MSCs as Tumor-Specific Vectors for the Delivery of Anticancer Agents—A Potential Therapeutic Strategy in Cancer Diseases: Perspectives for Quinazoline Derivatives
Abstract
:1. Introduction
2. General Properties of MSCs
3. Therapeutic Strategies Based on MSCs
3.1. Interactions of MSCs with Normal and Tumor Cells
3.2. MSCs Can Affect PI3K/AKT and Wnt Signaling Pathways in the Tumor Microenvironment
3.3. The Use of MSCs as Vehicles of Various Therapies
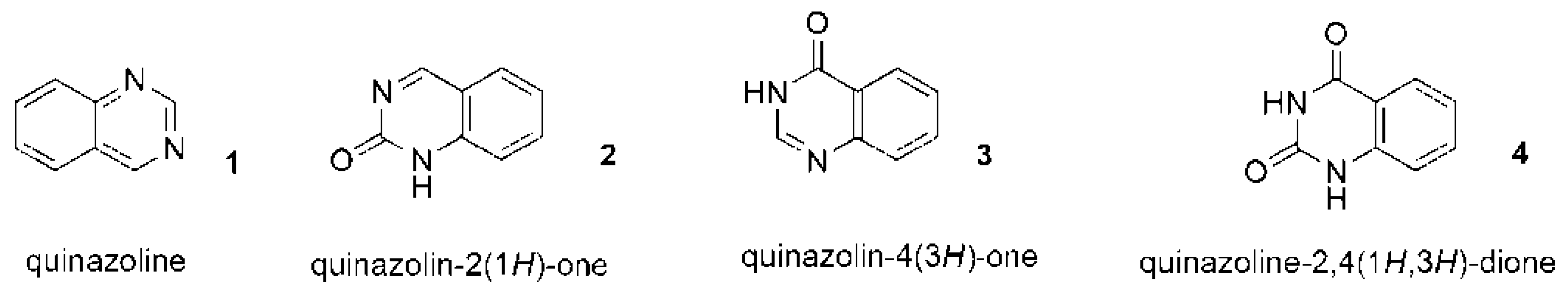
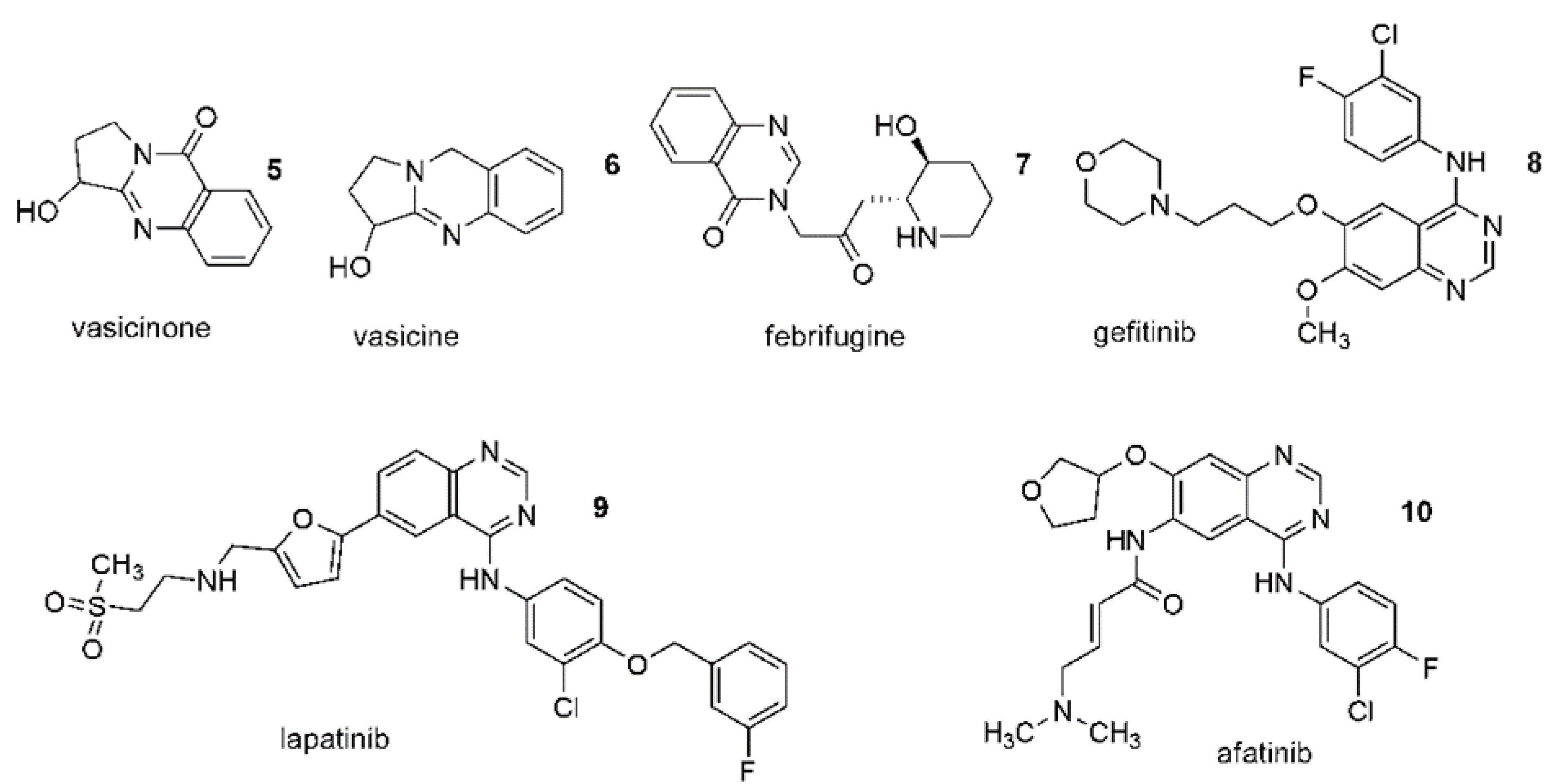
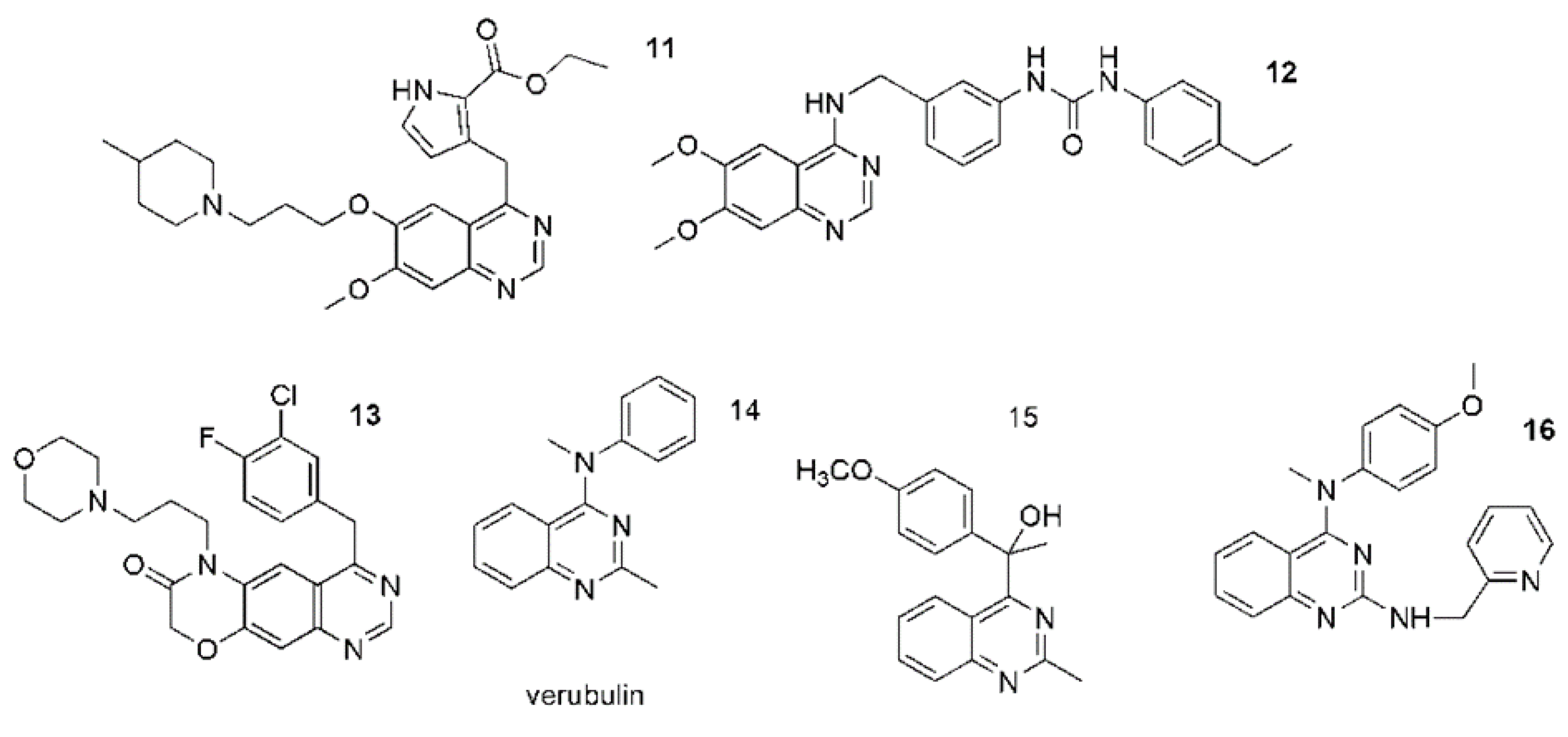
4. Are anticancer Quinazoline Derivatives Good Candidates to Be Delivered with MSCs?
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.; Li, T.; Wu, W.; Ding, G. Interplay between mesenchymal stem cell and tumor and potential application. Hum. Cell 2020, 33, 444–458. [Google Scholar] [CrossRef]
- Timanera, M.; Tsaib, K.K.; Shakeda, Y. The multifaceted role of mesenchymal stem cells in cancer. Semin. Cancer Biol. 2020, 60, 225–237. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Piatetzky-Shapiro, I.I.; Petrakova, K.V. Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 1966, 16, 381–390. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblastcolonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Prolif. 1970, 3, 393–403. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef]
- Goshima, J.; Goldberg, V.M.; Caplan, A.I. The osteogenic potential of culture-expanded rat marrow mesenchymal cells assayed in vivo in calcium phosphate phosphate ceramic. Biomaterials 1991, 12, 253–258. [Google Scholar] [CrossRef]
- Salem, H.K.; Thiemermann, C. Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells 2010, 28, 585–596. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Yang, X.; Han, Z.P.; Qu, F.F.; Shao, L.; Shi, Y.F. Mesenchymal stem cells: A new trend for cell therapy. Acta Pharmacol. Sin. 2013, 34, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Niess, H.; Thomas, M.N.; Schiergens, T.S.; Kleespies, A.; Jauch, K.W.; Bruns, C.; Werner, J.; Nelson, P.J.; Angele, M.K. Genetic engineering of mesenchymal stromal cells for cancer therapy: Turning partners in crime into Trojan Horses. Innov. Surg. Sci. 2016, 1, 19–32. [Google Scholar] [CrossRef]
- Dominici, M.L.B.K.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Kauer, J.; Schwartz, K.; Tandler, C.; Hinterleitner, C.; Roerden, M.; Jung, G.; Salih, H.R.; Heitmann, J.S.; Märklin, M. CD105 (Endoglin) as negative prognostic factor in AML. Sci. Rep. 2019, 9, 18337. [Google Scholar] [CrossRef]
- Yang, J.; Liao, X.; Yu, J.; Zhou, P. Role of CD73 in Disease: Promising Prognostic Indicator and Therapeutic Target. Curr. Med. Chem. 2018, 25, 2260–2271. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bhanja, A.; Bhattacharyya, J.; Jaganathan, B.G. Multiple roles of CD90 in cancer. Tumour Biol. 2016, 37, 11611–11622. [Google Scholar] [CrossRef] [PubMed]
- Moses, H.L.; Roberts, A.B.; Derynck, R. The Discovery and Early Days of TGF-β: A Historical Perspective. Cold Spring Harb. Perspect. Biol. 2016, 8, a021865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roh, M.; Wainwright, D.A.; Wu, J.D.; Wan, Y.; Zhang, B. Targeting CD73 to augment cancer immunotherapy. Curr. Opin. Pharmacol. 2020, 53, 66–76. [Google Scholar] [CrossRef]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells-current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef]
- Mosna, F.; Sensebé, L.; Krampera, M. Human bone marrow and adipose tissue mesenchymal stem cells: A user’s guide. Stem Cells Dev. 2010, 19, 1449–1470. [Google Scholar] [CrossRef]
- Dazzi, F.; Ramasamy, R.; Glennie, S.; Jones, S.P.; Roberts, I. The role of mesenchymal stem cells in haemopoiesis. Blood Rev. 2006, 20, 161–171. [Google Scholar] [CrossRef]
- Miana, V.V.; Gonzalez, E.A.P. Adipose tissue stem cells in regenerative medicine. Ecancermedicalscience 2018, 12, 822. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Sun, Z.; Cui, D.; Xu, X.; Zhu, H.; Wang, L.; Qian, W.; Han, X. Isolation and characterization of lung resident mesenchymal stem cells capable of differentiating into alveolar epithelial type II cells. Cell Biol. Int. 2014, 38, 405–411. [Google Scholar] [CrossRef]
- Lecourt, S.; Marolleau, J.P.; Fromigue, O.; Vauchez, K.; Andriamanalijaona, R.; Ternaux, B.; Lacassagne, M.-N.; Robert, I.; Boumédiene, K.; Chéreau, F.; et al. Characterization of distinct mesenchymallike cell populations from human skeletal muscle in situ and in vitro. Exp. Cell Res. 2010, 316, 2513–2526. [Google Scholar] [CrossRef]
- Trivanovic, D.; Kocic, J.; Mojsilovic, S.; Krstic, A.; Ilic, V.; Djordjevic, I.O.; Santibanez, J.F.; Jovcić, G.; Terzić, M.; Bugarski, D. Mesenchymal stem cells isolated from peripheral blood and umbilical cord Wharton’s jelly. Srp. Arh. Celok. Lek. 2013, 141, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Delo, D.M.; de Coppi, P.; Bartsch, G., Jr.; Atala, A. Amniotic fluid and placental stem cells. Methods Enzymol. 2006, 419, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Pelekanos, R.A.; Sardesai, V.S.; Futrega, K.; Lott, W.B.; Kuhn, M.; Doran, M.R. Isolation and expansion of mesenchymal Stem/Stromal cells derived from human placenta tissue. J. Vis. Exp. 2016, 112, e54204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leuning, D.G.; Beijer, N.R.M.; du Fosse, N.A.; Vermeulen, S.; Lievers, E.; van Kooten, C.; Rabelink, T.J.; de Boer, J. The cytokine secretion profile of mesenchymal stromal cells is determined by surface structure of the microenvironment. Sci. Rep. 2018, 8, 7716. [Google Scholar] [CrossRef]
- Klingemann, H.; Matzilevich, D.; Marchand, J. Mesenchymal stem cells-sources and clinical applications. Transfus. Med. Hemother. 2008, 35, 272–277. [Google Scholar] [CrossRef]
- Hwang, N.S.; Zhang, C.; Hwang, Y.S.; Varghese, S. Mesenchymal stem cell differentiation and roles in regenerative medicine. Wiley Interdiscip. Rev. Syst. Biol. Med. 2009, 1, 97–106. [Google Scholar] [CrossRef]
- Almeida-Porada, G.; Atala, A.J.; Porada, C.D. Therapeutic Mesenchymal Stromal Cells for Immunotherapy and for Gene and Drug Delivery. Mol. Ther. Methods Clin. Dev. 2020, 16, 204–224. [Google Scholar] [CrossRef] [Green Version]
- Chulpanova, D.S.; Kitaeva, K.V.; Tazetdinova, L.G.; James, V.; Rizvanov, A.A.; Solovyeva, V.V. Application of Mesenchymal Stem Cells for Therapeutic Agent Delivery in Anti-tumor Treatment. Front. Pharmacol. 2018, 9, 259. [Google Scholar] [CrossRef]
- Gil-Kulik, P.; Chomik, P.; Krzyżanowski, A.; Radzikowska-Büchner, E.; Maciejewski, R.; Kwaśniewska, A.; Rahnama, M.; Kocki, J. Influence of the Type of Delivery, Use of Oxytocin, and Maternal Age on POU5F1 Gene Expression in Stem Cells Derived from Wharton’s Jelly within the Umbilical Cord. Oxid. Med. Cell. Longev. 2019, 2019, 1027106. [Google Scholar] [CrossRef]
- Tang, X.J.; Lu, J.T.; Tu, H.J.; Huang, K.M.; Fu, R.; Cao, G.; Huang, M.; Cheng, L.H.; Dai, L.J.; Zhang, L. TRAIL-engineered bone marrow-derived mesenchymal stem cells: TRAIL expression and cytotoxic effects on C6 glioma cells. Anticancer Res. 2014, 34, 729–734. [Google Scholar] [PubMed]
- Ge, X.; Shi, K.; Hou, J.; Fu, Y.; Xiao, H.; Chi, F.; Xu, J.; Cai, F.; Bai, C. Galectin-1 secreted by bone marrow-derived mesenchymal stem cells mediates anti-inflammatory responses in acute airway disease. Exp. Cell Res. 2021, 407, 112788. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, E.; Abnous, K.; Farzad, S.A.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Targeted doxorubicin-loaded mesenchymal stem cells-derived exosomes as a versatile platform for fighting against colorectal cancer. Life Sci. 2020, 261, 118369. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, A.; Sordi, V.; Dugnani, E.; Ceserani, V.; Dossena, M.; Coccè, V.; Cavicchini, L.; Ciusani, E.; Bondiolotti, G.; Piovani, G.; et al. Gemcitabine-releasing mesenchymal stromal cells inhibit in vitro proliferation of human pancreatic carcinoma cells. Cytotherapy 2015, 17, 1687–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Gao, J.; Ouyang, X.; Wang, J.; Sun, X.; Lv, Y. Mesenchymal stem cells loaded with paclitaxel-poly(lactic-co-glycolic acid) nanoparticles for glioma-targeting therapy. Int. J. Nanomed. 2018, 13, 5231–5248. [Google Scholar] [CrossRef] [Green Version]
- Pessina, A.; Leonetti, C.; Artuso, S.; Benetti, A.; Dessy, E.; Pascucci, L.; Passeri, D.; Orlandi, A.; Berenzi, A.; Bonomi, A.; et al. Drug-releasing mesenchymal cells strongly suppress B16 lung metastasis in a syngeneic murine model. J. Exp. Clin. Cancer Res. 2015, 34, 82. [Google Scholar] [CrossRef] [Green Version]
- Pascucci, L.; Coccè, V.; Bonomi, A.; Ami, D.; Ceccarelli, P.; Ciusani, E.; Viganò, L.; Locatelli, A.; Sisto, F.; Doglia, S.M.; et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J. Control. Release 2014, 192, 262–270. [Google Scholar] [CrossRef]
- Dembinski, J.L.; Wilson, S.M.; Spaeth, E.L.; Studeny, M.; Zompetta, C.; Samudio, I.; Roby, K.; Andreeff, M.; Marini, F.C. Tumor stroma engraftment of gene-modified mesenchymal stem cells as anti-tumor therapy against ovarian cancer. Cytotherapy 2013, 15, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Xu, W.; Xu, G. Transplantation of CX3CL1-expressing mesenchymal stem cells provides neuroprotective and immunomodulatory effects in a rat model of retinal degeneration. Ocul. Immunol. Inflamm. 2013, 21, 276–285, Erratum in Ocul. Immunol. Inflamm. 2015, 23, 470. [Google Scholar] [CrossRef]
- Kumagai, G.; Tsoulfas, P.; Toh, S.; McNiece, I.; Bramlett, H.M.; Dietrich, W.D. Genetically modified mesenchymal stem cells (MSCs) promote axonal regeneration and prevent hypersensitivity after spinal cord injury. Exp. Neurol. 2013, 248, 369–380. [Google Scholar] [CrossRef]
- He, J.G.; Li, H.R.; Li, B.B.; Xie, Q.L.; Yan, D.; Wang, X.J. Bone marrow mesenchymal stem cells overexpressing GATA-4 improve cardiac function following myocardial infarction. Perfusion 2019, 34, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, J.; Li, Y.; Cao, W.; Wang, Y.; Ma, Z.; Li, F. Mesenchymal stem cells expressing interleukin-18 inhibit breast cancer in a mouse model. Oncol. Lett. 2018, 15, 6265–6274. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Z.; Ding, T.; Chen, Z.; Zhang, T. Mesenchymal stem cells overexpressing PEDF decrease the angiogenesis of gliomas. Biosci. Rep. 2013, 33, e00019. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.B.; He, X.W.; Zhang, L.J.; Qin, H.B.; Lin, X.T.; Liu, X.H.; Zhou, C.; Liu, H.S.; Hu, T.; Cheng, H.C.; et al. Bone marrow-derived CXCR4-overexpressing MSCs display increased homing to intestine and ameliorate colitis-associated tumorigenesis in mice. Gastroenterol. Rep. 2019, 7, 127–138. [Google Scholar] [CrossRef]
- Hoban, D.B.; Howard, L.; Dowd, E. GDNF-secreting mesenchymal stem cells provide localized neuroprotection in an inflammation-driven rat model of Parkinson’s disease. Neuroscience 2015, 303, 402–411. [Google Scholar] [CrossRef]
- He, H.; Liu, L.; Chen, Q.; Liu, A.; Cai, S.; Yang, Y.; Lu, X.; Qiu, H. Mesenchymal Stem Cells Overexpressing Angiotensin-Converting Enzyme 2 Rescue Lipopolysaccharide-Induced Lung Injury. Cell Transplant. 2015, 24, 1699–1715. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Sung, D.K.; Chang, Y.S.; Sung, S.I.; Kim, Y.E.; Kim, H.J.; Lee, S.M.; Park, W.S. BDNF-Overexpressing Engineered Mesenchymal Stem Cells Enhances Their Therapeutic Efficacy against Severe Neonatal Hypoxic Ischemic Brain Injury. Int. J. Mol. Sci. 2021, 22, 11395. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Liu, C.; Yan, M.; Raman, I.; Du, Y.; Fang, X.; Zhou, X.J.; Mohan, C.; Li, Q.Z. Delivering Oxidation Resistance-1 (OXR1) to Mouse Kidney by Genetic Modified Mesenchymal Stem Cells Exhibited Enhanced Protection against Nephrotoxic Serum Induced Renal Injury and Lupus Nephritis. J. Stem Cell Res. Ther. 2014, 4, 231. [Google Scholar] [CrossRef]
- Silva, D.N.; Souza, B.S.F.; Azevedo, C.M.; Vasconcelos, J.F.; de Jesus, P.G.; Feitoza, M.S.; Meira, C.S.; Carvalho, G.B.; Cavalcante, B.R.; Ribeiro-Dos-Santos, R.; et al. IGF-1-Overexpressing Mesenchymal Stem/Stromal Cells Promote Immunomodulatory and Proregenerative Effects in Chronic Experimental Chagas Disease. Stem Cells Int. 2018, 2018, 9108681. [Google Scholar] [CrossRef]
- Gao, L.; Bledsoe, G.; Yin, H.; Shen, B.; Chao, L.; Chao, J. Tissue kallikrein-modified mesenchymal stem cells provide enhanced protection against ischemic cardiac injury after myocardial infarction. Circ. J. 2013, 77, 2134–2144. [Google Scholar] [CrossRef] [Green Version]
- Pucułek, M.; Baj, J.; Portincasa, P.; Sitarz, M.; Grochowski, C.; Radzikowska, E. The morphology and application of stem cells in digestive system surgery. Folia Morphol. 2021, 80, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Kidd, S.; Spaeth, E.; Dembinski, J.L.; Dietrich, M.; Watson, K.; Klopp, A.; Battula, V.L.; Weil, M.; Andreeff, M.; Marini, F.C. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells 2009, 27, 2614–2623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007, 449, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, H.F. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Kidd, S.; Spaeth, E.; Watson, K.; Burks, J.; Lu, H.; Klopp, A.; Andreeff, M.; Marini, F.C. Origins of the tumor microenvironment: Quantitative assessment of adipose-derived and bone marrowderived stroma. PLoS ONE 2012, 7, e30563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, B.J.; Gilissen, C.; Roelofs, H.; Schaap-Oziemlak, A.; Veltman, J.A.; Raymakers, R.A.; Jansen, J.H.; Kögler, G.; Figdor, C.G.; Torensma, R.; et al. Functional differences between mesenchymal stem cell populations are reflected by their transcriptome. Stem Cells Dev. 2010, 19, 481–490. [Google Scholar] [CrossRef]
- Spaeth, E.L.; Dembinski, J.L.; Sasser, A.K.; Watson, K.; Klopp, A.; Hall, B.; Andreeff, M.; Marini, F. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS ONE 2009, 4, e4992. [Google Scholar] [CrossRef] [Green Version]
- Quante, M.; Tu, S.P.; Tomita, H.; Gonda, T.; Wang, S.S.; Takashi, S.; Baik, G.H.; Shibata, W.; Diprete, B.; Betz, K.S.; et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 2011, 19, 257–272. [Google Scholar] [CrossRef] [Green Version]
- Zischek, C.; Niess, H.; Ischenko, I.; Conrad, C.; Huss, R.; Jauch, K.W.; Nelson, P.J.; Bruns, C. Targeting tumor stroma using engineered mesenchymal stem cells reduces the growth of pancreatic carcinoma. Ann. Surg. 2009, 250, 747–753. [Google Scholar] [CrossRef]
- Niess, H.; Bao, Q.; Conrad, C.; Zischek, C.; Notohamiprodjo, M.; Schwab, F.; Schwarz, B.; Huss, R.; Jauch, K.-W.; Nelson, P.J.; et al. Selective targeting of genetically engineered mesenchymal stem cells to tumor stroma microenvironments using tissue-specific suicide gene expression suppresses growth of hepatocellular carcinoma. Ann. Surg. 2011, 254, 767–774. [Google Scholar] [CrossRef]
- Komarova, S.; Kawakami, Y.; Stoff-Khalili, M.A.; Curiel, D.T.; Pereboeva, L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol. Cancer Ther. 2006, 5, 755–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, Y.; Kim, J.K.; Shiozawa, Y.; Wang, J.; Mishra, A.; Joseph, J.; Berry, J.E.; McGee, S.; Lee, E.; Sun, H.; et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat. Commun. 2013, 4, 1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, V.K.; Shih, H.H.; Parveen, F.; Lenzen, D.; Ito, E.; Chan, T.F.; Ke, L.Y. Identifying the Therapeutic Significance of Mesenchymal Stem Cells. Cells 2020, 9, 1145. [Google Scholar] [CrossRef]
- Rastegar, F.; Shenaq, D.; Huang, J.; Zhang, W.; Zhang, B.Q.; He, B.C.; Chen, L.; Zuo, G.W.; Luo, Q.; Shi, Q.; et al. Mesenchymal stem cells: Molecular characteristics and clinical applications. World J. Stem Cells 2010, 2, 67–80. [Google Scholar] [CrossRef]
- Sharma, R.R.; Pollock, K.; Hubel, A.; McKenna, D. Mesenchymal stem or stromal cells: A review of clinical applications and manufacturing practices. Transfusion 2014, 54, 1418–1437. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y. The Role of MSCs in the Tumor Microenvironment and Tumor Progression. Anticancer Res. 2020, 40, 3039–3047. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, S.; Alt, E.; Khalek, F.J.A.; Jotzu, C.; Muehlberg, F.; Beckmann, C.; Song, Y.-H. Tissue resident stem cells produce CCL5 under the influence of cancercells and thereby promote breast cancer cell invasion. Cancer Lett. 2009, 284, 80–85. [Google Scholar] [CrossRef]
- Huang, W.H.; Chang, M.C.; Tsai, K.S.; Hung, M.C.; Chen, H.L.; Hung, S.C. Mesenchymal stem cells promote growth and angiogenesis of tumors in mice. Oncogene 2013, 32, 4343–4354. [Google Scholar] [CrossRef] [Green Version]
- Cousin, B.; Ravet, E.; Poglio, S.; de Toni, F.; Bertuzzi, M.; Lulka, H.; Touil, I.; André, M.; Grolleau, J.-L.; Péron, J.-M.; et al. Adult stromal cells derived from human adipose tissue provoke pancreatic cancer cell death both in vitro and in vivo. PLoS ONE 2009, 4, e6278. [Google Scholar] [CrossRef] [Green Version]
- Chanda, D.; Isayeva, T.; Kumar, S.; Hensel, J.A.; Sawant, A.; Ramaswamy, G.; Siegal, G.P.; Beatty, M.S.; Ponnazhagan, S. Therapeutic potential of adult bone marrow-derived mesenchymal stem cells in prostate cancer bone metastasis. Clin. Cancer Res. 2009, 15, 7175–7185. [Google Scholar] [CrossRef] [Green Version]
- Ohlsson, L.B.; Varas, L.; Kjellman, C.; Edvardsen, K.; Lindvall, M. Mesenchymal progenitor cellmediated inhibition of tumor growth in vivo and in vitro in gelatin matrix. Exp. Mol. Pathol. 2003, 75, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Otsu, K.; Das, S.; Houser, S.D.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Concentrationdependent inhibition of angiogenesis by mesenchymal stem cells. Blood 2009, 113, 4197–4205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, L.; Xu, Z.L.; Zhao, T.J.; Ye, L.H.; Zhang, X.D. Dkk-1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signaling. Cancer Lett. 2008, 269, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Dasari, V.R.; Velpula, K.K.; Kaur, K.; Fassett, D.; Klopfenstein, J.D.; Dinh, D.H.; Gujrati, M.; Rao, J.S. Cord blood stem cell-mediated induction of apoptosis in glioma downregulates X-linked inhibitor of apoptosis protein (XIAP). PLoS ONE 2010, 5, e11813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, L.; Wang, X.; Zhou, Y.; Ma, H.; Wang, Z.; He, J.; Hu, H.; Gu, W.; Ma, J. Inhibitory effect and mechanism of mesenchymal stem cells on liver cancer cells. Tumour Biol. 2014, 35, 1239–1250. [Google Scholar] [CrossRef]
- Li, J.; Ji, L.; Chen, J.; Zhang, W.; Ye, Z. Wnt/β-catenin signaling pathway in skin carcinogenesis and therapy. Biomed. Res. Int. 2015, 2015, 964842. [Google Scholar] [CrossRef] [Green Version]
- Xia, P.; Xu, X.Y. PI3K/Akt/mTOR signaling pathway in cancer stem cells: From basic research to clinical application. Am. J. Cancer Res. 2015, 5, 1602–1609. [Google Scholar]
- Torsvik, A.; Bjerkvig, R. Mesenchymal stem cell signaling in cancer progression. Cancer Treat. Rev. 2013, 39, 180–188. [Google Scholar] [CrossRef]
- Yulyana, Y.; Ho, I.A.W.; Sia, K.C.; Newman, J.P.; Toh, X.Y.; Endaya, B.B.; Chan, J.K.Y.; Gnecchi, M.; Huynh, H.; Chung, A.Y.F.; et al. Paracrine factors of human fetal MSCs inhibit liver cancer growth through reduced activation of IGF-1R/PI3K/Akt signaling. Mol. Ther. 2015, 23, 746–756. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Chen, G.; Yang, J.; Ma, Z.; Yang, Y.; Hu, Y.; Lu, Y.; Cao, Z.; Wang, Y.; Wang, X. Bone marrow mesenchymal stem cells suppress growth and promote the apoptosis of glioma U251 cells through downregulation of the PI3K/AKT signaling pathway. Biomed. Pharmacother. 2019, 112, 108625. [Google Scholar] [CrossRef]
- Koziński, K.; Dobrzyń, A. Wnt signaling pathway—Its role in regulation of cell metabolism. Postepy Hig. Med. Dosw. 2013, 67, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhong, W.; Yuan, J.; Yan, C.; Hu, S.; Tong, Y.; Mao, Y.; Hu, T.; Zhang, B.; Song, G. Involvement of Wnt/β-catenin signaling in the mesenchymal stem cells promote metastatic growth and chemoresistance of cholangiocarcinoma. Oncotarget 2015, 6, 42276–42289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Zhao, G.; Zhang, Y.; Jiang, H.; Wang, W.; Zhao, D.; Hong, J.; Yu, H.; Qi, L. Mesenchymal stem cell-derived exosomal microRNA-133b suppresses glioma progression via Wnt/β-catenin signaling pathway by targeting EZH2. Stem Cell Res. Ther. 2019, 10, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Z.; Zhu, H.; Sun, H.; Hua, Y.; Zhang, G.; Jiang, J.; Wang, X. Adipose Mesenchymal Stem Cell-Derived Exosomal microRNA-1236 Reduces Resistance of Breast Cancer Cells to Cisplatin by Suppressing SLC9A1 and the Wnt/β-Catenin Signaling. Cancer Manag. Res. 2020, 12, 8733–8744. [Google Scholar] [CrossRef]
- Babajani, A.; Soltani, P.; Jamshidi, E.; Farjoo, M.H.; Nikneja, H. Recent Advances on Drug-Loaded Mesenchymal Stem Cells with Anti-neoplastic Agents for Targeted Treatment of Cancer. Front. Bioeng. Biotechnol. 2020, 8, 748. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Brennen, W.N.; Han, E.; Rosen, D.M.; Musabeyezu, J.; Safaee, H.; Ranganath, S.; Ngai, J.; Heinelt, M.; Milton, Y.; et al. A prodrug-doped cellular Trojan Horse for the potential treatment of prostate cancer. Biomaterials 2016, 91, 140–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krueger, T.E.G.; Thorek, D.L.J.; Denmeade, S.R.; Isaacs, J.T.; Brennen, W.N. Concise review: Mesenchymal stem cell-based drug delivery: The good, the bad, the ugly, and the promise. Stem Cells Transl. Med. 2018, 7, 651–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pessina, A.; Bonomi, A.; Coccè, V.; Invernici, G.; Navone, S.; Cavicchini, L.; Sisto, F.; Ferrari, M.; Viganò, L.; Locatelli, A.; et al. Mesenchymal stromal cells primed with paclitaxel provide a newapproach for cancer therapy. PLoS ONE 2011, 6, e28321. [Google Scholar] [CrossRef] [Green Version]
- Duchi, S.; Dambruoso, P.; Martella, E.; Sotgiu, G.; Guerrini, A.; Lucarelli, E.; Pessina, A.; Coccè, V.; Bonomi, A.; Varchi, G. Thiophene-based compounds as fluorescent tags to study mesenchymalstem cell uptake and release of taxanes. Bioconj. Chem. 2014, 25, 649–655. [Google Scholar] [CrossRef]
- Brini, A.T.; Coccè, V.; Ferreira, L.M.J.; Giannasi, C.; Cossellu, G.; Giannì, A.B.; Angiero, F.; Bonomi, A.; Pascucci, L.; Falchetti, M.L.; et al. Cell-mediated drug delivery by gingival interdentalpapilla mesenchymal stromal cells (GinPa-MSCs) loaded with paclitaxel. Expert Opin. Drug Deliv. 2016, 13, 789–798. [Google Scholar] [CrossRef]
- Pessina, A.; Coccè, V.; Pascucci, L.; Bonomi, A.; Cavicchini, L.; Sisto, F.; Ferrari, M.; Ciusani, E.; Crovace, A.; Falchetti, M.L.; et al. Mesenchymal stromal cells primed with P aclitaxel attract and killleukaemia cells, inhibit angiogenesis and improve survival of leukaemia-bearingmice. Br. J. Haematol. 2013, 160, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, A.; Steimberg, N.; Benetti, A.; Berenzi, A.; Alessandri, G.; Pascucci, L.; Boniotti, J.; Coccè, V.; Sordi, V.; Pessina, A.; et al. Paclitaxel-releasing mesenchymal stromalcells inhibit the growth of multiple myeloma cells in a dynamic3D culture system. Hematol. Oncol. 2017, 35, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M. Extracellular vesicle-mediated transport of non-coding RNAsbetween stem cells and cancer cells: Implications in tumor progression andtherapeutic resistance. Stem Cell Investig. 2017, 4, 83. [Google Scholar] [CrossRef] [Green Version]
- Fatima, F.; Nawaz, M. Vesiculated long non-coding RNAs: Offshorepackages deciphering trans-regulation between cells, cancer progressionand resistance to therapies. Noncoding RNA 2017, 3, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucerova, L.; Altanerova, V.; Matuskova, M.; Tyciakova, S.; Altaner, C. Adipose tissuederived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res. 2007, 67, 6304–6313. [Google Scholar] [CrossRef] [Green Version]
- Cavarretta, I.T.; Altanerova, V.; Matuskova, M.; Kucerova, L.; Culig, Z.; Altaner, C. Adipose tissue-derived mesenchymal stem cells expressing prodrug-converting enzyme inhibit human prostate tumor growth. Mol. Ther. 2010, 18, 223–231. [Google Scholar] [CrossRef]
- Studeny, M.; Marini, F.C.; Champlin, R.E.; Zompetta, C.; Fidler, I.J.; Andreeff, M. Bone marrowderived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002, 62, 3603–3608. [Google Scholar]
- Lu, J.H.; Peng, B.Y.; Chang, C.C.; Dubey, N.K.; Lo, W.C.; Cheng, H.C.; Wang, J.R.; Wei, H.-J.; Deng, W.-P. Tumor-targeted immunotherapy by using primary adipose-derived stem cells and an antigen-specific protein vaccine. Cancers 2018, 10, 446. [Google Scholar] [CrossRef] [Green Version]
- Spano, C.; Grisendi, G.; Golinelli, G.; Rossignoli, F.; Prapa, M.; Bestangno, M.; Candini, O.; Petrachi, T.; Recchia, A.; Miselli, F.; et al. Soluble TRAIL armed human MSC as gene therapy for pancreatic cancer. Sci. Rep. 2019, 9, 1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, K. Mesenchymal stem cells engineered for cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 739–748. [Google Scholar] [CrossRef] [Green Version]
- Levy, O.; Zhao, W.; Mortensen, L.J.; Leblanc, S.; Tsang, K.; Fu, M.; Phillips, J.A.; Sagar, V.; Anandakumaran, P.; Ngai, J.; et al. mRNA-engineered mesenchymal stem cells for targeted delivery of interleukin-10 to sites of inflammation. Blood 2013, 122, e23–e32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altanerova, U.; Babincova, M.; Babinec, P.; Benejova, K.; Jakubechova, J.; Altanerova, V.; Zduriencikova, M.; Repiska, V.; Altaner, C. Human mesenchymal stem cell-derived iron oxide exosomes allow targeted ablation of tumor cells via magnetic hyperthermia. Int. J. Nanomed. 2017, 12, 7923–7936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, S.; Srivastava, S. Biological activity of Quinazoline: A Review. Int. J. Pharm. Sci. Res. 2015, 6, 1206–1213. [Google Scholar]
- Mahato, A.K.; Srivastava, B.; Nithya, S. Chemistry structure activity relationship and biological activity of quinazoline-4(3H)-one derivatives. Inventi. Rapid Med. Chem. 2011, 2, 13–19. [Google Scholar]
- Nagar, A.A.; Patel, A.; Rajesh, K.S.; Danao, K.R.; Rathi, L.G. Solvent Free One Pot Microwave Synthesis of Quinazolin 4-(3H)-One derivatives with their Antibacterial and Antifungal Activity. Pharmagene 2013, 1, 1–6. [Google Scholar]
- Mohamed, M.S.; Kamel, M.M.; Kassem, E.M.M.; Abotaleb, N.; Kherd, M.; Ahmed, M.F. Synthesis, Biological Evaluation and Molecular Dockingof Quinazoline-4(1h)-one Derivatives as Anti-inflammatory and Analgesic Agents. Acta Pol. Pharm. 2011, 68, 665–675. [Google Scholar] [PubMed]
- Patel, H.U.; Patel, R.S.; Patel, C.N. Synthesis and Antihypertensive Activity of Some Quinazoline Derivatives. J. App. Pharm. Sci. 2013, 3, 171–174. [Google Scholar]
- Mohamed, Y.A.; Amr, A.E.G.E.; Mohamed, S.F.; Abdalla, M.M.; Al-Omar, M.A.; Shfik, S.H. Cytotoxicity and anti-HIV evaluations of some new synthesized quinazoline and thioxopyrimidine derivatives using 4-(thiophen-2yl)3,4,5,6tetrahydrobenzo[h]quinazoline-2(1H)-thione as synthon. J. Chem. Sci. 2012, 124, 693–702. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, D.; Mukhopadhyay, A.; Shridhara, K.B.; Shridhara, A.M.; Rao, K.S. Synthesis, Characterization And Anticonvulsant Activity Of Substituted 4-Chloro-2-(4-Piperazin-1-Yl) Quinazolines. Int. J. Pharm. Pharm. Sci. 2014, 6, 567–571. [Google Scholar]
- Sen, D.; Banerjee, A.; Ghosh, A.K.; Chatterjee, T.K. Synthesis And Antimalarial Evaluation Of Some 4-Quinazolinone Derivatives Based On Febrifugine. J. Adv. Pharm. Technol. Res. 2010, 1, 401–405. [Google Scholar] [CrossRef] [PubMed]
- El-Azab, A.S.; Al-Omar, M.A.; Abdel-Aziz, A.A.M.; Abdel-Aziz, N.I.; El-Sayed, M.A.A.; Aleisa, A.M.; Sayed-Ahmed, M.M.; Abdel-Hamide, S.G. Design, Synthesis And Biological Evaluation Of Novel Quinazoline Derivatives As Potential Antitumor Agents: Molecular Docking Study. Eur. J. Med. Chem. 2010, 45, 4188–4198. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, M.K.; Shantakumar, S.M. Design and Synthesis of Novel 2-Trichloromethyl-4Substituted Quinazoline Derivatives as Anti-tubercular Agents. Chem. Sci. Trans. 2013, 2, 1056–1062. [Google Scholar] [CrossRef] [Green Version]
- Al-Omar, M.A.; El-Azab, A.S.; El-Obeid, H.A.; Hamide, S.G.A. Sythesis of Some New 4-(3H)quinazoline Analogs as Potential Antioxidant Agents. J. Saudi Chem. Soc. 2006, 10, 1131. [Google Scholar]
- Bathula, R.; Mondal, P.; Raparla, R.; Satla, S.R. Evaluation of antitumor potential of synthesized novel 2-substituted 4-anilinoquinazolines as quinazoline-pyrrole hybrids in MCF-7 human breast cancer cell line and A-549 human lung adenocarcinoma cell lines. Future J. Pharm. Sci. 2020, 6, 44. [Google Scholar] [CrossRef]
- Alqasoumi, S.I.; Al-Taweel, A.M.; Alafeefy, A.M.; Ghorab, M.M.; Noaman, E. Discovering some novel tetrahydroquinoline derivatives bearing the biologically active sulfonamide moiety as a new class of antitumor agents. Eur. J. Med. Chem. 2010, 45, 1849–1853. [Google Scholar] [CrossRef]
- Peng, W.; Tu, Z.C.; Long, Z.J.; Liu, Q.; Lu, G. Discovery of 2-(2-aminopyrimidin-5-yl)-4morpholino-N-(pyridin-3-yl)quinazolin-7-amines as novel PI3K/mTOR inhibitors and anticancer agents. Eur. J. Med. Chem. 2016, 108, 644–654. [Google Scholar] [CrossRef]
- Zwick, E.; Bange, J.; Ullrich, A. Receptor tyrosine kinase signalling as a target for cancer intervention strategies. Endocr. Relat. Cancer 2001, 8, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Porter, A.C.; Vaillancourt, R.R. Tyrosine kinase receptor-activated signal transduction pathways which lead to oncogenesis. Oncogene 1998, 17, 1343–1352. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, I. An insight into the therapeutic potential of quinazoline derivatives as anticancer agents. MedChemComm 2017, 8, 871–885. [Google Scholar] [CrossRef]
- Yang, L.L.; Liu, S.S.; Chu, J.J.; Miao, S.; Wang, K.; Zhang, Q.W.; Wang, Y.; Xiao, Y.; Wu, L.; Liu, Y.; et al. Novel anilino quinazoline-based EGFR tyrosine kinase inhibitors for treatment of non-small cell lung cancer. Biomater. Sci. 2021, 9, 443–455. [Google Scholar] [CrossRef]
- Bhatia, P.; Sharma, V.; Alam, O.; Manaithiya, A.; Alam, P.; Alam, M.T.; Imran, M. Novel quinazolinebased EGFR kinase inhibitors: A review focussing on SAR and molecular docking studies (2015–2019). Eur. J. Med. Chem. 2020, 204, 112640. [Google Scholar] [CrossRef] [PubMed]
- Chinigo, G.M.; Paige, M.; Grindrod, S.; Hamel, E.; Dakshanamurthy, S.; Chruszcz, M.; Minor, W.; Brown, M.L. Asymmetric synthesis of 2,3-dihydro-2 arylquinazolin-4-ones: Methodology and application to a potent fluorescent tubulin inhibitor with anticancer activity. J. Med. Chem. 2008, 51, 4620–4631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirisoma, N.; Pervin, A.; Zhang, H.; Jiang, S.; Willardsen, J.A.; Anderson, M.B.; Mather, G.; Pleiman, C.M.; Kasibhatla, S.; Tseng, B.; et al. Discovery of Nmethyl-4-(4-methoxyanilino)quinazolines as potent apoptosis inducers. Structureactivity relationship of the quinazoline ring. Bioorg. Med. Chem. Lett. 2010, 20, 2330–2334. [Google Scholar] [CrossRef] [PubMed]
- Al-Obeed, O.; Vaali-Mohammed, M.A.; Eldehna, W.M.; Al-Khayal, K.; Mahmood, A.; Abdel-Aziz, H.A.; Zubaidi, A.; Alafeefy, A.; Abdulla, M.; Ahmad, R. Novel quinazoline-based sulfonamide derivative (3D) induces apoptosis in colorectal cancer by inhibiting JAK2-STAT3 pathway. OncoTargets Ther. 2018, 11, 3313–3322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, L.Y.; Zhang, Y.L.; Yang, R.; Wang, Z.C.; Lu, Y.D.; Wang, B.Z.; Zhu, H.-L. Tubulin Inhibitors binding to colchicine-site: A review from 2015 to 2019. Curr. Med. Chem. 2020, 27, 6787–6814. [Google Scholar] [CrossRef]
- Li, W.; Yin, Y.; Shuai, W.; Xu, F.; Yao, H.; Liu, J.; Cheng, K.; Xu, J.; Zhu, Z.; Xu, S. Discovery of novel quinazolines as potential anti-tubulin agents occupying three zones of colchicine domain. Bioorg. Chem. 2019, 83, 380–390. [Google Scholar] [CrossRef]
- Zayed, M.F.; Rateb, H.S.; Ahmed, S.; Khaled, O.A.; Ibrahim, S.R.M. Quinazolinone-amino acid hybrids as dual inhibitors of EGFR kinase and tubulin polymerization. Molecules 2018, 23, 1699. [Google Scholar] [CrossRef] [Green Version]
- Sonawane, V.; Siddique, M.U.M.; Jadav, S.S.; Sinha, B.N.; Jayaprakash, V.; Chaudhuri, B. Cink4T, a quinazolinone-based dual inhibitor of Cdk4 and tubulin polymerization, identified via ligand-based virtual screening, for efficient anticancer therapy. Eur. J. Med. Chem. 2019, 165, 115–132. [Google Scholar] [CrossRef]
- Alafeefy, A.M.; Kadi, A.A.; Al-Deeb, O.A.; El-Tahir, K.E.H.; Al-Jaber, N.A. Synthesis, analgesic and anti-inflammatory evaluation of some novel quinazoline derivatives. Eur. J. Med. Chem. 2010, 45, 4947–4952. [Google Scholar] [CrossRef]
- Nilius, B.; Droogmans, G. A role for K+ channels in cell proliferation. Physiology 1994, 9, 105–110. [Google Scholar] [CrossRef]
- Fraser, S.; Grimes, J.; Djamgo, M. Effects of voltage-gated ion channel modulators on rat prostatic cancer cell proliferation: Comparison of strongly and weakly metastatic cell lines. Prostate 2000, 44, 61–76. [Google Scholar] [CrossRef]
- Wilson, D.; Fanning, L.T.D.; Krenitsky, P.; Termin, A.; Joshi, P.; Sheth, U. Quinazolines Useful as Modulators of Voltage Gated Ion Channels. U.S. Patent 8,158,637, 17 April 2012. [Google Scholar]
- Liu, F.; Chen, X.; Allali-Hassani, A.; Quinn, A.M.; Wasney, G.A.; Dong, A.P.; Barsyte, D.; Kozieradzki, I.; Senisterra, G.; Chau, I.; et al. Discovery of a 2,4diamino-7-aminoalkoxyquinazoline as a potent and selective inhibitor of histone lysine methyltransferase G9a. J. Med. Chem. 2009, 52, 7950–7953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hei, Y.Y.; Xin, M.H.; Zhang, H.; Xie, X.X.; Mao, S.; Zhang, S.Q. Synthesis and antitumor activity evaluation of 4,6-disubstituted quinazoline derivatives as novel PI3K inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 4408–4413. [Google Scholar] [CrossRef] [PubMed]
- Krapf, M.K.; Gallus, J.; Spindler, A.; Wiese, M. Synthesis and biological evaluation of quinazoline derivatives—A SAR study of novel inhibitors of ABCG2. Eur. J. Med. Chem. 2019, 161, 506–525. [Google Scholar] [CrossRef] [PubMed]
- Krapf, M.K.; Gallus, J.; Wiese, M. Synthesis and biological investigation of 2,4-substituted quinazolines as highly potent inhibitors of breast cancer resistance protein (ABCG2). Eur. J. Med. Chem. 2017, 139, 587–611. [Google Scholar] [CrossRef]
- Ding, C.; Chen, S.; Zhang, C.; Hu, G.; Zhang, W.; Li, L.; Chen, Y.Z.; Tan, C.; Jiang, Y. Synthesis and investigation of novel 6-(1,2,3-triazol-4-yl)-4-aminoquinazolin derivatives possessing hydroxamic acid moiety for cancer therapy. Bioorg. Med. Chem. 2017, 25, 27–37. [Google Scholar] [CrossRef]
- Abuelizz, H.A.; Marzouk, M.; Ghabbour, H.; Al-Salahi, R. Synthesis and anticancer activity of new quinazoline derivatives. Saudi Pharm. J. 2017, 25, 1047–1054. [Google Scholar] [CrossRef]
- Madhavi, S.; Sreenivasulu, R.; Yazala, J.P.; Raju, R.R. Synthesis of chalcone incorporated quinazoline derivatives as anticancer agents. Saudi Pharm. J. 2017, 25, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Regin, G.L.; Bai, R.; Coluccia, A.; Famiglini, V.; Pelliccia, S.; Passacantilli, S.; Mazzoccoli, C.; Ruggieri, V.; Sisinni, L.; Bolognesi, A.; et al. New pyrrole derivatives with potent tubulin polymerization inhibiting activity as anticancer agents including hedgehog-dependent cancer. J. Med. Chem. 2014, 57, 6531–6552. [Google Scholar] [CrossRef]
- Auti, P.S.; George, G.; Paul, A.T. Recent advances in the pharmacological diversification of quinazoline/quinazolinone hybrids. RSC Adv. 2020, 10, 41353. [Google Scholar] [CrossRef]
- Osipov, V.N.; Khachatryan, D.S.; Balaev, A.N. Biologically active quinazoline-based hydroxamic acids. Med. Chem. Res. 2020, 29, 831–845. [Google Scholar] [CrossRef]
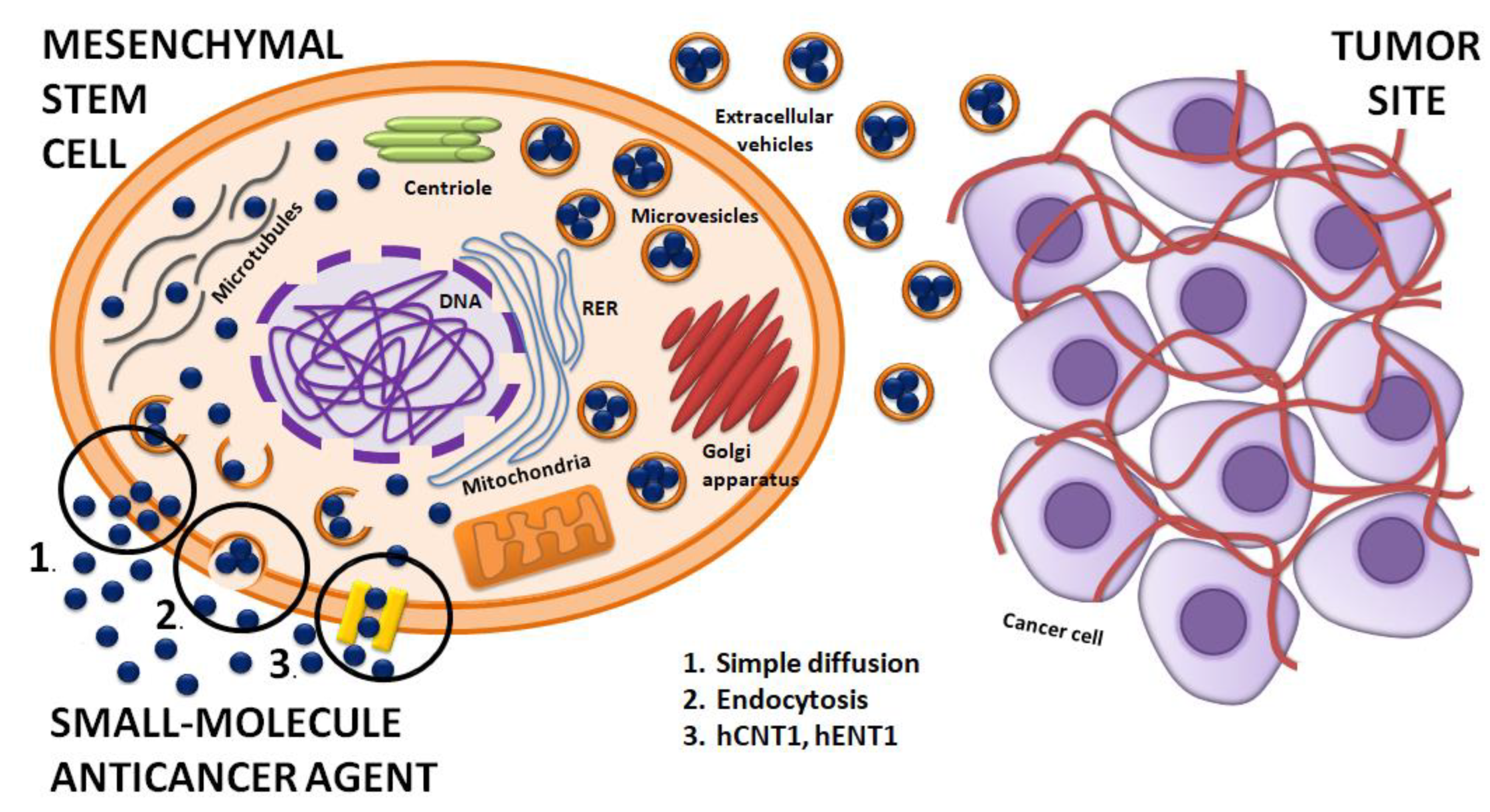

| Loaded Drug/Expressed Transgene | Target Disease | Experimental Model | Therapeutic Effect | Study |
|---|---|---|---|---|
| TNF-related apoptosis-inducing ligand (TRAIL) | Glioma | Glioblastoma cells (C6) | Apoptosis of tumor cells | Tang X.J. et al. [31] |
| Galectin-1 | Allergic Airway Disease (AAD) | Mouse model | Anti-inflammatory effect | Ge X. et al. [32] |
| Doxorubicin (DOX) | Colorectal cancer | Female BALB/c mice (4–6weeks), C26 and MCF7 cell lines | Significant tumor growth inhibition in comparison with free DOX | Bagheri E. et al. [33] |
| Gemcitabine | Pancreatic cancer | Human pancreatic carcinoma (pCa) cells | Growth inhibition of a human pCa cell line in vitro | Bonomi A. et al. [34] |
| Ptx-PLGA NPs | Glioma | Rat model | Tumor cell death, prolonged survival | Wang X. et al. [35] |
| Paclitaxel (PTX) | Melanoma lung metastasis | Syngeneic murine model | Inhibition of the formation of lung metastasis | Pessina A. et al. [36] |
| Paclitaxel (PTX) | Pancreatic cancer | Human pancreatic cell line CFPAC-1 | Strong anti-proliferative effect | Pascucci L. et al. [37] |
| Interferon-β (IFN-β) | Ovarian cancer | Syngeneic mouse tumors (ID8-R) and human xenograft (OVCAR3, SKOV3) tumor models | Modulation of tumor kinetics resulting in prolonged survival | Dembinski J.L. et al. [38] |
| (C-X3-C motif) ligand 1 (CX3CL1) | Light-induced retinal degeneration | Rat model | Neuroprotective and immunomodulatory effects | Huang L. et al. [39] |
| Multineurotrophin MNTS1 | Spinal Cord Injury (SCI) | Rat model | Promotion of cell growth and improvement of sensory function after SCI | Kumagai G. et al. [40] |
| GATA binding protein 4 (GATA-4) | Myocardial infarction | Cardiomyocytes | A significant increase the number of blood vessels, a decrease the proportion of apoptotic cells, and an increase the mean number of cardiac c-kit-positive cells | He J.G. et al. [41] |
| Interleukin (IL)-18 | Breast cancer | Mouse model | Inhibition of tumor cell proliferation and tumor angiogenesis, induction of a more pronounced and better therapeutic effect at tumor sites, especially in early tumors | Liu X. et al. [42] |
| Pigment epithelium-derived factor (PEDF) | Glioma | Mouse model | Apoptosis of glioma cells and prolonged the survival | Wang Q. et al. [43] |
| C-X-C chemokine receptor type 4 (CXCR4) | Inflammatory bowel disease (IBD) and IBD-induced cancer | Mouse model | Anti-tumor effect | Zheng X.B. et al. [44] |
| Glial cell line-derived neurotrophic factor (GDNF) | Parkinson’s disease | Rat model | Localized neuroprotective effect | Hoban D.B. et al. [45] |
| Angiotensin-converting enzyme 2 (ACE2) gene | Lipopolysaccharide-Induced Lung Injury | Mouse model | Improvement of the lung histopathology; anti-inflammatory effects; reduction of pulmonary vascular permeability; improvement of endothelial barrier integrity, and normalization of lung eNOS | He H. et al. [46] |
| Brain-derived neurotropic factor (BDNF) gene | Severe Neonatal Hypoxic Ischemic Brain Injury | Rat model | Supression of the increase in cytotoxicity, oxidative stress, and cell death in vitro; significant attenuating effects on severe neonatal HI-induced short-term brain injury scores, long-term progress of brain infarct, increased apoptotic cell death, astrogliosis and inflammatory responses, and impaired negative geotaxis and rotarod tests in vivo | Ahn S.Y. et al. [47] |
| Oxidation Resistance 1 (OXR1) gene | Immune-mediated nephritis | Mouse model | Protective effect on nephritis by suppressing inflammation and oxidative stress | Li Y. et al. [48] |
| Insulin-like growth factor-1 (IGF-1) | Chronic Chagas disease | Mouse model | Immunomodulatory and proregenerative effects to the cardiac and skeletal muscles | Silva D. N. et al. [49] |
| Human tissue kallikrein (TK) gene | Cardiac injury | Rat model | Protect against cardiac injury, apoptosis and inflammation, and promote neovascularization to improve cardiac function | Gao L. et al. [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szewc, M.; Radzikowska-Bűchner, E.; Wdowiak, P.; Kozak, J.; Kuszta, P.; Niezabitowska, E.; Matysiak, J.; Kubiński, K.; Masłyk, M. MSCs as Tumor-Specific Vectors for the Delivery of Anticancer Agents—A Potential Therapeutic Strategy in Cancer Diseases: Perspectives for Quinazoline Derivatives. Int. J. Mol. Sci. 2022, 23, 2745. https://doi.org/10.3390/ijms23052745
Szewc M, Radzikowska-Bűchner E, Wdowiak P, Kozak J, Kuszta P, Niezabitowska E, Matysiak J, Kubiński K, Masłyk M. MSCs as Tumor-Specific Vectors for the Delivery of Anticancer Agents—A Potential Therapeutic Strategy in Cancer Diseases: Perspectives for Quinazoline Derivatives. International Journal of Molecular Sciences. 2022; 23(5):2745. https://doi.org/10.3390/ijms23052745
Chicago/Turabian StyleSzewc, Monika, Elżbieta Radzikowska-Bűchner, Paulina Wdowiak, Joanna Kozak, Piotr Kuszta, Ewa Niezabitowska, Joanna Matysiak, Konrad Kubiński, and Maciej Masłyk. 2022. "MSCs as Tumor-Specific Vectors for the Delivery of Anticancer Agents—A Potential Therapeutic Strategy in Cancer Diseases: Perspectives for Quinazoline Derivatives" International Journal of Molecular Sciences 23, no. 5: 2745. https://doi.org/10.3390/ijms23052745
APA StyleSzewc, M., Radzikowska-Bűchner, E., Wdowiak, P., Kozak, J., Kuszta, P., Niezabitowska, E., Matysiak, J., Kubiński, K., & Masłyk, M. (2022). MSCs as Tumor-Specific Vectors for the Delivery of Anticancer Agents—A Potential Therapeutic Strategy in Cancer Diseases: Perspectives for Quinazoline Derivatives. International Journal of Molecular Sciences, 23(5), 2745. https://doi.org/10.3390/ijms23052745






