Acute Prenatal Hypoxia in Rats Affects Physiology and Brain Metabolism in the Offspring, Dependent on Sex and Gestational Age
Abstract
1. Introduction
2. Results
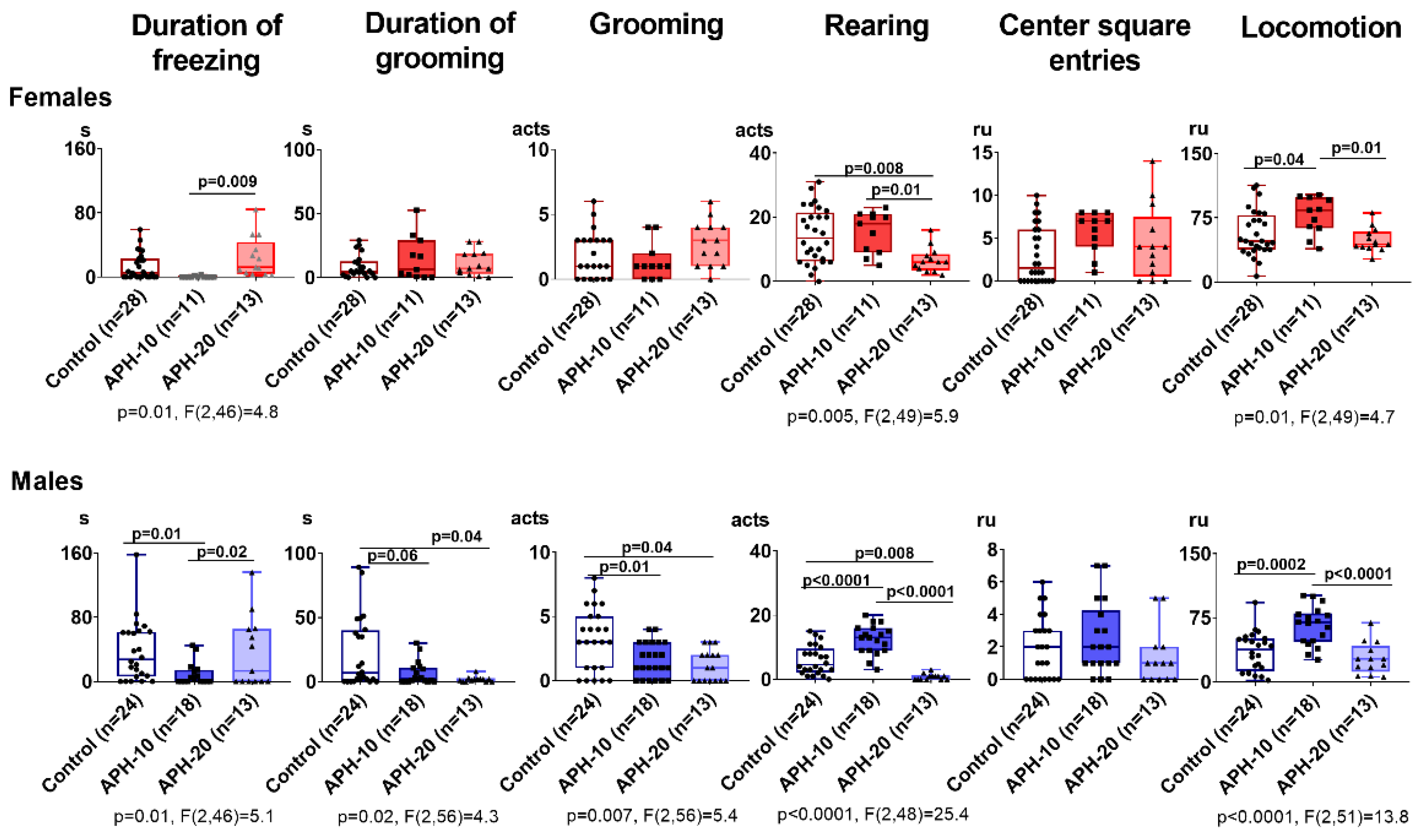
2.1. Behavioral Parameters Are Affected by APH, Dependent on the Offspring Sex and Gestational Age at the Insult Exposure
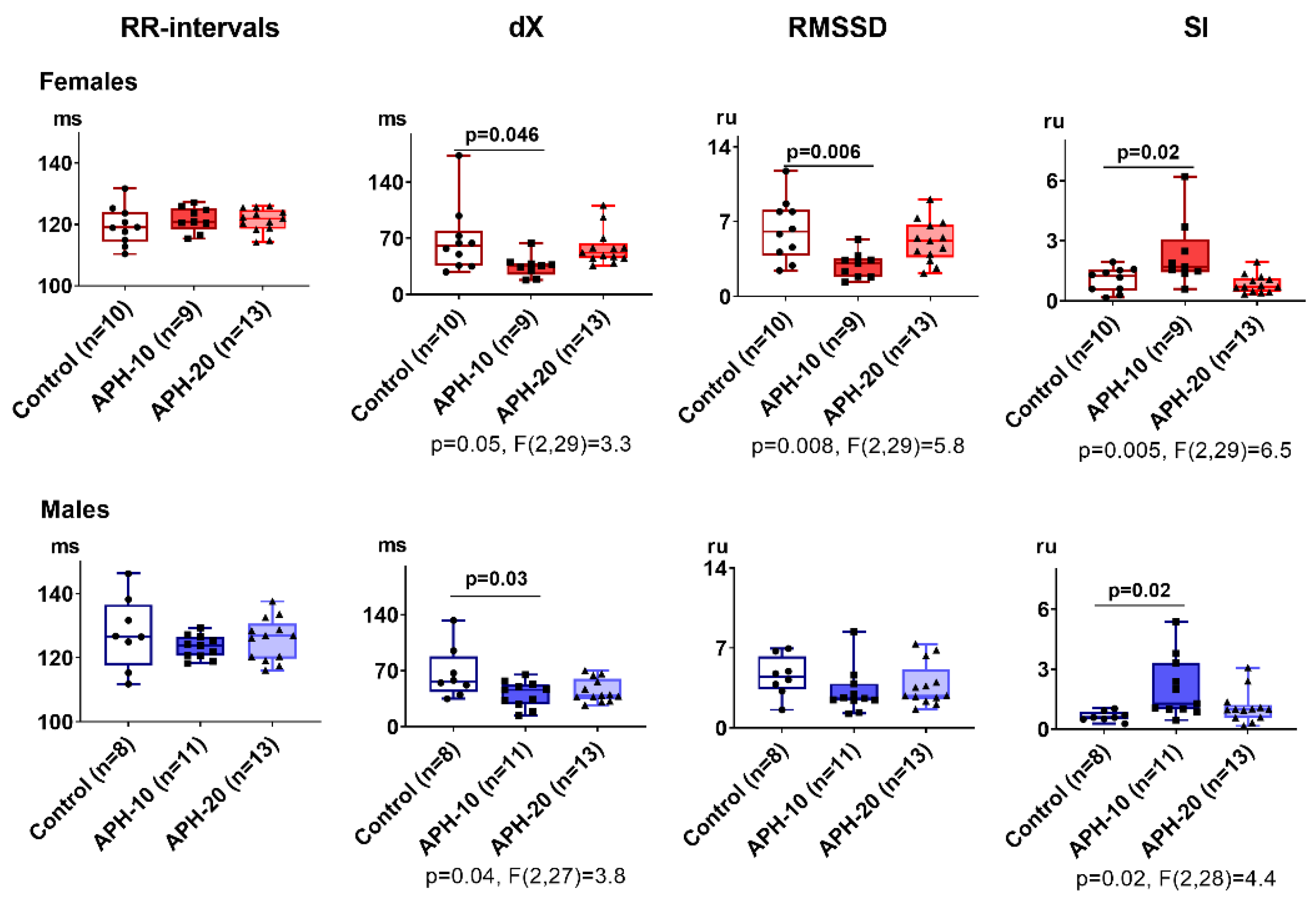
2.2. Only APH-10 Affects ECG, with the ECG Changes Similar in the Female and Male Offspring
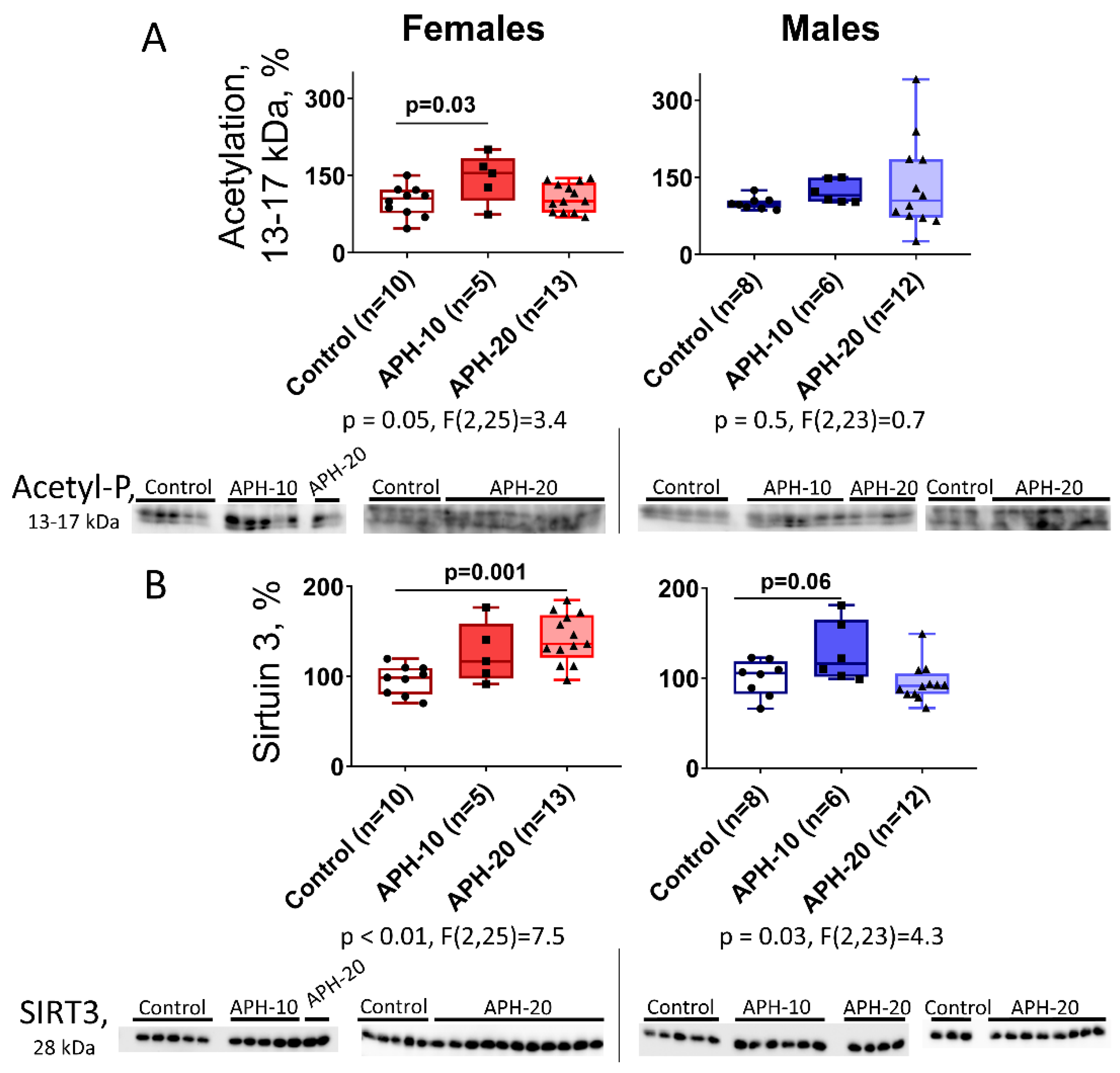
2.3. Levels of Acetylation of 13–17 kDa Proteins and Expression of Mitochondrial Sirtuin 3 in the Offspring Brain Depend on the Offspring Sex and Gestational Age during the Acute Prenatal Hypoxia
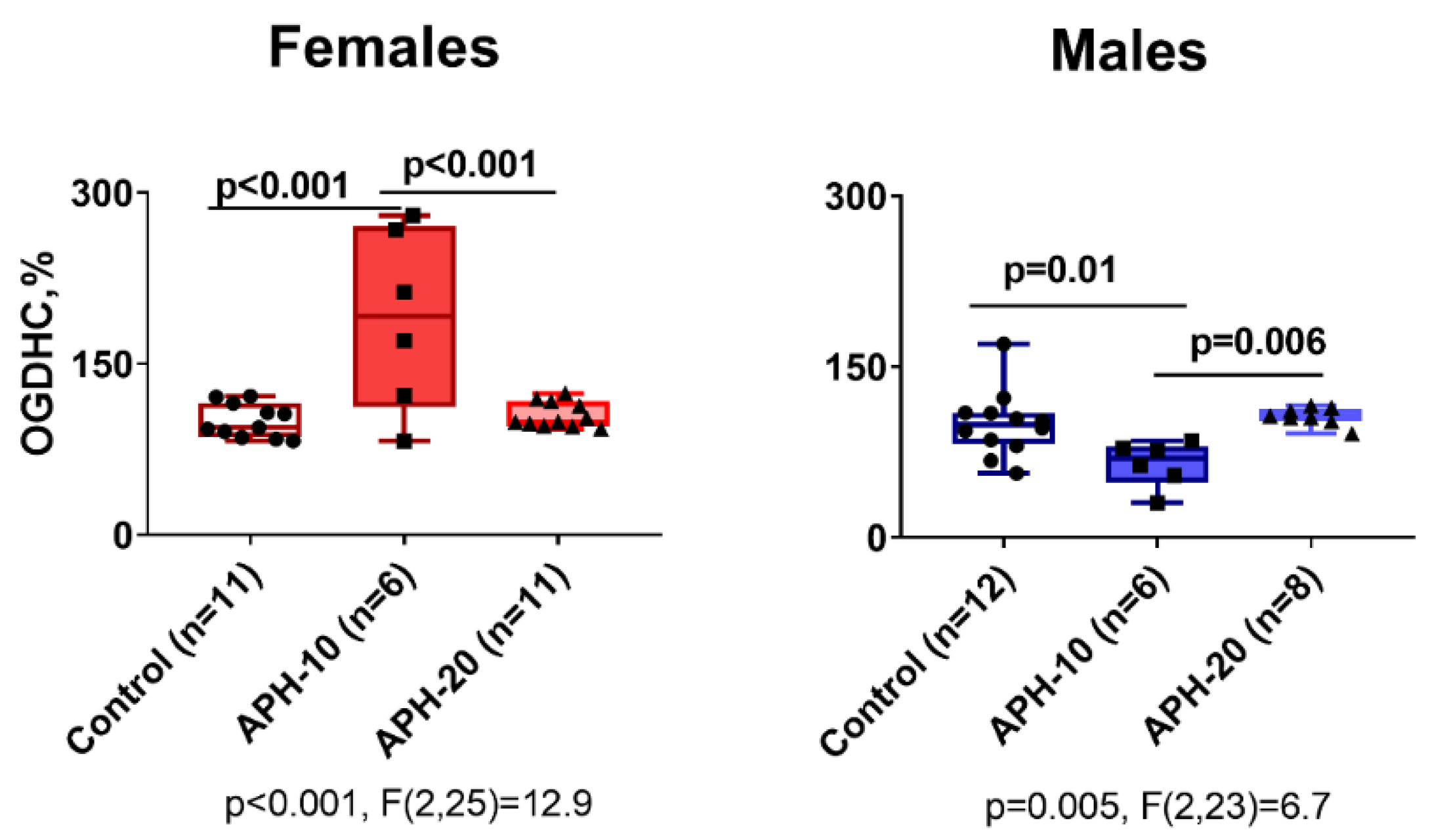
2.4. Effects of APH on the Activity of OGDHC Critical for the Neurotransmitter Metabolism, in Cerebral Cortex of Offspring
2.5. Alterations in Amino Acids, Dipeptides and Enzymes in the Cerebral Cortex of the Offspring Exposed to APH-20
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. Acute Hypobaric Hypoxia
4.3. Behavioral Parameters
4.4. Animal Electrocardiography (ECG)
4.5. Enzyme Assays
4.6. Assay of Protein Lysine Acetylation and Sirtuin 3 by Western Blotting
4.7. Ninhydrine Quantification of Amino Acids
4.8. Data Acquisition and Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APH | acute prenatal hypoxia |
| CNS | central nervous system |
| dX | range of RR intervals |
| ECG | electrocardiogram/electrocardiography |
| GDH | glutamate dehydrogenase |
| GOT2 | glutamic oxaloacetic transaminase |
| MDH | malate dehydrogenase |
| ME | malic enzyme |
| OGDHC | 2-oxoglutarate dehydrogenase complex |
| PDHC | pyruvate dehydrogenase complex |
| RMSSD | parasympathetic or relaxation index of the nervous systems state |
| SI | sympathetic or stress index of the nervous systems state stress index. |
References
- Patterson, A.; Zhang, L. Hypoxia and Fetal Heart Development. Curr. Mol. Med. 2010, 10, 653–666. [Google Scholar] [CrossRef]
- Dey, D.; Joshi, D.; Shrivastava, V.; Singal, C.M.S.; Tyagi, S.; Bhat, M.A.; Jaiswal, P.; Sharma, J.B.; Palanichamy, J.K.; Sinha, S.; et al. Hypoxia Induces Early Neurogenesis in Human Fetal Neural Stem Cells by Activating the WNT Pathway. bioRxiv 2021, 1–19. [Google Scholar] [CrossRef]
- Giaccia, A.J.; Simon, M.C.; Johnson, R. The biology of hypoxia: The role of oxygen sensing in development, normal function, and disease. Genes Dev. 2004, 18, 2183–2194. [Google Scholar] [CrossRef]
- Volpe, J.J. Hypoxic Ischemic Encephalopathy: Clinical aspects. In Neurology of the Newborn, 3rd ed.; W B Saunders: London, UK, 1995; pp. 314–369. [Google Scholar]
- Golan, H.; Kashtuzki, I.; Hallak, M.; Sorokin, Y.; Huleihel, M. Maternal hypoxia during pregnancy induces fetal neurodevelopmental brain damage: Partial protection by magnesium sulfate. J. Neurosci. Res. 2004, 78, 430–441. [Google Scholar] [CrossRef]
- Bhat, R.Y.; Broughton, S.; Khetriwal, B.; Rafferty, G.; Hannam, S.; Milner, A.D.; Greenough, A. Dampened ventilatory response to added dead space in newborns of smoking mothers. Arch. Dis. Child.-Fetal Neonatal Ed. 2005, 90, F316–FF319. [Google Scholar] [CrossRef][Green Version]
- Hutter, D.; Kingdom, J.; Jaeggi, E. Causes and Mechanisms of Intrauterine Hypoxia and Its Impact on the Fetal Cardiovascular System: A Review. Int. J. Pediatr. 2010, 2010, 401323. [Google Scholar] [CrossRef]
- Giussani, D. The fetal brain sparing response to hypoxia: Physiological mechanisms. J. Physiol. 2016, 594, 1215–1230. [Google Scholar] [CrossRef]
- Giussani, D.A.; Davidge, S.T. Developmental Programming of Cardiovascular Disease by Prenatal Hypoxia. J. Dev. Orig. Health Dis. 2013, 4, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Hugh, A.; Hugh, R.; Schneider, H.; Rooth, G. Continuous Transcutaneous Monitoring of Fetal Oxygen Tension during Labour. BJOG Int. J. Obstet. Gynaecol. 1977, 84, 1–39. [Google Scholar] [CrossRef]
- Graf, A.; Kabysheva, M.; Klimuk, E.; Trofimova, L.; Dunaeva, T.; Zündorf, G.; Kahlert, S.; Reiser, G.; Storozhevykh, T.; Pinelis, V.; et al. Role of 2-oxoglutarate dehydrogenase in brain pathologies involving glutamate neurotoxicity. J. Mol. Catal. B Enzym. 2009, 61, 80–87. [Google Scholar] [CrossRef]
- Ream, M.; Ray, A.M.; Chandra, R.; Chikaraishi, D.M. Early fetal hypoxia leads to growth restriction and myocardial thinning. Am. J. Physiol. Integr. Comp. Physiol. 2008, 295, R583–R595. [Google Scholar] [CrossRef]
- Coles, C. Critical Periods for Prenatal Alcohol Exposure: Evidence from Animal and Human Studies. Alcohol Health Res. World 1994, 18, 22. [Google Scholar]
- Moore, K.L.; Persaud, T.V.N.; Torchia, M.G. The Developing Human-e-Book: Clinically Oriented Embryology; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Chan, L.; Chiu, P.; Siu, S.; Lau, T. A study of diclofenac-induced teratogenicity during organogenesis using a whole rat embryo culture model. Hum. Reprod. 2001, 16, 2390–2393. [Google Scholar] [CrossRef][Green Version]
- Ostadal, B.; Ostadalova, I.; Szarszoi, O.; Netuka, I.; Olejnickova, V.; Hlavackova, M. Sex-dependent effect of perinatal hypoxia on cardiac tolerance to oxygen deprivation in adults. Can. J. Physiol. Pharmacol. 2021, 99, 1–8. [Google Scholar] [CrossRef]
- Wood, C.E.; Keller-Wood, M. Current paradigms and new perspectives on fetal hypoxia: Implications for fetal brain development in late gestation. Am. J. Physiol. Integr. Comp. Physiol. 2019, 317, R1–R13. [Google Scholar] [CrossRef]
- Hellgren, K.T.; Premanandhan, H.; Quinn, C.J.; Trafford, A.W.; Galli, G.L. Sex-dependent effects of developmental hypoxia on cardiac mitochondria from adult murine offspring. Free Radic. Biol. Med. 2021, 162, 490–499. [Google Scholar] [CrossRef]
- Grojean, S.; Schroeder, H.; Pourié, G.; Charriaut-Marlangue, C.; Koziel, V.; Desor, D.; Vert, P.; Daval, J.-L. Histopathological alterations and functional brain deficits after transient hypoxia in the newborn rat pup: A long term follow-up. Neurobiol. Dis. 2003, 14, 265–278. [Google Scholar] [CrossRef]
- Nyakas, C.; Buwald, B.; Luiten, P.G. Hypoxia and brain development. Prog. Neurobiol. 1996, 49, 1–51. [Google Scholar] [CrossRef]
- Gardner, F.C.; Adkins, C.S.; Hart, S.E.; Travagli, R.A.; Doheny, K.K. Preterm Stress Behaviors, Autonomic Indices, and Maternal Perceptions of Infant Colic. Adv. Neonatal Care 2018, 18, 49–57. [Google Scholar] [CrossRef]
- Hashiguchi, K.; Kuriyama, N.; Koyama, T.; Matsui, D.; Ozaki, E.; Hasegawa, T.; Tokuda, S.; Niwa, F.; Iwasa, K.; Watanabe, I.; et al. Validity of stress assessment using heart-rate variability in newborns. Pediatr. Int. 2020, 62, 694–700. [Google Scholar] [CrossRef]
- Frasch, M.G. Heart Rate Variability Code: Does It Exist and Can We Hack It? arXiv 2020, arXiv:2001.08264. [Google Scholar]
- Lobmaier, S.M.; Müller, A.; Zelgert, C.; Shen, C.; Su, P.C.; Schmidt, G.; Haller, B.; Berg, G.; Fabre, B.; Weyrich, J.; et al. Fetal heart rate variability responsiveness to maternal stress, non-invasively detected from maternal transabdominal ECG. Arch. Gynecol. Obstet. 2019, 301, 405–414. [Google Scholar] [CrossRef]
- Huang, L.; Chen, X.; Dasgupta, C.; Chen, W.; Song, R.; Wang, C.; Zhang, L. Foetal hypoxia impacts methylome and transcriptome in developmental programming of heart disease. Cardiovasc. Res. 2018, 115, 1306–1319. [Google Scholar] [CrossRef]
- Li, G.; Xiao, Y.; Estrella, J.L.; Ducsay, C.A.; Gilbert, R.D.; Zhang, L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J. Soc. Gynecol. Investig. 2003, 10, 265–274. [Google Scholar] [CrossRef]
- Cannon, T.D.; Van Erp, T.G.M.; Rosso, I.M.; Huttunen, M.; Lönnqvist, J.; Pirkola, T.; Salonen, O.; Valanne, L.; Poutanen, V.-P.; Standertskjöld-Nordenstam, C.-G. Fetal Hypoxia and Structural Brain Abnormalities in Schizophrenic Patients, Their Siblings, and Controls. Arch. Gen. Psychiatry 2002, 59, 35–41. [Google Scholar] [CrossRef]
- Chiera, M.; Cerritelli, F.; Casini, A.; Barsotti, N.; Boschiero, D.; Cavigioli, F.; Corti, C.G.; Manzotti, A. Heart Rate Variability in the Perinatal Period: A Critical and Conceptual Review. Front. Neurosci. 2020, 14, 999. [Google Scholar] [CrossRef]
- Ostadal, B.; Drahota, Z.; Houstek, J.; Milerova, M.; Ostadalova, I.; Hlavackova, M.; Kolar, F. Developmental and Sex Differences in Cardiac Tolerance to Ischemia–Reperfusion Injury: The Role of Mitochondria. Can. J. Physiol. Pharmacol. 2019, 97, 808–814. [Google Scholar] [CrossRef]
- Grimm, V.E.; Frieder, B. Differential Vulnerability of Male and Female Rats to the Timing of Various Perinatal Insults. Int. J. Neurosci. 1985, 27, 155–164. [Google Scholar] [CrossRef]
- Netuka, I.; Szárszoi, O.; Malý, J.; Říha, H.; Turek, D.; Ošťádalová, I.; Ošťádal, B. Late Effect of Early Hypoxic Disturbance in the Rat Heart: Gender Differences. Physiol. Res. 2010, 59, 127–131. [Google Scholar] [CrossRef]
- Ross, E.J.; Graham, D.L.; Money, K.M.; Stanwood, G.D. Developmental Consequences of Fetal Exposure to Drugs: What We Know and What We Still Must Learn. Neuropsychopharmacology 2015, 40, 61–87. [Google Scholar] [CrossRef]
- Bunik, V.I.; Fernie, A.R. Metabolic control exerted by the 2-oxoglutarate dehydrogenase reaction: A cross-kingdom comparison of the crossroad between energy production and nitrogen assimilation. Biochem. J. 2009, 422, 405–421. [Google Scholar] [CrossRef]
- Graf, A.; Ksenofontov, A.; Bunik, V. Inhibition of 2-Oxoglutarate Dehydrogenase as a Chemical Model of Acute Hypobaric Hypoxia. Front. Med. 2021, 8, 751639. [Google Scholar] [CrossRef]
- Graf, A.; Trofimova, L.; Ksenofontov, A.; Baratova, L.; Bunik, V. Hypoxic Adaptation of Mitochondrial Metabolism in Rat Cerebellum Decreases in Pregnancy. Cells 2020, 9, 139. [Google Scholar] [CrossRef]
- Artiukhov, A.V.; Graf, A.V.; Bunik, V.I. Directed Regulation of Multienzyme Complexes of 2-Oxo Acid Dehydrogenases Using Phosphonate and Phosphinate Analogs of 2-Oxo Acids. Biochemistry 2016, 81, 1498–1521. [Google Scholar] [CrossRef]
- Rice, D.; Barone, S., Jr. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ. Health Perspect. 2000, 108 (Suppl. 3), 511–533. [Google Scholar] [CrossRef]
- Mehta, S.K.; Super, D.M.; Connuck, D.; Salvator, A.; Singer, L.; Fradley, L.G.; Harcar-Sevcik, R.A.; Kirchner, H.; Kaufman, E.S. Heart rate variability in healthy newborn infants. Am. J. Cardiol. 2002, 89, 50–53. [Google Scholar] [CrossRef]
- Danson, E.; Li, D.; Wang, L.; Dawson, T.; Paterson, D. Targeting cardiac sympatho-vagal imbalance using gene transfer of nitric oxide synthase. J. Mol. Cell. Cardiol. 2009, 46, 482–489. [Google Scholar] [CrossRef]
- Bristow, M.R. β-Adrenergic Receptor Blockade in Chronic Heart Failure. Circulation 2000, 101, 558–569. [Google Scholar] [CrossRef]
- Valkonen-Korhonen, M.; Tarvainen, M.P.; Ranta-Aho, P.; Karjalainen, P.A.; Partanen, J.; Karhu, J.; Lehtonen, J. Heart Rate Variability in Acute Psychosis. Psychophysiology 2003, 40, 716–726. [Google Scholar] [CrossRef]
- Henry, B.L.; Minassian, A.; Paulus, M.; Geyer, M.A.; Perry, W. Heart rate variability in bipolar mania and schizophrenia. J. Psychiatr. Res. 2010, 44, 168–176. [Google Scholar] [CrossRef]
- Toichi, M.; Kubota, Y.; Murai, T.; Kamio, Y.; Sakihama, M.; Toriuchi, T.; Inakuma, T.; Sengoku, A.; Miyoshi, K. The influence of psychotic states on the autonomic nervous system in schizophrenia. Int. J. Psychophysiol. 1999, 31, 147–154. [Google Scholar] [CrossRef]
- Pham, T.; Lau, Z.; Chen, S.; Makowski, D. Heart Rate Variability in Psychology: A Review of HRV Indices and an Analysis Tutorial. Sensors 2021, 21, 3998. [Google Scholar] [CrossRef]
- Thayer, J.F.; Åhs, F.; Fredrikson, M.; Sollers, J.J., III; Wager, T.D. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef]
- Jennings, J.R.; Allen, B.; Gianaros, P.J.; Thayer, J.F.; Manuck, S.B. Focusing Neurovisceral Integration: Cognition, Heart Rate Variability, and Cerebral Blood Flow. Psychophysiology 2015, 52, 214–224. [Google Scholar] [CrossRef]
- Schneider, U.; Bode, F.; Schmidt, A.; Nowack, S.; Rudolph, A.; Doelcker, E.-M.; Schlattmann, P.; Götz, T.; Hoyer, D. Developmental milestones of the autonomic nervous system revealed via longitudinal monitoring of fetal heart rate variability. PLoS ONE 2018, 13, e0200799. [Google Scholar] [CrossRef]
- Oliveira, V.; von Rosenberg, W.; Montaldo, P.; Adjei, T.; Mendoza, J.; Shivamurthappa, V.; Mandic, D.; Thayyil, S. Early Postnatal Heart Rate Variability in Healthy Newborn Infants. Front. Physiol. 2019, 10, 922. [Google Scholar] [CrossRef]
- Graf, A.; Trofimova, L.; Loshinskaja, A.; Mkrtchyan, G.; Strokina, A.; Lovat, M.; Tylicky, A.; Strumilo, S.; Bettendorff, L.; Bunik, V.I. Up-regulation of 2-oxoglutarate dehydrogenase as a stress response. Int. J. Biochem. Cell Biol. 2013, 45, 175–189. [Google Scholar] [CrossRef]
- Bunik, V.I.; Schloss, J.V.; Pinto, J.T.; Gibson, G.E.; Cooper, A.J.L. Enzyme-Catalyzed Side Reactions with Molecular Oxygen may Contribute to Cell Signaling and Neurodegenerative Diseases. Neurochem. Res. 2007, 32, 871–891. [Google Scholar] [CrossRef]
- Tsepkova, P.M.; Artiukhov, A.; Boyko, A.I.; Aleshin, V.; Mkrtchyan, G.V.; Zvyagintseva, M.A.; Ryabov, S.I.; Ksenofontov, A.L.; Baratova, L.A.; Graf, A.V.; et al. Thiamine induces long-term changes in amino acid profiles and activities of 2-oxoglutarate and 2-oxoadipate dehydrogenases in rat brain. Biochemistry 2017, 82, 723–736. [Google Scholar] [CrossRef]
- Trofimova, L.; Lovat, M.; Groznaya, A.; Efimova, E.; Dunaeva, T.; Maslova, M.; Graf, A.; Bunik, V. Behavioral Impact of the Regulation of the Brain 2-Oxoglutarate Dehydrogenase Complex by Synthetic Phosphonate Analog of 2-Oxoglutarate: Implications into the Role of the Complex in Neurodegenerative Diseases. Int. J. Alzheimers. Dis. 2010, 2020, 749061. [Google Scholar] [CrossRef]
- Aleshin, V.A.; Mkrtchyan, G.V.; Kaehne, T.; Graf, A.V.; Maslova, M.V.; Bunik, V.I. Diurnal Regulation of the 612 Function of the Rat Brain Glutamate Dehydrogenase by Acetylation and Its Dependence on Thiamine 613 Administration. J. Neurochem. 2020, 153, 80–102. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Zhang, X.; Xie, Y.; Jiang, S.; Liu, Y.; Liu, X.; Xie, W.; Jia, X.; Bade, R.; Shi, R.; et al. 5-Aza-2′-Deoxycytidine Increases Hypoxia Tolerance-Dependent Autophagy in Mouse Neuronal Cells by Initiating the TSC1/MTOR Pathway. Biomed. Pharmacother. 2019, 118, 109219. [Google Scholar] [CrossRef]
- Huang, Q.; Zhan, L.; Cao, H.; Li, J.; Lyu, Y.; Guo, X.; Zhang, J.; Ji, L.; Ren, T.; An, J.; et al. Increased Mitochondrial Fission Promotes Autophagy and Hepatocellular Carcinoma Cell Survival through the ROS-Modulated Coordinated Regulation of the NFKB and TP53 Pathways. Autophagy 2016, 12, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Gao, D.; Yang, X.; Hua, X.; Li, S.; Sun, H. Exogenous Netrin-1 Inhibits Autophagy of Ischemic Brain Tissues and Hypoxic Neurons via PI3K/MTOR Pathway in Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2019, 28, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Raman, L.; Kong, X.; Gilley, J.A.; Kernie, S.G. Chronic Hypoxia Impairs Murine Hippocampal Development and Depletes the Postnatal Progenitor Pool by Attenuating Mammalian Target of Rapamycin Signaling. Pediatr. Res. 2011, 70, 159–165. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mundo, W.; Wolfson, G.; Moore, L.G.; Houck, J.A.; Park, D.; Julian, C.G. Hypoxia-Induced Inhibition of MTORC1 Activity in the Developing Lung: A Possible Mechanism for the Developmental Programming of Pulmonary Hypertension. Am. J. Physiol. Circ. Physiol. 2021, 320, H980–H990. [Google Scholar] [CrossRef]
- Xia, S.; Lv, J.; Gao, Q.; Li, L.; Chen, N.; Wei, X.; Xiao, J.; Chen, J.; Tao, J.; Sun, M.; et al. Prenatal Exposure to Hypoxia Induced Beclin 1 Signaling-Mediated Renal Autophagy and Altered Renal Development in Rat Fetuses. Reprod. Sci. 2015, 22, 156–164. [Google Scholar] [CrossRef][Green Version]
- Li, K.; Zheng, Y.; Wang, X. The Potential Relationship Between HIF-1α and Amino Acid Metabolism After Hypoxic Ischemia and Dual Effects on Neurons. Front. Neurosci. 2021, 15, 676553. [Google Scholar] [CrossRef]
- Shah, R.; Courtiol, E.; Castellanos, F.X.; Teixeira, C.M. Abnormal Serotonin Levels During Perinatal 597 Development Lead to Behavioral Deficits in Adulthood. Front. Behav. Neurosci. 2018, 12, 114. [Google Scholar] [CrossRef]
- Hashimoto, K.; Sawa, A.; Iyo, M. Increased Levels of Glutamate in Brains from Patients with Mood Disorders. Biol. Psychiatry 2007, 62, 1310–1316. [Google Scholar] [CrossRef]
- Madeira, C.; Vargas-Lopes, C.; Brandão, C.O.; Reis, T.; Laks, J.; Panizzutti, R.; Ferreira, S.T. Elevated Glutamate 602 and Glutamine Levels in the Cerebrospinal Fluid of Patients With Probable Alzheimer’s Disease and 603 Depression. Front. Psychiatry 2018, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Korman, S.H.; Gutman, A. Pitfalls in the Diagnosis of Glycine Encephalopathy (Non-Ketotic Hyperglycinemia). Dev. Med. Child Neurol. 2007, 44, 712–720. [Google Scholar] [CrossRef]
- Almannai, M.; Al Mahmoud, R.A.; Mekki, M.; El-Hattab, A.W. Metabolic Seizures. Front. Neurol. 2021, 12, 985. [Google Scholar] [CrossRef] [PubMed]
- Von Essen, C.; Rydenhag, B.; Nyström, B.; Mozzi, R.; Van Gelder, N.; Hamberger, A. High Levels of Glycine 609 and Serine as a Cause of the Seizure Symptoms of Cavernous Angiomas? J. Neurochem. 2002, 67, 260–264. [Google Scholar] [CrossRef]
- Maslova, M.V.; Graf, A.V.; Maklakova, A.S.; Krushinskaya, Y.V.; Sokolova, N.A.; Koshelev, V.B. Acute 615 Hypoxia during Organogenesis Affects Cardiac Autonomic Balance in Pregnant Rats. Bull. Exp. Biol. Med. 2005, 139, 180–182. [Google Scholar] [CrossRef]
- Graf, A.V.; Maklakova, A.S.; Maslova, M.V.; Krushinskaya, Y.V.; Sokolova, N.A. Effect of Prenatal Hypoxia 618 during Organogenesis on Albino Rat Behavior during Postnatal Period. Biol. Bull. 2006, 33, 619. [Google Scholar] [CrossRef]
- Lukyanova, L.D.; Germanova, E.L.; Kopaladze, R.A. Development of Resistance of an Organism under 621 Various Conditions of Hypoxic Preconditioning: Role of the Hypoxic Period and Reoxygenation. Bull. Exp. Biol. Med. 2009, 147, 400. [Google Scholar] [CrossRef]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The Open Field Test; Humana Press: Totowa, NJ, USA, 2009; pp. 1–20. [Google Scholar] [CrossRef]
- Sestakova, N.; Puzserova, A.; Kluknavsky, M.; Bernatova, I. Determination of Motor Activity and Anxiety-Related Behaviour in Rodents: Methodological Aspects and Role of Nitric Oxide. Interdiscip. Toxicol. 2013, 6, 126–135. [Google Scholar] [CrossRef]
- Aleshin, V.A.; Graf, A.V.; Artiukhov, A.V.; Boyko, A.I.; Ksenofontov, A.L.; Maslova, M.V.; Nogués, I.; di Salvo, M.L.; Bunik, V.I. Physiological and Biochemical Markers of the Sex-Specific Sensitivity to Epileptogenic Factors, Delayed Consequences of Seizures and Their Response to Vitamins B1 and B6 in a Rat Model. Pharm. 2021, 14, 737. [Google Scholar] [CrossRef]
- Baevsky, R.M.; Chernikova, A.G. Heart Rate Variability Analysis: Physiological Foundations and Main Methods. Cardiometry 2017, 10, 66–76. [Google Scholar] [CrossRef]
- Suckow, M.A.; Stevens, K.A.; Wilson, R.P. The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Hinman, L.M.; Blass, J.P. An NADH-Linked Spectrophotometric Assay for Pyruvate Dehydrogenase Complex in Crude Tissue Homogenates. J. Biol. Chem. 1981, 256, 6583–6586. [Google Scholar] [CrossRef]
- Schwab, M.A.; Kolker, S.; van den Heuvel, L.P.; Sauer, S.; Wolf, N.I.; Rating, D.; Hoffmann, G.F.; Smeitink, J.A.; Okun, J.G. Optimized Spectrophotometric Assay for the Completely Activated Pyruvate Dehydrogenase Complex in Fibroblasts. Clin. Chem. 2005, 51, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Levintow, L.; Meister, A. Reversibility of the Enzymatic Synthesis of Glutamine. J. Biol. Chem. 1954, 209, 265–280. [Google Scholar] [CrossRef]
- Boyko, A.; Tsepkova, P.; Aleshin, V.; Artiukhov, A.; Mkrtchyan, G.; Ksenofontov, A.; Baratova, L.; Ryabov, S.; Graf, A.; Bunik, V. Severe Spinal Cord Injury in Rats Induces Chronic Changes in the Spinal Cord and Cerebral Cortex Metabolism, Adjusted by Thiamine That Improves Locomotor Performance. Front. Mol. Neurosci. 2021, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Trofimova, L.; Ksenofontov, A.; Mkrtchyan, G.; Graf, A.; Baratova, L.; Bunik, V. Quantification of Rat Brain Amino Acids: Analysis of the Data Consistency. Curr. Anal. Chem. 2016, 12, 349–356. [Google Scholar] [CrossRef]

| Females | Males | |||||
|---|---|---|---|---|---|---|
| Control n = 5 | APH-20 n = 11 | p | Control n = 6 | APH-20 n = 8 | p | |
| Ala | 0.513 ± 0.030 | 0.574 ± 0.012 | 0.786 | 0.520 ± 0.011 | 0.534 ± 0.021 | 0.662 |
| Anserine | 0.006 ± 0.000 | 0.017 ± 0.006 | 0.441 | 0.011 ± 0.002 | 0.019 ± 0.008 | 0.852 |
| Arg | 0.100 ± 0.010 | 0.085 ± 0.004 | 0.071 | 0.091 ± 0.012 | 0.102 ± 0.007 | 0.414 |
| Asn | 0.031 ± 0.002 | 0.035 ± 0.002 | 0.036 | 0.035 ± 0.002 | 0.033 ± 0.002 | 0.662 |
| Asp | 2.430 ± 0.220 | 2.447 ± 0.042 | 0.393 | 2.414 ± 0.098 | 2.372 ± 0.099 | 0.755 |
| Carnosine | 0.012 ± 0.001 | 0.017 ± 0.001 | 0.036 | 0.030 ± 0.007 | 0.015 ± 0.001 | 0.181 |
| Citrulline | 0.024 ± 0.003 | 0.028 ± 0.002 | 0.071 | 0.029 ± 0.002 | 0.030 ± 0.002 | 0.662 |
| Cystine | 0.005 ± 0.001 | 0.012 ± 0.003 | 0.571 | 0.004 ± 0.001 | 0.011 ± 0.004 | 0.142 |
| GABA | 1.270 ± 0.166 | 1.404 ± 0.034 | 0.786 | 1.317 ± 0.023 | 1.359 ± 0.053 | 0.573 |
| Gln | 4.473 ± 0.478 | 4.596 ± 0.123 | 0.571 | 4.003 ± 0.183 | 4.389 ± 0.248 | 0.181 |
| Glu | 9.783 ± 0.486 | 10.776 ± 0.23 | 0.009 | 9.625 ± 0.237 | 10.203 ± 0.462 | 0.282 |
| Gly | 0.560 ± 0.031 | 0.639 ± 0.014 | 0.000 | 0.616 ± 0.019 | 0.604 ± 0.025 | 0.950 |
| His | 0.058 ± 0.006 | 0.061 ± 0.003 | 0.583 | 0.055 ± 0.004 | 0.066 ± 0.005 | 0.181 |
| Ile | 0.029 ± 0.005 | 0.030 ± 0.001 | 0.913 | 0.029 ± 0.002 | 0.030 ± 0.001 | 0.852 |
| Leu | 0.058 ± 0.008 | 0.060 ± 0.002 | 0.827 | 0.054 ± 0.003 | 0.060 ± 0.003 | 0.081 |
| Lys | 0.122 ± 0.009 | 0.144 ± 0.009 | 0.145 | 0.114 ± 0.011 | 0.117 ± 0.008 | 0.950 |
| Met | 0.043 ± 0.006 | 0.038 ± 0.001 | 0.180 | 0.043 ± 0.003 | 0.046 ± 0.003 | 0.282 |
| Phe | 0.035 ± 0.004 | 0.039 ± 0.001 | 0.009 | 0.033 ± 0.002 | 0.044 ± 0.002 | 0.003 |
| Pro | 0.047 ± 0.008 | 0.056 ± 0.004 | 0.221 | 0.053 ± 0.005 | 0.063 ± 0.011 | 0.662 |
| Ser | 0.814 ± 0.058 | 0.949 ± 0.025 | 0.003 | 0.818 ± 0.026 | 0.838 ± 0.039 | 0.491 |
| Thr | 0.431 ± 0.036 | 0.592 ± 0.027 | 0.000 | 0.389 ± 0.008 | 0.438 ± 0.022 | 0.020 |
| Trp | 0.035 ± 0.001 | 0.044 ± 0.001 | 0.001 | 0.025 ± 0.002 | 0.043 ± 0.001 | 0.000 |
| Tyr | 0.056 ± 0.006 | 0.056 ± 0.005 | 0.913 | 0.052 ± 0.003 | 0.067 ± 0.005 | 0.108 |
| Val | 0.077 ± 0.008 | 0.085 ± 0.002 | 0.115 | 0.087 ± 0.003 | 0.083 ± 0.002 | 0.345 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graf, A.V.; Maslova, M.V.; Artiukhov, A.V.; Ksenofontov, A.L.; Aleshin, V.A.; Bunik, V.I. Acute Prenatal Hypoxia in Rats Affects Physiology and Brain Metabolism in the Offspring, Dependent on Sex and Gestational Age. Int. J. Mol. Sci. 2022, 23, 2579. https://doi.org/10.3390/ijms23052579
Graf AV, Maslova MV, Artiukhov AV, Ksenofontov AL, Aleshin VA, Bunik VI. Acute Prenatal Hypoxia in Rats Affects Physiology and Brain Metabolism in the Offspring, Dependent on Sex and Gestational Age. International Journal of Molecular Sciences. 2022; 23(5):2579. https://doi.org/10.3390/ijms23052579
Chicago/Turabian StyleGraf, Anastasia V., Maria V. Maslova, Artem V. Artiukhov, Alexander L. Ksenofontov, Vasily A. Aleshin, and Victoria I. Bunik. 2022. "Acute Prenatal Hypoxia in Rats Affects Physiology and Brain Metabolism in the Offspring, Dependent on Sex and Gestational Age" International Journal of Molecular Sciences 23, no. 5: 2579. https://doi.org/10.3390/ijms23052579
APA StyleGraf, A. V., Maslova, M. V., Artiukhov, A. V., Ksenofontov, A. L., Aleshin, V. A., & Bunik, V. I. (2022). Acute Prenatal Hypoxia in Rats Affects Physiology and Brain Metabolism in the Offspring, Dependent on Sex and Gestational Age. International Journal of Molecular Sciences, 23(5), 2579. https://doi.org/10.3390/ijms23052579







