Brain Mechanisms of Exercise-Induced Hypoalgesia: To Find a Way Out from “Fear-Avoidance Belief”
Abstract
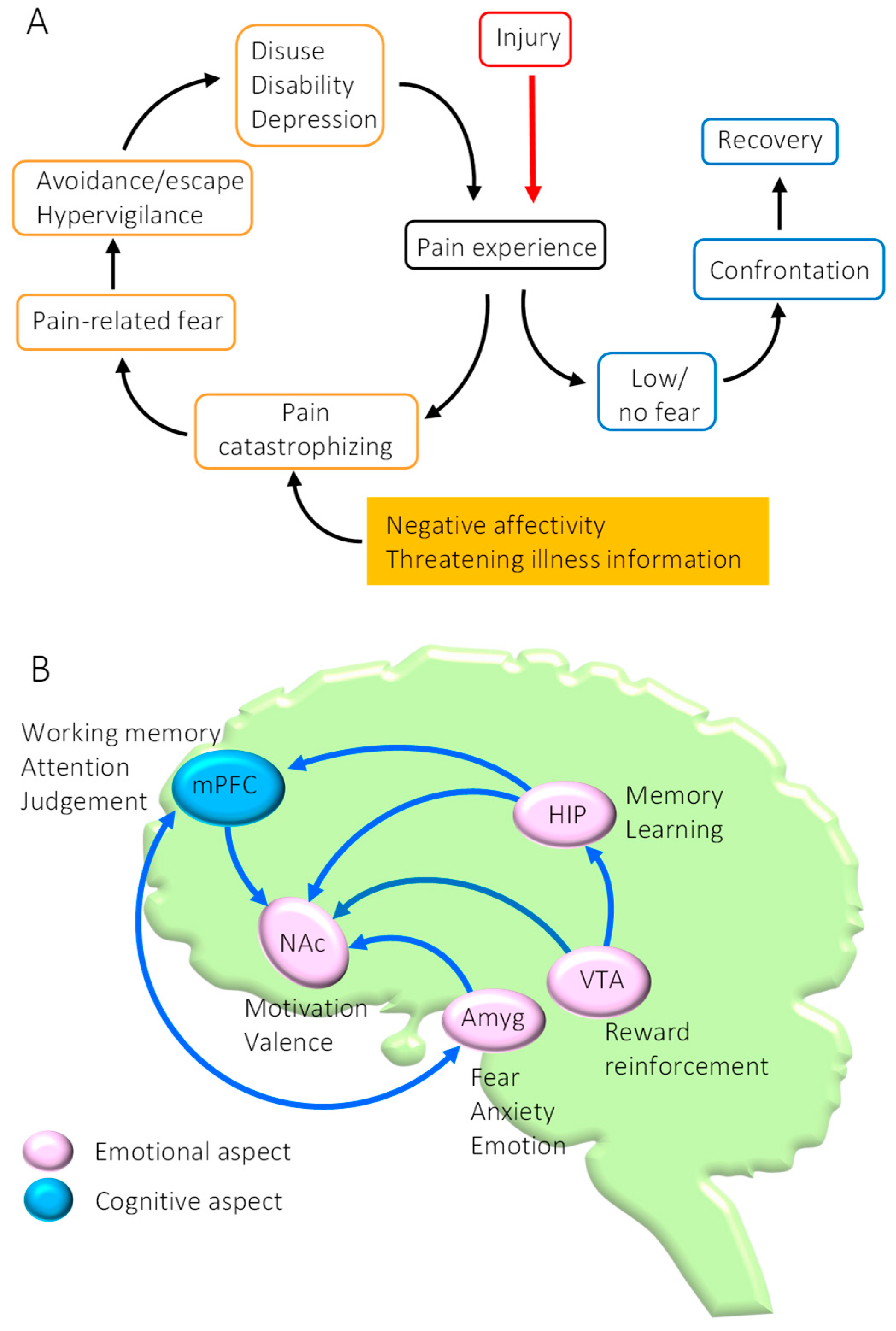
:1. Introduction
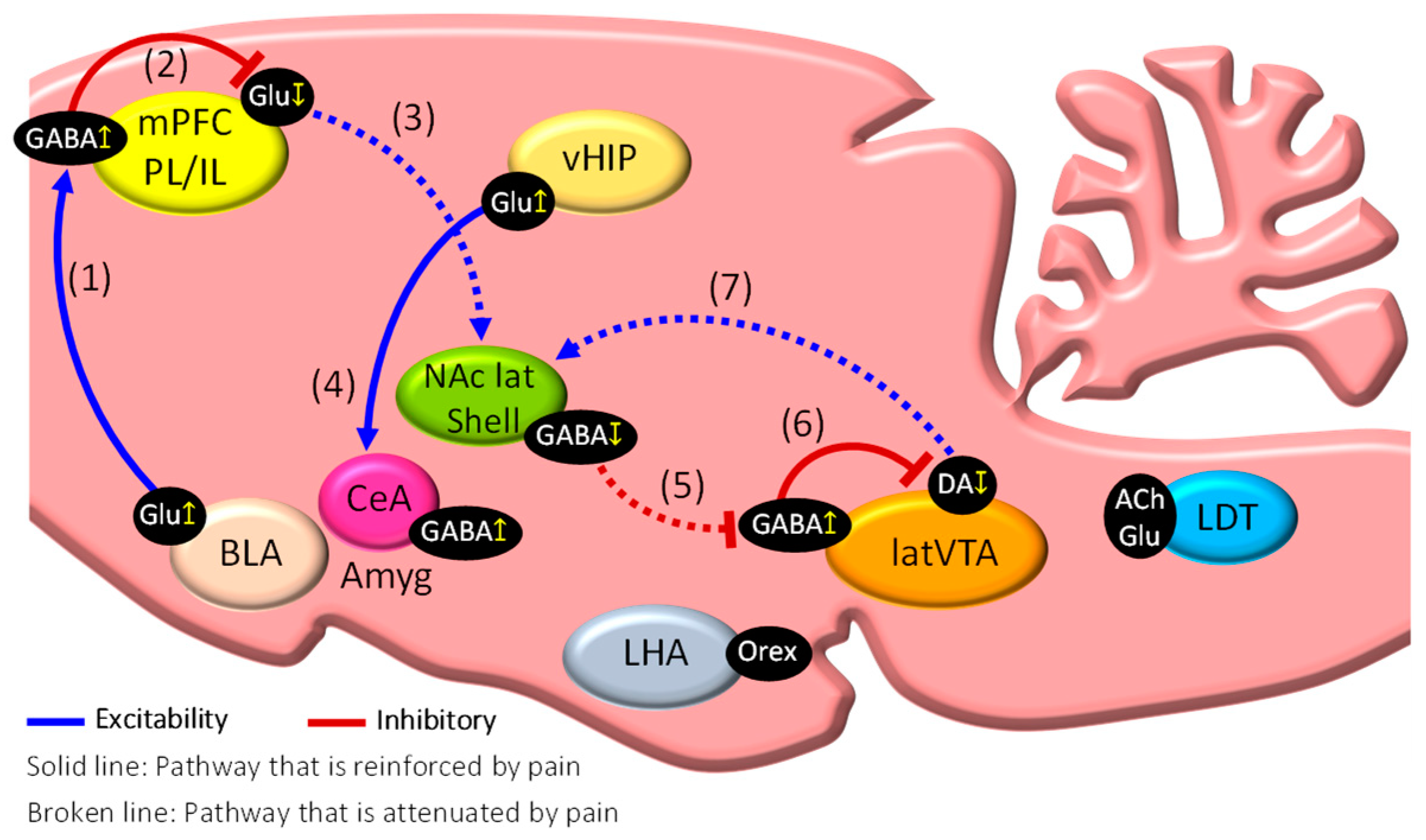
2. Dysfunction of the Mesocorticolimbic System in Chronic Pain State
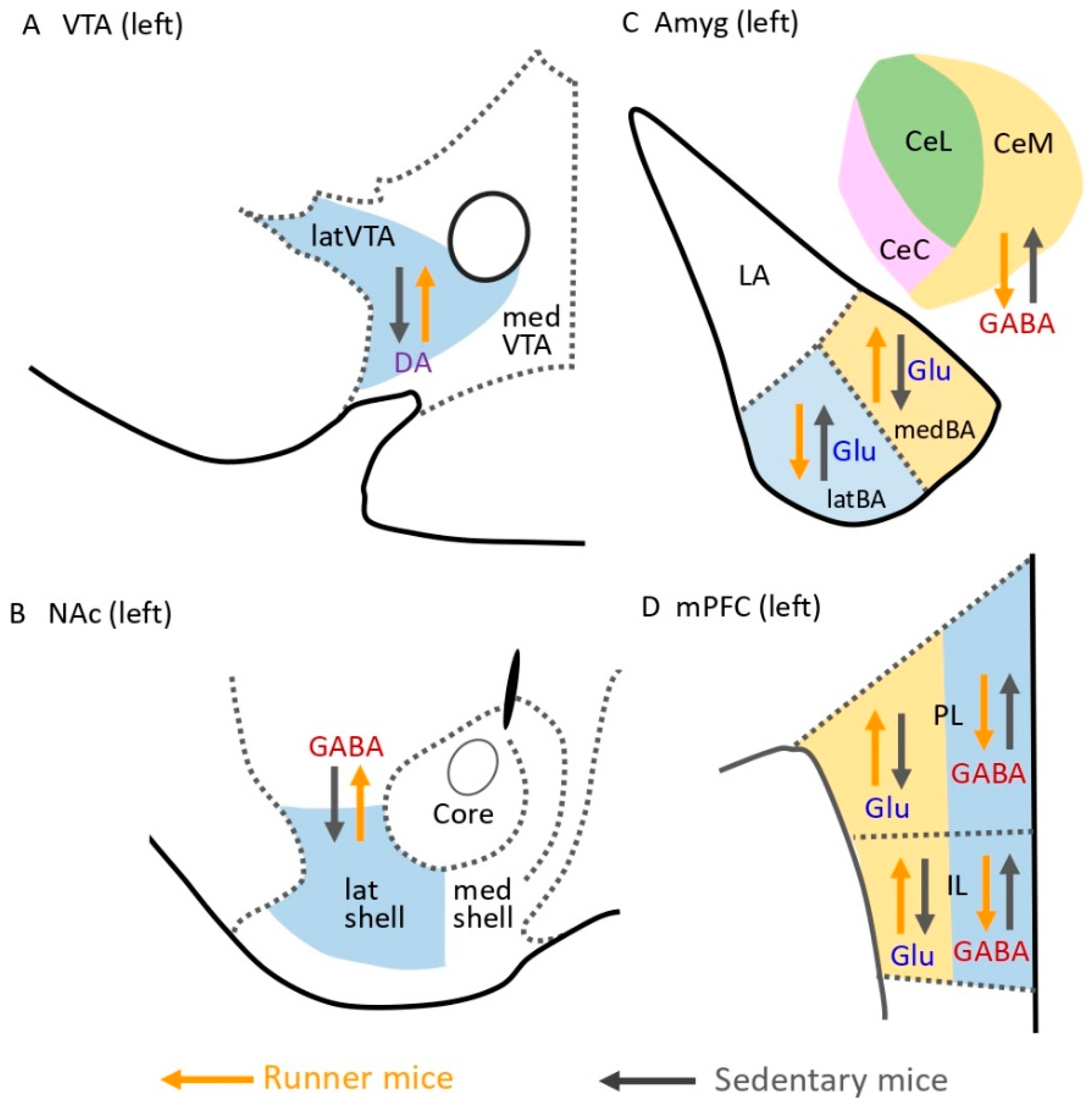
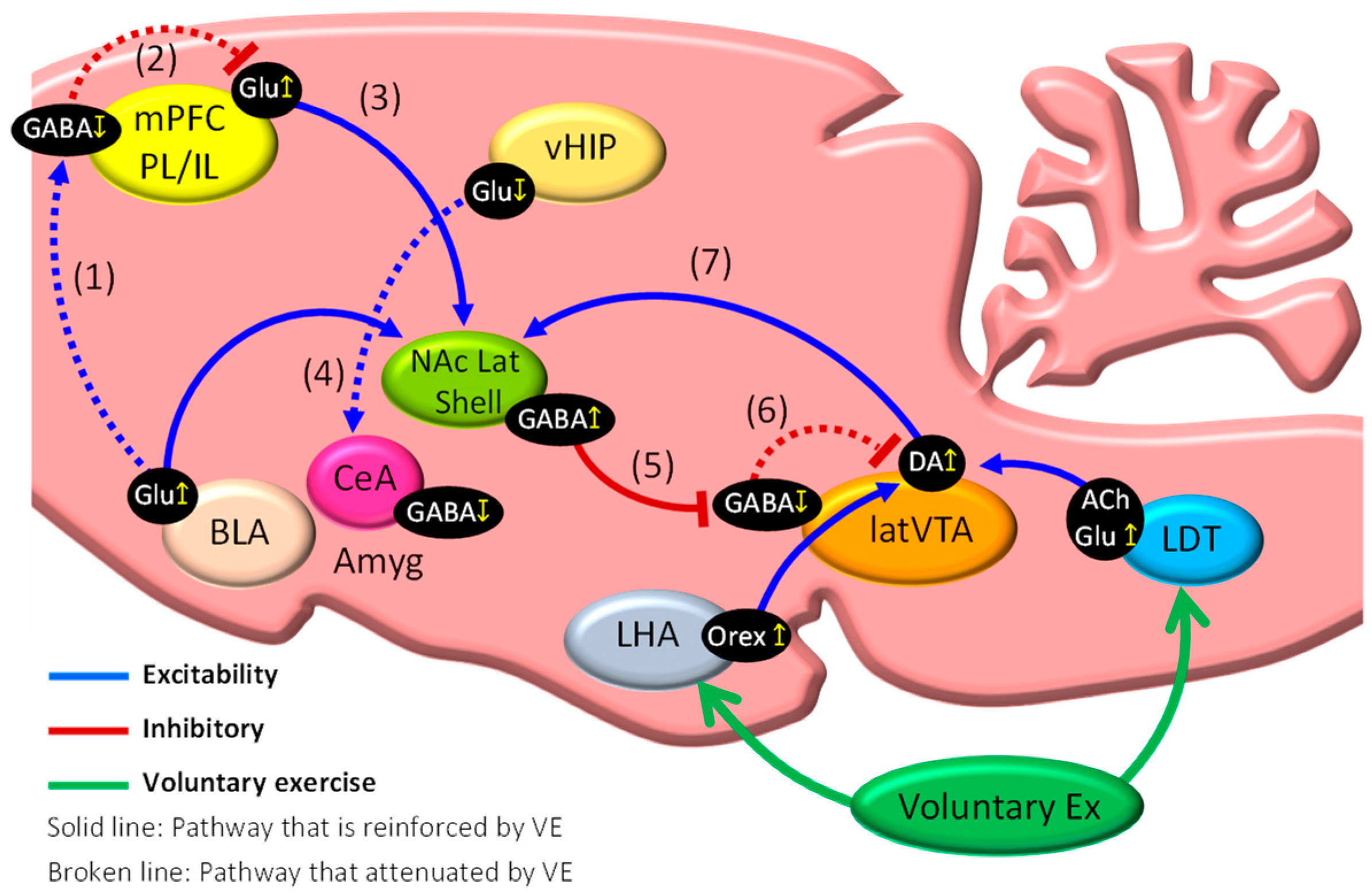
3. Brain Mechanisms of Exercise-Induced Hypoalgesia (EIH)
4. Attenuation of Fear Memories Contributes to EIH Effects
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ettlin, D.A.; Napimoga, M.H.; Meira E Cruz, M.; Clemente-Napimoga, J.T. Orofacial musculoskeletal pain: An evidence-based bio-psycho-social matrix model. Neurosci. Biobehav. Rev. 2021, 128, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Meints, S.M.; Edwards, R.R. Evaluating psychosocial contributions to chronic pain outcomes. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 87, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Slawek, D.E.; Syed, M.; Cunningham, C.O.; Zhang, C.; Ross, J.; Herman, M.; Sohler, N.; Minami, H.; Levin, F.R.; Arnsten, J.H.; et al. Pain catastrophizing and mental health phenotypes in adults with refractory chronic pain: A latent class analysis. J. Psychiatr. Res. 2021, 145, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Baradaran Mahdavi, S.; Riahi, R.; Vahdatpour, B.; Kelishadi, R. Association between sedentary behavior and low back pain; A systematic review and meta-analysis. Health Promot. Perspect 2021, 11, 393–410. [Google Scholar] [CrossRef]
- Lindell, M.; Grimby-Ekman, A. Stress, non-restorative sleep, and physical inactivity as risk factors for chronic pain in young adults: A cohort study. PLoS ONE 2022, 17, e0262601. [Google Scholar] [CrossRef]
- Lethem, J.; Slade, P.D.; Troup, J.D.; Bentley, G. Outline of a Fear-Avoidance Model of exaggerated pain perception-I. Behav. Res. 1983, 21, p401–p408. [Google Scholar] [CrossRef]
- Leeuw, M.; Goossens, M.E.J.B.; Linton, S.J.; Crombez, G.; Boersma, K.; Vlaeyen, J.W.S. The fear-avoidance model of musculoskeletal pain: Current state of scientific evidence. J. Behav. Med. 2007, 30, 77–94. [Google Scholar] [CrossRef] [Green Version]
- Kuner, R.; Kuner, T. Cellular Circuits in the Brain and Their Modulation in Acute and Chronic Pain. Physiol. Rev. 2021, 101, p213–p258. [Google Scholar] [CrossRef]
- Mercer Lindsay, N.; Chen, C.; Gilam, G.; Mackey, S.; Scherrer, G. Brain circuits for pain and its treatment. Sci. Transl. Med. 2021, 13, eabj7360. [Google Scholar] [CrossRef]
- Tan, L.L.; Kuner, R. Neocortical circuits in pain and pain relief. Nat. Rev. Neurosci. 2021, 22, 458–471. [Google Scholar] [CrossRef]
- Kami, K.; Tajima, F.; Senba, E. Activation of mesolimbic reward system via laterodorsal tegmental nucleus and hypothalamus in exercise-induced hypoalgesia. Sci Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ji, G.; Sun, H.; Fu, Y.; Li, Z.; Pais-Vieira, M.; Galhardo, V.; Neugebauer, V. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J. Neurosci. 2010, 30, 5451–5464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gadotti, V.M.; Chen, L.; Souza, I.A.; Stemkowski, P.L.; Zamponi, G.W. Role of Prelimbic GABAergic Circuits in Sensory and Emotional Aspects of Neuropathic Pain. Cell Rep. 2015, 12, 752–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitrić, M.; Seewald, A.; Moschetti, G.; Sacerdote, P.; Ferraguti, F.; Kummer, K.K.; Kress, K. Layer- and subregion-specific electrophysiological and morphological changes of the medial prefrontal cortex in a mouse model of neuropathic pain. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Manders, T.R.; Eberle, S.E.; Su, C.; D’amour, J.; Yang, R.; Lin, H.Y.; Deisseroth, K.; Froemke, R.C.; Wang, J. Activation of corticostriatal circuitry relieves chronic neuropathic pain. J. Neurosci. 2015, 35, 5247–5259. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.M.; Neugebauer, V. Amygdala Plasticity and Pain. Pain Res. Manag. 2017, 8296501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, J.M.; Neugebauer, V. Cortico-limbic pain mechanisms. Neurosci. Lett. 2019, 702, p15–p23. [Google Scholar] [CrossRef]
- Cai, Y.Q.; Wang, W.; Paulucci-Holthauzen, A.; Pan, Z.Z. Brain Circuits Mediating Opposing Effects on Emotion and Pain. J. Neurosci. 2018, 38, 6340–6349. [Google Scholar] [CrossRef]
- Beyeler, A.; Chang, C.J.; Silvestre, M.; Lévêque, C.; Namburi, P.; Wildes, C.P.; Tye, K.M. Organization of Valence-Encoding and Projection-Defined Neurons in the Basolateral Amygdala. Cell Rep. 2018, 22, 905–918. [Google Scholar] [CrossRef] [Green Version]
- Mutso, A.A.; Radzicki, D.; Baliki, M.N.; Huang, L.; Banisadr, G.; Centeno, M.V.; Radulovic, J.; Martina, M.; Miller, R.J.; Apkarian, A.V. Abnormalities in hippocampal functioning with persistent pain. J. Neurosci. 2012, 32, 5747–5756. [Google Scholar] [CrossRef] [Green Version]
- Ren, W.J.; Liu, Y.; Zhou, L.J.; Li, W.; Zhong, Y.; Pang, R.P.; Xin, W.J.; Wei, X.H.; Wang, J.; Zhu, H.Q.; et al. Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-alpha in rodents. Neuropsychopharmacology 2011, 36, 979–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navratilova, E.; Atcherley, C.W.; Porreca, F. Brain circuits encoding reward from pain relief. Trends Neurosci. 2015, 38, 741–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, W.; Centeno, M.V.; Berger, S.; Wu, Y.; Na, X.; Liu, X.; Kondapalli, J.; Apkarian, A.V.; Martina, M.; Surmeier, D.J. The indirect pathway of the nucleus accumbens shell amplifies neuropathic pain. Nat. Neurosci. 2016, 19, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Sarkis, R.; Saadé, N.; Atweh, S.; Jabbur, S.; Al-Amin, H. Chronic dizocilpine or apomorphine and development of neuropathy in two rat models I: Behavioral effects and role of nucleus accumbens. Exp. Neurol. 2011, 228, 19–29. [Google Scholar] [CrossRef]
- Yang, H.; de Jong, J.W.; Tak, Y.; Peck, J.; Bateup, H.S.; Lammel, S. Nucleus accumbens subnuclei regulate motivated behavior via direct inhibition and disinhibition of VTA dopamine subpopulations. Neuron 2018, 97, 434–449. [Google Scholar] [CrossRef]
- Rice, D.; Nijs, J.; Kosek, E.; Wideman, T.; Hasenbring, M.I.; Koltyn, K.; Graven-Nielsen, T.; Polli, A. Exercise-Induced Hypoalgesia in Pain-Free and Chronic Pain Populations: State of the Art and Future Directions. J. Pain 2019, 20, 1249–1266. [Google Scholar] [CrossRef] [Green Version]
- Da Silva Santos, R.; Galdino, G. Endogenous systems involved in exercise-induced analgesia. J. Physiol. Pharm. 2018, 69, 3–13. [Google Scholar]
- Zhang, Y.H.; Hu, H.Y.; Xiong, Y.C.; Peng, C.; Hu, L.; Kong, Y.Z.; Wang, Y.L.; Guo, J.B.; Bi, S.; Li, T.S.; et al. Exercise for Neuropathic Pain: A Systematic Review and Expert Consensus. Front. Med. 2021, 8, 2239. [Google Scholar] [CrossRef]
- Kami, K.; Tajima, F.; Senba, E. Exercise-induced hypoalgesia: Potential mechanisms in animal models of neuropathic pain. Anat. Sci. Int. 2017, 93, 79–90. [Google Scholar] [CrossRef]
- Bobinski, F.; Teixeira, J.M.; Sluka, K.A.; Santos, A.R.S. Interleukin-4 mediates the analgesia produced by low-intensity exercise in mice with neuropathic pain. Pain 2018, 159, p437–p450. [Google Scholar] [CrossRef]
- Zheng, J.; Jiang, Y.Y.; Xu, L.C.; Ma, L.Y.; Liu, F.Y.; Cui, S.; Cai, J.; Liao, F.F.; Wan, Y.; Yi, M. Adult Hippocampal Neurogenesis along the Dorsoventral Axis Contributes Differentially to Environmental Enrichment Combined with Voluntary Exercise in Alleviating Chronic Inflammatory Pain in Mice. J. Neurosci. 2017, 37, 4145–4157. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Nakagawa, S.; Kitaichi, Y.; An, Y.; Omiya, Y.; Song, N.; Koga, M.; Kato, A.; Inoue, T.; Kusumi, I. The role of medial prefrontal corticosterone and dopamine in the antidepressant-like effect of exercise. Psychoneuroendocrinology 2016, 69, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitcher, M.H.; Tarum, F.; Rauf, I.Z.; Low, L.A.; Bushnell, C. Modest Amounts of Voluntary Exercise Reduce Pain- and Stress-Related Outcomes in a Rat Model of Persistent Hind Limb Inflammation. J. Pain 2017, 18, 687–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senba, E.; Kami, K. A new aspect of chronic pain as a lifestyle-related disease. Neurobiol. Pain 2017, 1, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Stagg, N.J.; Mata, H.P.; Ibrahim, M.M.; Henriksen, E.J.; Porreca, F.; Vanderah, T.W.; Philip Malan, T., Jr. Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: Role of endogenous opioids. Anesthesiology 2011, 114, 940–948. [Google Scholar] [CrossRef] [Green Version]
- Bobinski, F.; Ferreira, T.A.A.; Córdova, M.M.; Dombrowski, P.A.; da Cunha, C.; Santo, C.C.D.E.; Poli, A.; Pires, R.G.W.; Martins-Silva, C.; Sluka, K.A.; et al. Role of brainstem serotonin in analgesia produced by low-intensity exercise on neuropathic pain after sciatic nerve injury in mice. Pain 2015, 156, 2595–2606. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Alvarez, V.M.; Puigdomenech, M.; Navarro, X.; Cobianchi, S. Monoaminergic descending pathways contribute to modulation of neuropathic pain by increasing intensity treadmill exercise after peripheral nerve injury. Exp. Neurol. 2018, 299, 42–55. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wu, Z.; Wang, D.; Huang, C.; Xu, J.; Liu, C.; Yang, C. Muscle-brain communication in pain: The key role of myokines. Brain Res. Bull. 2022, 179, 25–35. [Google Scholar] [CrossRef]
- Spanagel, R.; Herz, A.; Shippenberg, T.S. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc. Natl Acad. Sci. USA 1992, 89, 2046–2050. [Google Scholar] [CrossRef] [Green Version]
- Van Bockstaele, E.J.; Cestari, D.M.; Pickel, V.M. Synaptic structure and connectivity of serotonin terminals in the ventral tegmental area: Potential sites for modulation of mesolimbic dopamine neurons. Brain Res. 1994, 647, 307–322. [Google Scholar] [CrossRef]
- Wakaizumi, K.; Kondo, T.; Hamada, Y.; Narita, M.; Kawabe, R.; Narita, H.; Watanabe, M.; Kato, S.; Senba, E.; Kobayashi, K.; et al. Involvement of mesolimbic dopaminergic network in neuropathic pain relief by treadmill exercise: A study for specific neural control with Gi-DREADD in mice. Mol. Pain 2016, 12, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenwood, B.N.; Foley, T.E.; Le, T.V.; Strong, P.V.; Loughridge, A.B.; Day, H.E.; Fleshner, M. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav. Brain Res. 2011, 217, 354–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kami, K.; Tajima, F.; Senba, E. Plastic changes in amygdala subregions by voluntary running contribute to exercise-induced hypoalgesia in neuropathic pain model mice. Mol. Pain 2020, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.G.; LeDoux, J.E. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992, 106, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Krabbe, S.; Gründemann, J.; Botta, P.; Fadok, J.P.; Osakada, F.; Saur, D.; Grewe, B.F.; Schnitzer, M.J.; Callaway, E.M.; et al. Distinct Hippocampal Pathways Mediate Dissociable Roles of Context in Memory Retrieval. Cell 2016, 167, 961–972. [Google Scholar] [CrossRef] [Green Version]
- Brellenthin, A.G.; Crombie, K.M.; Cook, D.B.; Sehgal, N.; Koltyn, K.F. Psychosocial Influences on Exercise-Induced Hypoalgesia. Pain Med. 2017, 18, 538–550. [Google Scholar] [CrossRef] [Green Version]
- Naugle, K.M.; Naugle, K.E.; Fillingim, R.B.; Riley, J.L., 3rd. Isometric exercise as a test of pain modulation: Effects of experimental pain test, psychological variables, and sex. Pain Med. 2014, 15, 692–701. [Google Scholar] [CrossRef] [Green Version]
- Weissman-Fogel, I.; Sprecher, E.; Pud, D. Effects of catastrophizing on pain perception and pain modulation. Exp. Brain Res. 2008, 186, 79–85. [Google Scholar] [CrossRef]
- Hoeger Bement, M.K.; Weyer, A.; Hartley, S.; Drewek, B.; Harkins, A.L.; Hunter, S.K. Pain perception after isometric exercise in women with fibromyalgia. Arch. Phys. Med. Rehabil. 2011, 92, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Vaegter, H.B.; Handberg, G.; Graven-Nielsen, T. Hypoalgesia After Exercise and the Cold Pressor Test is Reduced in Chronic Musculoskeletal Pain Patients With High Pain Sensitivity. Clin. J. Pain 2016, 32, 58–69. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kami, K.; Tajima, F.; Senba, E. Brain Mechanisms of Exercise-Induced Hypoalgesia: To Find a Way Out from “Fear-Avoidance Belief”. Int. J. Mol. Sci. 2022, 23, 2886. https://doi.org/10.3390/ijms23052886
Kami K, Tajima F, Senba E. Brain Mechanisms of Exercise-Induced Hypoalgesia: To Find a Way Out from “Fear-Avoidance Belief”. International Journal of Molecular Sciences. 2022; 23(5):2886. https://doi.org/10.3390/ijms23052886
Chicago/Turabian StyleKami, Katsuya, Fumihiro Tajima, and Emiko Senba. 2022. "Brain Mechanisms of Exercise-Induced Hypoalgesia: To Find a Way Out from “Fear-Avoidance Belief”" International Journal of Molecular Sciences 23, no. 5: 2886. https://doi.org/10.3390/ijms23052886
APA StyleKami, K., Tajima, F., & Senba, E. (2022). Brain Mechanisms of Exercise-Induced Hypoalgesia: To Find a Way Out from “Fear-Avoidance Belief”. International Journal of Molecular Sciences, 23(5), 2886. https://doi.org/10.3390/ijms23052886





