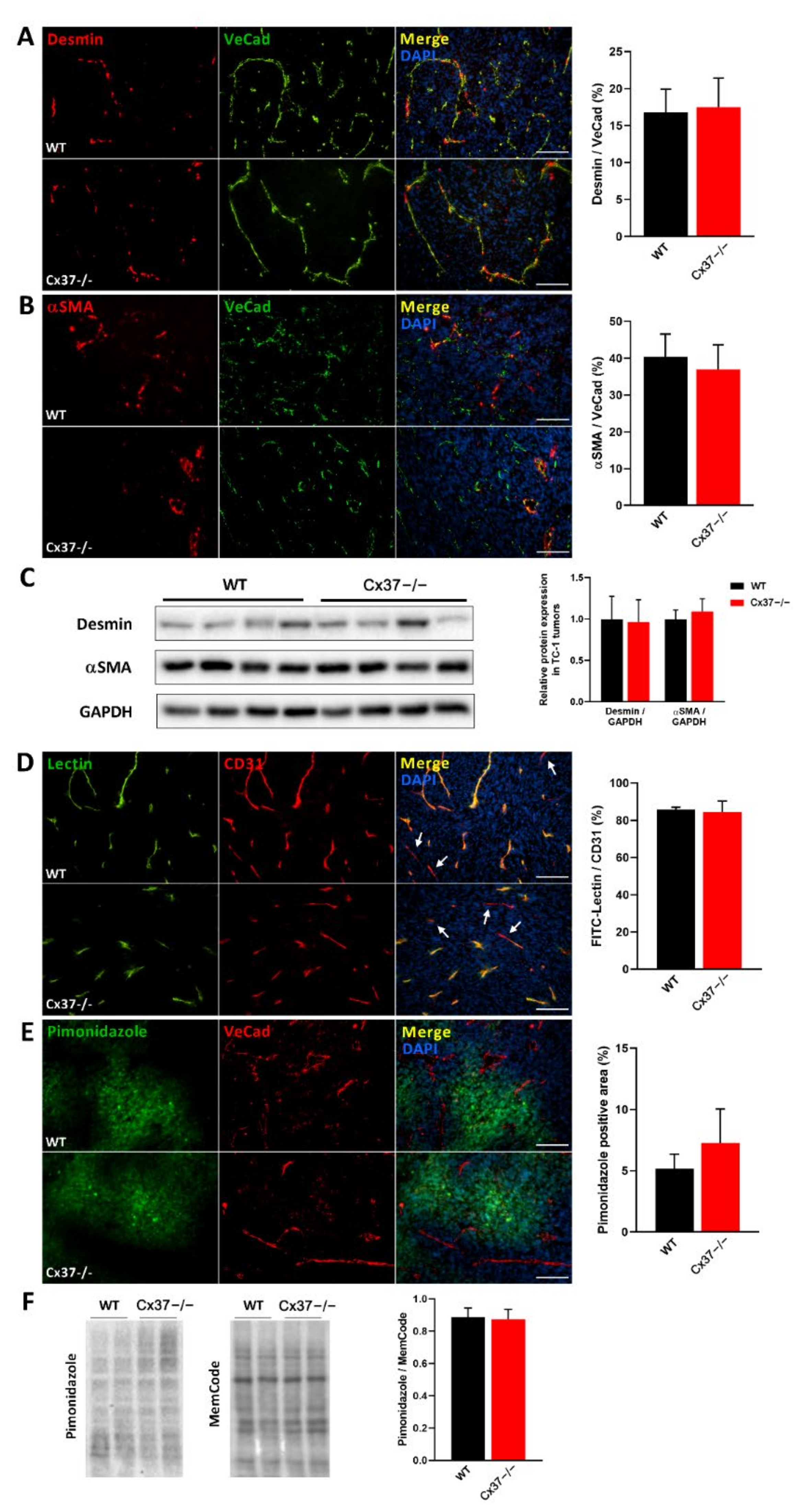
2.4. Loss of Cx37 Does Not Alter the Perfusion and the Maturation of Vessels in theTC-1 Tumors
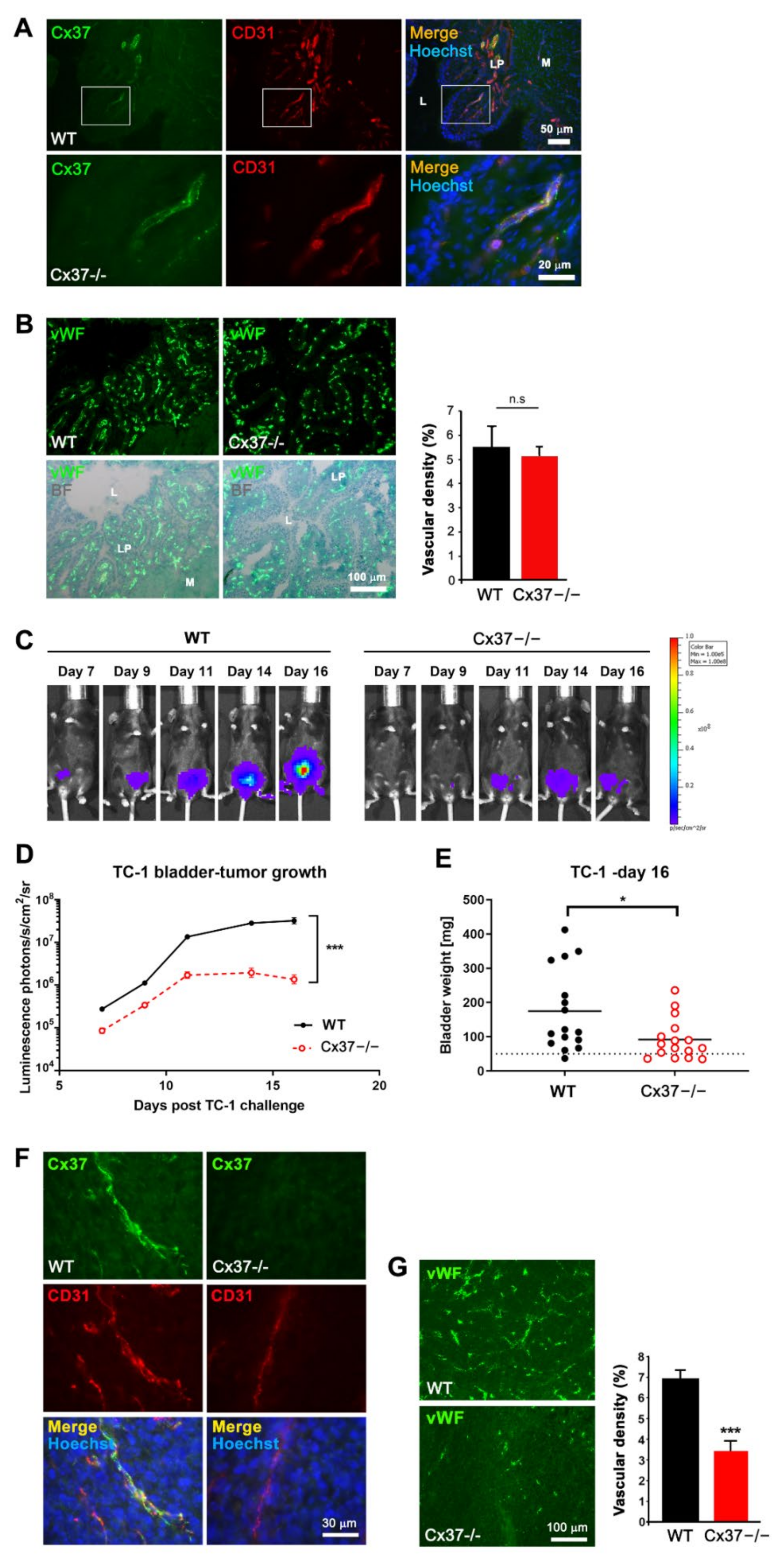
2.5. Loss of Cx37 Decreases Angiogenesis of TC-1 Tumors Induced within the Bladder
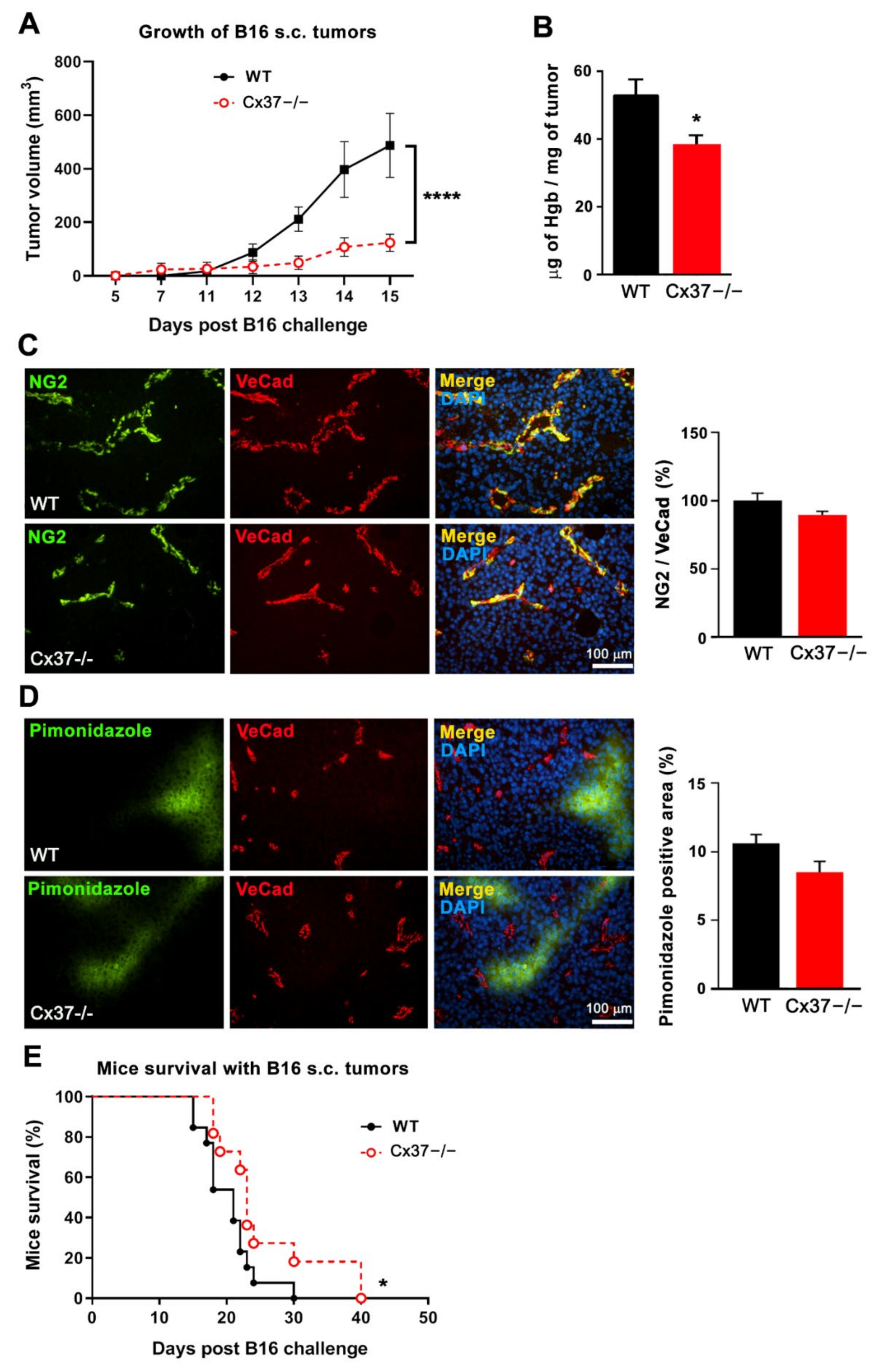
2.6. Loss of Cx37 Decreases the Growth and Angiogenesis of B16-F10 Tumors
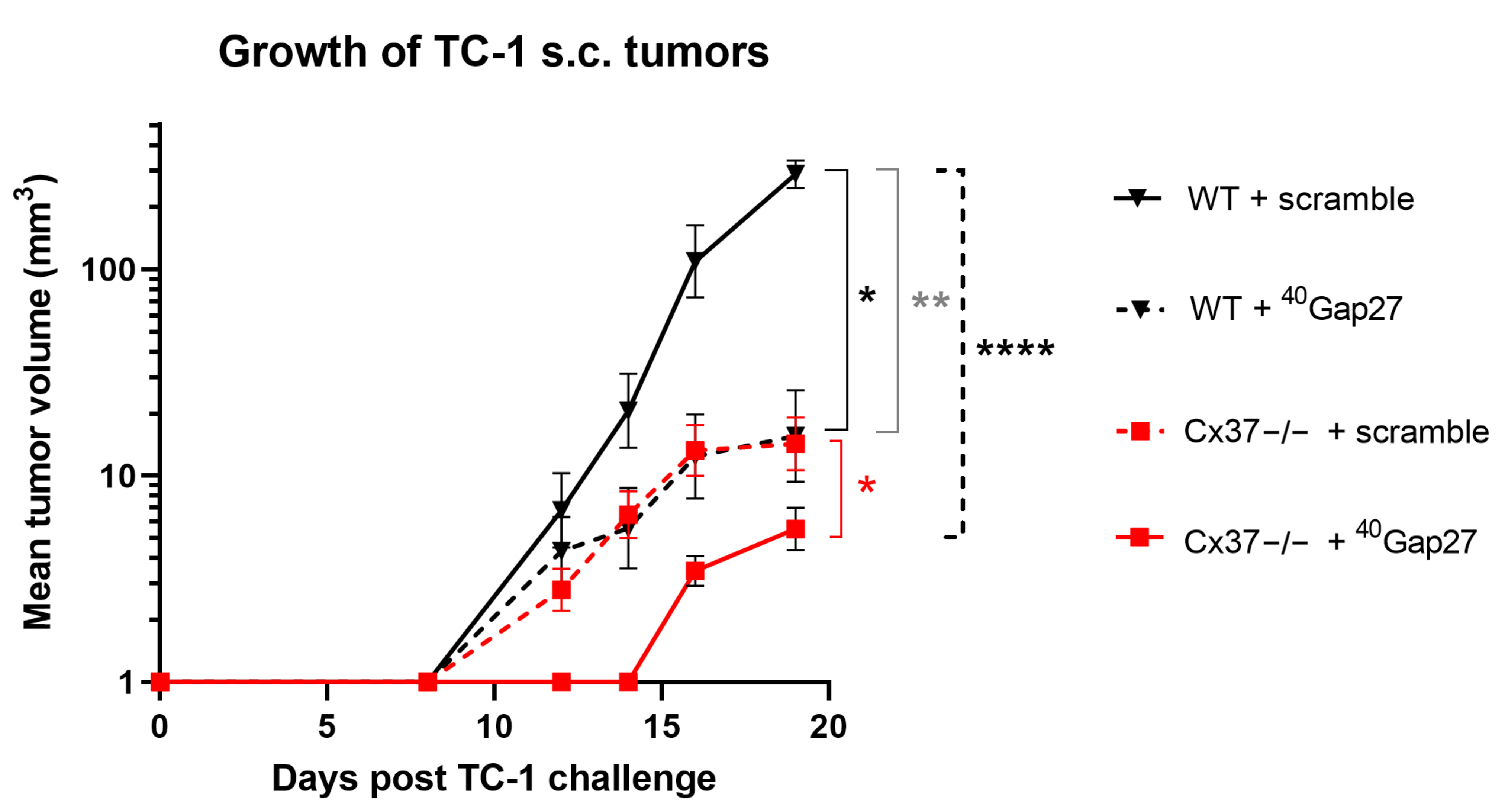
2.7. A Peptide Targeting Cx40 Decreases Tumoral Growth in Cx37−/− Mice
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Transfections
4.2. Connexin-Mimetic Peptides
4.3. Animals
4.4. Tumor Models
4.5. Matrigel Plug Assay
4.6. Immunostaining
4.7. Hemoglobin Content
4.8. Tumor Perfusion and Hypoxia Assays
4.9. Protein Analysis
4.10. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Li, Y.; Lu, W.; Chen, M.; Ye, W.; Zhang, D. The Tumor Vessel Targeting Strategy: A Double-Edged Sword in Tumor Metastasis. Cells 2019, 8, 1602. [Google Scholar] [CrossRef]
- Chung, A.S.; Ferrara, N. Developmental and pathological angiogenesis. Annu. Rev. Cell Dev. Biol. 2011, 27, 563–584. [Google Scholar] [CrossRef]
- Goel, S.; Duda, D.G.; Xu, L.; Munn, L.L.; Boucher, Y.; Fukumura, D.; Jain, R.K. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 2011, 91, 1071–1121. [Google Scholar] [CrossRef]
- Tahergorabi, Z.; Khazaei, M. Imbalance of angiogenesis in diabetic complications: The mechanisms. Int. J. Prev. Med. 2012, 3, 827–838. [Google Scholar] [CrossRef]
- Nohata, N.; Uchida, Y.; Stratman, A.N.; Adams, R.H.; Zheng, Y.; Weinstein, B.M.; Mukouyama, Y.-S.; Gutkind, J.S. Temporal-specific roles of Rac1 during vascular development and retinal angiogenesis. Dev. Biol. 2016, 411, 183–194. [Google Scholar] [CrossRef]
- Campochiaro, P.A. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog. Retin. Eye Res. 2015, 49, 67–81. [Google Scholar] [CrossRef]
- Cabral, T.; Mello, L.G.M.; Lima, L.H.; Polido, J.; Regatieri, C.V.; Belfort, R., Jr.; Mahajan, V.B. Retinal and choroidal angiogenesis: A review of new targets. Int. J. Retin. Vitreous. 2017, 3, 1. [Google Scholar] [CrossRef]
- Phng, L.K.; Gerhardt, H. Angiogenesis: A team effort coordinated by notch. Dev. Cell 2009, 16, 196–208. [Google Scholar] [CrossRef]
- Rohlenova, K.; Veys, K.; Miranda-Santos, I.; De Bock, K.; Carmeliet, P. Endothelial Cell Metabolism in Health and Disease. Trends Cell Biol. 2018, 28, 224–236. [Google Scholar] [CrossRef]
- Gaengel, K.; Genove, G.; Armulik, A.; Betsholtz, C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 630–638. [Google Scholar] [CrossRef]
- Cuervo, H.; Pereira, B.; Nadeem, T.; Lin, M.; Lee, F.; Kitajewski, J.; Lin, C.S. PDGFRbeta-P2A-CreER(T2) mice: A genetic tool to target pericytes in angiogenesis. Angiogenesis 2017, 20, 655–662. [Google Scholar] [CrossRef]
- Siemann, D.W. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by Tumor-Vascular Disrupting Agents. Cancer Treat. Rev. 2011, 37, 63–74. [Google Scholar] [CrossRef]
- Baluk, P.; Hashizume, H.; McDonald, D.M. Cellular abnormalities of blood vessels as targets in cancer. Curr. Opin. Genet. Dev. 2005, 15, 102–111. [Google Scholar] [CrossRef]
- Belotti, D.; Pinessi, D.; Taraboletti, G. Alternative Vascularization Mechanisms in Tumor Resistance to Therapy. Cancers 2021, 13, 1912. [Google Scholar] [CrossRef]
- Zheng, R.; Li, F.; Li, F.; Gong, A. Targeting tumor vascularization: Promising strategies for vascular normalization. J. Cancer Res. Clin. Oncol. 2021, 147, 2489–2505. [Google Scholar] [CrossRef]
- Meda, P.; Haefliger, J.A. Connexins and pannexins: From biology towards clinical targets. Swiss Med. Wkly. 2016, 146, w14365. [Google Scholar] [CrossRef]
- Bosco, D.; Haefliger, J.A.; Meda, P. Connexins: Key mediators of endocrine function. Physiol. Rev. 2011, 91, 1393–1445. [Google Scholar] [CrossRef]
- Meens, M.J.; Kwak, B.R.; Duffy, H.S. Role of connexins and pannexins in cardiovascular physiology. Cell. Mol. Life Sci. 2015, 72, 2779–2792. [Google Scholar] [CrossRef]
- Meens, M.J.; Pfenniger, A.; Kwak, B.R.; Delmar, M. Regulation of cardiovascular connexins by mechanical forces and junctions. Cardiovasc. Res. 2013, 99, 304–314. [Google Scholar] [CrossRef]
- Alonso, F.; Krattinger, N.; Mazzolai, L.; Simon, A.; Waeber, G.; Meda, P.; Haefliger, J.-A. An angiotensin II- and NF-kappaB-dependent mechanism increases connexin 43 in murine arteries targeted by renin-dependent hypertension. Cardiovasc. Res. 2010, 87, 166–176. [Google Scholar] [CrossRef]
- Meens, M.J.; Alonso, F.; Le Gal, L.; Kwak, B.R.; Haefliger, J.A. Endothelial Connexin37 and Connexin40 participate in basal but not agonist-induced NO release. Cell. Commun. Signal 2015, 13, 34. [Google Scholar] [CrossRef]
- Le Gal, L.; Alonso, F.; Mazzolai, L.; Meda, P.; Haefliger, J.A. Interplay between connexin40 and nitric oxide signaling during hypertension. Hypertension 2015, 65, 910–915. [Google Scholar] [CrossRef]
- Le Gal, L.; Alonso, F.; Wagner, C.; Germain, S.; Haefliger, D.N.; Meda, P.; Meda, P.; Haefliger, J. Restoration of Connexin 40 (Cx40) in Renin-Producing Cells Reduces the Hypertension of Cx40 Null Mice. Hypertension 2014, 63, 1198–1204. [Google Scholar] [CrossRef][Green Version]
- Boittin, F.X.; Alonso, F.; Le Gal, L.; Allagnat, F.; Beny, J.L.; Haefliger, J.A. Connexins and M3 muscarinic receptors contribute to heterogeneous Ca(2+) signaling in mouse aortic endothelium. Cell. Physiol. Biochem. 2013, 31, 166–178. [Google Scholar] [CrossRef]
- Jobs, A.; Schmidt, K.; Schmidt, V.J.; Lübkemeier, I.; Van Veen, T.A.; Kurtz, A.; Willecke, K.; De Wit, C. Defective Cx40 maintains Cx37 expression but intact Cx40 is crucial for conducted dilations irrespective of hypertension. Hypertension 2012, 60, 1422–1429. [Google Scholar] [CrossRef]
- Simon, A.M.; McWhorter, A.R. Vascular abnormalities in mice lacking the endothelial gap junction proteins connexin37 and connexin40. Dev. Biol. 2002, 251, 206–220. [Google Scholar] [CrossRef]
- Simon, A.M.; McWhorter, A.R. Decreased intercellular dye-transfer and downregulation of non-ablated connexins in aortic endothelium deficient in connexin37 or connexin40. J. Cell. Sci. 2003, 116, 2223–2236. [Google Scholar] [CrossRef]
- Alonso, F.; Domingos-Pereira, S.; Le Gal, L.; Derré, L.; Meda, P.; Jichlinski, P.; Nardelli-Haefliger, D.; Haefliger, J.-A. Targeting endothelial connexin40 inhibits tumor growth by reducing angiogenesis and improving vessel perfusion. Oncotarget 2016, 7, 14015–14028. [Google Scholar] [CrossRef]
- Haefliger, J.-A.; Allagnat, F.; Hamard, L.; Le Gal, L.; Meda, P.; Nardelli-Haefliger, D.; Génot, E.; Alonso, F. Targeting Cx40 (Connexin40) Expression or Function Reduces Angiogenesis in the Developing Mouse Retina. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2136–2146. [Google Scholar] [CrossRef]
- Hamard, L.; Santoro, T.; Allagnat, F.; Meda, P.; Nardelli-Haefliger, D.; Alonso, F.; Haefliger, J. Targeting connexin37 alters angiogenesis and arteriovenous differentiation in the developing mouse retina. FASEB J. 2020, 34, 8234–8249. [Google Scholar] [CrossRef]
- Haefliger, J.A.; Meda, P.; Alonso, F. Endothelial Connexins in Developmental and Pathological Angiogenesis. Cold Spring Harb. Perspect. Med. 2022, a041158. [Google Scholar] [CrossRef] [PubMed]
- Hadizadeh, M.; Mohaddes Ardebili, S.M.; Salehi, M.; Young, C.; Mokarian, F.; McClellan, J.; Xu, Q.; Kazemi, M.; Moazam, E.; Mahak, B.; et al. GJA4/Connexin 37 Mutations Correlate with Secondary Lymphedema Following Surgery in Breast Cancer Patients. Biomedicines 2018, 6, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Ugwu, N.; Atzmony, L.; Ellis, K.T.; Panse, G.; Jain, D.; Ko, C.J.; Nassiri, N.; Choate, K.A. Cutaneous and hepatic vascular lesions due to a recurrent somatic GJA4 mutation reveal a pathway for vascular malformation. HGG Adv. 2021, 2, 100028. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.M.; Goodenough, D.A.; Li, E.; Paul, D.L. Female infertility in mice lacking connexin 37. Nature 1997, 385, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Le Gal, L.; Pellegrin, M.; Santoro, T.; Mazzolai, L.; Kurtz, A.; Meda, P.; Wagner, C.; Haefliger, J. Connexin37-Dependent Mechanisms Selectively Contribute to Modulate Angiotensin II -Mediated Hypertension. J. Am. Heart Assoc. 2019, 8, e010823. [Google Scholar] [CrossRef]
- Pacheco-Costa, R.; Kadakia, J.R.; Atkinson, E.G.; Wallace, J.M.; Plotkin, L.I.; Reginato, R.D. Connexin37 deficiency alters organic bone matrix, cortical bone geometry, and increases Wnt/beta-catenin signaling. Bone 2017, 97, 105–113. [Google Scholar] [CrossRef]
- Pacheco-Costa, R.; Hassan, I.; Reginato, R.; Davis, H.M.; Bruzzaniti, A.; Allen, M.R.; Plotkin, L.I. High bone mass in mice lacking Cx37 because of defective osteoclast differentiation. J. Biol. Chem. 2014, 289, 8508–8520. [Google Scholar] [CrossRef]
- Allagnat, F.; Dubuis, C.; Lambelet, M.; Le Gal, L.; Alonso, F.; Corpataux, J.M.; Sébastien, D.; Haefliger, J.-A. Connexin37 reduces smooth muscle cell proliferation and intimal hyperplasia in a mouse model of carotid artery ligation. Cardiovasc. Res. 2017, 113, 805–816. [Google Scholar] [CrossRef]
- Chaytor, A.T.; Martin, P.E.; Evans, W.H.; Randall, M.D.; Griffith, T.M. The endothelial component of cannabinoid-induced relaxation in rabbit mesenteric artery depends on gap junctional communication. J. Physiol. 1999, 520, 539–550. [Google Scholar] [CrossRef]
- Vaiyapuri, S.; Moraes, L.A.; Sage, T.; Ali, M.S.; Lewis, K.R.; Mahaut-Smith, M.P.; Oviedo-Orta, E.; Simon, A.M.; Gibbins, J.M. Connexin40 regulates platelet function. Nat. Commun. 2013, 4, 2564. [Google Scholar] [CrossRef]
- Bol, M.; Van Geyt, C.; Baert, S.; Decrock, E.; Wang, N.; De Bock, M.; Gadicherla, A.K.; Randon, C.; Evans, W.H.; Beele, H.; et al. Inhibiting connexin channels protects against cryopreservation-induced cell death in human blood vessels. Eur. J. Vasc. Endovasc. Surg. 2013, 45, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Gartner, C.; Ziegelhoffer, B.; Kostelka, M.; Stepan, H.; Mohr, F.W.; Dhein, S. Knock-down of endothelial connexins impairs angiogenesis. Pharmacol. Res. 2012, 65, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Tahergorabi, Z.; Khazaei, M. A review on angiogenesis and its assays. Iran J. Basic Med. Sci. 2012, 15, 1110–1126. [Google Scholar] [PubMed]
- Cai, J.; Kehoe, O.; Smith, G.M.; Hykin, P.; Boulton, M.E. The angiopoietin/Tie-2 system regulates pericyte survival and recruitment in diabetic retinopathy. Invest Ophthalmol. Vis. Sci. 2008, 49, 2163–2171. [Google Scholar] [CrossRef]
- Ito, A.; Katoh, F.; Kataoka, T.R.; Okada, M.; Tsubota, N.; Asada, H.; Yoshikawa, K.; Maeda, S.; Kitamura, Y.; Yamasaki, H.; et al. A role for heterologous gap junctions between melanoma and endothelial cells in metastasis. J. Clin. Invest 2000, 105, 1189–1197. [Google Scholar] [CrossRef]
- Alonso, F.; Boittin, F.X.; Beny, J.L.; Haefliger, J.A. Loss of connexin40 is associated with decreased endothelium-dependent relaxations and eNOS levels in the mouse aorta. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1365–H1373. [Google Scholar] [CrossRef]
- Duan, C.Y.; Chen, K.; Yang, G.M.; Li, T.; Liu, L.M. HIF-1 alpha regulates Cx40-dependent vasodilatation following hemorrhagic shock in rats. Am. J. Transl. Res. 2017, 9, 1277–1286. [Google Scholar]
- Hariprabu, K.N.G.; Sathya, M.; Vimalraj, S. CRISPR/Cas9 in cancer therapy: A review with a special focus on tumor angiogenesis. Int. J. Biol. Macromol. 2021, 192, 913–930. [Google Scholar] [CrossRef]
- Van Campenhout, R.; Cooreman, A.; Leroy, K.; Rusiecka, O.M.; Van Brantegem, P.; Annaert, P.; Muyldermans, S.; Devoogdt, N.; Cogliati, B.; Kwak, B.R.; et al. Non-canonical roles of connexins. Prog. Biophys. Mol. Biol. 2020, 153, 35–41. [Google Scholar] [CrossRef]
- Okamoto, T.; Usuda, H.; Tanaka, T.; Wada, K.; Shimaoka, M. The Functional Implications of Endothelial Gap Junctions and Cellular Mechanics in Vascular Angiogenesis. Cancers 2019, 11, 237. [Google Scholar] [CrossRef]
- Hautefort, A.; Pfenniger, A.; Kwak, B.R. Endothelial connexins in vascular function. Vasc. Biol. 2019, 1, H117–H124. [Google Scholar] [CrossRef] [PubMed]
- Zecchin, A.; Kalucka, J.; Dubois, C.; Carmeliet, P. How Endothelial Cells Adapt Their Metabolism to Form Vessels in Tumors. Front. Immunol. 2017, 8, 1750. [Google Scholar] [CrossRef] [PubMed]
- Dubuis, C.; May, L.; Alonso, F.; Luca, L.; Mylonaki, I.; Meda, P.; Delie, F.; Jordan, O.; Déglise, S.; Corpataux, J.-M.; et al. Atorvastatin-loaded hydrogel affects the smooth muscle cells of human veins. J. Pharmacol. Exp. Ther. 2013, 347, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Longchamp, A.; Allagnat, F.; Alonso, F.; Kuppler, C.; Dubuis, C.; Ozaki, C.-K.; Mitchell, J.R.; Berceli, S.; Corpataux, J.-M.; Déglise, S.; et al. Connexin43 Inhibition Prevents Human Vein Grafts Intimal Hyperplasia. PLoS ONE 2015, 10, e0138847. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Matchkov, V.V.; Rahman, A.; Peng, H.; Nilsson, H.; Aalkjaer, C. Junctional and nonjunctional effects of heptanol and glycyrrhetinic acid derivates in rat mesenteric small arteries. Br. J. Pharmacol. 2004, 142, 961–972. [Google Scholar] [CrossRef]
- Griffith, T.M. Endothelium-dependent smooth muscle hyperpolarization: Do gap junctions provide a unifying hypothesis? Br. J. Pharmacol. 2004, 141, 881–903. [Google Scholar] [CrossRef]
- Chaytor, A.T.; Martin, P.E.; Edwards, D.H.; Griffith, T.M. Gap junctional communication underpins EDHF-type relaxations evoked by ACh in the rat hepatic artery. Am. J. Physiol. 2001, 280, H2441–H2450. [Google Scholar] [CrossRef]
- Martin, P.E.; Wall, C.; Griffith, T.M. Effects of connexin-mimetic peptides on gap junction functionality and connexin expression in cultured vascular cells. Br. J. Pharmacol. 2005, 144, 617–627. [Google Scholar] [CrossRef]
- Lang, N.N.; Luksha, L.; Newby, D.E.; Kublickiene, K. Connexin 43 mediates endothelium-derived hyperpolarizing factor-induced vasodilatation in subcutaneous resistance arteries from healthy pregnant women. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1026–H1032. [Google Scholar] [CrossRef][Green Version]
- De Bock, M.; Culot, M.; Wang, N.; Bol, M.; Decrock, E.; De Vuyst, E.; da Costa, A.; Dauwe, I.; Vinken, M.; Simon, A.M.; et al. Connexin channels provide a target to manipulate brain endothelial calcium dynamics and blood-brain barrier permeability. J. Cereb. Blood Flow Metab. 2011, 31, 1942–1957. [Google Scholar] [CrossRef] [PubMed]
- Chaytor, A.T.; Bakker, L.M.; Edwards, D.H.; Griffith, T.M. Connexin-mimetic peptides dissociate electrotonic EDHF-type signalling via myoendothelial and smooth muscle gap junctions in the rabbit iliac artery. Br. J. Pharmacol. 2005, 144, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Peacock, H.M.; Tabibian, A.; Criem, N.; Caolo, V.; Hamard, L.; Deryckere, A.; Haefliger, J.-A.; Brenda, R.K.; Zwijsen, A.; Jones, E.A.V.; et al. Impaired SMAD1/5 Mechanotransduction and Cx37 (Connexin37) Expression Enable Pathological Vessel Enlargement and Shunting. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e87–e104. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.Y.; Guarnieri, F.G.; Staveley-O’Carroll, K.F.; Levitsky, H.I.; August, J.T.; Pardoll, D.M.; Wu, T.C. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996, 56, 21–26. [Google Scholar] [PubMed]
- Overwijk, W.W.; Tsung, A.; Irvine, K.R.; Parkhurst, M.R.; Goletz, T.J.; Tsung, K.; Miles, W.; Chunlei, L.; Bernard, M.; Rosenberg, S.A.; et al. gp100/pmel 17 is a murine tumor rejection antigen: Induction of self-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J. Exp. Med. 1998, 188, 277–286. [Google Scholar] [CrossRef]
- Domingos-Pereira, S.; Derré, L.; Warpelin-Decrausaz, L.; Haefliger, J.-A.; Romero, P.; Jichlinski, P.; Nardelli-Haefliger, D. Intravaginal and subcutaneous immunization induced vaccine specific CD8 T cells, and tumor regression in the bladder. J. Urol. 2014, 191, 814–822. [Google Scholar] [CrossRef]
- Domingos-Pereira, S.; Cesson, V.; Chevalier, M.F.; Derre, L.; Jichlinski, P.; Nardelli-Haefliger, D. Preclinical efficacy and safety of the Ty21a vaccine strain for intravesical immunotherapy of non-muscle-invasive bladder cancer. Oncoimmunology 2017, 6, e1265720. [Google Scholar] [CrossRef]
- Peng, S.; Lyford-Pike, S.; Akpeng, B.; Wu, A.; Hung, C.-F.; Hannaman, D.; Saunders, J.R.; Wu, T.-C.; Pai, S.I. Low-dose cyclophosphamide administered as daily or single dose enhances the antitumor effects of a therapeutic HPV vaccine. Cancer Immunol. Immunother. 2013, 62, 171–182. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sathiyanadan, K.; Alonso, F.; Domingos-Pereira, S.; Santoro, T.; Hamard, L.; Cesson, V.; Meda, P.; Nardelli-Haefliger, D.; Haefliger, J.-A. Targeting Endothelial Connexin37 Reduces Angiogenesis and Decreases Tumor Growth. Int. J. Mol. Sci. 2022, 23, 2930. https://doi.org/10.3390/ijms23062930
Sathiyanadan K, Alonso F, Domingos-Pereira S, Santoro T, Hamard L, Cesson V, Meda P, Nardelli-Haefliger D, Haefliger J-A. Targeting Endothelial Connexin37 Reduces Angiogenesis and Decreases Tumor Growth. International Journal of Molecular Sciences. 2022; 23(6):2930. https://doi.org/10.3390/ijms23062930
Chicago/Turabian StyleSathiyanadan, Karthik, Florian Alonso, Sonia Domingos-Pereira, Tania Santoro, Lauriane Hamard, Valérie Cesson, Paolo Meda, Denise Nardelli-Haefliger, and Jacques-Antoine Haefliger. 2022. "Targeting Endothelial Connexin37 Reduces Angiogenesis and Decreases Tumor Growth" International Journal of Molecular Sciences 23, no. 6: 2930. https://doi.org/10.3390/ijms23062930
APA StyleSathiyanadan, K., Alonso, F., Domingos-Pereira, S., Santoro, T., Hamard, L., Cesson, V., Meda, P., Nardelli-Haefliger, D., & Haefliger, J.-A. (2022). Targeting Endothelial Connexin37 Reduces Angiogenesis and Decreases Tumor Growth. International Journal of Molecular Sciences, 23(6), 2930. https://doi.org/10.3390/ijms23062930





