The Targeting of Nuclear Factor Kappa B by Drugs Adopted for the Prevention and Treatment of Preeclampsia
Abstract
1. Introduction
2. NFĸB and Its Relationship with Preeclampsia Development
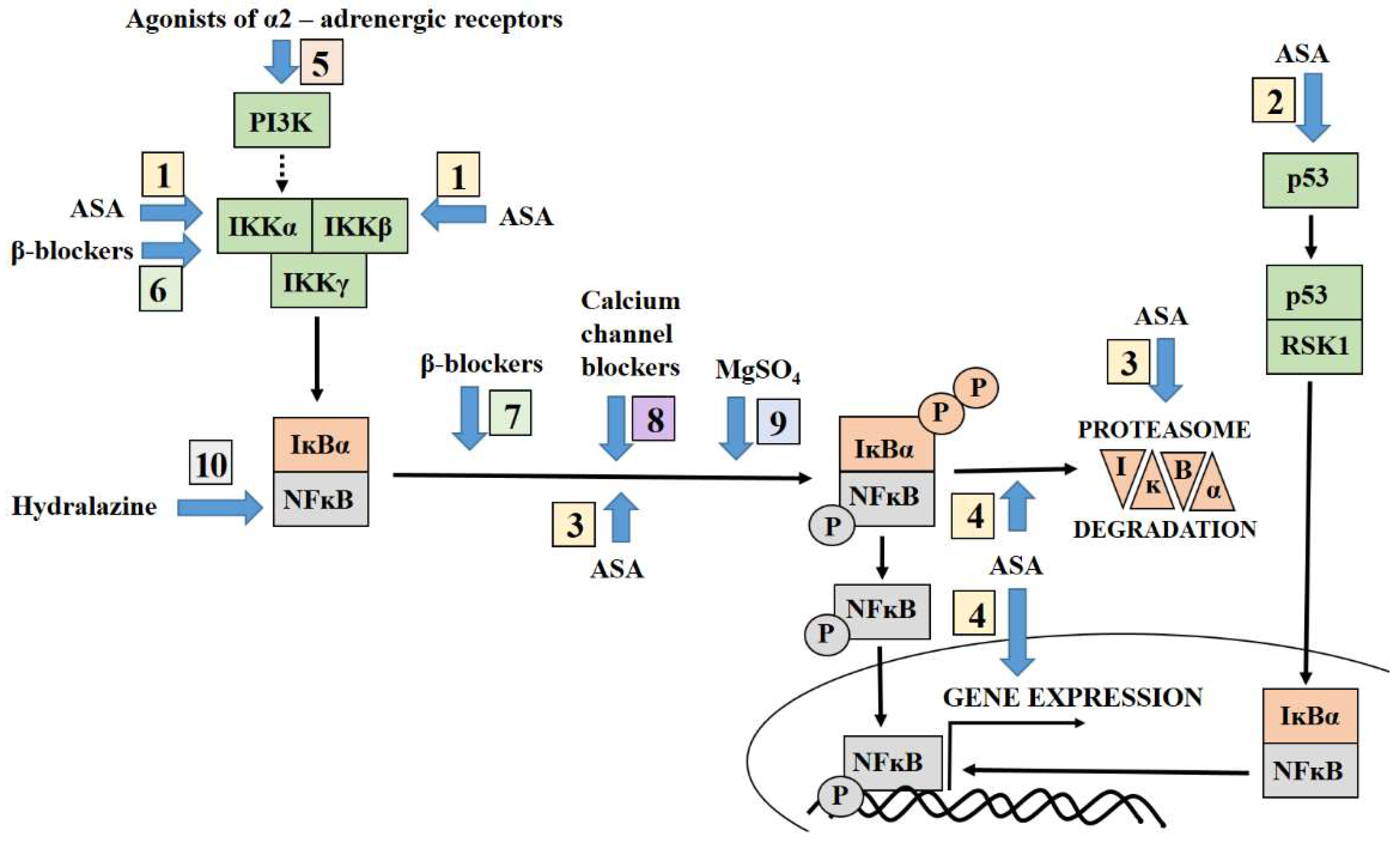
3. Targeting NFĸB by Aspirin
4. Antihypertensive Therapy Modulates the NFĸB Pathway
4.1. Agonist of Alpha-2 Adrenergic Receptors and NFĸB
4.2. Beta-Blockers Modulate NFĸB Activation Pathways
4.3. Calcium Channel Blockers Inhibit the NFĸB Activation Pathways
4.4. Hydralazine and Its Association with NFĸB
5. Targeting NFĸB by Magnesium Sulphate Adopted for Prevention of Neurological Complication of Preeclampsia
6. Directions for Future Research into the Treatment of Preeclampsia
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clark, T.P. Late-onset postpartum preeclampsia: A case study. Nurse Pract. 2014, 39, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.; Griffiths, M.; Nelson-Piercy, C.; Sinnamon, K. Pre-eclampsia before 20-week gestation: Diagnosis, investigation and management. Clin. Kidney J. 2012, 5, 597–599. [Google Scholar] [CrossRef] [PubMed][Green Version]
- ACOG Practice Bulletin No. 222 Clinical Management Guidelines for Obstetrician—Gynecologists Gestational Hypertension and Preeclampsia. Obstet. Gynecol. 2020, 135, e237–e260. [CrossRef]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Wageh, A.; Nagib, R.M.; Eid, M. Placenta Of Late Onset Preeclampsia Without Fetal Growth Restriction: Is It Different From The Normal? Evid. Based Women’s Health J. 2019, 9, 399–406. [Google Scholar] [CrossRef][Green Version]
- van Der Merwe, J.L.; Hall, D.R.; Wright, C.; Schubert, P.; Grové, D. Are early and late preeclampsia distinct subclasses of the diseasewhat does the placenta reveal. Hypertens. Pregnancy 2010, 29, 457–467. [Google Scholar] [CrossRef]
- Socha, M.W.; Malinowski, B.; Puk, O.; Wart, M.; Kazdepka-ziemi, A. The Role of NF-κB in Uterine Spiral Arteries Remodeling, Insight into the Cornerstone of Preeclampsia. Int. J. Mol. Sci. 2021, 22, 704. [Google Scholar] [CrossRef] [PubMed]
- Armistead, B.; Kadam, L.; Drewlo, S.; Kohan-Ghadr, H.R. The role of NFκB in healthy and preeclamptic placenta: Trophoblasts in the spotlight. Int. J. Mol. Sci. 2020, 21, 1775. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Kimura, T.; Ogita, K.; Koyama, S.; Tsujie, T.; Tsutsui, T.; Shimoya, K.; Koyama, M.; Kaneda, Y.; Murata, Y. Alteration of the timing of implantation by in vivo gene transfer: Delay of implantation by suppression of nuclear factor κB activity and partial rescue by leukemia inhibitory factor. Biochem. Biophys. Res. Commun. 2004, 321, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.W.; Ashworth, M.D.; Mathew, D.; Reagan, P.; Ritchey, J.W.; Hayashi, K.; Spencer, T.E.; Lucy, M.; Geisert, R.D. Activation of the transcription factor, nuclear factor kappa-B, during the estrous cycle and early pregnancy in the pig. Reprod. Biol. Endocrinol. 2010, 8, 39. [Google Scholar] [CrossRef]
- King, A.E.; Critchley, H.O.; Kelly, R.W. The NF-kappaB pathway in human endometrium and first trimester decidua. Mol. Hum. Reprod. 2001, 7, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Dekel, N.; Gnainsky, Y.; Granot, I.; Racicot, K.; Mor, G. The role of inflammation for a successful implantation. Am. J. Reprod. Immunol. 2014, 72, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Veerbeek, J.H.W.; Rana, T.K.; van Rijn, B.B.; Burton, G.J.; Yung, H.W. Role of Endoplasmic Reticulum Stress in pro-inflammatory Cytokine–Mediated Inhibition of Trophoblast Invasion in Placenta-Related Complications of Pregnancy. Am. J. Pathol. 2019, 189, 467–478. [Google Scholar] [CrossRef] [PubMed]
- van Mourik, M.S.M.; Macklon, N.S.; Heijnen, C.J. Embryonic implantation: Cytokines, adhesion molecules, and immune cells in establishing an implantation environment. J. Leukoc. Biol. 2009, 85, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Deb, K.; Chaturvedi, M.M.; Jaiswal, Y.K. A “minimum dose” of lipopolysaccharide required for implantation failure: Assessment of its effect on the maternal reproductive organs and interleukin-1α expression in the mouse. Reproduction 2004, 128, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Cotechini, T.; Komisarenko, M.; Sperou, A.; Macdonald-Goodfellow, S.; Adams, M.A.; Graham, C.H. Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J. Exp. Med. 2014, 211, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Litang, Z.; Hong, W.; Weimin, Z.; Xiaohui, T.; Qian, S. Serum NF-κBp65, TLR4 as biomarker for diagnosis of preeclampsia. Open Med. 2017, 12, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, J.E.; Walsh, S.W. Activation of NF-κB in Placentas of Women with Preeclampsia. Hypertens. Pregnancy 2012, 31, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Schreck, R.; Rieberl, P.; Baeuerle, P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-ĸB transcription factor and HIV-1. Embo 1991, 10, 2247–2258. [Google Scholar] [CrossRef]
- Pueyo, M.E.; Gonzalez, W.; Nicoletti, A.; Savoie, F.; Arnal, J.; Michel, J. Angiotensin II Stimulates Endothelial Vascular Cell Adhesion Molecule-1 via Nuclear Factor-ĸB Activation Induced by Intracellular Oxidative Stress. Arter. Thromb. Vasc. Biol. 2000, 20, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Sakowicz, A.; Bralewska, M.; Pietrucha, T.; Habrowska-Górczyńska, D.E.; Piastowska-Ciesielska, A.W.; Gach, A.; Rybak-Krzyszkowska, M.; Witas, P.J.; Huras, H.; Grzesiak, M.; et al. Canonical, non-canonical and atypical pathways of nuclear factor ĸB activation in preeclampsia. Int. J. Mol. Sci. 2020, 21, 5574. [Google Scholar] [CrossRef] [PubMed]
- Canty, T.G.; Boyle, E.M.; Farr, A.; Morgan, E.N.; Verrier, E.D.; Pohlman, T.H. Oxidative stress induces NF-κB nuclear translocation without degradation of IκBα. Circulation 1999, 100, 361–365. [Google Scholar] [CrossRef]
- Sakowicz, A.; Bralewska, M.; Pietrucha, T.; Figueras, F.; Habrowska-górczyńska, D.E.; Piastowska-ciesielska, A.W.; Gach, A.; Sakowicz, B.; Rybak-krzyszkowska, M.; Huras, H.; et al. The preeclamptic environment promotes the activation of transcription factor kappa b by p53/RSK1 complex in a HTR8/SVneo trophoblastic cell line. Int. J. Mol. Sci. 2021, 22, 10200. [Google Scholar] [CrossRef]
- Sakowicz, A.; Lisowska, M.; Biesiada, L.; Płuciennik, E.; Gach, A.; Rybak-Krzyszkowska, M.; Huras, H.; Sakowicz, B.; Romanowicz, H.; Piastowska-Ciesielska, A.W.; et al. Placental Expression of NEMO Protein in Normal Pregnancy and Preeclampsia. Dis. Markers 2019, 2019, 8418379. [Google Scholar] [CrossRef] [PubMed]
- Sankaralingam, S.; Xu, H.; Davidge, S.T. Arginase contributes to endothelial cell oxidative stress in response to plasma from women with preeclampsia. Cardiovasc. Res. 2010, 85, 194–203. [Google Scholar] [CrossRef]
- Guerby, P.; Tasta, O.; Swiader, A.; Pont, F.; Bujold, E.; Parant, O.; Vayssiere, C.; Salvayre, R.; Negre-Salvayre, A. Role of oxidative stress in the dysfunction of the placental endothelial nitric oxide synthase in preeclampsia. Redox Biol. 2021, 40, 101861. [Google Scholar] [CrossRef]
- Fiore, G.; Florio, P.; Micheli, L.; Nencini, C.; Rossi, M.; Cerretani, D.; Ambrosini, G.; Giorgi, G.; Petraglia, F. Endothelin-1 triggers placental oxidative stress pathways: Putative role in preeclampsia. J. Clin. Endocrinol. Metab. 2005, 90, 4205–4210. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Quehenberger, P.; Bierhaus, A.; Fasching, P.; Muellner, C.; Klevesath, M.; Hong, M.; Stier, G.; Sattler, M.; Schleicher, E.; Speiser, W.; et al. Endothelin 1 transcription is controlled by nuclear factor-κB in AGE-stimulated cultured endothelial cells. Diabetes 2000, 49, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.E.; Min, J.Y.; Merchan, J.; Lim, K.H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.G.; Sargent, I.L. Placental Debris, Oxidative Stress and Pre-eclampsia. Placenta 2000, 21, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Tolcher, M.C.; Sangi-Haghpeykar, H.; Mendez-Figueroa, H.; Aagaard, K.M. Low-dose aspirin for preeclampsia prevention: Efficacy by ethnicity and race. Am. J. Obstet. Gynecol. MFM 2020, 2, 100184. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Huai, J.; Li, B.; Zhu, Y.; Juan, J.; Zhang, M.; Cui, S.; Zhao, X.; Ma, Y.; Zhao, Y.; et al. A randomized controlled trial of low-dose aspirin for the prevention of preeclampsia in women at high risk in China. Am. J. Obstet. Gynecol. 2022, 226, 251.e1–251.e12. [Google Scholar] [CrossRef]
- van Doorn, R.; Mukhtarova, N.; Flyke, I.P.; Lasarev, M.; Kim, K.M.; Hennekens, C.H.; Hoppe, K.K. Dose of aspirin to prevent preterm preeclampsia in women with moderate or high-risk factors: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0247782. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia; WHO: Geneva, Switzerland, 2011; ISBN 9789241548335. [Google Scholar]
- Stepan, H.; Kuse-Föhl, H.; Klockenbusch, W.; Rath, W.; Schauf, B.; Walther, T.; Schlembach, D. Diagnosis and treatment guideline of hypertensive disorders in pregnancy (2015). Geburtshilfe Frauenheilkd. 2015, 75, 900–914. [Google Scholar] [CrossRef] [PubMed]
- Mounier-Vehier, C.; Amar, J.; Boivin, J.M.; Denolle, T.; Fauvel, J.P.; Plu-Bureau, G.; Tsatsaris, V.; Blacher, J. Hypertension and pregnancy: Expert consensus statement from the French Society of Hypertension, an affiliate of the French Society of Cardiology. Fundam. Clin. Pharmacol. 2017, 31, 83–103. [Google Scholar] [CrossRef]
- ACOG Committee Opinion No. 743 Low-Dose Aspirin Use During Pregnancy. Obstet. Gynecol. 2018, 132, e44–e52. [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- New Zealand Committee of The Royal Australian & New Zealand College of Obstetricians & Gynaecologists. Guidance Regarding the Use of Low-Dose Aspirin in the Prevention of Pre-Eclampsia in High-Risk Women; RANZCOG: Melbourne, Australia, 2018. [Google Scholar]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018, 13, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; da Silva Costa, F.; von Dadelszen, P.; McIntyre, H.D.; et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynecol. Obstet. 2019, 145, 1–33. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Hypertension in Pregnancy: Diagnosis and Management (NG133); NICE Guideline; National Institute for Health and Care Excellence: London, UK, 2019; pp. 1–55. Available online: https://www.nice.org.uk/guidance/ng133.html (accessed on 7 February 2022).
- Polish Society of Hypertension. Management of hypertension in pregnancy—Prevention, diagnosis, treatment and long-term prognosis Ginekol. Perinatol. Prakt. 2019, 4, 43–111. (In Polish) [Google Scholar] [CrossRef]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef] [PubMed]
- Combs, C.A.; Montgomery, D.M. Society for Maternal-Fetal Medicine Special Statement: Checklists for preeclampsia risk-factor screening to guide recommendations for prophylactic low-dose aspirin. Am. J. Obstet. Gynecol. 2020, 223, B7–B11. [Google Scholar] [CrossRef]
- Atallah, A.; Lecarpentier, E.; Goffinet, F.; Doret-Dion, M.; Gaucherand, P.; Tsatsaris, V. Aspirin for Prevention of Preeclampsia. Drugs 2017, 77, 1819–1831. [Google Scholar] [CrossRef] [PubMed]
- Kopp, E.; Ghosh, S. Inhibition of NF-κB by sodium salicylate and aspirin. Science 1994, 265, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Sehnert, B.; Burkhardt, H.; Dübel, S.; Voll, R.E. Cell-Type Targeted NF-kappaB Inhibition for the Treatment of Inflammatory Diseases. Cells 2020, 9, 1627. [Google Scholar] [CrossRef]
- D’Acquisto, F.; Iuvone, T.; Rombolà, L.; Sautebin, L.; Di Rosa, M.; Carnuccio, R. Involvement of NF-κB in the regulation of cyclooxygenase-2 protein expression in LPS-stimulated J774 macrophages. FEBS Lett. 1997, 418, 175–178. [Google Scholar] [CrossRef]
- Sakowicz, A. The role of NFκB in the three stages of pregnancy—Implantation, maintenance, and labour: A review article. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Zhong, L.; Duan, T.; Zhang, R.H.; Wang, X.; Wang, G.; Hu, K.; Lv, X.; Kang, T. Aspirin Suppresses the Growth and Metastasis of Osteosarcoma through the NF-κB Pathway. Clin. Cancer Res. 2015, 21, 5349–5359. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.G.; Lee, S.; Lee, C.T.; Kim, Y.W.; Han, S.K.; Shim, Y.S. Effect of acetylsalicylic acid on endogenous IκB kinase activity in lung epithelial cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2001, 280, L3–L9. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.J.; Yamamoto, Y.; Gaynor, R.B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature 1998, 396, 77–80. [Google Scholar] [CrossRef]
- Kim, S.Y.; Ryu, H.M.; Jae, H.Y.; Kim, M.Y.; Ahn, H.K.; Lim, H.J.; Shin, J.S.; Woo, H.J.; Park, S.Y.; Kim, Y.M.; et al. Maternal serum levels of VCAM-1, ICAM-1 and E-selectin in preeclampsia. J. Korean Med. Sci. 2004, 19, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Austgulen, R.; Lien, E.; Vince, G.; Redman, C.W.G. Increased maternal plasma levels of soluble adhesion molecules (ICAM-1, VCAM-1, E-selectin) in preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 1997, 71, 53–58. [Google Scholar] [CrossRef]
- Weber, C.; Erl, W.; Pietsch, A.; Weber, P.C. Aspirin Inhibits Nuclear Factor–κB Mobilization and Monocyte Adhesion in Stimulated Human Endothelial Cells. Circulation 1995, 91, 1914–1917. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.W.; Read, M.A.; Ding, H.; Luscinskas, F.W.; Collins, T. Salicylates inhibit IκB-α phosphorylation, endothelial-leukocyte adhesion molecule expression, and neutrophil transmigration. J. Immunol. 1996, 156, 3961–3969. [Google Scholar] [PubMed]
- Walsh, S.W.; Reep, D.T.; Alam, S.M.K.; Washington, S.L.; Al Dulaimi, M.; Lee, S.M.; Springel, E.H.; Strauss, J.F.; Stephenson, D.J.; Chalfant, C.E. Placental Production of Eicosanoids and Sphingolipids in Women Who Developed Preeclampsia on Low-Dose Aspirin. Reprod. Sci. 2020, 27, 2158–2169. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.W.; Strauss, J.F. The road to low-dose aspirin therapy for the prevention of preeclampsia began with the placenta. Int. J. Mol. Sci. 2021, 22, 6985. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ma, L.; Lin, L.; Wang, Y.L.; Yang, H. The intervention effect of aspirin on a lipopolysaccharide-induced preeclampsia-like mouse model by inhibiting the nuclear factor-κB pathway. Biol. Reprod. 2018, 99, 422–432. [Google Scholar] [CrossRef]
- Zuo, Q.; Zou, Y.; Huang, S.; Wang, T.; Xu, Y.; Zhang, T.; Zhang, M.; Ge, Z.; Jiang, Z. Aspirin reduces sFlt-1-mediated apoptosis of trophoblast cells in preeclampsia. Mol. Hum. Reprod. 2021, 27, gaaa089. [Google Scholar] [CrossRef]
- Panagodage, S.; Yong, H.E.J.; Da Silva Costa, F.; Borg, A.J.; Kalionis, B.; Brennecke, S.P.; Murthi, P. Low-Dose Acetylsalicylic Acid Treatment Modulates the Production of Cytokines and Improves Trophoblast Function in an in Vitro Model of Early-Onset Preeclampsia. Am. J. Pathol. 2016, 186, 3217–3224. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, X.; Zhu, L.; Shen, Y.; Chengedza, S.; Feng, H.; Wang, L.; Jung, J.U.; Gutkind, J.S.; Feng, P. IKK epsilon kinase is crucial for viral G protein-coupled receptor tumorigenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 11139–11144. [Google Scholar] [CrossRef]
- Bohuslav, J.; Chen, L.F.; Kwon, H.; Mu, Y.; Greene, W.C. p53 induces NF-κB activation by an IκB kinase-independent mechanism involving phosphorylation of p65 by ribosomal S6 kinase 1. J. Biol. Chem. 2004, 279, 26115–26125. [Google Scholar] [CrossRef]
- Costanzo, V.; Bardelli, A.; Siena, S.; Abrignani, S. Exploring the links between cancer and placenta development. Open Biol. 2018, 8, 180081. [Google Scholar] [CrossRef]
- Kutuk, O.; Basaga, H. Aspirin inhibits TNFα- and IL-1-induced NF-κB activation and sensitizes HeLa cells to apoptosis. Cytokine 2004, 25, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Kutuk, O.; Basaga, H. Aspirin prevents apoptosis and NF-κB activation induced by H2O2 in HeLa cells. Free Radic. Res. 2003, 37, 1267–1276. [Google Scholar] [CrossRef]
- Din, F.V.N.; Dunlop, M.G.; Stark, L.A. Evidence for colorectal cancer cell specificity of aspirin effects on NFkB signalling and apoptosis. Br. J. Cancer 2004, 91, 381–388. [Google Scholar] [CrossRef]
- Stark, L.A.; Din, F.V.N.; Zwacka, R.M.; Dunlop, M.G. Aspirin-induced activation of the NF-kB signaling pathway: A novel mechanism for aspirin-mediated apoptosis in colon cancer cells 1. FASEB J. 2001, 15, 1273–1275. [Google Scholar] [CrossRef]
- Din, F.V.N.; Stark, L.A.; Dunlop, M.G. Aspirin-induced nuclear translocation of NFkB and apoptosis in colorectal cancer is independent of p53 status and DNA mismatch repair proficiency. Br. J. Cancer 2005, 92, 1137–1143. [Google Scholar] [CrossRef]
- Stark, L.A.; Reid, K.; Sansom, O.J.; Din, V.; Guichard, S.; Mayer, I.; Jodrell, D.I.; Clarke, A.R.; Dunlop, M.G. Aspirin activates the NF-kB signalling pathway and induces apoptosis in intestinal neoplasia in two in vivo models of human colorectal cancer. Carcinogenesis 2007, 28, 968–976. [Google Scholar] [CrossRef]
- Wang, W.; Xie, X.; Yuan, T.; Wang, Y.; Zhao, F.; Zhou, Z.; Zhang, H. Epidemiological trends of maternal hypertensive disorders of pregnancy at the global, regional, and national levels: A population - based study. BMC Pregnancy Childbirth 2021, 21, 364. [Google Scholar] [CrossRef] [PubMed]
- Magee, L.A.; Dadelszen, P. Von Management of Hypertension in Pregnancy. Matern. Med. 2021, 3, 124–135. [Google Scholar] [CrossRef]
- Rabi, D.M.; Mcbrien, K.A.; Sapir-pichhadze, R.; Nakhla, M.; Ahmed, B.; Dumanski, S.M.; Butalia, S.; Leung, A.A.; Harris, K.C.; Cloutier, L.; et al. Hypertension Canada’s 2020 Comprehensive Guidelines for the Prevention, Diagnosis, Risk Assessment, and Treatment of Hypertension in Adults and Children. Can. J. Cardiol. 2020, 36, 596–624. [Google Scholar] [CrossRef]
- Lowe, S.A.; Bowyer, L.; Lust, K.; Mcmahon, L.P.; Morton, M.; North, R.A.; Paech, M.; Said, J.M. SOMANZ guidelines for the management of hypertensive disorders of pregnancy 2014. Aust. N. Z. J. Obstet. Gynaecol. 2015, 55, e1–e29. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.M.; Garovic, V.D. Drug treatment of hypertension in pregnancy. Drugs 2014, 74, 283–296. [Google Scholar] [CrossRef]
- Lymperopoulos, A.; Karkoulias, G.; Koch, W.J.; Flordellis, C.S. α2-Adrenergic receptor subtype-specific activation of NF-kB in PC12 cells. Neurosci. Lett. 2006, 402, 210–215. [Google Scholar] [CrossRef]
- Bailey, L.J.; Alahari, S.; Tagliaferro, A.; Post, M.; Caniggia, I. Augmented trophoblast cell death in preeclampsia can proceed via ceramide-mediated necroptosis. Cell Death Dis. 2017, 8, e2590. [Google Scholar] [CrossRef] [PubMed]
- Safi, S.Z.; Shah, H.; Qvist, R.; Bindal, P.; Mansor, M.; Yan, G.O.S.; Ismail, I.S. Bin Beta Adrenergic Receptors Stimulation Attenuates Phosphorylation of NF-κB and IκBα in Hyperglycemic Endothelial Cells. Cell. Physiol. Biochem. 2018, 51, 1429–1436. [Google Scholar] [CrossRef]
- Yang, S.; Ho, L.; Lin, Y.; Cheng, S.; Tsao, T.; Chang, D.; Hsu, Y.; Shih, C.; Juan, T.; Lai, J. Carvedilol, a new antioxidative β-blocker, blocks in vitro human peripheral blood T cell activation by downregulating NFĸB activity. Cardiovasc. Res. 2003, 59, 776–787. [Google Scholar] [CrossRef]
- ACOG Committee Opinion No 767 Emergent Therapy for Acute-Onset, Severe Hypertension during Pregnancy and the Postpartum Period. Obstet. Gynecol. 2019, 133, 409–412. [CrossRef]
- Matsumori, A.; Nunokawa, Y.; Sasayama, S. Nifedipine inhibits activation of transcription factor NF-kB. Life Sci. 2000, 67, 2655–2661. [Google Scholar] [CrossRef]
- Takase, H.; Toriyama, T.; Sugiyama, M.; Nakazawa, A.; Hayashi, K.; Goto, T.; Sugiura, T.; Ikeda, K.; Sato, K.; Ueda, R.; et al. Effect of Nifedipine on C-Reactive Protein Levels in the Coronary Sinus and on Coronary Blood Flow in Response to Acetylcholine in Patients With Stable Angina Pectoris Having Percutaneous Coronary Intervention. Am. J. Cardiol. 2005, 95, 1235–1237. [Google Scholar] [CrossRef] [PubMed]
- Sarada, S.K.S.; Himadri, P.; Mathew, T.; Saumya, S.; Chitharanjan, M. Respiratory Physiology & Neurobiology Nifedipine inhibits hypoxia induced transvascular leakage through down regulation of NFkB. Respir. Physiol. Neurobiol. 2012, 183, 26–34. [Google Scholar] [CrossRef]
- Chen, Q.; Guo, F.; Liu, S.; Xiao, J.; Wang, C.; Snowise, S.; Stone, P.R.; Chamley, L.W. Calcium channel blockers prevent endothelial cell activation in response to necrotic trophoblast debris: Possible relevance to pre-eclampsia. Cardiovasc. Res. 2012, 96, 484–493. [Google Scholar] [CrossRef]
- Karna, E.; Szoka, L.; Palka, J.A. The mechanism of hydralazine-induced collagen biosynthesis in cultured fibroblasts. Naunyn-Schmiedebergs Arch. Pharmacol. 2013, 386, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.N.; Mervaala, E.M.A.; Schmidt, F.; Park, J.-K.; Dechend, R.; Genersch, E.; Breu, V.; Loffler, B.-M.; Ganten, D.; Schneider, W.; et al. Effect of Bosentan on NF-B, Inflammation, and Tissue Factor in Angiotensin II—Induced End-Organ Damage. Hypertension 2000, 36, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Bouguerne, B.; Belkheiri, N.; Bedos-Belval, F.; Vindis, C.; Uchida, K.; Duran, H.; Grazide, M.-H.; Baltas, M.; Salvayre, R.; Nègre-Salvayre, A. Antiatherogenic Effect of Bisvanillyl-Hydralazone, a New Hydralazine Derivative with Antioxidant, Carbonyl Scavenger, and Antiapoptotic Properties. Antioxid. Redox Signal 2011, 14, 2093–2106. [Google Scholar] [CrossRef]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef]
- Beloosesky, R.; Khatib, N.; Anabusi, S.; Ginsberg, Y.; Ross, M.G.; Weiner, Z. Maternal magnesium sulphate (Mg) fetal neuroprotective effects to the fetus: Inhibition of nNOS and NFkB activation. Am. J. Obstet. Gynecol. 2016, 18, s76. [Google Scholar] [CrossRef]
- Gao, F.; Ding, B.; Zhou, L.; Gao, X.; Guo, H.; Xu, H. Magnesium sulfate provides neuroprotection in lipopolysaccharide-activated primary microglia by inhibiting NF-kB pathway. J. Surg. Res. 2013, 184, 944–950. [Google Scholar] [CrossRef]
- Sugimoto, J.; Romani, A.M.; Valentin-Torres, A.M.; Luciano, A.A.; Kitchen, C.M.R.; Funderburg, N.; Mesiano, S.; Bernstein, H.B. Magnesium Decreases Inflammatory Cytokine Production: A Novel Innate Immunomodulatory Mechanism. J. Immunol. 2012, 188, 6338–6346. [Google Scholar] [CrossRef]
- Zhang, L.W.; Warrington, J.P. Magnesium Sulfate Prevents Placental Ischemia-Induced Increases in Brain Water Content and Cerebrospinal Fluid Cytokines in Pregnant Rats. Front. Neurosci. 2016, 10, 561. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Xu, L.; Huang, Y.; Chen, T.; Zhou, T.; Yang, L. Magnesium sulphate can alleviate oxidative stress and reduce inflammatory cytokines in rat placenta of intrahepatic cholestasis of pregnancy model. Arch. Gynecol. Obstet. 2018, 298, 631–638. [Google Scholar] [CrossRef]
- Kovo, M.; Mevorach-zussman, N.; Khatib, N.; Ginsberg, Y.; Divon, M.; Weiner, Z.; Bar, J.; Beloosesky, R. The Effects of Magnesium Sulfate on the Inflammatory Response of Placentas Perfused With Lipopolysaccharide: Using the Ex Vivo Dual-Perfused Human Single-Cotyledon Model. Reprod. Sci. 2017, 25, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-K.; Liu, L.; Odouli, R. Infarction: Exposure to non-steroidal anti-inflammatory drugs during pregnancy and risk of miscarriage: Population based cohort study. BMJ 2003, 327, 368. [Google Scholar] [CrossRef] [PubMed]
- Keim, S.A.; Klebanoff, M.A. Aspirin use and miscarriage risk. Epidemiology 2006, 17, 435–439. [Google Scholar] [CrossRef]
- Borges, A.L.V.; Borges do Nascimento Chofakian, C.; Sayuri Sato, A.P. Pregnancy and Non-Sexually Transmitted Infections; Oxford Research Encyclopedia of Global Public Health: Oxford, UK, 2020; ISBN 978-019-063-236-6. [Google Scholar]
- Espinoza, J.; Romero, R.; Yeon, M.K.; Kusanovic, J.P.; Hassan, S.; Erez, O.; Gotsch, F.; Than, N.G.; Papp, Z.; Chong, J.K. Normal and abnormal transformation of the spiral arteries during pregnancy. J. Perinat. Med. 2006, 34, 447–458. [Google Scholar] [CrossRef]
- Wang, Y.; Alexander, J.S. Placental pathophysiology in preeclampsia. Pathophysiology 2000, 6, 261–270. [Google Scholar] [CrossRef]
- Roberge, S.; Nicolaides, K.; Demers, S.; Hyett, J.; Chaillet, N.; Bujold, E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: Systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2017, 216, 110–120.e6. [Google Scholar] [CrossRef] [PubMed]
| Selected Word Organisation (Year) | ASA Dose | Initiation ASA Supplement | Bibliograph |
|---|---|---|---|
| World Health Organisation (WHO) (2011) | 75 mg/day | <week 20 | [35] |
| German Society of Gynaecology and Obstetrics (DGGG) (2015) | 100 mg/day | no data | [36] |
| French Society of Cardiology (FESC)/French Society of Hypertension (2016) | 75–160 mg/day | <week 20 | [37] |
| The American College of Obstetricians and Gynaecologists (ACOG) (2018) | 81 mg/day | week 12–28 Optimum <16 | [38] |
| European Society of cardiology (ESC)/European Society of Hypertension (ESH) (2018) | 100–150 mg/day | week 12 | [39] |
| New Zealand Committee of the Royal Australian & New Zealand College of Obstetricians & Gynaecologists (RANZCOG) New Zealand College of Midwives (NZCOM) (2018) | ≥75 mg/day optimum 100 mg/day | week 12 | [40] |
| The International Society for the Study of Hypertension in Pregnancy (ISSHP) (2018) | 75–162 mg/day | <week 20 Optimum <16 | [41] |
| International Federation of Gynaecology and Obstetrics (FIGO) (2019) | 150 mg/day (at night) | week 11–14 | [42] |
| National Institute for Health and Care Excellence (NICE) (2019) | 75–150 mg/day ** | week 12 | [43] |
| Polish Society of Hypertension (PTNT), Polish Cardiac Society (PTK) and Polish Society of Gynaecologists and Obstetricians (PTGiP) (2019) | 100–150 mg/day | <week 16 | [44] |
| International Society of Hypertension (ISH) (2020) | 75–162 mg/day | week 12 | [45] |
| Society for Maternal-Foetal Medicine (SMFM) (2020) | 81 mg/day | week 12–28 Optimum <16 | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakowicz, A. The Targeting of Nuclear Factor Kappa B by Drugs Adopted for the Prevention and Treatment of Preeclampsia. Int. J. Mol. Sci. 2022, 23, 2881. https://doi.org/10.3390/ijms23052881
Sakowicz A. The Targeting of Nuclear Factor Kappa B by Drugs Adopted for the Prevention and Treatment of Preeclampsia. International Journal of Molecular Sciences. 2022; 23(5):2881. https://doi.org/10.3390/ijms23052881
Chicago/Turabian StyleSakowicz, Agata. 2022. "The Targeting of Nuclear Factor Kappa B by Drugs Adopted for the Prevention and Treatment of Preeclampsia" International Journal of Molecular Sciences 23, no. 5: 2881. https://doi.org/10.3390/ijms23052881
APA StyleSakowicz, A. (2022). The Targeting of Nuclear Factor Kappa B by Drugs Adopted for the Prevention and Treatment of Preeclampsia. International Journal of Molecular Sciences, 23(5), 2881. https://doi.org/10.3390/ijms23052881






