Amelioration of Endotoxin-Induced Acute Lung Injury and Alveolar Epithelial Cells Apoptosis by Simvastatin Is Associated with Up-Regulation of Survivin/NF-kB/p65 Pathway
Abstract
1. Introduction
2. Results
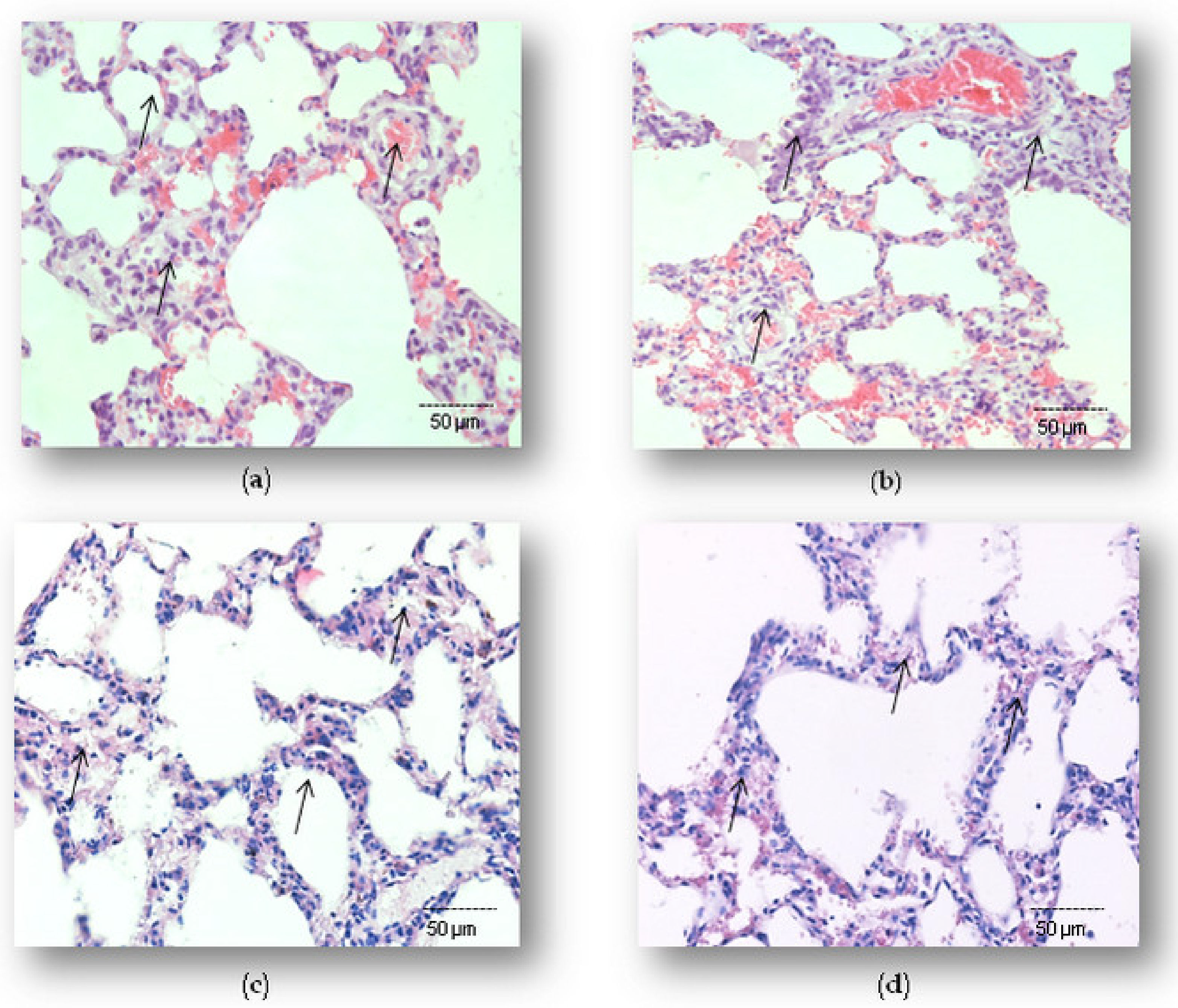
2.1. Effects of Simvastatin on Lung Histopathology in LPS-Induced Inflammation
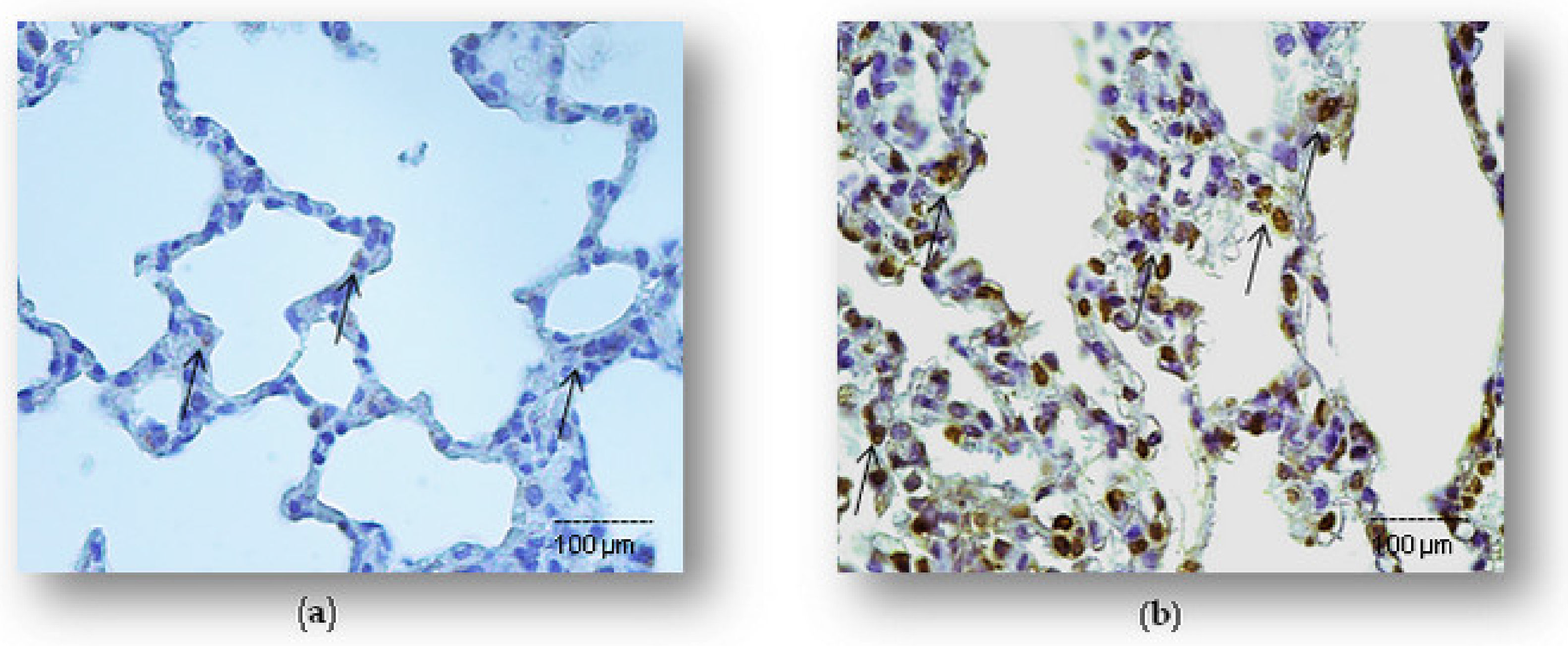
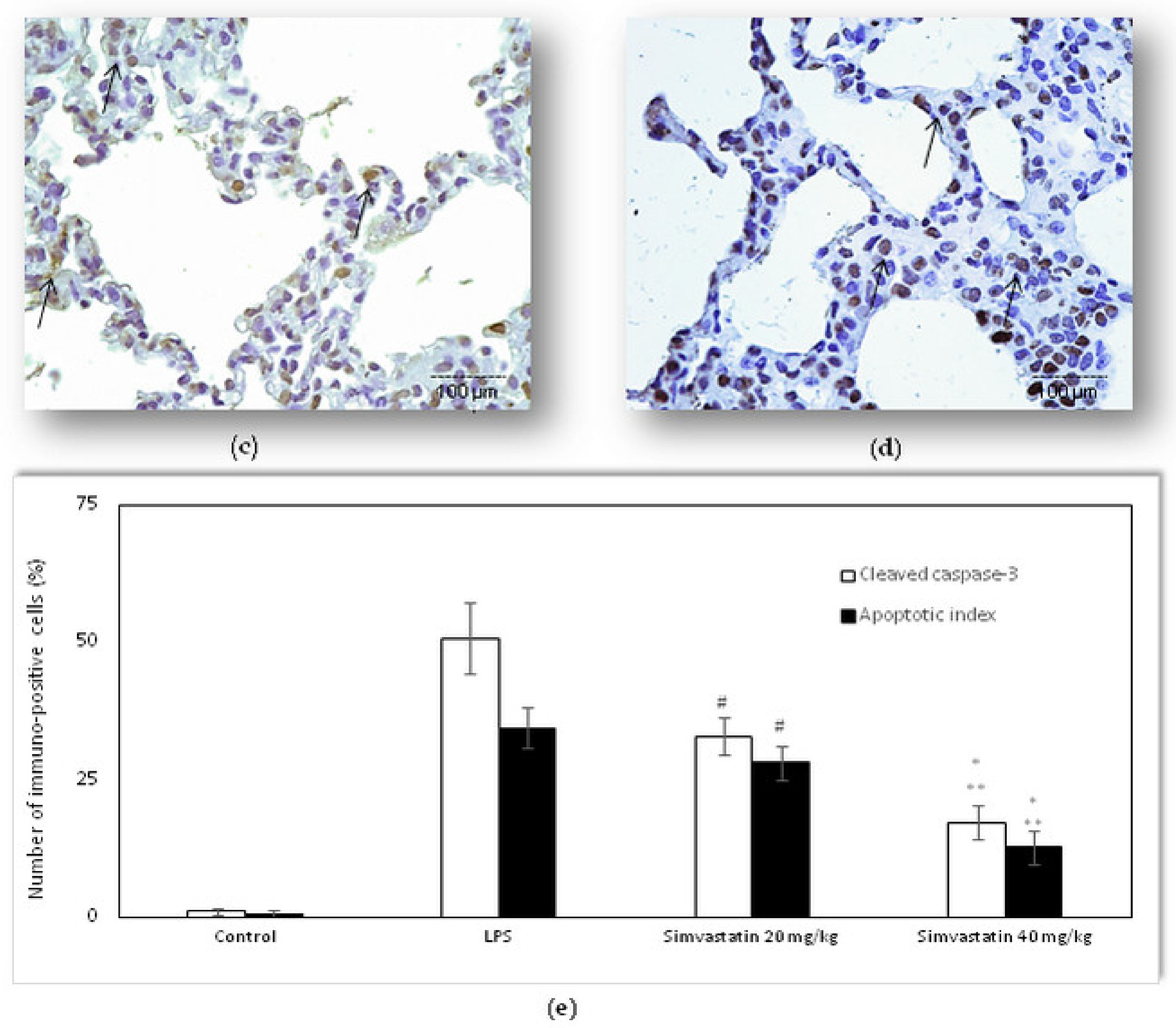
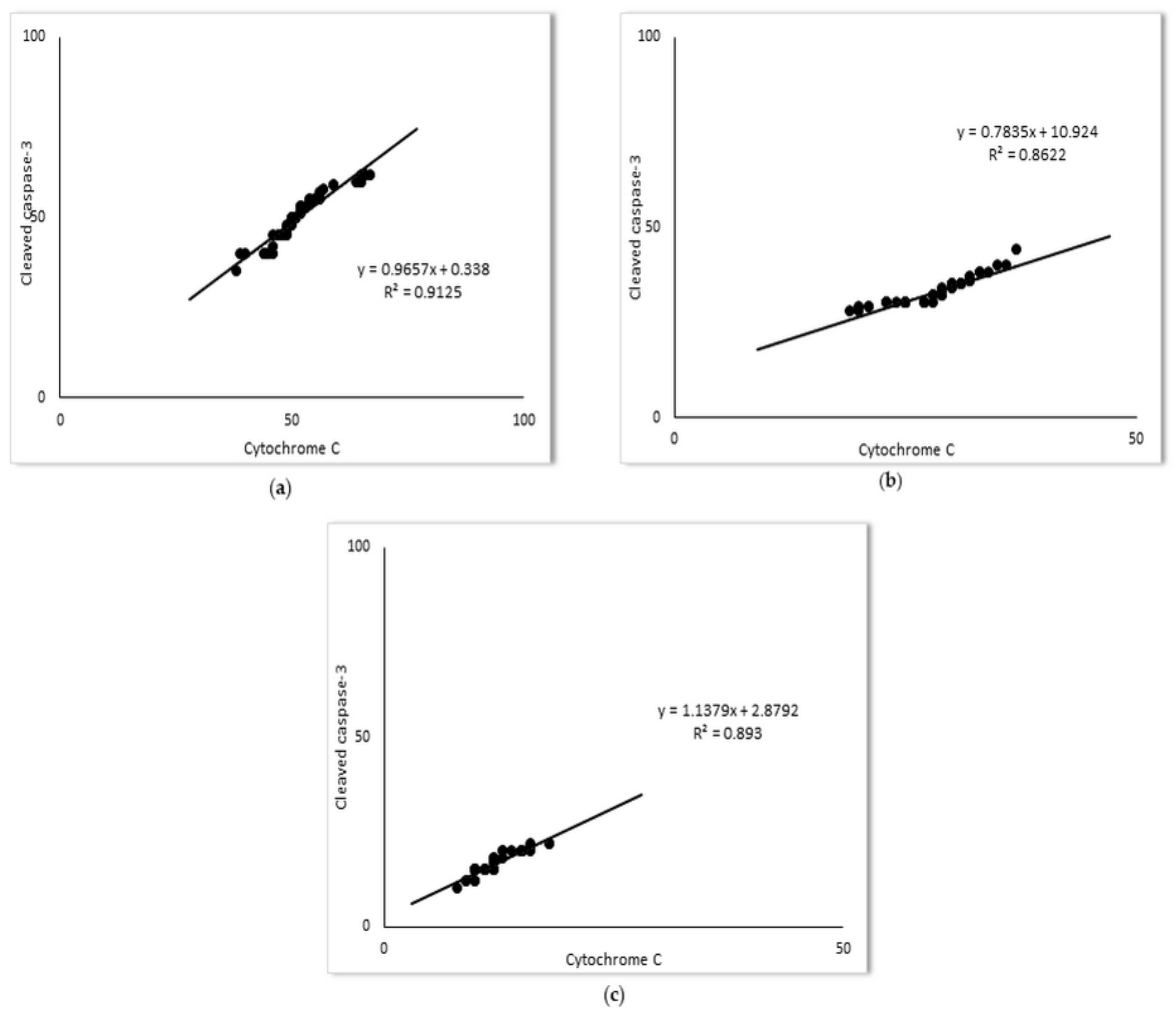
2.2. Simvastatin Attenuated Cytochrome C and Cleaved Caspase-3 Expression and LPS-Induced Apoptosis of Alveolar Epithelial Cells
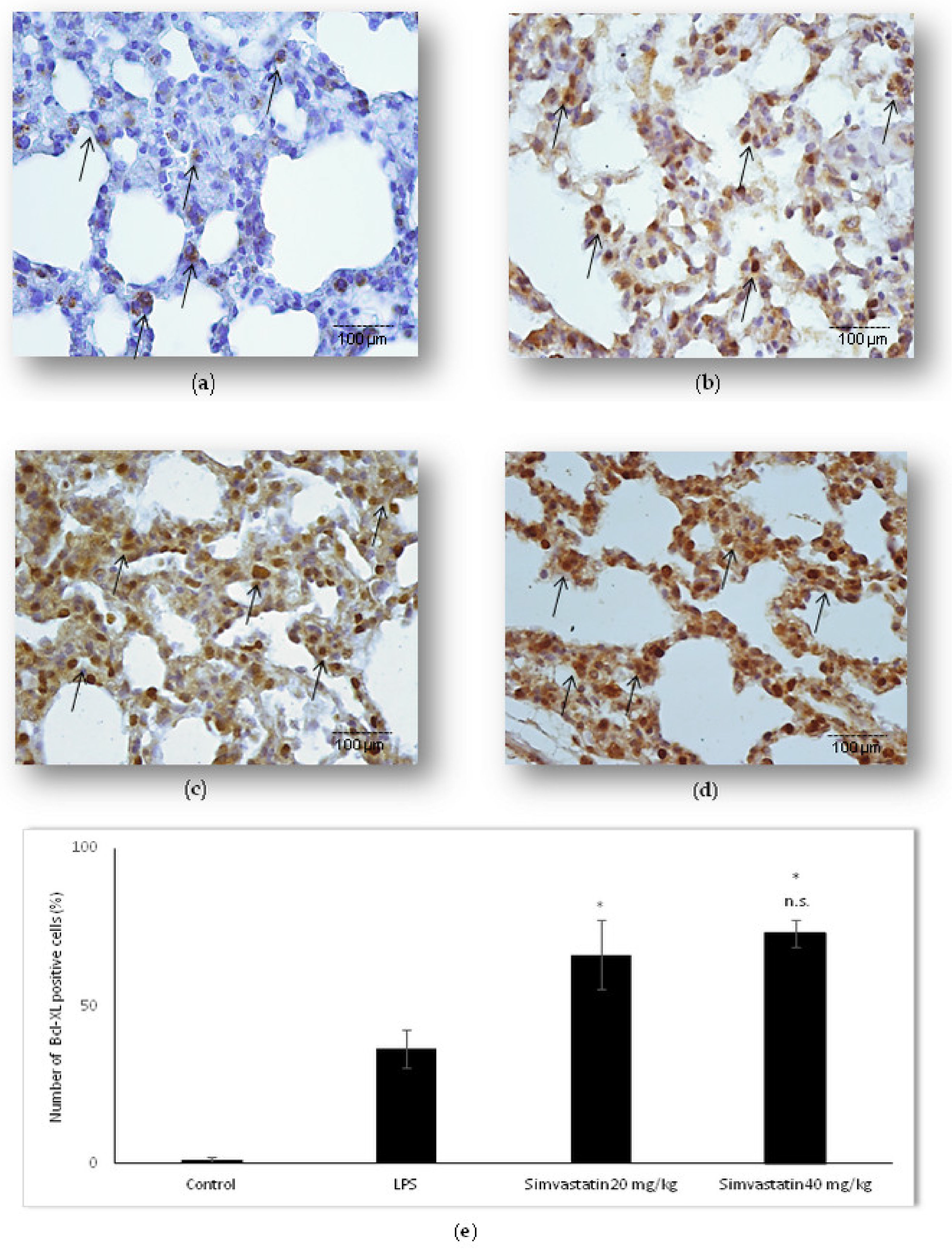
2.3. Simvastatin Up-Regulated Bcl-xL Expression in Pulmonary Parenchyma in LPS-Induced Inflammatory Injury
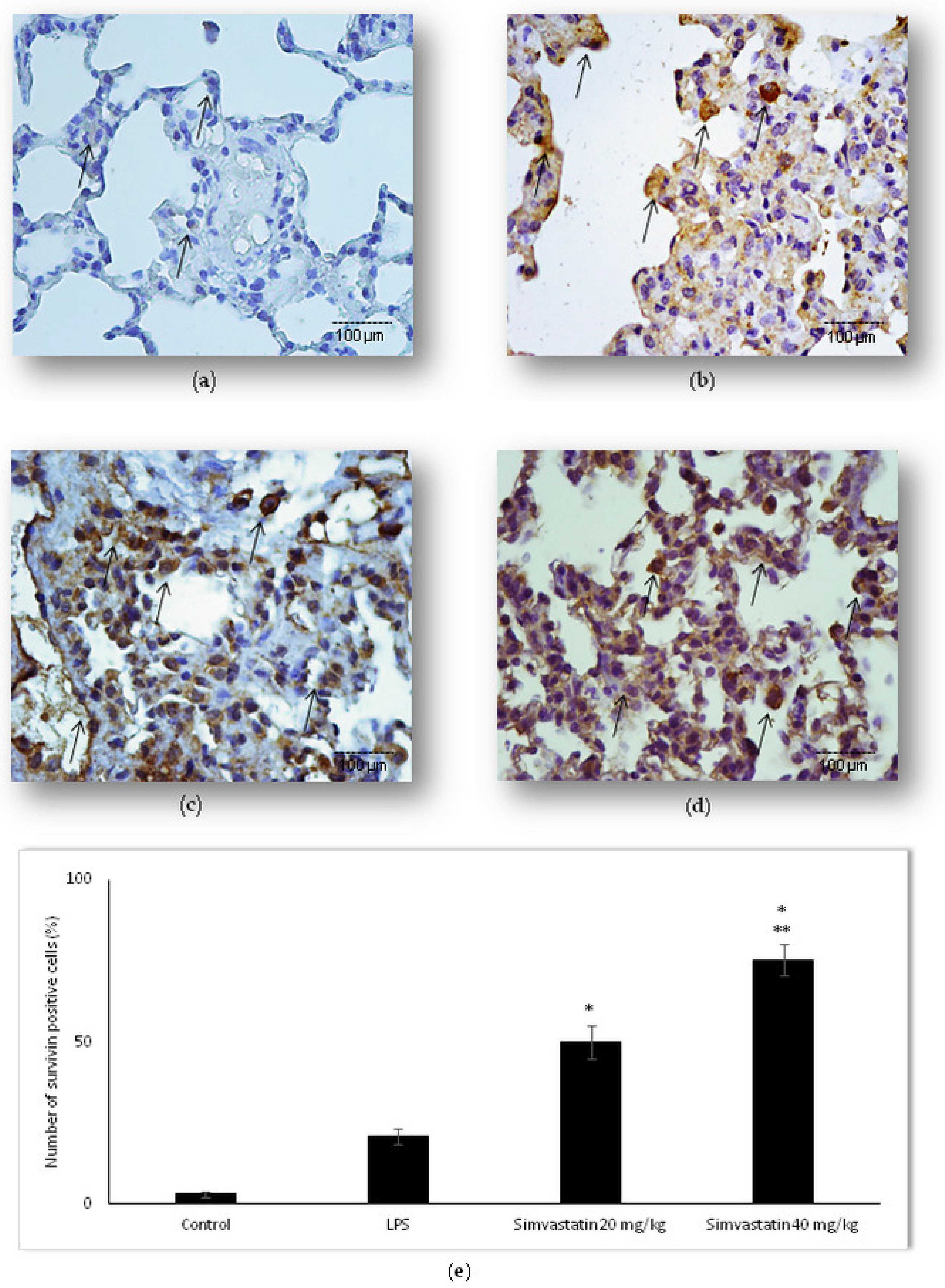
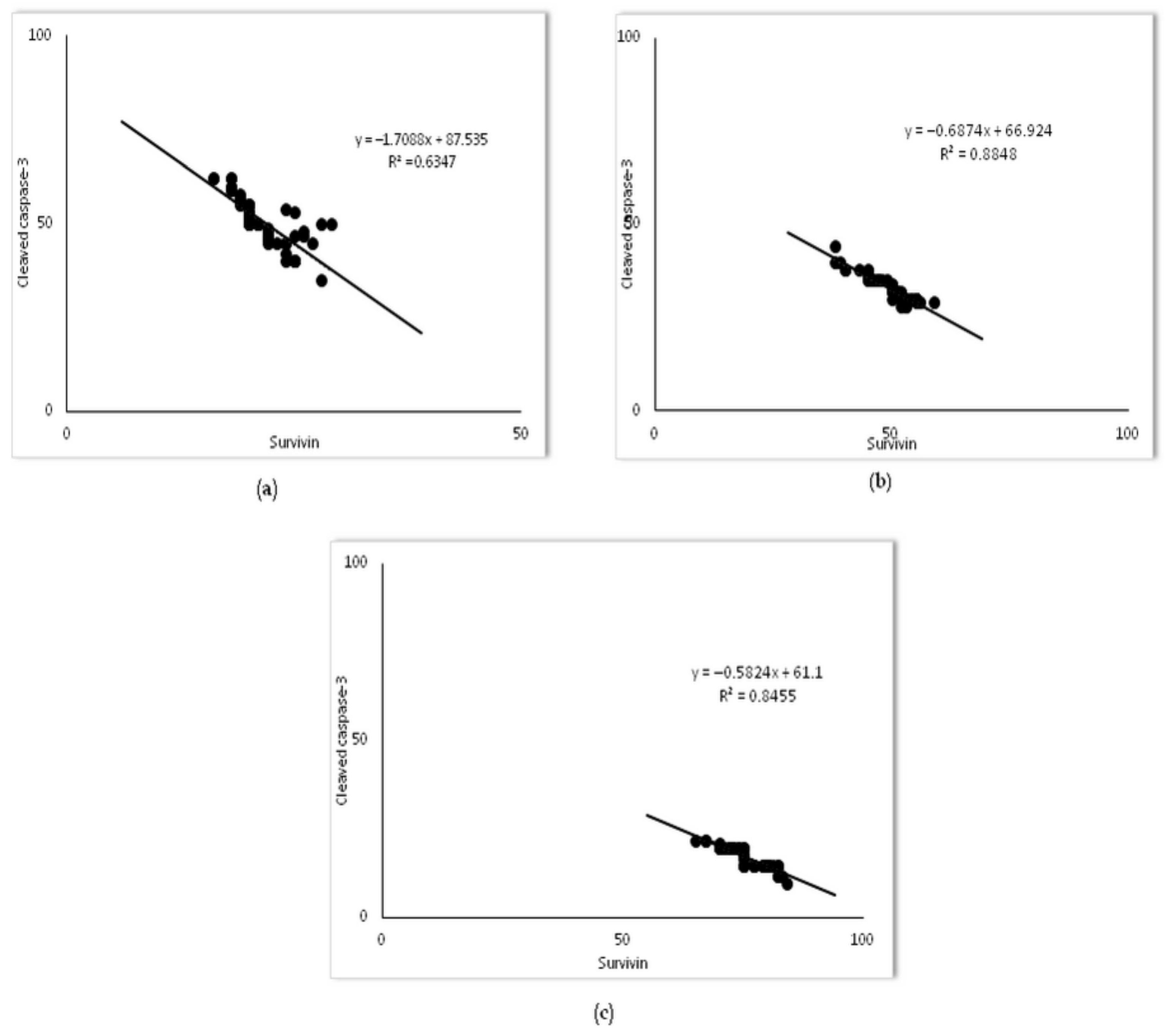
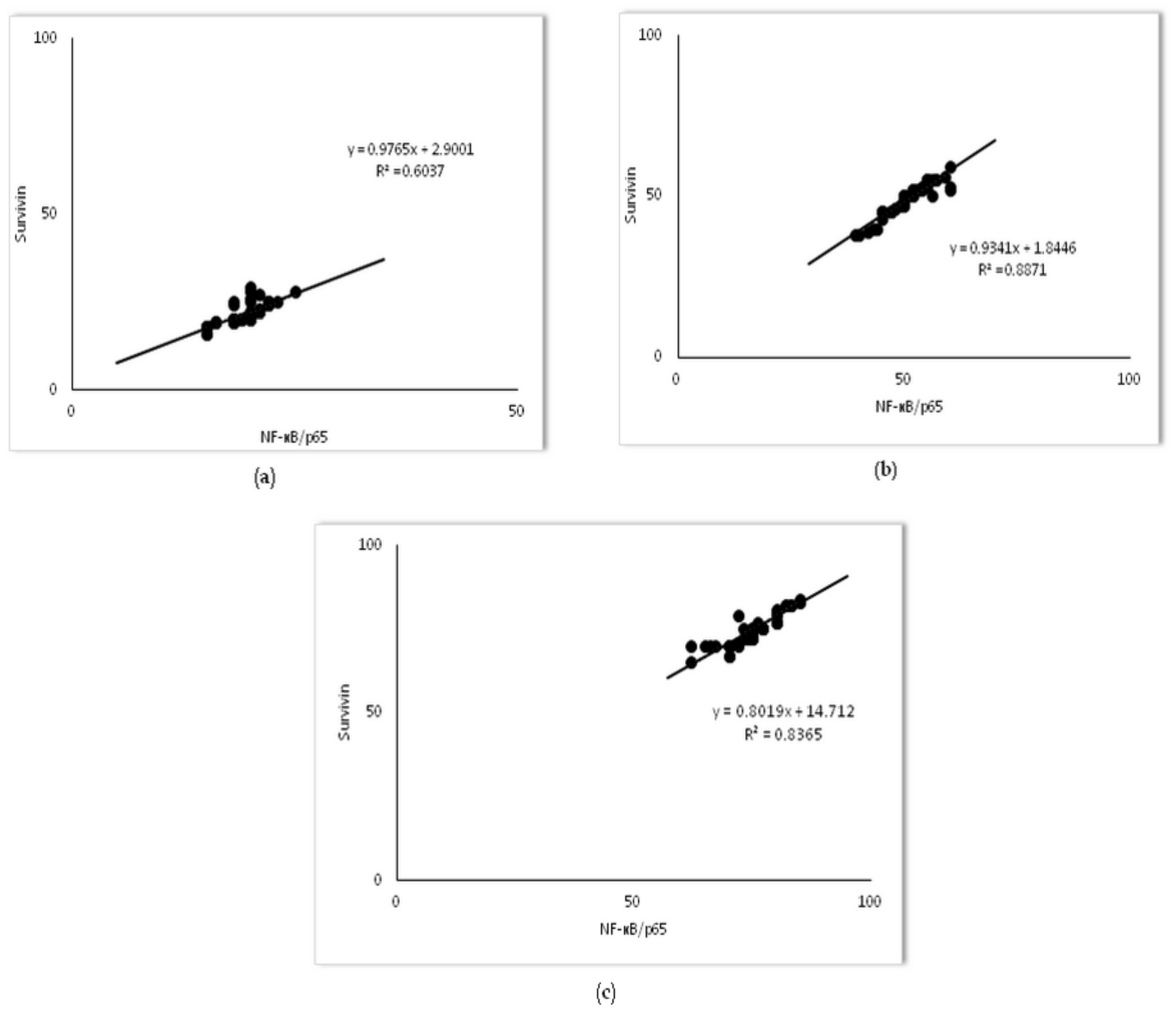
2.4. Survivin Was Involved in Simvastatin-Ameliorated Apoptosis of Alveolar Epithelial Cells Induced by LPS
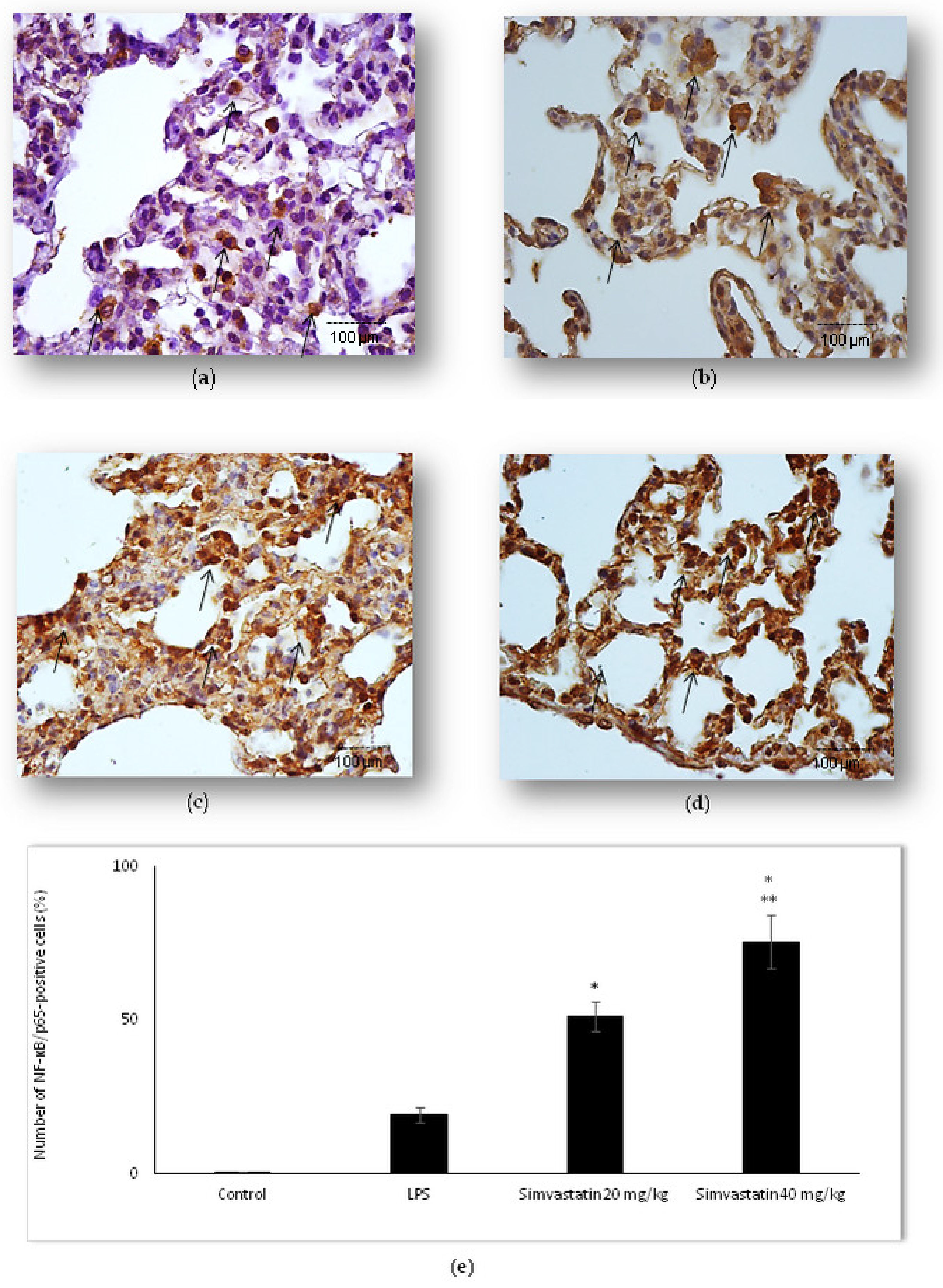
2.5. Changes of NF-kB Expression in Pulmonary Parenchyma after Simvastatin and LPS Administration
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Pharmacological Agents
4.3. Experimental Protocol
- (1)
- Control group (0.5% methylcellulose 1 mL/kg i.p.);
- (2)
- LPS group (non-lethal dose as 0.25 LD50/kg i.p., which is equal to 5.5 mg/kg of LPS i.p.);
- (3)
- 10 mg/kg of simvastatin group (10 mg/kg of simvastatin p.o. + 0.25 LD50/kg LPS i.p.);
- (4)
- 20 mg/kg of simvastatin group (20 mg/kg of simvastatin p.o. + 0.25 LD50/kg LPS i.p.);
- (5)
- 40 mg/kg of simvastatin group (40 mg/kg of simvastatin p.o. + 0.25 LD50/kg LPS i.p.).
4.4. Histopathological Evaluation and Semi-Quantitative Analysis of Pulmonary Damage Score
4.5. Semiquantitative Analyses
4.6. Detection and Quantification of Cell Apoptosis In Situ by the TUNEL Method
4.7. Immunohistochemistry in the Detection of Apoptosis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chopra, M.; Reuben, J.S.; Sharma, A.C. Acute lung injury: Apoptosis and signalling mechanisms. Exp. Biol. Med. 2009, 234, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Bai, C.; Wang, X. The value of the lipopolysaccharide-induced acute lung injury model in respiratory medicine. Expert Rev. Respir. Med. 2010, 4, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Nova, Z.; Skovierova, H.; Calkovska, A. Alveolar-Capillary Membrane-Related Pulmonary Cells as a Target in Endotoxin-Induced Acute Lung Injury. Int. J. Mol. Sci. 2019, 20, 831. [Google Scholar] [CrossRef] [PubMed]
- Zarrilli, G.; Angerilli, V.; Businello, G.; Sbaraglia, M.; Traverso, G.; Fortarezza, F.; Rizzo, S.; De Gaspari, M.; Basso, C.; Calabrese, F.; et al. The Immunopathological and Histological Landscape of COVID-19-Mediated Lung Injury. Int. J. Mol. Sci. 2021, 22, 974. [Google Scholar] [CrossRef]
- Chuang, C.Y.; Chen, T.L.; Cherng, Y.G.; Tai, Y.T.; Chen, T.G.; Chen, R.M. Lipopolysaccharide induces apoptotic insults to human alveolar epithelial A549 cells through reactive oxygen species-mediated activation of an intrinsic mitochondrion-dependent pathway. Arch. Toxicol. 2011, 85, 209–218. [Google Scholar] [CrossRef]
- Flusberg, D.A.; Sorger, P.K. Survivin apoptosis: Life-death signalling in single cells. Trends Cell. Biol. 2015, 25, 446–458. [Google Scholar] [CrossRef]
- Jiang, K.; Zhang, T.; Yin, N.; Ma, X.; Zhao, G.; Wu, H.; Qiu, C.; Deng, G. Geraniol alleviates LPS-induced acute lung injury in mice via inhibiting inflammation and apoptosis. Oncotarget 2017, 8, 71038–71053. [Google Scholar] [CrossRef]
- Xu, W.J.; Wang, X.X.; Jin, J.J.; Zou, Q.; Wu, L.; Lv, T.F.; Wan, B.; Zhan, P.; Zhu, S.H.; Liu, H.B.; et al. Inhibition of GGPPS1 attenuated LPS-induced acute lung injury and was associated with NLRP3 inflammasome suppression. Am. J. Physiol. Lung. Cell Mol. Physiol. 2019, 316, L567–L577. [Google Scholar] [CrossRef]
- Zhang, M.; Lin, L.; Lee, S.J.; Mo, L.; Cao, J.; Ifedigbo, E.; Jin, Y. Deletion of caveolin-1 protects hyperoxia-induced apoptosis via survivin-mediated pathways. Am. J. Physiol. Lung. Cell Mol Physiol. 2009, 297, L945–L953. [Google Scholar] [CrossRef][Green Version]
- Khan, Z.; Khan, A.A.; Yadav, H.; Prasad, G.B.K.S.; Bisen, P.S. Survivin, a molecular target for therapeutic interventions in squamous cell carcinoma. Cell. Mol. Biol. Lett. 2017, 22, 8. [Google Scholar] [CrossRef]
- Terasaki, Y.; Terasaki, M.; Urushiyama, H.; Nagasaka, S.; Takahashi, M.; Kunugi, S.; Ishikawa, A.; Wakamatsu, K.; Kuwahara, N.; Miyake, K.; et al. Role of survivin in acute lung injury: Epithelial cells of mice and humans. Lab. Invest. 2013, 93, 1147–1163. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.F.; Moussa, R.A.; Eldemerdash, R.S.; Zakaria, M.M.; Abdel-Gaber, S.A. Ameliorative effects of silymarin on HCl-induced acute lung injury in rats; the role of the Nrf-2/HO-1 pathway. Iran. J. Basic. Med. Sci. 2019, 22, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Nežić, L.; Škrbić, R.; Dobrić, S.; Stojiljković, M.P.; Jaćević, V.; Stoisavljević, S.; Milovanović, Z.A.; Stojaković, N. Simvastatin and indomethacin have similar anti-inflammatory activity in a rat model of acute local inflammation. Basic Clin. Pharm. Toxicol. 2009, 104, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.C.; Valença, S.S.; Gitirana, L.B.; Santos, J.C.; Ribeiro, M.L.; Machado, M.N.; Magalhães, C.B.; Zin, W.A.; Porto, L.C. Redox markers and inflammation are differentially affected by atorvastatin, pravastatin or simvastatin administered before endotoxin-induced acute lung injury. Int. Immunopharmacol. 2013, 17, 57–64. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, W.; Zhao, X.; Zhang, R. Experimental study of the protective effect of simvastatin on lung injury in rats with sepsis. Inflammation 2018, 41, 104–113. [Google Scholar] [CrossRef]
- Wang, C.Y.; Liu, P.Y.; Liao, J.K. Pleiotropic effects of statin therapy: Molecular mechanisms and clinical results. Trends Mol. Med. 2008, 14, 37–44. [Google Scholar] [CrossRef]
- Murphy, C.; Deplazes, E.; Cranfield, C.G.; Garcia, A. The Role of Structure and Biophysical Properties in the Pleiotropic Effects of Statins. Int. J. Mol. Sci. 2020, 21, 8745. [Google Scholar] [CrossRef]
- Ren, Y.; Li, L.; Wang, M.M.; Cao, L.P.; Sun, Z.R.; Yang, Z.Z.; Zhang, W.; Zhang, P.; Nie, S.N. Pravastatin attenuates sepsis-induced acute lung injury through decreasing pulmonary microvascular permeability via inhibition of Cav-1/eNOS pathway. Int. Immunopharmacol. 2021, 100, 108077. [Google Scholar] [CrossRef]
- Nežić, L.; Škrbić, R.; Dobrić, S.; Stojiljković, M.P.; Šatara, S.S.; Milovanović, Z.A.; Stojaković, N. Effect of simvastatin on proinflammatory cytokines production during lipopolysaccharide-induced inflammation in rats. Gen. Physiol. Biophys. 2009, 28, 119–126. [Google Scholar]
- Nežić, L.; Škrbić, R.; Amidžić, L.; Gajanin, R.; Kuča, K.; Jaćević, V. Simvastatin Protects Cardiomyocytes Against Endotoxin-induced Apoptosis and Up-regulates Survivin/NF-kB/p65 Expression. Sci. Rep. 2018, 8, 14652. [Google Scholar] [CrossRef]
- Nežić, L.; Amidžić, L.; Škrbić, R.; Gajanin, R.; Nepovimova, E.; Vališ, M.; Kuča, K.; Jaćević, V. Simvastatin Inhibits Endotoxin-Induced Apoptosis in Liver and Spleen Through Up-Regulation of Survivin/NF-kB/p65 Expression. Front. Pharmacol. 2019, 10, 54. [Google Scholar] [CrossRef]
- Nežić, L.; Škrbić, R.; Amidžić, L.; Gajanin, R.; Milovanović, Z.; Nepovimova, E.; Kuča, K.; Jaćević, V. Protective Effects of Simvastatin on Endotoxin-Induced Acute Kidney Injury through Activation of Tubular Epithelial Cells’ Survival and Hindering Cytochrome C-mediated Apoptosis. Int. J. Mol. Sci. 2020, 21, 7236. [Google Scholar] [CrossRef] [PubMed]
- Chopra, V.; Rogers, M.A.; Buist, M.; Govindan, S.; Lindenauer, P.K.; Saint, S.; Flanders, S.A. Is statin use associated with reduced mortality after pneumonia? A systematic review and meta-analysis. Am. J. Med. 2012, 125, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Kruger, P.; Bailey, M.; Bellomo, R.; Cooper, D.J.; Harward, M.; Higgins, A.; Howe, B.; Jones, D.; Joyce, C.; Kostner, K.; et al. ANZ-STATInS Investigators–ANZICS Clinical Trials Group. A multicenter randomized trial of atorvastatin therapy in intensive care patients with severe sepsis. Am. J. Respir. Crit. Care Med. 2013, 187, 743–750. [Google Scholar] [CrossRef]
- Calfee, C.S.; Delucchi, K.L.; Sinha, P.; Matthay, M.A.; Hackett, J.; Shankar-Hari, M.; McDowell, C.; Laffey, J.G.; O’Kane, C.M.; McAuley, D.F. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: Secondary analysis of a randomised controlled trial. Lancet Respir. Med. 2018, 6, 691–698. [Google Scholar] [CrossRef]
- Craig, T.R.; Duffy, M.J.; Shyamsundar, M.; McDowell, C.; O’Kane, C.M.; Elborn, J.S.; McAuley, D.F. A randomized clinical trial of hydroxymethylglutaryl- coenzyme a reductase inhibition for acute lung injury (The HARP Study). Am. J. Respir. Crit. Care Med. 2011, 183, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Singla, S.; Jacobson, J.R. Statins as a novel therapeutic strategy in acute lung injury. Pulm. Circ. 2012, 2, 397–406. [Google Scholar] [CrossRef]
- Takano, K.; Yamamoto, S.; Tomita, K.; Takashina, M.; Yokoo, H.; Matsuda, N.; Takano, Y.; Hattori, Y. Successful treatment of acute lung injury with pitavastatin in septic mice: Potential role of glucocorticoid receptor expression in alveolar macrophages. J. Pharmacol. Exp. Ther. 2011, 336, 381–390. [Google Scholar] [CrossRef]
- Lee, E.F.; Fairlie, W.D. The Structural Biology of Bcl-xL. Int. J. Mol. Sci. 2019, 20, 2234. [Google Scholar] [CrossRef]
- Parikh, S.M.; Yang, Y.; He, L.; Tang, C.; Zhan, M.; Dong, Z. Mitochondrial function and disturbances in the septic kidney. Semin. Nephrol. 2015, 35, 108–119. [Google Scholar] [CrossRef]
- Bao, X.C.; Mao, A.R.; Fang, Y.Q.; Fan, Y.H.; Wang, F.F.; Ma, J.; You, P. Simvastatin decreases hyperbaric oxygen-induced acute lung injury by upregulating eNOS. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L287–L297. [Google Scholar] [CrossRef] [PubMed]
- Tsang, T.J.; Hsueh, Y.C.; Wei, E.I.; Lundy, D.J.; Cheng, B.; Chen, Y.T.; Wang, S.S.; Hsieh, P.C.H. Subcellular localization of survivin determines its function in cardiomyocytes. Theranostics 2017, 7, 4577–4590. [Google Scholar] [CrossRef] [PubMed]
- Amenomori, S.; Terasaki, Y.; Terasaki, M.; Urusiyama, H.; Takahashi, M.; Kunugi, S.; Fukuida, Y. The increased expression of survivin on lipopolysaccharide (LPS)-induced acute lung injury (ALI) in mice. Am. J. Respir. Crit. Care Med. 2011, 183, A2098. [Google Scholar] [CrossRef]
- Wilson, R.L.; Selvaraju, V.; Lakshmanan, R.; Thirunavukkarasu, M.; Campbell, J.; McFadden, D.W.; Maulik, D.W. Thioredoxin-1 attenuates sepsis-induced cardiomyopathy after cecal ligation and puncture in mice. J. Surg. Res. 2017, 220, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Kita, T.; Brown, M.S.; Goldstein, J.L. Feedback regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in livers of mice treated with mevinolin, a competitive inhibitor of the reductase. J. Clin. Inv. 1980, 66, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Morel, J.; Hargreaves, I.; Brealey, D.; Neergheen, V.; Backman, J.T.; Lindig, S.; Bläss, M.; Bauer, M.; McAuley, D.F.; Singer, M. Simvastatin pre-treatment improves survival and mitochondrial function in a 3-day fluid-resuscitated rat model of sepsis. Clin. Sci. 2017, 131, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Jaćević, V.; Jović, D.; Kuča, K.; Dragojević-Simić, V.; Dobrić, S.; Trajković, S.; Borisev, I.; Šegrt, Z.; Milovanovic, Z.; Bokonjic, D.; et al. Effects of Fullerenol nanoparticles and Amifostine on radiation-induced tissue damages: Histopathological analysis. J. Appl. Biomed. 2016, 14, 285–297. [Google Scholar] [CrossRef]
- Jaćević, V.; Djordjević, A.; Srdjenović, B.; Milic-Tores, V.; Šegrt, Z.; Dragojević-Simić, V.; Kuca, K. Fullerenol nanoparticles prevent doxorubicin-induced acute hepatotoxicity in rats. Exp. Mol. Pathol. 2017, 102, 360–369. [Google Scholar] [CrossRef]
- Jaćević, V.; Dragojević-Simić, V.; Tatomirović, Ž.; Dobrić, S.; Bokonjić, D.; Kovačević, A.; Nepovimova, E.; Vališ, M.; Kuča, K. The effcacy of amifostine against multiple-dose doxorubicin-induced toxicity in rats. Int. J. Mol. Sci. 2018, 19, 2370. [Google Scholar] [CrossRef]
- Jaćević, V.; Nepovimova, E.; Kuča, K. Toxic injury to the muscle tissue of rats following acute oximes exposure. Sci. Rep. 2019, 9, 1457. [Google Scholar] [CrossRef]
- Jaćević, V.; Nepovimova, E.; Kuča, K. Acute toxic injuries of rat’s visceral tissues induced by different oximes. Sci. Rep. 2019, 9, 16425. [Google Scholar] [CrossRef] [PubMed]
- Jaćević, V.; Wu, Q.; Nepovimova, E.; Kuča, K. Efficacy of methylprednisolone on T-2 toxin-induced cardiotoxicity in vivo: A pathohistological study. Environ. Toxicol. Pharm. 2019, 71, 103221. [Google Scholar] [CrossRef] [PubMed]
- Jaćević, V.; Wu, Q.; Nepovimova, E.; Kuča, K. Cardiomiopythy induced by T-2 toxin. Food Chem. Toxicol. 2020, 137, 111138. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Brems, J.J.; Gamelli, R.L.; Holterman, X.A. Survivin signalling is regulated through the nuclear factor-kappa B pathway during glycochenodeoxycholate-induced hepatocyte apoptosis. Biochim. Biophys. Acta 2010, 1803, 1368–1375. [Google Scholar] [CrossRef]



| Treatment (mg/kg) | Pulmonary Damage Score (6 Lung/Group × 6 Slices/Lung) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ± SD | |
| Control | 31 | 5 | 0 | 0 | 0 | 0 | 0.60 ± 0.11 |
| LPS group | 0 | 0 | 4 | 20 | 12 | 0 | 3.33 ± 0.48 a1 |
| 10 mg/kg of simvastatin group | 0 | 0 | 24 | 10 | 2 | 0 | 2.33 ± 0.48 a2 |
| 20 mg/kg of simvastatin group | 0 | 6 | 24 | 6 | 0 | 0 | 2.00 ± 0.59 a2,b1 |
| 40 mg/kg of simvastatin group | 0 | 23 | 13 | 0 | 0 | 0 | 1.33 ± 0.40 b1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nežić, L.; Amidžić, L.; Škrbić, R.; Gajanin, R.; Mandić, D.; Dumanović, J.; Milovanović, Z.; Jaćević, V. Amelioration of Endotoxin-Induced Acute Lung Injury and Alveolar Epithelial Cells Apoptosis by Simvastatin Is Associated with Up-Regulation of Survivin/NF-kB/p65 Pathway. Int. J. Mol. Sci. 2022, 23, 2596. https://doi.org/10.3390/ijms23052596
Nežić L, Amidžić L, Škrbić R, Gajanin R, Mandić D, Dumanović J, Milovanović Z, Jaćević V. Amelioration of Endotoxin-Induced Acute Lung Injury and Alveolar Epithelial Cells Apoptosis by Simvastatin Is Associated with Up-Regulation of Survivin/NF-kB/p65 Pathway. International Journal of Molecular Sciences. 2022; 23(5):2596. https://doi.org/10.3390/ijms23052596
Chicago/Turabian StyleNežić, Lana, Ljiljana Amidžić, Ranko Škrbić, Radoslav Gajanin, Danijela Mandić, Jelena Dumanović, Zoran Milovanović, and Vesna Jaćević. 2022. "Amelioration of Endotoxin-Induced Acute Lung Injury and Alveolar Epithelial Cells Apoptosis by Simvastatin Is Associated with Up-Regulation of Survivin/NF-kB/p65 Pathway" International Journal of Molecular Sciences 23, no. 5: 2596. https://doi.org/10.3390/ijms23052596
APA StyleNežić, L., Amidžić, L., Škrbić, R., Gajanin, R., Mandić, D., Dumanović, J., Milovanović, Z., & Jaćević, V. (2022). Amelioration of Endotoxin-Induced Acute Lung Injury and Alveolar Epithelial Cells Apoptosis by Simvastatin Is Associated with Up-Regulation of Survivin/NF-kB/p65 Pathway. International Journal of Molecular Sciences, 23(5), 2596. https://doi.org/10.3390/ijms23052596






