Expression of Aquaglyceroporins in Spermatozoa from Wild Ruminants Is Influenced by Photoperiod and Thyroxine Concentrations
Abstract
:1. Introduction
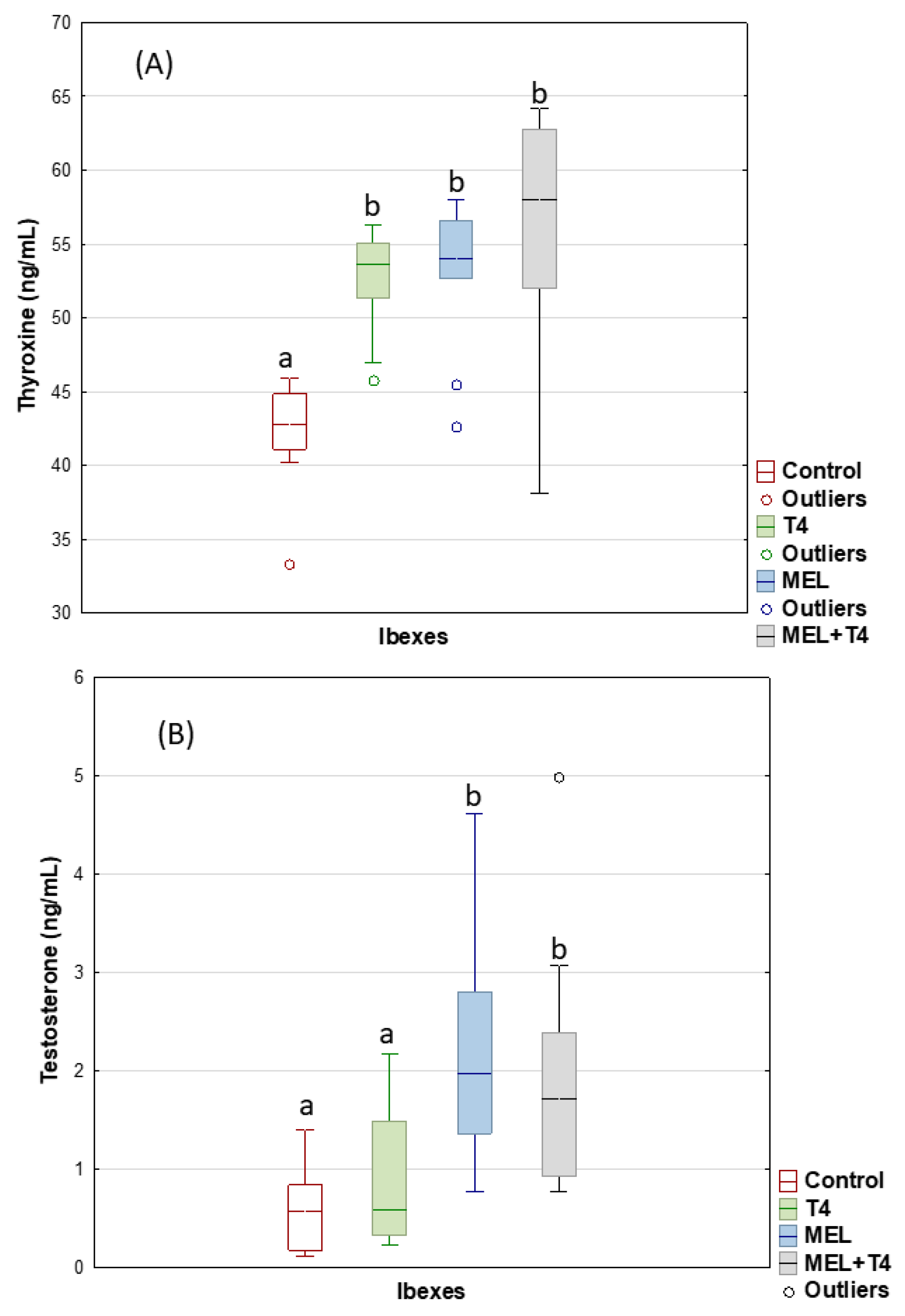
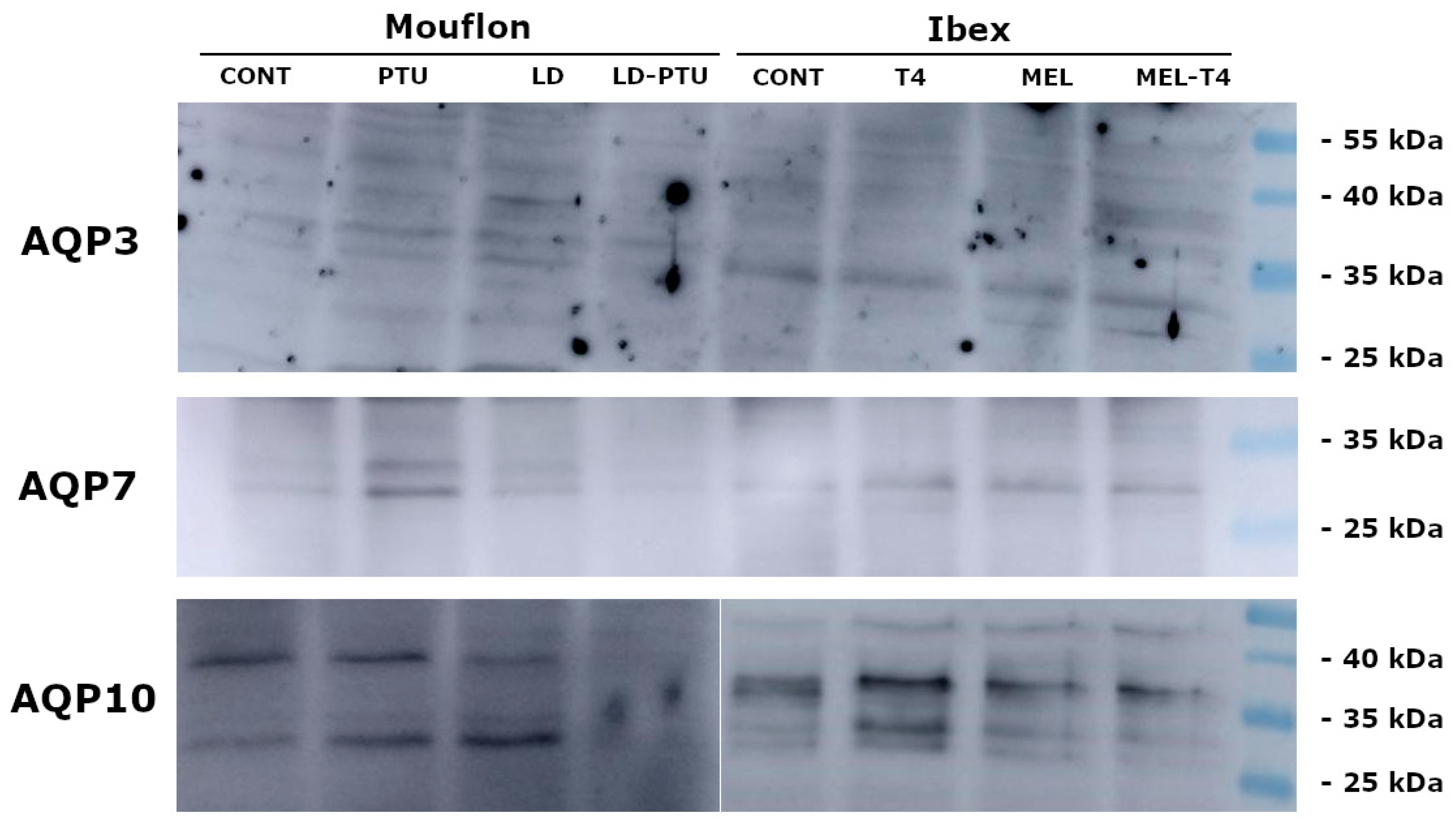
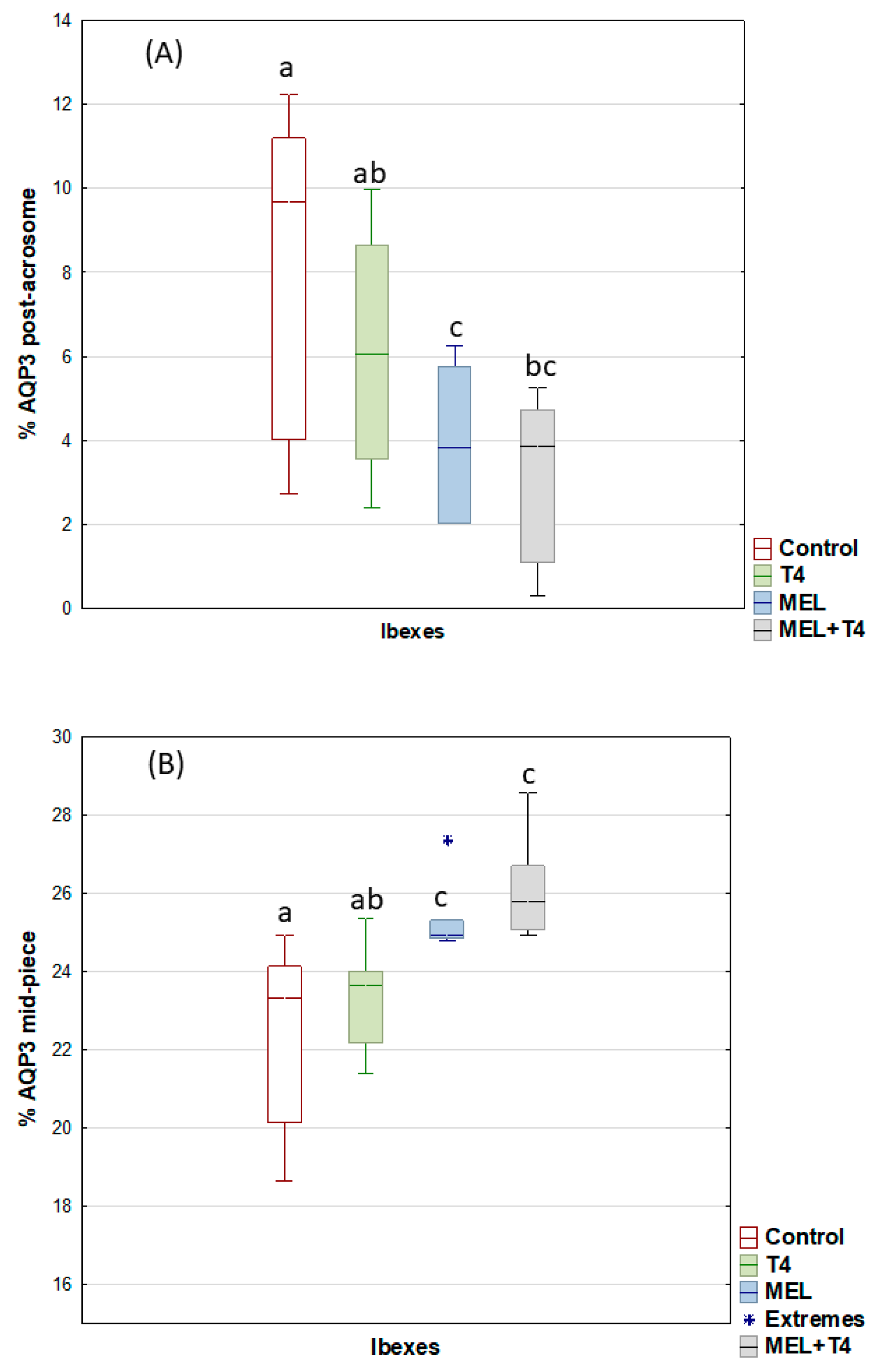
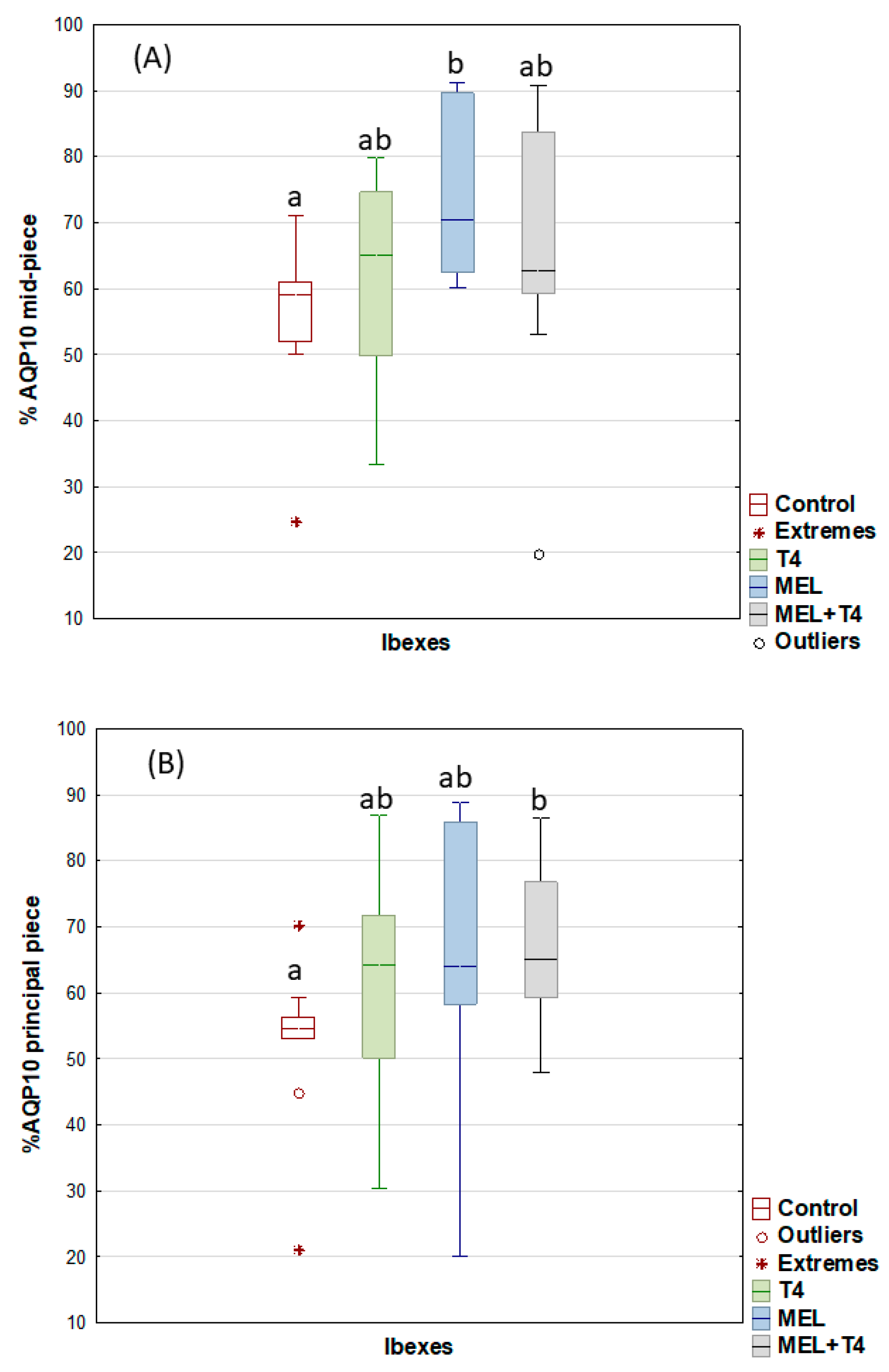
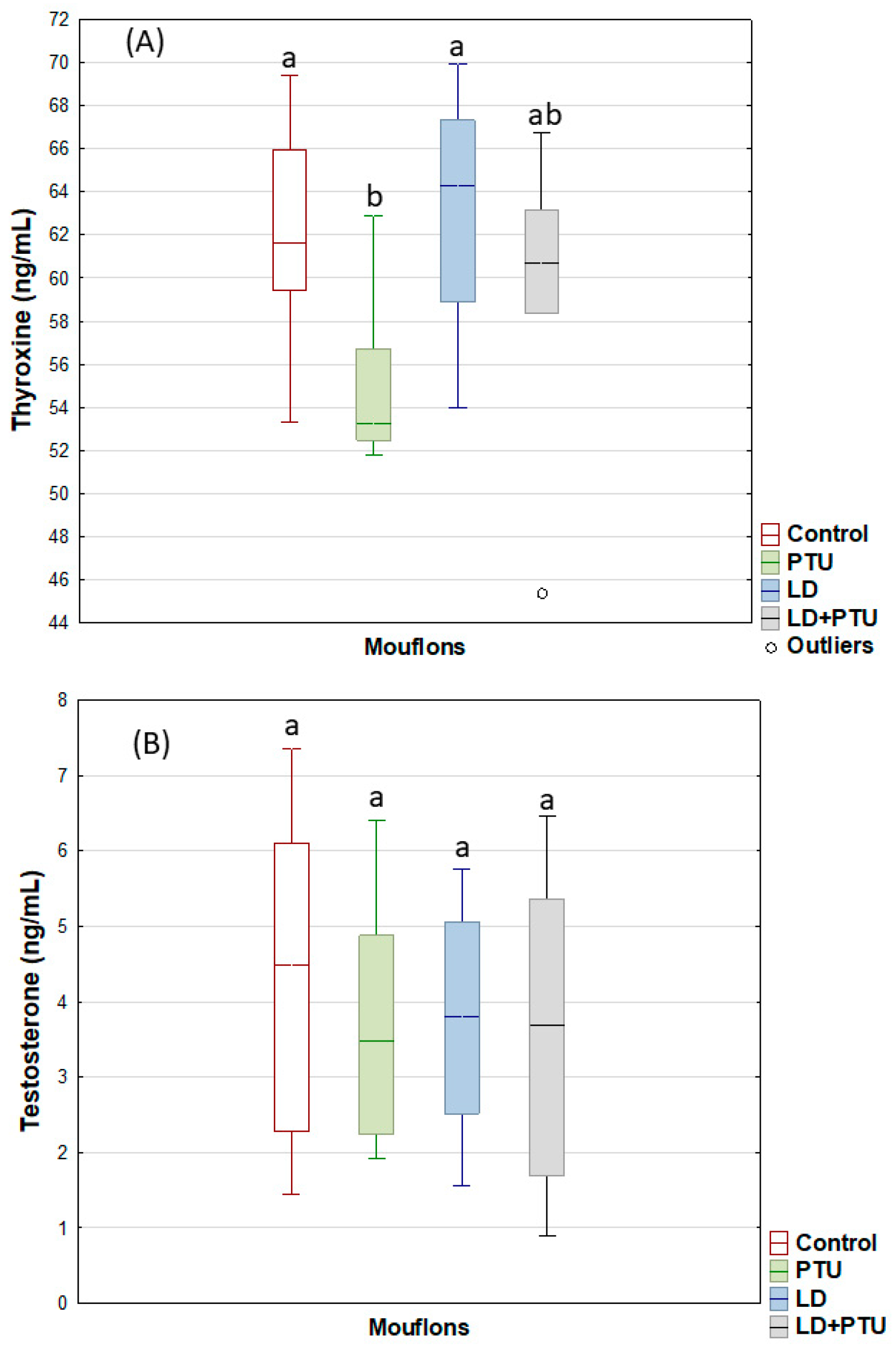
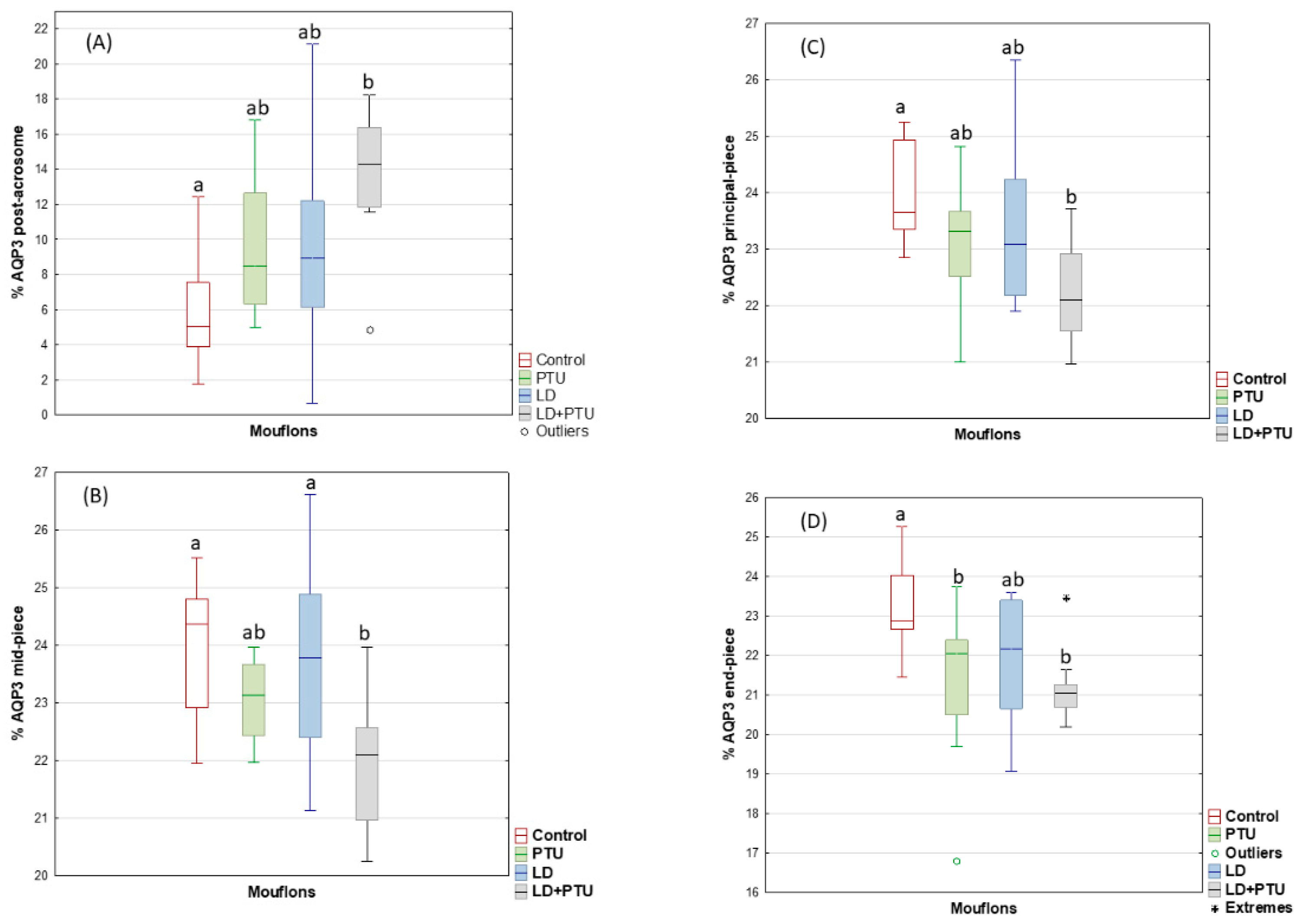
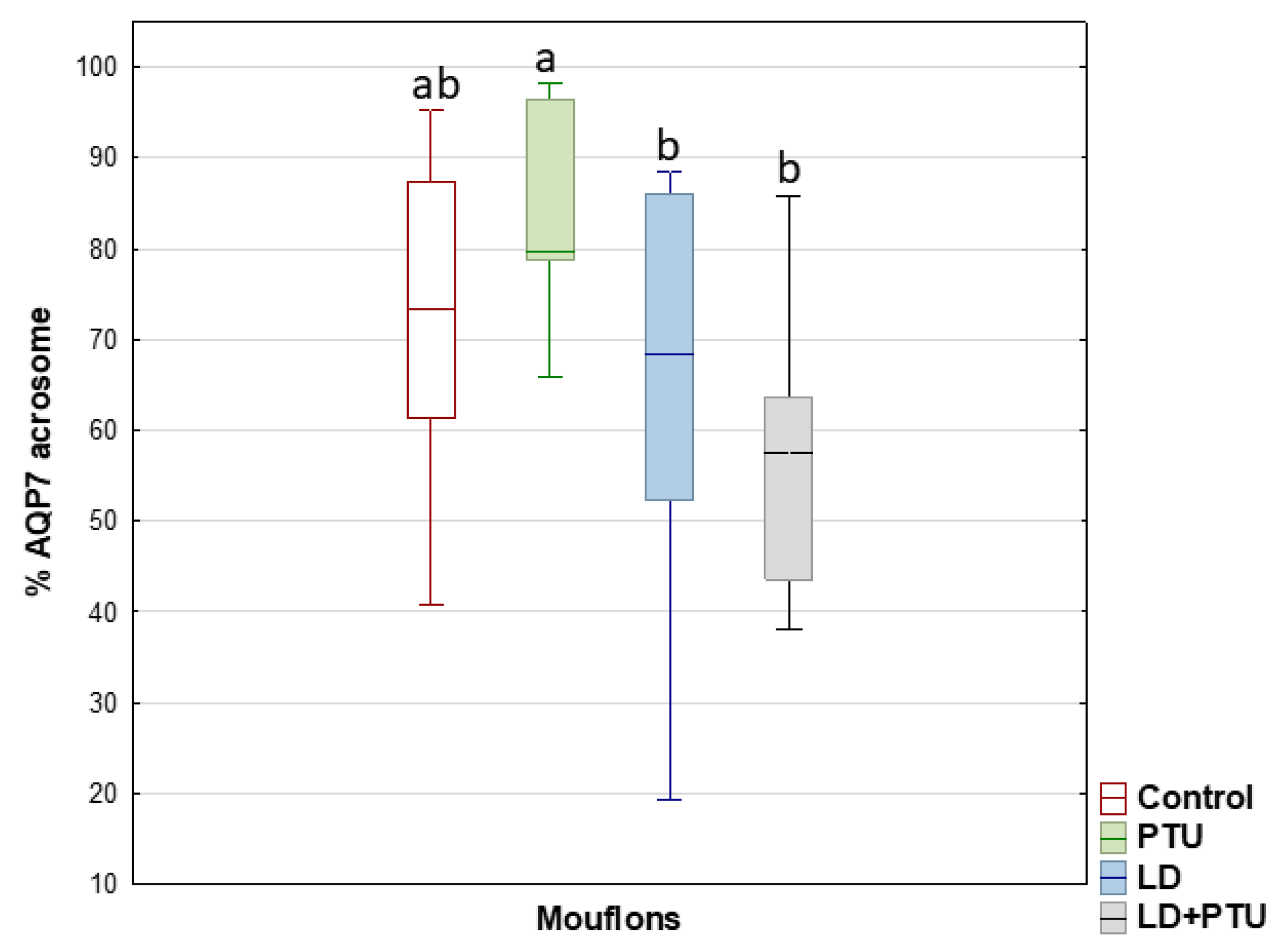
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Procedure
- (1)
- Thyroxine group (T4 group): composed of four ibexes kept under natural photoperiod conditions (natural variations in day length from 15 h light/day at the summer solstice to 9 h/day at the winter solstice) that received a continuous infusion of T4 from 1 January to 25 February (i.e., coinciding with the period of natural reduction in the blood plasma testosterone concentration [41]), using Model 2ML2 2 mL Alcet®® osmotic pumps (Durect Corporation, Cupertino, CA, USA). These were implanted (under anesthesia) under the skin in the lateral shoulder area and contained 2.8 mg of T4 (L-Thyroxine T1775, Lot BCBV1017) (Sigma-Aldrich, St. Louis, MO, USA), 0.1 g BSA, 10.6 mg Na2CO3, and 40 µL NaOH (1N) dissolved in 2.0 mL normal saline solution (0.9%). They delivered 164 µg/day of T4 for 14 days (5.0 µL/h, 14 days), and therefore had to be replaced three times to cover the total infusion period of 56 days. This protocol was previously tested at our laboratory as a means of increasing plasma T4 concentrations within the physiological range; no pathological hyperthyroidism was induced.
- (2)
- Melatonin group (MEL group): composed of four ibexes kept under natural photoperiod conditions but which received two subcutaneous (s.c.) melatonin implants (Melovine®®) (Ceva Salud Animal, Barcelona, Spain), each of 18 mg, at the base of an ear on 23 December (the winter solstice). This provided for the continuous release of melatonin at a rate maintaining high daytime levels for about 70 days [42], thus establishing a continuous short day-signal from the winter solstice onward, i.e., the photoperiod that stimulates reproductive activity in small ruminants.
- (3)
- Thyroxine + melatonin group (T4+MEL group): composed of four ibexes kept under natural photoperiod conditions but receiving continuous infusion of T4 from 1st January to 25 February via the same pumps as described above, and which received two s.c. melatonin implants (as above) at the base of an ear on 23 December.
- (4)
- Control group: composed of four ibexes kept under natural photoperiod.
- (1)
- Propylthiouracil-treated group (PTU group): composed of five mouflons kept under natural photoperiod conditions and administered 35 mg/kg propylthiouracil orally in 10 mL of propylene glycol from 1 November to 31 December. This protocol was previously checked at our laboratory as a means of reducing the plasma concentration of thyroxine.
- (2)
- Long day photoperiod group (LD group): composed of four mouflons kept in an open stable exposed to long days of 15 h light:9 h dark (15L:9D, equivalent to the summer solstice photoperiod) from 1 November to 31 December. This photoperiod was regulated using an electric clock that operated fluorescent tubes providing an artificial light intensity of approximately 350 lux at floor level. Artificial long days inhibit reproductive activity in wild ruminants [40].
- (3)
- Long day photoperiod group (LD+PTU group): composed of five mouflons kept in an open stable exposed to long days (15L:9D) from 1 November to 31 December, and administered 35 mg/kg PTU orally in 10 mL of propylene glycol from 1 November to 31 December.
- (4)
- Control group: composed of five mouflons kept under natural photoperiod conditions and administered 10 mL of propylene glycol orally from 1 November to 31 December.
4.3. Collection of Samples and Measurements
4.4. Hormone Analyses
4.5. Sperm Quality Analysis
4.6. Sperm Cryopreservation
4.7. AQP Assay
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martínez-Fresneda, L.; O’Brien, E.; Velázquez, R.; Toledano-Díaz, A.; Martínez-Cáceres, C.M.; Tesfaye, D.; Schellander, K.; García-Vázquez, F.A.; Santiago-Moreno, J. Seasonal variation in sperm freezability associated with changes in testicular germinal epithelium in domestic (Ovis aries) and wild (Ovis musimon) sheep. Reprod. Fertil. Dev. 2019, 31, 1545–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Fresneda, L.; O’Brien, E.; Sebastián, A.L.; Velázquez, R.; Toledano-Díaz, A.; Tesfaye, D.; Schellander, K.; García-Vázquez, F.; Santiago-Moreno, J. In vitro supplementation of testosterone or prolactin affects spermatozoa freezability in small ruminants. Domest. Anim. Endocrinol. 2019, 72, 106372. [Google Scholar] [CrossRef] [PubMed]
- Flores-Gil, V.; de la Blanca, M.M.; Velázquez, R.; Toledano-Díaz, A.; Santiago-Moreno, J.; López-Sebastián, A. Influence of testosterone administration at the end of the breeding season on sperm cryoresistance in rams (Ovis aries) and bucks (Capra hircus). Domest. Anim. Endocrinol. 2020, 72, 106425. [Google Scholar] [CrossRef]
- Hafez, E.S.E. Studies on the breeding season and reproduction of the ewe. J. Agric. Sci. 1952, 42, 189–265. [Google Scholar] [CrossRef]
- Lincoln, G.A. Correlation with changes in horns and pelage, but not reproduction, of seasonal cycles in the secretion of prolactin in rams of wild, feral and domesticated breeds of sheep. Reproduction 1990, 90, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, G. Reproductive seasonality and maturation throughout the complete life-cycle in the mouflon ram (Ovis musimon). Anim. Reprod. Sci. 1998, 53, 87–105. [Google Scholar] [CrossRef]
- Karsch, F.J.; Bittman, E.L.; Foster, D.L.; Goodman, R.L.; Legan, S.J.; Robinson, J.E. Neuroendocrine basis of seasonal re-production. Recent Prog. Horm. Res. 1984, 40, 185–232. [Google Scholar]
- Casao, A.; Mendoza, N.; Pérez-Pé, R.; Grasa, P.; Abecia, J.A.; Forcada, F.; Cebrián-Pérez, J.A.; Muiño-Blanco, T. Melatonin prevents capacitation and apoptotic-like changes of ram spermatozoa and increases fertility rate. J. Pineal Res. 2010, 48, 39–46. [Google Scholar] [CrossRef]
- Casao, A.; Gallego, M.; Abecia, J.A.; Forcada, F.; Pérez-Pé, R.; Muiño-Blanco, T.; Cebrián-Pérez, J. Identification and immunolocalisation of melatonin MT1 and MT2 receptors in Rasa Aragonesa ram spermatozoa. Reprod. Fertil. Dev. 2012, 24, 953–961. [Google Scholar] [CrossRef]
- Gallego-Calvo, L.; Gatica, M.C.; Santiago-Moreno, J.; Guzmán, J.L.; Zarazaga, L.A. Exogenous melatonin does not improve the freezability of Blanca Andaluza goat semen over exposure to two months of short days. Anim. Reprod. Sci. 2015, 157, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Succu, S.; Berlinguer, F.; Pasciu, V.; Satta, V.; Leoni, G.G.; Naitana, S. Melatonin protects ram spermatozoa from cryo-preservation injuries in a dose-dependent manner. J. Pineal Res. 2011, 50, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.D.; Barrell, G.K. Requirement of thyroid function for the expression of seasonal reproductive and related changes in red deer (Cervus elaphus) stags. Reproduction 1992, 94, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Karsch, F.J.; Dahl, G.E.; Hachigian, T.M.; Thrun, L.A. Involvement of thyroid hormones in seasonal reproduction. J. Reprod. Fertil. Suppl. Only 2019, 49, 409–422. [Google Scholar] [CrossRef]
- Nicholls, T.J.; Follett, B.K.; Goldsmith, A.R.; Pearson, H. Possible homologies between photorefractoriness in sheep and birds: The effect of thyroidectomy on the length of the ewe’s breeding season. Reprod. Nutr. Dev. 1988, 28, 375–386. [Google Scholar] [CrossRef] [Green Version]
- Webster, J.R.; Moenter, S.M.; Woodfill, C.J.I.; Karsch, F.J. Role of the Thyroid Gland in Seasonal Reproduction. II. Thyroxine Allows a Season-Specific Suppression of Gonadotropin Secretion in Sheep. Endocrinology 1991, 129, 176–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loudon, A.S.I.; Milne, J.A.; Curlewis, J.D.; Mc Neilly, A.S. A comparison of the seasonal hormone changes and patterns of growth, voluntary food intake and reproduction in juvenile and adult red deer (Cervus elaphus) and Père David’s deer (Elaphurus davidianus) hinds. J. Endocrinol. 1989, 122, 733–745. [Google Scholar] [CrossRef]
- Maran, R.R.M.; Arunakaran, J.; Aruldhas, M.M. T3 stimulates basal and modulates LH induced testosterone and oestradiol production by rat Leydig cells. Endocr. J. 2000, 47, 417–428. [Google Scholar] [CrossRef] [Green Version]
- Buzzard, J.J.; Morrison, J.R.; O’Bryan, M.K.; Song, Q.; Wreford, N.G. Developmental expression of thyroid hormone receptors in the rat testis. Biol. Reprod. 2000, 62, 664–669. [Google Scholar] [CrossRef] [Green Version]
- Wagner, M.S.; Wajner, S.M.; Maia, A.L. The role of thyroid hormone in testicular development and function. J. Endocrinol. 2008, 199, 351–365. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Shekhar, S.; Dhole, B. Thyroid and male reproduction. Indian J. Endocrinol. Metab. 2014, 18, 23–31. [Google Scholar] [CrossRef]
- Choudhury, S.; Chainy, G.B.; Mishro, M.M. Experimentally induced hypo- and hyperthyroidism influence on the anti-oxidant defence system in adult rat testis. Andrologia 2003, 35, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Madrid, B.; Castaño, C.; Ureña, L.P.; Flix, E.; Velázquez, R.; López-Sebastián, A.; Ungerfeld, R.; Arrebola, F.A.; Santiago-Moreno, J. Seasonal changes in testosterone and thyroxine concentrations in Mediterranean rams and bucks and their relationship with sperm cryoresistance. Livest. Sci. 2021, 249, 104513. [Google Scholar] [CrossRef]
- Yeste, M.; Morató, R.; Rodríguez-Gil, J.E.; Bonet, S.; Prieto-Martínez, N. Aquaporins in the male reproductive tract and sperm: Functional implications and cryobiology. Reprod. Domest. Anim. 2017, 52, 12–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-C.; Cadnapaphornchai, M.A.; Yang, J.; Summer, S.N.; Falk, S.; Li, C.; Wang, W.; Schrier, R.W. Nonosmotic release of vasopressin and renal aquaporins in impaired urinary dilution in hypothyroidism. Am. J. Physiol. Ren. Physiol. 2005, 289, F672–F678. [Google Scholar] [CrossRef]
- Cadnapaphornchai, M.A.; Kim, Y.-W.; Gurevich, A.K.; Summer, S.N.; Falk, S.; Thurman, J.M.; Schrier, R.W. Urinary Concentrating Defect in Hypothyroid Rats: Role of Sodium, Potassium, 2-Chloride Co-Transporter, and Aquaporins. J. Am. Soc. Nephrol. 2003, 14, 566–574. [Google Scholar] [CrossRef] [Green Version]
- Prieto-Martínez, N.; Morató, R.; Muiño, R.; Hidalgo, C.O.; Rodríguez-Gil, J.E.; Bonet, S.; Yeste, M. Aquaglyceroporins 3 and 7 in bull spermatozoa: Identification, localisation and their relationship with sperm cryotolerance. Reprod. Fertil. Dev. 2017, 29, 1249. [Google Scholar] [CrossRef]
- Pastor-Soler, N.; Bagnis, C.; Sabolic, I.; Tyszkowski, R.; McKee, M.; Van Hoek, A.; Breton, S.; Brown, D. Aquaporin 9 expression along the male reproductive tract. Biol. Reprod. 2001, 65, 384–393. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Kamiie, J.; Morishita, Y.; Yoshida, Y.; Yaoita, E.; Ishibashi, K.; Yamamoto, T. Expression and localization of two isoforms of AQP10 in human small intestine. Biol. Cell 2005, 97, 823–829. [Google Scholar] [CrossRef] [Green Version]
- Pool, K.R.; Rickard, J.P.; Pini, T.; De Graaf, S.P. Exogenous melatonin advances the ram breeding season and increases testicular function. Sci. Rep. 2020, 10, 9711. [Google Scholar] [CrossRef]
- Bóveda, P.; Esteso, M.C.; Velázquez, R.; Castaño, C.; Toledano-Díaz, A.; López-Sebastián, A.; Mejía, O.; de la Blanca, M.G.M.; Ungerfeld, R.; Santiago-Moreno, J. Influence of circulating testosterone concentration on sperm cryoresistance: The ibex as an experimental model. Andrology 2021, 9, 1242–1253. [Google Scholar] [CrossRef]
- Prieto-Martínez, N.; Vilagran, I.; Morató, R.; del Álamo, M.M.R.; Rodríguez-Gil, J.E.; Bonet, S.; Yeste, M. Relationship of aquaporins 3 (AQP3), 7 (AQP7), and 11 (AQP11) with boar sperm resilience to withstand freeze-thawing procedures. Andrology 2017, 5, 1153–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonilla-Correal, S.; Noto, F.; Garcia-Bonavila, E.; Rodríguez-Gil, J.; Yeste, M.; Miro, J. First evidence for the presence of aquaporins in stallion sperm. Reprod. Domest. Anim. 2017, 52, 61–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edashige, K.; Ohta, S.; Tanaka, M.; Kuwano, T.; Valdez, D.M., Jr.; Hara, T.; Jin, B.; Takahashi, S.-I.; Seki, S.; Koshimoto, C.; et al. The Role of Aquaporin 3 in the Movement of Water and Cryoprotectants in Mouse Morulae. Biol. Reprod. 2007, 77, 365–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prieto-Martínez, N.; Vilagran, I.; Morató, R.; Rodríguez-Gil, J.E.; Yeste, M.; Bonet, S. Aquaporins 7 and 11 in boar spermatozoa: Detection, localisation and relationship with sperm quality. Reprod. Fertil. Dev. 2016, 28, 663–672. [Google Scholar] [CrossRef] [Green Version]
- Du Plessis, S.S.; Agarwal, A.; Mohanty, G.; van der Linde, M.; Agarwal, A.; Mohanty, G.; van der Linde, M. Oxidative phosphorylation versus glycolysis: What fuel do spermatozoa use? Asian J. Androl. 2015, 17, 230–235. [Google Scholar] [CrossRef]
- Berlinguer, F.; Madeddu, M.; Pasciu, V.; Succu, S.; Spezzigu, A.; Satta, V.; Mereu, P.; Leoni, G.G.; Naitana, S. Semen molecular and cellular features: These parameters can reliably predict subsequent ART outcome in a goat model. Reprod. Biol. Endocrinol. 2009, 7, 125. [Google Scholar] [CrossRef] [Green Version]
- Carbrey, J.M.; Gorelick-Feldman, D.A.; Kozono, D.; Praetorius, J.; Nielsen, S.; Agre, P. Aquaglyceroporin AQP9: Solute permeation and metabolic control of expression in liver. Proc. Natl. Acad. Sci. USA 2003, 100, 2945–2950. [Google Scholar] [CrossRef] [Green Version]
- Arena, S.; Arena, F.; Maisano, D.; Di Benedetto, V.; Romeo, C.; Nicòtina, P.A. Aquaporin-9 immunohistochemistry in varicocele testes as a consequence of hypoxia in the sperm production site. Andrologia 2010, 43, 34–37. [Google Scholar] [CrossRef]
- Mendeluk, G.R.; Rosales, M. Thyroxin Is Useful to Improve Sperm Motility. Int. J. Fertil. Steril. 2016, 10, 208–214. [Google Scholar] [CrossRef]
- Santiago-Moreno, J.; Toledano-Díaz, A.; Castaño, C.; Coloma, M.A.; Esteso, M.C.; Prieto, M.T.; Delgadillo, J.A.; López-Sebastián, A. Photoperiod and melatonin treatments for controlling sperm parameters, testicular and accessory sex glands size in male Iberian ibex: A model for captive mountain ruminants. Anim. Reprod. Sci. 2013, 139, 45–52. [Google Scholar] [CrossRef]
- Coloma, M.A.; Toledano-Díaz, A.; Castaño, C.; Velázquez, R.; Gómez-Brunet, A.; López-Sebastián, A.; Santia-go-Moreno, J. Seasonal variation in reproductive physiological status in the Iberian ibex (Capra pyrenaica) and its relationship with sperm freezability. Theriogenology 2011, 76, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Chemineau, P.; Malpaux, B.; Delgadillo, J.A.; Guérin, Y.; Ravault, J.P.; Thimonier, J.; Pelletier, J. Control of sheep and goat reproduction: Use of light and melatonin. Anim. Reprod. Sci. 1992, 30, 157–184. [Google Scholar] [CrossRef]
- Santiago-Moreno, J.; Castaño, C.; Toledano-Díaz, A.; Esteso, M.C.; López-Sebastián, A.; Guerra, R.; Ruiz, M.J.; Mendoza, N.; Luna, C.; Cebrián-Pérez, J.A.; et al. Cryopreservation of aoudad (Ammotragus lervia sahariensis) sperm obtained by transrectal ultrasound-guided massage of the accessory sex glands and electroejaculation. Theriogenology 2013, 79, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Moreno, J.; Gómez-Brunet, A.; González-Bulnes, A.; Toledano-Díaz, A.; Malpaux, B.; López-Sebastián, A. Differences in reproductive pattern between wild and domestic rams are not associated with inter-specific annual variations in plasma prolactin and melatonin concentrations. Domest. Anim. Endocrinol. 2005, 28, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Pradiee, J.; Esteso, M.C.; Castaño, C.; Toledano-Diaz, A.; Lopez-Sebastian, A.; Guerra, R.; Santiago-Moreno, J. Conventional slow freezing cryopreserves mouflon spermatozoa better than vitrification. Andrologia 2017, 49, e12629. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, A.V.; Ekwall, H.; Alvarez-Rodriguez, M.; Rodríguez-Martínez, H. Membrane Stress During Thawing Elicits Redistribution of Aquaporin 7 But Not of Aquaporin 9 in Boar Spermatozoa. Reprod. Domest. Anim. 2016, 51, 665–679. [Google Scholar] [CrossRef] [Green Version]



| Fresh Sperm Variables | Control | T4 | MEL | T4+MEL |
|---|---|---|---|---|
| Motile sperm (%) | 41.7 ± 8.7 | 51.9 ± 7.9 | 59.3 ± 14.1 | 64.4 ± 7.3 |
| Intact acrosome (%) | 69.0 ± 15.7 a | 79.0 ± 11.6 ab | 88.1 ± 5.1 ab | 96.78 ± 2.1 b |
| Viable sperm (%) | 42.8 ± 13.2 | 55.9 ± 10.5 | 56.1 ± 6.7 | 56.8 ± 7.2 |
| Cryoresistance ratio | ||||
| CR-Motile sperm | 53.3 ± 5.6 | 61.0 ± 12.4 | 38.9 ± 7.3 | 44.3 ± 5.7 |
| CR-Intact acrosome | 89.1 ± 15.3 a | 75.5 ± 4.7 a | 49.5 ± 11.0 b | 64.8 ± 3.1 ab |
| CR-Viable sperm | 51.7 ± 7.1 ab | 57.1 ± 13.0 a | 28.5 ± 6.4 b | 48.7 ± 9.0 ab |
| Fresh Sperm Variables | Control | PTU | LD | LD+PTU |
|---|---|---|---|---|
| Motile sperm (%) | 66.6 ± 5.7 a | 51.5 ± 5.8 b | 73.1 ± 4.3 a | 77.1 ± 3.4 a |
| Intact acrosome (%) | 90.4 ± 2.8 a | 79.6 ± 5.2 b | 92.7 ± 3.1 a | 97.3 ± 0.8 a |
| Viable sperm (%) | 68.7 ± 3.3 a | 47.1 ± 8.0 b | 67.1 ± 4.0 a | 77.4 ± 4.0 a |
| Cryoresistance ratio | ||||
| CR-Motile sperm | 45.9 ± 9.8 | 45.6 ± 6.7 | 28.3 ± 8.0 | 37.9 ± 10.0 |
| CR-Intact acrosome | 67.7 ± 9.1 | 70.8 ± 3.9 | 59.3 ± 6.8 | 67.4 ± 6.8 |
| CR-Viable sperm | 32.7 ± 8.1 | 37.6 ± 7.8 | 22.0 ± 6.1 | 32.1 ± 13.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago-Moreno, J.; Pequeño, B.; Martinez-Madrid, B.; Castaño, C.; Bóveda, P.; Velázquez, R.; Toledano-Díaz, A.; Álvarez-Rodríguez, M.; Rodríguez-Martínez, H. Expression of Aquaglyceroporins in Spermatozoa from Wild Ruminants Is Influenced by Photoperiod and Thyroxine Concentrations. Int. J. Mol. Sci. 2022, 23, 2903. https://doi.org/10.3390/ijms23062903
Santiago-Moreno J, Pequeño B, Martinez-Madrid B, Castaño C, Bóveda P, Velázquez R, Toledano-Díaz A, Álvarez-Rodríguez M, Rodríguez-Martínez H. Expression of Aquaglyceroporins in Spermatozoa from Wild Ruminants Is Influenced by Photoperiod and Thyroxine Concentrations. International Journal of Molecular Sciences. 2022; 23(6):2903. https://doi.org/10.3390/ijms23062903
Chicago/Turabian StyleSantiago-Moreno, Julián, Belén Pequeño, Belen Martinez-Madrid, Cristina Castaño, Paula Bóveda, Rosario Velázquez, Adolfo Toledano-Díaz, Manuel Álvarez-Rodríguez, and Heriberto Rodríguez-Martínez. 2022. "Expression of Aquaglyceroporins in Spermatozoa from Wild Ruminants Is Influenced by Photoperiod and Thyroxine Concentrations" International Journal of Molecular Sciences 23, no. 6: 2903. https://doi.org/10.3390/ijms23062903
APA StyleSantiago-Moreno, J., Pequeño, B., Martinez-Madrid, B., Castaño, C., Bóveda, P., Velázquez, R., Toledano-Díaz, A., Álvarez-Rodríguez, M., & Rodríguez-Martínez, H. (2022). Expression of Aquaglyceroporins in Spermatozoa from Wild Ruminants Is Influenced by Photoperiod and Thyroxine Concentrations. International Journal of Molecular Sciences, 23(6), 2903. https://doi.org/10.3390/ijms23062903







