Bone Fragility in Gastrointestinal Disorders
Abstract
1. Introduction
2. Inflammatory Bowel Disease (IBD)
2.1. Epidemiology of Osteoporosis and Fractures in IBD
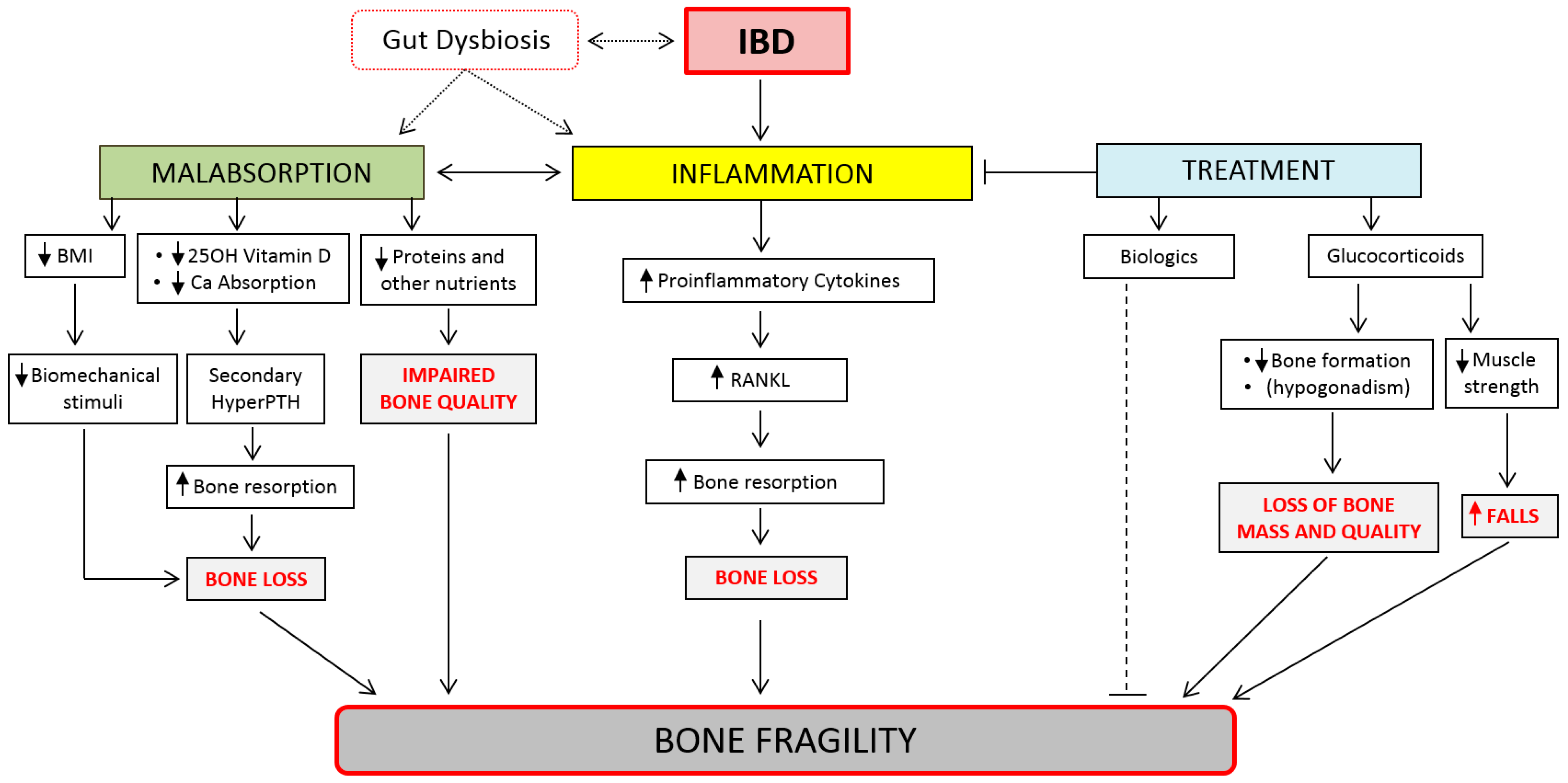
2.2. Pathogenesis of Bone Fragility in IBD
2.3. Management of Bone Fragility in IBD
3. Celiac Disease
3.1. Epidemiology of Bone Fragility in Celiac Disease
3.2. Pathogenesis of Bone Fragility in Celiac Disease
3.3. Management of Bone Fragility in IBD
4. Gastric Disorders
4.1. Helicobacter Pylori Infection
4.2. Chronic Gastritis and Peptic Ulcer Disease
4.3. Gastric Cancer and Gastrectomy
5. Microbiome, Dysbiosis, and Bone Health
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sambrook, P.; Cooper, C. Osteoporosis. Lancet 2006, 367, 2010–2018. [Google Scholar] [CrossRef]
- Cummings, S.R.; Melton, L.J. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002, 359, 1761–1767. [Google Scholar] [CrossRef]
- Martin, T.J.; Seeman, E. Bone remodelling: Its local regulation and the emergence of bone fragility. Best Pr. Res. Clin. Endocrinol. Metab. 2008, 22, 701–722. [Google Scholar] [CrossRef]
- Seeman, E.; Delmas, P.D. Bone Quality—The Material and Structural Basis of Bone Strength and Fragility. N. Engl. J. Med. 2006, 354, 2250–2261. [Google Scholar] [CrossRef]
- Bellido, T. Osteocyte-Driven Bone Remodeling. Calcif. Tissue Res. 2013, 94, 25–34. [Google Scholar] [CrossRef]
- Raisz, L.G. Pathogenesis of osteoporosis: Concepts, conflicts, and prospects. J. Clin. Investig. 2005, 115, 3318–3325. [Google Scholar] [CrossRef] [PubMed]
- Riggs, B.L.; Khosla, S.; Melton, L.J., III. A Unitary Model for Involutional Osteoporosis: Estrogen Deficiency Causes Both Type I and Type II Osteoporosis in Postmenopausal Women and Contributes to Bone Loss in Aging Men. J. Bone Miner. Res. 1998, 13, 763–773. [Google Scholar] [CrossRef]
- Khosla, S.; Melton, L.J., III; Riggs, B.L. Clinical review 144: Estrogen and the male skeleton. J. Clin. Endocrinol. Metab. 2002, 87, 1443–1450. [Google Scholar] [CrossRef]
- Gennari, L.; Nuti, R.; Bilezikian, J.P. Aromatase Activity and Bone Homeostasis in Men. J. Clin. Endocrinol. Metab. 2004, 89, 5898–5907. [Google Scholar] [CrossRef] [PubMed]
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef]
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef]
- Rosen, M.J.; Dhawan, A.; Saeed, S.A. Inflammatory Bowel Disease in Children and Adolescents. JAMA Pediatr. 2015, 169, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Abraham, C.; Cho, J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.B.; Xavier, R.J. Pathway paradigms revealed from the genetics of inflammatory bowel disease. Nature 2020, 578, 527–539. [Google Scholar] [CrossRef]
- Sairenji, T.; Collins, K.L.; Evans, D.V. An Update on Inflammatory Bowel Disease. Prim. Care 2017, 44, 673–692. [Google Scholar] [CrossRef]
- Pigot, F.; Roux, C.; Chaussade, S.; Hardelin, D.; Pelleter, O.; Montbrun, T.D.P.; Listrat, V.; Dougados, M.; Couturier, D.; Amor, B. Low bone mineral density in patients with inflammatory bowel disease. Am. J. Dig. Dis. 1992, 37, 1396–1403. [Google Scholar] [CrossRef]
- Arden, N.K. Osteoporosis in patients with inflammatory bowel disease. Gut 2002, 50, 9–10. [Google Scholar] [CrossRef][Green Version]
- Lima, C.A.; Lyra, A.C.; Rocha, R.; Santana, G.O. Risk factors for osteoporosis in inflammatory bowel disease patients. World J. Gastrointest. Pathophysiol. 2015, 6, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Szafors, P.; Che, H.; Barnetche, T.; Morel, J.; Gaujoux-Viala, C.; Combe, B.; Lukas, C. Risk of fracture and low bone mineral density in adults with inflammatory bowel diseases. A systematic literature review with meta-analysis. Osteoporos. Int. 2018, 29, 2389–2397. [Google Scholar] [CrossRef]
- Komaki, Y.; Komaki, F.; Sakuraba, A. Tu1084 Risk of Fracture in Inflammatory Bowel Disease; A Systematic Review and Meta-Analysis. Gastroenterology 2016, 150, S837. [Google Scholar] [CrossRef]
- Vestergaard, P.; Mosekilde, L. Fracture Risk in Patients with Celiac Disease, Crohn’s Disease, and Ulcerative Colitis: A Nationwide Follow-up Study of 16,416 Patients in Denmark. Am. J. Epidemiol. 2002, 156, 1–10. [Google Scholar] [CrossRef]
- Laakso, S.; Valta, H.; Verkasalo, M.; Toiviainen-Salo, S.; Viljakainen, H.; Mäkitie, O. Impaired Bone Health in Inflammatory Bowel Disease: A Case–Control Study in 80 Pediatric Patients. Calcif. Tissue Res. 2012, 91, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Pepe, J.; Zawadynski, S.; Herrmann, F.R.; Juillerat, P.; Michetti, P.; Ferrari-Lacraz, S.; Belli, D.; Ratib, O.; Rizzoli, R.; Chevalley, T.; et al. Structural basis of bone fragility in young subjects with inflammatory bowel disease: A high-resolution pQCT study of the SWISS IBD Cohort (SIBDC). Inflamm. Bowel Dis. 2017, 23, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.; Mehta, P.; Mittal, M.; Rajender, S.; Chattopadhyay, N. Human Relevance of Preclinical Studies on the Skeletal Impact of Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Calcif. Tissue Res. 2021, 108, 708–724. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, D.; Rodacki, T.; Domagała-Rodacka, R.; Cibor, D.; Mach, T. Diet and nutritional factors in inflammatory bowel diseases. World J. Gastroenterol. 2016, 22, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Donnellan, C.F.; Yann, L.H.; Lal, S. Nutritional management of Crohn’s disease. Ther. Adv. Gastroenterol. 2013, 6, 231–242. [Google Scholar] [CrossRef]
- Van Bodegraven, A.A.; Bravenboer, N. Perspective on skeletal health in inflammatory bowel disease. Osteoporos. Int. 2019, 31, 637–646. [Google Scholar] [CrossRef]
- Ratajczak, A.E.; Szymczak-Tomczak, A.; Rychter, A.M.; Zawada, A.; Dobrowolska, A.; Krela-Ka´zmierczak, I. Impact of Cigarette Smoking on the Risk of Osteoporosis in Inflammatory Bowel Diseases. J. Clin. Med. 2021, 10, 1515. [Google Scholar] [CrossRef]
- Krela-Ka´zmierczak, I.; Wysocka, E.; Szymczak, A.; Eder, P.; Michalak, M.; Łykowska-Szuber, L.; Stawczyk-Eder, K.; Klimczak, K.; Linke, K.; Horst-Sikorska, W. Osteoprotegerin, s-RANKL, and Selected Interleukins in the Pathology of Bone Metabolism in Patients with Crohn’s Disease. Przeglad Gastroenterol. 2016, 11, 30–34. [Google Scholar]
- Moschen, A.; Kaser, A.; Enrich, B.; Ludwiczek, O.; Gabriel, M.; Obrist, P.; Wolf, A.M.; Tilg, H. The RANKL/OPG system is activated in inflammatory bowel disease and relates to the state of bone loss. Gut 2005, 54, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Blaschke, M.; Koepp, R.; Cortis, J.; Komrakova, M.; Schieker, M.; Hempel, U.; Siggelkow, H. IL-6, IL-1, and TNF-Only in Combination Influence the Osteoporotic Phenotype in Crohn’s Patients via Bone Formation and Bone Resorption. Adv. Clin. Exp. Med. Off. Organ. Wroclaw Med. Univ. 2018, 27, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Yoon, V.; Maalouf, N.M.; Sakhaee, K. The Effects of Smoking on Bone Metabolism. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2012, 23, 2081–2092. [Google Scholar]
- Patankar, J.V.; Müller, T.M.; Kantham, S.; Acera, M.G.; Mascia, F.; Scheibe, K.; Mahapatro, M.; Heichler, C.; Yu, Y.; Li, W.; et al. E-type prostanoid receptor 4 drives resolution of intestinal inflammation by blocking epithelial necroptosis. Nature 2021, 23, 796–807. [Google Scholar] [CrossRef]
- Von Tirpitz, C.; Pischulti, G.; Klaus, J.; Rieber, A.; Brückel, J.; Böhm, B.O.; Adler, G.; Reinshagen, M. Pathological bone density in chronic inflammatory bowel diseases—prevalence and risk factors. Z Gastroenterol. 1999, 37, 5–12. [Google Scholar]
- Wadolowska, L.; Sobas, K.; Szczepanska, J.W.; Slowinska, M.A.; Czlapka-Matyasik, M.; Niedźwiedzka, E. Dairy Products, Dietary Calcium and Bone Health: Possibility of Prevention of Osteoporosis in Women: The Polish Experience. Nutrients 2013, 5, 2684–2707. [Google Scholar] [CrossRef]
- Warensjö, E.; Byberg, L.; Melhus, H.; Gedeborg, R.; Mallmin, H.; Wolk, A.; Michaëlsson, K. Dietary calcium intake and risk of fracture and osteoporosis: Prospective longitudinal cohort study. BMJ 2011, 342, d1473. [Google Scholar] [CrossRef]
- Krela-Kazmierczak, I.; Michalak, M.; Szymczak-Tomczak, A.; Czarnywojtek, A.; Wawrzyniak, A.; Lykowska-Szuber, L.; Stawczyk-Eder, K.; Dobrowolska, A.; Eder, P. Milk and dairy product consumption in patients with inflammatory bowel disease: Helpful or harmful to bone mineral density? Nutrition 2020, 110830, 79–80. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Rejnmark, L.; Moss, A.C. Role of Vitamin D in the Natural History of Inflammatory Bowel Disease. J. Crohn’s Colitis 2018, 12, 742–752. [Google Scholar] [CrossRef]
- Ryan, E.; McNicholas, M.D.; Creavin, B.; Kelly, M.M.E.; Walsh, F.T.; Beddy, F.D. Sarcopenia and Inflammatory Bowel Disease: A Systematic Review. Inflamm. Bowel Dis. 2018, 25, 67–73. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOPSarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Pizzoferrato, M.; De Sire, R.; Ingravalle, F.; Mentella, M.C.; Petito, V.; Martone, A.M.; Landi, F.; Miggiano, G.A.D.; Mele, M.C.; Lopetuso, L.R.; et al. Characterization of Sarcopenia in an IBD Population Attending an Italian Gastroenterology Tertiary Center. Nutrients 2019, 11, 2281. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Al-Jaouni, R.; Filippi, J.; Wiroth, J.-B.; Zeanandin, G.; Arab, K.; Hébuterne, X. Sarcopenia is prevalent in patients with Crohn’s disease in clinical remission. Inflamm. Bowel Dis. 2008, 14, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Marcell, T.J. Review Article: Sarcopenia: Causes, Consequences, and Preventions. J. Gerontol. Ser. A 2003, 58, M911–M916. [Google Scholar] [CrossRef]
- Chiodini, I.; Falchetti, A.; Merlotti, D.; Eller Vainicher, C.; Gennari, L. Updates in epidemiology, pathophysiology and man-agement strategies of glucocorticoid-induced osteoporosis. Expert Rev. Endocrinol. Metab. 2020, 15, 283–298. [Google Scholar]
- Chiodini, I.; Merlotti, D.; Falchetti, A.; Gennari, L. Treatment options for glucocorticoid-induced osteoporosis. Expert Opin. Pharmacother. 2020, 21, 721–732. [Google Scholar] [CrossRef]
- Van Staa, T.P.; Leufkens, H.G.; Abenhaim, L.; Zhang, B.; Cooper, C. Use of oral corticosteroids and risk of fractures. J. Bone Miner. Res. 2000, 15, 993–1000. [Google Scholar] [CrossRef]
- Sambrook, P.N.; Eisman, J.A.; Yeates, M.G.; Pocock, N.A.; Eberl, S.; Champion, G.D. Osteoporosis in rheumatoid arthritis: Safety of low dose corticosteroids. Ann. Rheum. Dis. 1986, 45, 950–953. [Google Scholar] [CrossRef]
- Franchimont, D.; Putzeys, V.; Collette, J.M.; Vermeire, S.; Rutgeerts, P.; De Vos, M.; Van Gossum, A.; Fiasse, R.; Pelckmans, P.; Malaise, M.; et al. Rapid improvement of bone metabolism after infliximab treatment in Crohn’s disease. Aliment. Pharmacol. Ther. 2004, 20, 607–614. [Google Scholar] [CrossRef]
- Abreu, M.T.; Geller, J.L.; Vasiliauskas, E.A.; Kam, L.Y.; Vora, P.; Martyak, L.A.; Yang, H.; Hu, B.; Lin, Y.-C.; Keenan, G.; et al. Treatment with Infliximab Is Associated with Increased Markers of Bone Formation in Patients With Crohn’s Disease. J. Clin. Gastroenterol. 2006, 40, 55–63. [Google Scholar] [CrossRef]
- Veerappan, S.G.; O’Morain, C.A.; Daly, J.S.; Ryan, B.M. Review article: The effects of antitumour necrosis factor-α on bone metabolism in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2011, 33, 1261–1272. [Google Scholar] [CrossRef]
- Krajcovicova, A.; Hlavaty, T.; Killinger, Z.; Miznerova, E.; Toth, J.; Letkovsky, J.; Nevidanska, M.; Cierny, D.; Koller, T.; Zelinkova, Z.; et al. Combination therapy with an immunomodulator and anti-TNF? agent improves bone mineral density in IBD patients. J. Crohn’s Colitis 2014, 8, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Veerappan, S.G.; Healy, M.; Walsh, B.; O’Morain, C.A.; Daly, J.S.; Ryan, B.M. A 1-year prospective study of the effect of in-fliximab on bone metabolism in inflammatory bowel disease patients. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Farraye, F.A.; Melmed, G.Y.; Lichtenstein, G.R.; Kane, S.V. ACG clinical guideline: Preventive care in inflammatory bowel disease. Am. J. Gastroenterol. 2017, 112, 241–258. [Google Scholar] [CrossRef]
- American Gastroenterological Association medical position statement: Guidelines on osteoporosis in gastrointestinal diseases. Gastroenterology 2003, 124, 791–794.
- Harbord, M.; Annese, V.; Vavricka, S.R.; Allez, M.; Acosta, M.B.-D.; Boberg, K.M.; Burisch, J.; De Vos, M.; De Vries, A.-M.; Dick, A.D.; et al. The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease. J. Crohn’s Colitis 2015, 10, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Melek, J.; Sakuraba, A. Efficacy and Safety of Medical Therapy for Low Bone Mineral Density in Patients with Inflammatory Bowel Disease: A Meta-analysis and Systematic Review. Clin. Gastroenterol. Hepatol. 2014, 12, 32–44. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, X.; Chen, X.; Zhang, S.; Jiang, T.; Chang, J.; Gao, Y. Bone Loss Prevention of Bisphosphonates in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Can. J. Gastroenterol. Hepatol. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Bai, J.C.; Biagi, F.; Card, T.R.; Ciacci, C.; Ciclitira, P.J.; Green, P.H.R.; Hadjivassiliou, M.; Holdoway, A.; van Heel, D.A.; et al. Diagnosis and management of adult coeliac disease: Guidelines from the British Society of Gastroenterology. Gut 2014, 63, 1210–1228. [Google Scholar] [CrossRef]
- Pintos Sanchez, M.; Premysl, B.; Verdu, E.; Bai, J.C. Extraintestinal manifestations of celiac disease. Dig. Dis. 2015, 33, 147–154. [Google Scholar] [CrossRef]
- Zanchetta, M.B.; Longobardi, V.; Bai, J.C. Bone and celiac disease. Curr. Osteoporos. Rep. 2016, 14, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Larussa, T.; Suraci, E.; Nazionale, I.; Abenavoli, L.; Imeneo, M.; Luzza, F. Bone Mineralization in Celiac Disease. Gastroenterol. Res. Pr. 2012, 2012, 198025. [Google Scholar] [CrossRef] [PubMed]
- Olmos, M.; Antelo, M.; Vazquez, H.; Smecuol, E.; Mauriño, E.; Bai, J. Systematic review and meta-analysis of observational studies on the prevalence of fractures in coeliac disease. Dig. Liver Dis. 2008, 40, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Heikkila, K.; Pearce, J.; Mäki, M.; Kaukinen, K. Celiac Disease and Bone Fractures: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2015, 100, 25–34. [Google Scholar] [CrossRef]
- Vasquez, H.; Mazure, R.; Gonzalez, D.; Flores, D.; Pedreira, S.; Niveloni, S.; Smecuol, E.; Mauriño, E.; Bai, J.C. Risk of fractures in celiac disease patients: A cross-sectional, case–control study. Am. J. Gastroenterol. 2000, 95, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Nuti, R.; Martini, G.; Valenti, R.; Giovani, S.; Salvadori, S.; Avanzati, A. Prevalence of undiagnosed coeliac syndrome in os-teoporotic women. J. Intern. Med. 2001, 250, 361–366. [Google Scholar] [CrossRef]
- Rios, L.P.; Khan, A.; Sultan, M.; McAssey, K.; Fouda, M.A.; Armstrong, D. Approach to diagnosing celiac disease in patients with low bone mineral density or fragility fractures: Multidisciplinary task force report. Can. Fam Phys. 2013, 59, 1055–1061. [Google Scholar]
- Jorde, R.; Saleh, F.; Sundsfjord, J.; Haug, E.; Skogen, B. Coeliac disease in subjects with secondary hyperparathyroidism. Scand. J. Gastroenterol. 2005, 40, 178–182. [Google Scholar] [CrossRef]
- De Bruin, I.J.A.; Vranken, L.; Wyers, C.E.; Van Der Velde, R.Y.; Trienekens, T.A.M.; Kaarsemaker, S.; Janzing, H.M.J.; Wolters, F.L.; Wouda, S.; Geusens, P.P.M.M.; et al. The Prevalence of Celiac Disease in a Fracture Liaison Service Population. Calcif. Tissue Res. 2020, 107, 327–334. [Google Scholar] [CrossRef]
- Fornari, C.; Pedreira, S.; Niveloni, S.; González, D.; Diez, R.; Vázquez, H.; Mazure, R.; Sugai, E.; Smecuol, E.; Boerr, L.; et al. Pre- and post-treatment serum levels of cytokines IL-1β, IL-6, and IL-1 receptor antagonist in celiac disease. Are they related to the associated osteopenia? Am. J. Gastroenterol. 1998, 93, 413–418. [Google Scholar] [CrossRef]
- Krupa-Kozak, U. Pathologic bone alterations in celiac disease: Etiology, epidemiology, and treatment. Nutrition 2014, 30, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Huang, Z.; Tang, S.; Wei, S.; Zhang, Z. An evaluation of homocysteine, C-reactive protein, lipid levels, neutrophils to lymphocyte ratio in postmenopausal osteopenic women. Gynecol. Endocrinol. 2016, 32, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya, M.; Dogan, Z.; Ergul, B.; Filik, L. Neutrophil-tolymphocyte ratio as a sensitive marker in diagnosis of celiac disease. Ann. Gastroenterol. 2014, 27, 431–432. [Google Scholar] [PubMed]
- Aydemir, Y.; Erdogan, B.; Türkeli, A. Vitamin D deficiency negatively affects both the intestinal epithelial integrity and bone metabolism in children with Celiac disease. Clin. Res. Hepatol. Gastroenterol. 2020, 45, 101523. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Shapira, Y.; Agmon-Levin, N.; Pacht, A.; Ben-Ami Shor, D.; López, H.M.; Sanchez-Castanon, M.; Shoenfeld, Y. The clinical significance of 25OH-Vitamin D status in celiac disease. Clin. Rev. Allergy Immunol. 2012, 42, 322–330. [Google Scholar] [CrossRef]
- Zanchetta, M.B.; Costa, F.; Longobardi, V.; Longarini, G.; Mazure, R.M.; Moreno, M.L.; Vázquez, H.; Silveira, F.; Niveloni, S.; Smecuol, E.; et al. Significant bone microarchitecture impairment in premenopausal women with active celiac disease. Bone 2015, 76, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.M.; Rogers, H.; Leib, A.; McMahon, D.J.; Young, P.; Nishiyama, K.; Guo, X.E.; Lewis, S.; Green, P.H.; Shane, E. Abnormal skeletal strength and microarchitecture in women with celiac disease. J. Clin. Endocrinol. Metab. 2015, 100, 2347–2353. [Google Scholar] [CrossRef]
- González, D.; Mazure, R.; Mautalen, C.; Vazquez, H.; Bai, J. Body composition and bone mineral density in untreated and treated patients with celiac disease. Bone 1995, 16, 231–234. [Google Scholar] [CrossRef]
- Mora, S.; Weber, G.; Barera, G.; Bellini, A.; Pasolini, D.; Prinster, C.; Bianchi, C.; Chiumello, G. Effect of gluten-free diet on bone mineral content in growing patients with celiac disease. Am. J. Clin. Nutr. 1993, 57, 224–228. [Google Scholar] [CrossRef]
- Duerksen, D.R.; Leslie, W.D. Longitudinal Evaluation of Bone Mineral Density and Body Composition in Patients with Positive Celiac Serology. J. Clin. Densitom. 2011, 14, 478–483. [Google Scholar] [CrossRef]
- Barera, G.; Beccio, S.; Proverbio, M.C.; Mora, S. Longitudinal changes in bone metabolism and bone mineral content in children with celiac disease during consumption of a gluten-free diet. Am. J. Clin. Nutr. 2004, 79, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.I.; Mohaidle, A.; Baistrocchi, A.; Matoso, D.; Vázquez, H.; González, A.; Mazure, R.; Maffei, E.; Ferrari, G.; Smecuol, E.; et al. Risk of fracture in celiac disease: Gender, dietary compliance, or both? World J. Gastroenterol. 2011, 17, 3035–3042. [Google Scholar] [CrossRef] [PubMed]
- Ciacci, C.; Bilancio, G.; Russo, I.; Iovino, P.; Cavallo, P.; Santonicola, A.; Bucci, C.; Cirillo, M.; Zingone, F. 25-Hydroxyvitamin D, 1,25-Dihydroxyvitamin D, and Peripheral Bone Densitometry in Adults with Celiac Disease. Nutrients 2020, 12, 929. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Michaelsson, K.; Ekbom, A.; Montgomery, S.M. Coeliac disease and the risk of fractures—a general popu-lation- based cohort study. Aliment. Pharmacol. Ther. 2007, 25, 273–285. [Google Scholar] [CrossRef]
- LaRussa, T.; Suraci, E.; Imeneo, M.; Marasco, R.; Luzza, F. Normal Bone Mineral Density Associates with Duodenal Mucosa Healing in Adult Patients with Celiac Disease on a Gluten-Free Diet. Nutrients 2017, 9, 98. [Google Scholar] [CrossRef]
- Zanchetta, M.B.; Longobardi, V.; Costa, F.; Longarini, G.; Mazure, R.M.; Moreno, M.L.; Vázquez, H.; Silveira, F.; Niveloni, S.; Smecuol, E.; et al. Impaired Bone Microarchitecture Improves After One Year on Gluten-Free Diet: A Prospective Longitudinal HRpQCT Study in Women with Celiac Disease. J. Bone Miner. Res. 2016, 32, 135–142. [Google Scholar] [CrossRef]
- Fouda, M.A.; Khan, A.A.; Sultan, M.S.; Rios, L.P.; McAssey, K.; Armstrong, D. Evaluation and management of skeletal health in celiac disease: Position statement. Can. J. Gastroenterol. 2012, 26, 819–829. [Google Scholar] [CrossRef]
- Kanis, J.A.; Johnell, O.; Oden, A.; Johansson, H.; McCloskey, E. FRAX and theassessment of fracture probability in men and women from the UK. Osteoporos. Int. 2008, 19, 385–397. [Google Scholar] [CrossRef]
- Tortora, R.; Imperatore, N.; Capone, P.; Gerbino, N.; Rea, M.; Affinito, G.; Caporaso, N.; Rispo, A. FRAX Score Can Be Used to Avoid Superfluous DXA Scans in Detecting Osteoporosis in Celiac Disease: Accuracy of the FRAX Score in Celiac Patients. J. Clin. Densitom. 2017, 21, 315–321. [Google Scholar] [CrossRef]
- Ding, C.; Parameswaran, V.; Udayan, R.; Burgess, J.; Jones, G. Circulating Levels of Inflammatory Markers Predict Change in Bone Mineral Density and Resorption in Older Adults: A Longitudinal Study. J. Clin. Endocrinol. Metab. 2008, 93, 1952–1958. [Google Scholar] [CrossRef]
- McColl, K.E.L. Helicobacter pylori Infection. N. Engl. J. Med. 2010, 362, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Everhart, J.E. Recent Developments in The Epidemiology of Helicobacter Pylori. Gastroenterol. Clin. N. Am. 2000, 29, 559–578. [Google Scholar] [CrossRef]
- Bravo, D.; Hoare, A.; Soto, C.; Valenzuela, M.A.; Quest, A.F. Helicobacter pylori in human health and disease: Mechanisms for local gastric and systemic effects. World J. Gastroenterol. 2018, 24, 3071–3089. [Google Scholar] [CrossRef]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- Blaser, M.J.; Atherton, J.C. Helicobacter pylori persistence: Biology and disease. J. Clin. Investig. 2004, 113, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, F.; Tortora, A.; Gasbarrini, G.; Gasbarrini, A. Helicobacter pylori and Extragastric Diseases. Helicobacter 2014, 19, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Mashimo, M.; Nishikawa, M.; Higuchi, K.; Hirose, M.; Wei, Q.; Haque, A.; Sasaki, E.; Shiba, M.; Tominaga, K.; Watanabe, T.; et al. Production of Reactive Oxygen Species in Peripheral Blood is Increased in Individuals with Helicobacter pylori Infection and Decreased after its Eradication. Helicobacter 2006, 11, 266–271. [Google Scholar] [CrossRef]
- Chmiela, M.; Gonciarz, W. Molecular mimicry in Helicobacter pylori infections. World J. Gastroenterol. 2017, 23, 3964–3977. [Google Scholar] [CrossRef]
- Schubert, M.L. Gastric exocrine and endocrine secretion. Curr. Opin. Gastroenterol. 2009, 25, 529–536. [Google Scholar] [CrossRef]
- Papamichael, K.X.; Papaioannou, G.; Karga, H.; Roussos, A.; Mantzaris, G.J. Helicobacter pylori infection and endocrine dis-orders: Is there a link? World J. Gastroenterol. 2009, 15, 2701–2707. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Kodama, T.; Gutierrez, O.; Kim, J.G.; Kashima, K.; Graham, D.Y. Relationship between Helicobacter pylori iceA, cagA, and vacA Status and Clinical Outcome: Studies in Four Different Countries. J. Clin. Microbiol. 1999, 37, 2274–2279. [Google Scholar] [CrossRef] [PubMed]
- Perez-Perez, G.I.; Peek, R.M.; Legath, A.J.; Heine, P.R.; Graff, L.B. The role of CagA status in gastric and extragastric compli-cations of Helicobacter pylori. J. Physiol. Pharmacol. 1999, 50, 833–845. [Google Scholar] [PubMed]
- Brandt, S.; Kwok, T.; Hartig, R.; Konig, W.; Backert, S. NF-kappa B activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc. Natl. Acad. Sci. USA 2005, 102, 9300–9305. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, X.; Zhang, Q.; Ge, B.; Zhang, J.; Yu, L.; Cai, T.; Zhang, Y.; Xiong, H. Relationship between Helicobacter pylori infection and osteoporosis: A systematic review and meta-analysis. BMJ Open 2019, 9, e027356. [Google Scholar] [CrossRef]
- Pan, B.-L.; Huang, C.-F.; Chuah, S.-K.; Chiang, J.-C.; Loke, S.-S. Relationship between Helicobacter pylori infection and bone mineral density: A retrospective cross-sectional study. BMC Gastroenterol. 2018, 18, 54. [Google Scholar] [CrossRef]
- Ozdem, S.; Akcam, M.; Yilmaz, A.; Gultekin, M.; Artan, R. Biochemical markers of bone metabolism in children with Helico-bacter pylori infection. Dig. Dis. Sci. 2007, 52, 967–972. [Google Scholar] [CrossRef]
- Akkaya, N.; Akkaya, S.; Polat, Y.; Turk, M.; Turk, T.; Ergur, S.; Sahin, F. Helicobacter Pylori Seropositivity in Patients with Postmenopausal Osteoporosis. J. Phys. Ther. Sci. 2011, 23, 61–64. [Google Scholar] [CrossRef][Green Version]
- Lin, S.-C.; Koo, M.; Tsai, K.-W. Association betweenHelicobacter pyloriInfection and Risk of Osteoporosis in Elderly Taiwanese Women with Upper Gastrointestinal Diseases: A Retrospective Patient Record Review. Gastroenterol. Res. Pr. 2014, 2014, 1–5. [Google Scholar] [CrossRef]
- Mizuno, S.; Matsui, D.; Watanabe, I.; Ozaki, E.; Kuriyama, N.; Watanabe, Y. Serologically Determined Gastric Mucosal Condition Is a Predictive Factor for Osteoporosis in Japanese Men. Am. J. Dig. Dis. 2015, 60, 2063–2069. [Google Scholar] [CrossRef]
- Asaoka, D.; Nagahara, A.; Shimada, Y.; Matsumoto, K.; Ueyama, H.; Matsumoto, K.; Nakagawa, Y.; Takeda, T.; Tanaka, I.; Sasaki, H.; et al. Risk factors for osteoporosis in Japan: Is it associated with Helicobacter pylori? Ther. Clin. Risk Manag. 2015, 11, 381–391. [Google Scholar] [CrossRef]
- Fotouk-Kiai, M.; Hoseini, S.R.; Meftah, N.; Ghadimi, R.; Bijani, A.; Noreddini, H.; Nematollahi, H.; Shokri-Shirvani, J. Rela-tionship between Helicobacter pylori infection (HP) and bone mineral density (BMD) in elderly people. Casp. J. Intern. Med. 2015, 6, 62–66. [Google Scholar]
- Chung, Y.H.; Gwak, J.S.; Hong, S.W.; Hyeon, J.H.; Lee, C.M.; Oh, S.W.; Kwon, H. Helicobacter pylori: A Possible Risk Factor for Bone Health. Korean J. Fam. Med. 2015, 36, 239–244. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kalantarhormozi, M.R.; Assadi, M.; Vahdat, K.; Asadipooya, K.; Ostovar, A.; Raissi, K.; Darabi, H.; Farrokhi, S.; Dobaradaran, S.; Farrokhnia, M.; et al. Chlamydia pneumonia and Helicobacter pylori IgG seropositivities are not predictors of osteopo-rosis-associated bone loss: A prospective cohort study. J. Bone Miner. Metab. 2016, 34, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Kakehasi, A.M.; Rodrigues, C.B.; Carvalho, A.V.; Barbosa, A.J.A. Chronic Gastritis and Bone Mineral Density in Women. Am. J. Dig. Dis. 2008, 54, 819–824. [Google Scholar] [CrossRef]
- Asaoka, D.; Nagahara, A.; Hojo, M.; Sasaki, H.; Shimada, Y.; Yoshizawa, T.; Osada, T.; Watanabe, S. The relationship between H. pylori infection and osteoporosis in Japan. Gastroenterol. Res. Pract. 2014, 2014, 340765. [Google Scholar] [CrossRef]
- Chinda, D.; Shimoyama, T.; Iino, C.; Matsuzaka, M.; Nakaji, S.; Fukuda, S. Decrease of estradiol and several lifestyle factors, but not Helicobacter pylori infection, are significant risks for osteopenia in Japanese females. Digestion 2017, 96, 103–109. [Google Scholar] [CrossRef]
- Lu, L.-J.; Hao, N.-B.; Liu, J.-J.; Li, X.; Wang, R.-L. Correlation between Helicobacter pylori Infection and Metabolic Abnormality in General Population: A Cross-Sectional Study. Gastroenterol. Res. Pr. 2018, 2018, 7410801. [Google Scholar] [CrossRef]
- Chinda, D.; Shimoyama, T.; Sawada, K.; Iino, C.; Sakuraba, H.; Nakaji, S.; Fukuda, S. Lifestyle Factors Rather Than Helicobacter pylori Infection or Estradiol Level are Associated With Osteopenia in Japanese Men. Am. J. Men’s Health 2019, 13, 1557988319848219. [Google Scholar] [CrossRef]
- Figura, N.; Gennari, L.; Merlotti, D.; Lenzi, C.; Campagna, M.S.; Franci, M.B.; Lucani, B.; Trabalzini, L.; Bianciardi, L.; Gonnelli, C.; et al. Prevalence of Helicobacter pylori Infection in Male Patients with Osteoporosis and Controls. Am. J. Dig. Dis. 2005, 50, 847–852. [Google Scholar] [CrossRef]
- Gennari, L.; Merlotti, D.; Figura, N.; Mingiano, C.; Franci, M.B.; Lucani, B.; Picchioni, T.; Alessandri, M.; Campagna, M.S.; Gonnelli, S.; et al. Infection by CagA-Positive Helicobacter pylori Strains and Bone Fragility: A Prospective Cohort Study. J. Bone Miner. Res. 2021, 36, 80–89. [Google Scholar] [CrossRef]
- Kim, T.J.; Lee, H.; Min, Y.W.; Min, B.; Lee, J.H.; Rhee, P.; Kim, J.J. Cohort study of Helicobacter pylori infection and the risk of incident osteoporosis in women. J. Gastroenterol. Hepatol. 2020, 36, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.M.; Hsu, T.Y.; Chen, C.Y.; Lin, C.L.; Kao, C.H.; Chen, C.H.; Yang, T.Y.; Chen, W.K. Analysis of Patients with Heli-cobacter pylori Infection and the Subsequent Risk of Developing Osteoporosis after Eradication Therapy: A Nationwide Popu-lation-Based Cohort Study. PLoS ONE 2016, 11, e0162645. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, Y.; Kato, M.; Asaka, M. Geographic Differences in Gastric Cancer Incidence Can be Explained by Differences be-tween Helicobacter pylori Strains. Intern. Med. 2008, 47, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; McMahon, A.D.; Patel, H.; Packard, C.J.; Rathbone, B.J.; Samani, N.J. Prospective analysis of the association of infection with CagA bearing strains of Helicobacter pylori and coronary heart disease. Heart 2002, 88, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Pasceri, V.; Patti, G.; Cammarota, G.; Pristipino, C.; Richichi, G.; Di Sciascio, G. Virulent strains of Helicobacter pylori and vascular diseases: A meta-analysis. Am. Hear. J. 2006, 151, 1215–1222. [Google Scholar] [CrossRef]
- Gunn, M.; Stephens, J.C.; Thompson, J.R.; Rathbone, B.J.; Samani, N.J. Significant association of cagA positive Helicobacter pylori strains with risk of premature myocardial infarction. Heart 2000, 84, 267–271. [Google Scholar] [CrossRef]
- Chen, Y.; Blaser, M.J. Association Between Gastric Helicobacter pylori Colonization and Glycated Hemoglobin Levels. J. Infect. Dis. 2012, 205, 1195–1202. [Google Scholar] [CrossRef]
- Barbour, K.E.; Boudreau, R.; Danielson, M.E.; Youk, A.O.; Wactawski-Wende, J.; Greep, N.C.; LaCroix, A.Z.; Jackson, R.D.; Wallace, R.B.; Bauer, D.C.; et al. Inflammatory markers and the risk of hip fracture: The women’s health initiative. J. Bone Miner. Res. 2012, 27, 1167–1176. [Google Scholar] [CrossRef]
- Schinke, T.; Schilling, A.F.; Baranowsky, A.; Seitz, S.; Marshall, R.P.; Linn, T.; Blaeker, M.; Huebner, A.K.; Schulz, A.; Simon, R.; et al. Impaired gastric acidification negatively affects calcium homeostasis and bone mass. Nat. Med. 2009, 15, 674–681. [Google Scholar] [CrossRef]
- Sipponen, P.; Härkönen, M. Hypochlorhydric stomach: A risk condition for calcium malabsorption and osteoporosis? Scand. J. Gastroenterol. 2010, 45, 133–138. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, Y.-H.; Han, K.; Nam, G.E.; Kim, G.S.; Han, B.; Lee, A.; Ahn, J.Y.; Ko, B.J. Atrophic Gastritis: A Related Factor for Osteoporosis in Elderly Women. PLoS ONE 2014, 9, e101852. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, R. Role of T cells in ovariectomy induced bone loss-revisited. J. Bone Miner. Res. 2012, 27, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Osawa, H.; Nakazato, M.; Date, Y.; Kita, H.; Ohnishi, H.; Ueno, H.; Shiiya, T.; Satoh, K.; Ishino, Y.; Sugano, K. Impaired Production of Gastric Ghrelin in Chronic Gastritis Associated withHelicobacter pylori. J. Clin. Endocrinol. Metab. 2005, 90, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Roper, J.; Francois, F.; Shue, P.L.; Mourad, M.S.; Pei, Z.; De Perez, A.Z.O.; Perez, G.P.; Tseng, C.-H.; Blaser, M.J. Leptin and Ghrelin in Relation toHelicobacter pyloriStatus in Adult Males. J. Clin. Endocrinol. Metab. 2008, 93, 2350–2357. [Google Scholar] [CrossRef]
- Fukushima, N.; Hanada, R.; Teranishi, H.; Fukue, Y.; Tachibana, T.; Ishikawa, H.; Takeda, S.; Takeuchi, Y.; Fukumoto, S.; Kangawa, K.; et al. Ghrelin Directly Regulates Bone Formation. J. Bone Miner. Res. 2004, 20, 790–798. [Google Scholar] [CrossRef]
- Ueyama, T.; Shirasawa, N.; Numazawa, M.; Yamada, K.; Shelangouski, M.; Ito, T.; Tsuruo, Y. Gastric Parietal Cells: Potent Endocrine Role in Secreting Estrogen as a Possible Regulator of Gastro-Hepatic Axis. Endocrinology 2002, 143, 3162–3170. [Google Scholar] [CrossRef]
- Neu, B.; Randlkofe, R.P.; Neuhofer, M.; Voland, P.; Mayerhofer, A.; Gerhard, M.; Schepp, W.; Prinz, C. Helicobacter pylori in-duces apoptosis of rat gastric parietal cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G309–G318. [Google Scholar] [CrossRef]
- Ueyama, T.; Shirasawa, N.; Ito, T.; Tsuruo, Y. Estrogen-producing steroidogenic pathways in parietal cells of the rat gastric mucosa. Life Sci. 2004, 74, 2327–2337. [Google Scholar] [CrossRef]
- Wong, I.P.; Baldock, P.A.; Herzog, H. Gastrointestinal peptides and bone health. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 44–50. [Google Scholar] [CrossRef]
- Targownik, L.E.; Lix, L.M.; Metge, C.J.; Prior, H.J.; Leung, S.; Leslie, W.D. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ 2008, 179, 319–326. [Google Scholar] [CrossRef]
- Yang, Y.-X.; Lewis, J.D.; Epstein, S.; Metz, D.C. Long-term Proton Pump Inhibitor Therapy and Risk of Hip Fracture. JAMA 2006, 296, 2947–2953. [Google Scholar] [CrossRef] [PubMed]
- Briganti, S.I.; Naciu, A.M.; Tabacco, G.; Cesareo, R.; Napoli, N.; Trimboli, P.; Castellana, M.; Manfrini, S.; Palermo, A. Proton Pump Inhibitors and Fractures in Adults: A Critical Appraisal and Review of the Literature. Int. J. Endocrinol. 2021, 2021, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Aasarød, K.M.; Mosti, M.P.; Stunes, A.K.; Reseland, J.E.; Basso, T.; Syversen, U.; Fossmark, R. Impaired skeletal health in patients with chronic atrophic gastritis. Scand. J. Gastroenterol. 2016, 51, 774–781. [Google Scholar] [CrossRef]
- Jacob, L.; Hadji, P.; Kostev, K. The use of proton pump inhibitors is positively associated with osteoporosis in postmenopausal women in Germany. Climacteric 2016, 19, 478–481. [Google Scholar] [CrossRef]
- Kim, A.-S.; Ko, H.-J. Atrophic Gastritis as a Risk Factor for Bone Loss in Premenopausal Women in Their 40s: A Retrospective Cohort Study. Calcif. Tissue Res. 2018, 104, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Goerss, J.B.; Kim, C.H.; Atkinson, E.J.; Eastell, R.; O’Fallon, W.M.; Melton, L.J. Risk of fractures in patients with pernicious anemia. J. Bone Miner. Res. 2009, 7, 573–579. [Google Scholar] [CrossRef]
- Merriman, N.A.; Putt, M.E.; Metz, D.C.; Yang, Y. Hip Fracture Risk in Patients with a Diagnosis of Pernicious Anemia. Gastroenterology 2010, 138, 1330–1337. [Google Scholar] [CrossRef]
- Yang, G.-T.; Zhao, H.-Y.; Kong, Y.; Sun, N.-N.; Dong, A.-Q. Correlation between serum vitamin B12 level and peripheral neuropathy in atrophic gastritis. World J. Gastroenterol. 2018, 24, 1343–1352. [Google Scholar] [CrossRef]
- Yoon, P.H.; An, S.J.; Jeong, S.-H.; Yang, Y.-J.; Hong, Y.-P. Association between Peptic Ulcer Disease and Osteoporosis: The Population-Based Longitudinal Cohort Study in Korea. Int. J. Environ. Res. Public Health 2019, 16, 2777. [Google Scholar] [CrossRef]
- Choi, H.G.; Rhim, C.C.; Yoon, J.Y.; Park, B.J.; Min, C.Y.; Lee, S.W. Increased risk of osteoporosis in patients with peptic ulcer: A follow-up study using a national sample cohort. Arch. Osteoporos. 2019, 14, 105. [Google Scholar] [CrossRef]
- Wu, C.-H.; Tung, Y.-C.; Chai, C.-Y.; Lu, Y.-Y.; Su, Y.-F.; Tsai, T.-H.; Kuo, K.-L.; Lin, C.-L. Increased Risk of Osteoporosis in Patients with Peptic Ulcer Disease. Medicine 2016, 95, e3309. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.; Fisher, A.; Smith, P.N. Helicobacter pylori Related Diseases and Osteoporotic Fractures (Narrative Review). J. Clin. Med. 2020, 9, 3253. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Lee, J.-I. Prevalence, Pathophysiology, Screening and Management of Osteoporosis in Gastric Cancer Patients. J. Gastric Cancer 2011, 11, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Lewallen, D.G. Association of peptic ulcer disease and pulmonary disease with risk of periprosthetic fracture after primary total knee arthroplasty. Arthritis Care Res. 2011, 63, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.J.; Lewallen, D.G. Peptic ulcer disease and heart disease are associated with periprosthetic fractures after total hip replacement. Acta Orthop. 2012, 83, 353–359. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, methods and major patterns in GLOBOCAN. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Zittel, T.T.; Zeeb, B.; Maier, G.W.; Kaiser, G.W.; Zwirner, M.; Liebich, H.; Starlinger, M.; Becker, H.D. High prevalence of bone disorders after gastrectomy. Am. J. Surg. 1997, 174, 431–438. [Google Scholar] [CrossRef]
- Lim, J.S.; Kim, S.B.; Bang, H.-Y.; Cheon, G.J.; Lee, J.-I. High prevalence of osteoporosis in patients with gastric adenocarcinoma following gastrectomy. World J. Gastroenterol. 2007, 13, 6492–6497. [Google Scholar] [CrossRef]
- Krupski, W.; Tatara, M.R.; Bury, P.; Szabelska, A.; Charuta, A.; Maciejewski, R.; Wallner, G.; Dabrowski, A. Negative Effects of Total Gastrectomy on Bone Tissue Metabolism and Volumetric Bone Mineral Density (vBMD) of Lumbar Spine in 1-Year Study in Men. Medicine 2016, 95, e2817. [Google Scholar] [CrossRef]
- Oh, H.J.; Lim, C.-H.; Yoon, B.-H.; Yoon, S.B.; Baeg, M.K.; Kim, W.C.; Cho, Y.K.; Park, J.M.; Choi, M.-G.; Yoo, H.M.; et al. Fracture after gastrectomy for gastric cancer: A long-term follow-up observational study. Eur. J. Cancer 2017, 72, 28–36. [Google Scholar] [CrossRef]
- Yoo, S.H.; Lee, J.A.; Kang, S.Y.; Kim, Y.S.; Sunwoo, S.; Kim, B.S.; Yook, J.-H. Risk of osteoporosis after gastrectomy in long-term gastric cancer survivors. Gastric Cancer 2017, 21, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.-M.; Yoo, J.-H.; Jeong, J.Y.; Park, Y.S. Bone mineral density after treatment for gastric cancer. Medicine 2018, 97, e9582. [Google Scholar] [CrossRef]
- Seo, G.H.; Kang, H.Y.; Choe, E.K. Osteoporosis and fracture after gastrectomy for stomach cancer: A nationwide claims study. Medicine 2018, 97, e0532. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-M.; Shin, N.W.; Lee, J.E.; Jin, S.-M.; Kim, S. Increased Risk of Osteoporosis in Gastric Cancer Survivors Compared to General Population Control: A Study with Representative Korean Population. Cancer Res. Treat. 2019, 51, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, Y.; Rino, Y.; Wada, H.; Kitani, Y.; Ozawa, Y.; Aoyama, T.; Oshima, T.; Yukawa, N.; Yoshikawa, T.; Masuda, M. Changes in bone metabolism after gastric cancer surgery in male patients: A prospective observational study. Gastric Cancer 2018, 22, 237–243. [Google Scholar] [CrossRef]
- Iki, M.; Fujita, Y.; Kouda, K.; Yura, A.; Tachiki, T.; Tamaki, J.; Sato, Y.; Moon, J.-S.; Hamada, M.; Kajita, E.; et al. Increased risk of osteoporotic fracture in community-dwelling elderly men 20 or more years after gastrectomy: The Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) Cohort Study. Bone 2019, 127, 250–259. [Google Scholar] [CrossRef]
- Shin, N.W.; Suh, B.; Lim, H.; Suh, Y.-S.; Choi, Y.J.; Jeong, S.-M.; Yun, J.M.; Song, S.O.; Park, Y. Increased Risk of Osteoporotic Fracture in Postgastrectomy Gastric Cancer Survivors Compared with Matched Controls: A Nationwide Cohort Study in Korea. Am. J. Gastroenterol. 2019, 114, 1735–1743. [Google Scholar] [CrossRef]
- Namikawa, T.; Yokota, K.; Iwabu, J.; Munekage, M.; Uemura, S.; Tsujii, S.; Maeda, H.; Kitagawa, H.; Karashima, T.; Kumon, M.; et al. Incidence and risk factors of osteoporotic status in outpatients who underwent gastrectomy for gastric cancer. JGH Open 2020, 4, 903–908. [Google Scholar] [CrossRef]
- Melton, L.J., 3rd; Crowson, C.S.; Khosla, S.; O’Fallon, W.M. Fracture risk after surgery for peptic ulcer disease: A population-based cohort study. Bone 1999, 25, 61–67. [Google Scholar] [CrossRef]
- Nishimura, O.; Furumoto, T.; Nosaka, K.; Kouno, K.; Sumikawa, M.; Hisaki, T.; Odachi, T.; Mizumoto, K.; Kishimoto, H.; Yamamoto, K.; et al. Bone disorder following partial and total gastrectomy with reference to bone mineral content. Surg. Today 1986, 16, 98–105. [Google Scholar] [CrossRef]
- Imamura, T.; Komatsu, S.; Ichikawa, D.; Kosuga, T.; Kubota, T.; Okamoto, K.; Konishi, H.; Shiozaki, A.; Fujiwara, H.; Otsuji, E. Reconstruction method as an independent risk factor for postoperative bone mineral density loss in gastric cancer. J. Gastroenterol. Hepatol. 2017, 33, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Rino, Y.; Aoyama, T.; Atsumi, Y.; Yamada, T.; Yukawa, N. Metabolic bone disorders after gastrectomy: Inevitable or preventable? Surg. Today 2021, 52, 182–188. [Google Scholar] [CrossRef]
- Rino, Y.; Oshima, T.; Yoshikawa, T. Changes in fat-soluble vitamin levels after gastrectomy for gastric cancer. Surg. Today 2016, 47, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, M.; Shiraki, Y.; Aoki, C.; Miura, M. Vitamin K2 (Menatetrenone) Effectively Prevents Fractures and Sustains Lumbar Bone Mineral Density in Osteoporosis. J. Bone Miner. Res. 2010, 15, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Rino, Y.; Yamamoto, Y.; Wada, N.; Yukawa, N.; Murakami, H.; Tamagawa, H.; Yamada, T.; Ohshima, T.; Masuda, M.; Imada, T. Changes in vitamin D after gastrectomy. Gastric Cancer 2007, 10, 228–233. [Google Scholar] [CrossRef]
- Johnston, I.; Welbourn, R.; Acheson, K. Gastrectomy and loss of weight. Lancet 1958, 271, 1242–1245. [Google Scholar] [CrossRef]
- Saeki, H.; Rino, Y.; Cho, H.; Sato, T.; Kawamoto, M.; Takanashi, Y.; Yamada, R.; Oshima, T.; Hatori, S.; Imada, T. Evaluation of calorie intake according to age and sex in patients undergoing surgery for gastric cancer. Hepatogastroenterology 2008, 55, 795–798. [Google Scholar]
- Aoyama, T.; Kawabe, T.; Hirohito, F.; Hayashi, T.; Yamada, T.; Tsuchida, K.; Sato, T.; Oshima, T.; Rino, Y.; Masuda, M.; et al. Body composition analysis within 1 month after gastrectomy for gastric cancer. Gastric Cancer 2015, 19, 645–650. [Google Scholar] [CrossRef]
- Takachi, K.; Doki, Y.; Ishikawa, O.; Miyashiro, I.; Sasaki, Y.; Ohigashi, H.; Murata, K.; Nakajima, H.; Hosoda, H.; Kangawa, K.; et al. Postoperative Ghrelin Levels and Delayed Recovery from Body Weight Loss after Distal or Total Gastrectomy. J. Surg. Res. 2006, 130, 1–7. [Google Scholar] [CrossRef]
- Cesari, M.; Pahor, M.; Lauretani, F.; Penninx, B.W.H.J.; Bartali, B.; Russo, R.; Cherubini, A.; Woodman, R.; Bandinelli, S.; Guralnik, J.M.; et al. Bone density and hemoglobin levels in older persons: Results from the InCHIANTI study. Osteoporos. Int. 2004, 16, 691–699. [Google Scholar] [CrossRef]
- Oh, Y.H.; Moon, J.H.; Cho, B. Association between Hemoglobin Level and Bone Mineral Density in Korean Adults. J. Bone Metab. 2017, 24, 161–173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alhava, E.M.; Aukee, S.; Karjalainen, P.; Kettunen, K.; Juuti, M. The Influence of Calcium and Calcium + Vitamin D2Treatment on Bone Mineral after Partial Gastrectomy. Scand. J. Gastroenterol. 1975, 10, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Rino, Y.; Imada, T.; Yamamoto, Y.; Takahashi, M.; Amano, T.; Takanashi, Y. The efficacy of 1α hydroxy vitamin D3 for treatment of the metabolic bone disorder in patients undergone gastrectomy for gastric cancer. Hepatogastroenterology 2000, 47, 1498–1500. [Google Scholar]
- Atsumi, Y.; Rino, Y.; Sato, T.; Cho, H.; Yoshikawa, T.; Yamamoto, N.; Oshima, T.; Yukawa, N.; Shiozawa, M.; Morinaga, S.; et al. Effectiveness of alendronate for bone disorder after gastrectomy for gastric cancer. Asian J. Surg. 2016, 40, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Bäckhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Surana, N.K.; Kasper, D.L. Deciphering the tete-a-tete between the microbiota and the immune system. J. Clin. Investig. 2014, 124, 4197–4203. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Bäckhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- McCabe, L.R.; Britton, R.A.; Parameswaran, N. Prebiotic and Probiotic Regulation of Bone Health: Role of the Intestine and its Microbiome. Curr. Osteoporos. Rep. 2015, 13, 363–371. [Google Scholar] [CrossRef]
- Hernandez, C.J.; Guss, J.D.; Luna, M.; Goldring, S.R. Links Between the Microbiome and Bone. J. Bone Miner. Res. 2016, 31, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Cronin, O.; Lanham-New, S.A.; Corfe, B.M.; Gregson, C.L.; Darling, A.L.; Ahmadi, K.R.; Gibson, P.S.; Tobias, J.H.; Ward, K.A.; Traka, M.H.; et al. Role of the Microbiome in Regulating Bone Metabolism and Susceptibility to Osteoporosis. Calcif. Tissue Res. 2022, 110, 273–284. [Google Scholar] [CrossRef]
- Li, J.Y.; Yu, M.; Pal, S.; Tyagi, A.M.; Dar, H.; Adams, J.; Weitzmann, M.N.; Jones, R.M.; Pacifici, R. Parathyroid hor-mone-dependent bone formation requires butyrate production by intestinal microbiota. J. Clin. Investig. 2020, 130, 1767–1781. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Herzog, J.W.; Tsang, K.; Brennan, C.A.; Bower, M.A.; Garrett, W.S.; Sartor, B.R.; Aliprantis, A.O.; Charles, J. FGut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl. Acad. Sci. USA 2016, 113, e7554–e7563. [Google Scholar] [CrossRef] [PubMed]
- McCabe, L.R.; Irwin, R.; Schaefer, L.; Britton, R.A. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J. Cell Physiol. 2013, 228, 1793–1798. [Google Scholar] [CrossRef]
- Chiang, S.-S.; Pan, T.-M. Antiosteoporotic Effects of Lactobacillus-Fermented Soy Skim Milk on Bone Mineral Density and the Microstructure of Femoral Bone in Ovariectomized Mice. J. Agric. Food Chem. 2011, 59, 7734–7742. [Google Scholar] [CrossRef]
- Ohlsson, C.; Engdahl, C.; Fåk, F.; Andersson, A.; Windahl, S.H.; Farman, H.H.; Movérare-Skrtic, S.; Islander, U.; Sjögren, K. Probiotics Protect Mice from Ovariectomy-Induced Cortical Bone Loss. PLoS ONE 2014, 9, e92368. [Google Scholar] [CrossRef]
- Britton, R.A.; Irwin, R.; Quach, D.; Schaefer, L.; Zhang, J.; Lee, T.; Parameswaran, N.; McCabe, L.R. ProbioticL. reuteriTreatment Prevents Bone Loss in a Menopausal Ovariectomized Mouse Model. J. Cell. Physiol. 2014, 229, 1822–1830. [Google Scholar] [CrossRef]
- Chen, K.L.; Madak-Erdogan, Z. Estrogen and Microbiota Crosstalk: Should We Pay Attention? Trends Endocrinol. Metab. 2016, 27, 752–755. [Google Scholar] [CrossRef]
- Malik, T.A.; Chassaing, B.; Tyagi, A.M.; Vaccaro, C.; Luo, T.; Adams, J.; Darby, T.; Weitzmann, M.N.; Mulle, J.G.; Gewirtz, A.T.; et al. Sex steroid deficiency–associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Investig. 2016, 126, 2049–2063. [Google Scholar] [CrossRef]
- Nilsson, A.G.; Sundh, D.; Backhed, F.; Lorentzon, M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: A randomized, placebo-controlled, double-blind, clinical trial. J. Intern. Med. 2018, 284, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, T.; Hatanaka, M.; Hoshino, T.; Takara, T.; Tanaka, K.; Shimizu, A.; Morita, H.; Nakamura, T. Efect of Bacillus subtilis C-3102 on bone mineral density in healthy postmenopausal Japanese women: A randomized, placebo-controlled, double-blind clinical trial. Biosci. Microbiota Food Health 2018, 37, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.N.T.; Thybo, C.B.; Lykkeboe, S.; Rasmussen, L.M.; Frette, X.; Christensen, L.P.; Jeppesen, P.B. Combined bioa-vailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 106, 909–920. [Google Scholar]
- Jafarnejad, S.; Djafarian, K.; Fazeli, M.R.; Yekaninejad, M.S.; Rostamian, A.; Keshavarz, S.A. Effects of a Multispecies Probiotic Supplement on Bone Health in Osteopenic Postmenopausal Women: A Randomized, Double-blind, Controlled Trial. J. Am. Coll. Nutr. 2017, 36, 497–506. [Google Scholar] [CrossRef]
- Jansson, P.-A.; Curiac, D.; Ahrén, I.L.; Hansson, F.; Niskanen, T.M.; Sjögren, K.; Ohlsson, C. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2019, 1, e154–e162. [Google Scholar] [CrossRef]
- Li, C.; Huang, Q.; Yang, R.; Dai, Y.; Zeng, Y.; Tao, L.; Li, X.; Zeng, J.; Wang, Q. Gut microbiota composition and bone mineral loss—epidemiologic evidence from individuals in Wuhan, China. Osteoporos. Int. 2019, 30, 1003–1013. [Google Scholar] [CrossRef]
- Wei, M.; Li, C.; Dai, Y.; Zhou, H.; Cui, Y.; Zeng, Y.; Huang, Q.; Wang, Q. High-Throughput Absolute Quantification Sequencing Revealed Osteoporosis-Related Gut Microbiota Alterations in Han Chinese Elderly. Front. Cell. Infect. Microbiol. 2021, 11, 630372. [Google Scholar] [CrossRef]
- Rettedal, E.A.; Ilesanmi-Oyelere, B.L.; Roy, N.C.; Coad, J.; Kruger, M.C. The Gut Microbiome Is Altered in Postmenopausal Women with Osteoporosis and Osteopenia. JBMR Plus 2020, 5, e10452. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Leslie, W.D.; Leboff, M.S. AGA technical review on osteoporosis in gastrointestinal diseases. Gastroenterology 2003, 124, 795–841. [Google Scholar] [CrossRef]
- Gennari, L.; Rotatori, S.; Bianciardi, S.; Nuti, R.; Merotti, D. Treatment needs and current options for postmenopausal osteo-porosis. Expert Opin. Pharmacother. 2016, 17, 1141–1152. [Google Scholar] [CrossRef] [PubMed]



| Reference | Country | Study Design | Sample | HP Cases | Detection Method | Summary of Results |
|---|---|---|---|---|---|---|
| Figura et al., 2005 [119] | Italy | CS | 240 men (55–82 years) | 80 | ELISA | Increased OP prevalence in CagA-HP pts (OR 2.1; 95% CIs, 1.0–4.4) |
| Kakehashi et al., 2009 [114] | Brazil | CS | 85 postmen. women | 34 | UBT/His | No differences in BMD |
| Akkaya et al., 2011 [107] | Turkey | CS | 108 postmen. women | 76 | ELISA | No association with OP diagnosis |
| Asaoka et 2014 [115] | Japan | CS | 200 men and women (>50 years) | 83 | UBT/ELISA | Increased HP prevalence in OP pts (OR 5.3; 95% CIs, 1.7–16.4) |
| Lin et 2014 [108] | Taiwan | CS, R | 365 women (>65 years) | 77 | UBT/His | Increased HP prevalence in OP pts (OR 2.0; 95% CIs, 1.1–3.6) |
| Fotouk-Kiai et al., 2015 [111] | Iran | CS | 967 men and women (>60 years) | 758 | ELISA | No differences in BMD or OP prevalence (OR 0.76; 95% CIs, 0.6–1.0) |
| Chung et al., 2015 [112] | Korea | CS | 1126 men | 469 | ELISA | Decreased LS-BMD in HP pts |
| Asaoka et al., 2015 [110] | Japan | CS | 255 men and women (>50 years) | 94 | UBT/ELISA | Increased HP prevalence in OP pts (OR 3.0; 95% CIs, 1.3–6.9) |
| Mizuno et al., 2015 [109] | Japan | CS | 230 men (50–60 years) | 99 | ELISA | Increased HP prevalence in OP pts (OR 1.8; 95% CIs, 1.0–3.2) |
| Kalantarhormozi et al., 2016 [113] | Iran | P | 250 postmen. women (55–82 years) | 143 | ELISA | No difference in OP incidence (OR 0.96; 95% CIs, 0.5–1.8) |
| Chinda et al., 2017 [116] | Japan | CS | 473 women (20–86 years) | 118 | ELISA | No difference in osteopenia prevalence (OR 0.95; 95% CIs, 0.5–1.6) |
| Lu et al., 2018 [117] | China | CS | 1867 men and women | 589 | UBT | No association with OP diagnosis |
| Pan et al., 2018 [105] | Taiwan | CS | 867 men and women (>20 years) | 381 | His | Increased HP prevalence in OP pts (OR 1.6; 95% CIs, 1.1–2.3) |
| Chinda et al., 2019 [118] | Japan | CS, R | 268 men (19–90 years) | 268 | ELISA | No association with the diagnosis of osteopenia |
| Gennari et al., 2021 [120] | Italy | P | 1149 men and women (50–80 years) | 566 | ELISA | Increased fracture incidence in HP pts (HR 1.9; 95% CIs, 1.1–2.9) Increased fracture incidence in CagA-HP pts (HR 2.0; 95% CIs, 1.2–2.9) |
| Kim et al., 2021 [121] | Korea | R | 10482 women (>20 years) | 6009 | ELISA | Increased OP incidence in HP pts (HR 1.2; 95% CIs, 1.0–1.4) |
| Disease | Screening Indications | Follow-Up Indications | Treatment Indications * |
|---|---|---|---|
| IBD | DXA at diagnosis if:
| Repeat DXA within 2 years if:
| Consider treatment with bone-active agents if:
|
| Celiac Disease | DXA at diagnosis if
| Repeat DXA within 1–2 years after GFD if:
| Consider treatment with bone-active agents if:
|
| Helicobacter Pylori Infection | DXA at diagnosis if:
| Repeat DXA within 1–2 years after eradication if:
| Consider treatment with bone-active agents if:
|
| Chronic Gastritis or Peptic Ulcer Disease | DXA at diagnosis if:
| Repeat DXA within 2 years if:
| Consider treatment with bone-active agents if:
|
| Post-gastrectomy | DXA within 1–2 years post-gastrectomy | Repeat DXA within 2 years if:
| Consider treatment with bone active agents if:
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merlotti, D.; Mingiano, C.; Valenti, R.; Cavati, G.; Calabrese, M.; Pirrotta, F.; Bianciardi, S.; Palazzuoli, A.; Gennari, L. Bone Fragility in Gastrointestinal Disorders. Int. J. Mol. Sci. 2022, 23, 2713. https://doi.org/10.3390/ijms23052713
Merlotti D, Mingiano C, Valenti R, Cavati G, Calabrese M, Pirrotta F, Bianciardi S, Palazzuoli A, Gennari L. Bone Fragility in Gastrointestinal Disorders. International Journal of Molecular Sciences. 2022; 23(5):2713. https://doi.org/10.3390/ijms23052713
Chicago/Turabian StyleMerlotti, Daniela, Christian Mingiano, Roberto Valenti, Guido Cavati, Marco Calabrese, Filippo Pirrotta, Simone Bianciardi, Alberto Palazzuoli, and Luigi Gennari. 2022. "Bone Fragility in Gastrointestinal Disorders" International Journal of Molecular Sciences 23, no. 5: 2713. https://doi.org/10.3390/ijms23052713
APA StyleMerlotti, D., Mingiano, C., Valenti, R., Cavati, G., Calabrese, M., Pirrotta, F., Bianciardi, S., Palazzuoli, A., & Gennari, L. (2022). Bone Fragility in Gastrointestinal Disorders. International Journal of Molecular Sciences, 23(5), 2713. https://doi.org/10.3390/ijms23052713








