A Deadly Embrace: Hemagglutination Mediated by SARS-CoV-2 Spike Protein at Its 22 N-Glycosylation Sites, Red Blood Cell Surface Sialoglycoproteins, and Antibody
Abstract
1. Introduction
1.1. Binding of Viruses to SA and Host Decoy Defense
1.2. Cleavage of Viral–SA Binding via Hemagglutinin Esterase (HE), an Enzyme Expressed by the Common Cold Betacoronavirus Strains but Not by SARS-CoV, SARS-CoV-2 and MERS
1.3. Multivalent Binding
2. Properties of SARS-CoV-2 Related to Its Array of Spike Glycoprotein Glycans
2.1. SARS-CoV-2 Binds to SA and CD147
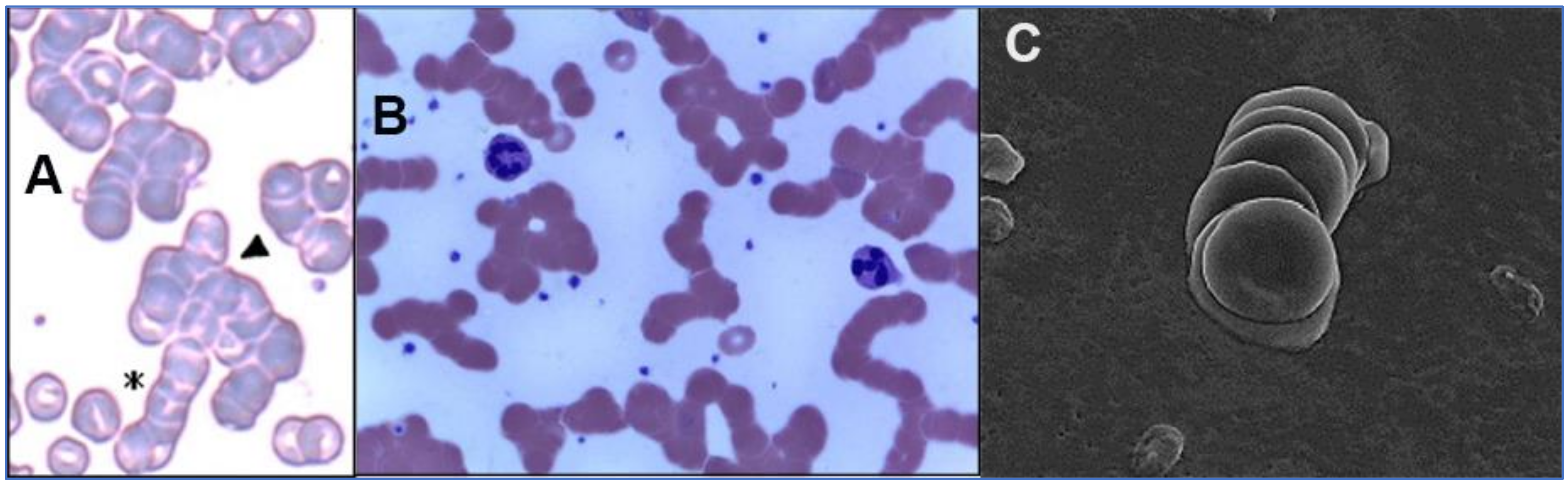
2.2. SARS-CoV-2 Attaches to RBCs, Other Blood Cells and Endothelial Cells
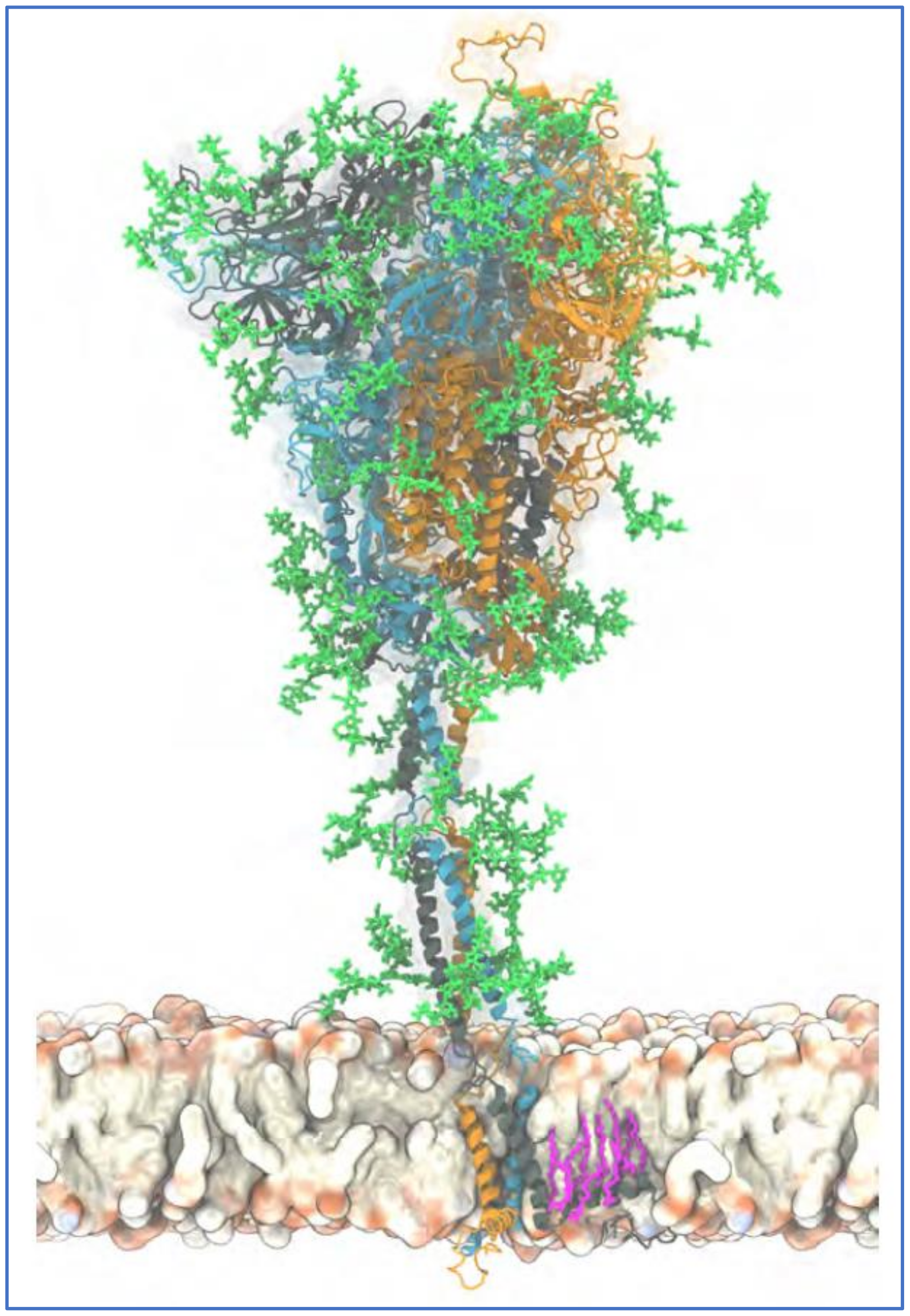
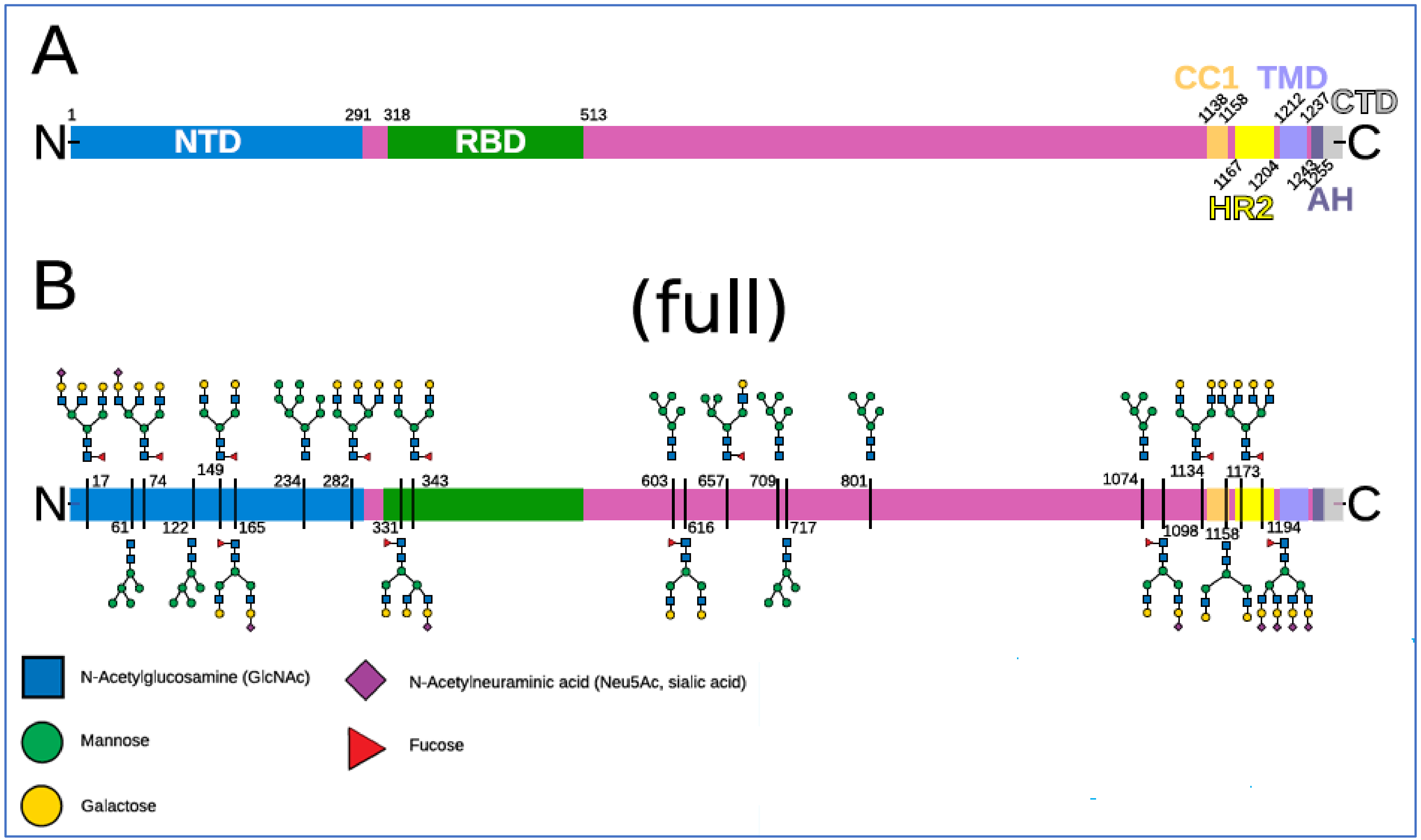
2.3. The Glycan Distribution and Composition at the 22 N-Glycosylation Sites of SARS-CoV-2 Spike Protein
3. Potential Scenarios for Virally Induced RBC Clumping and Damage to Endothelial Cells
Completing the Spike-Mediated Linkage of Two RBCs with IgG Antibody Targeting Spike RBD
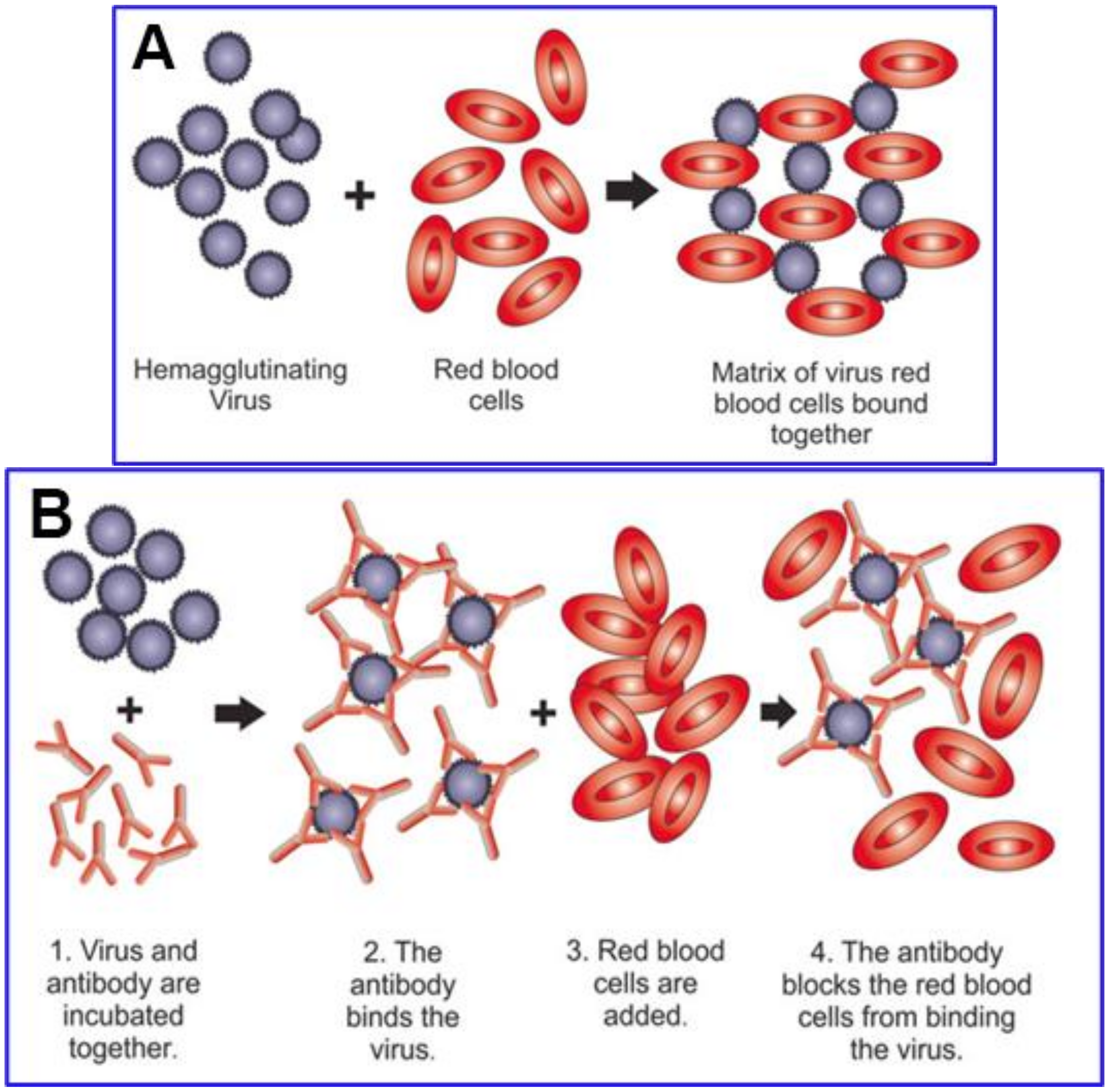
4. Testing for Hemagglutination Caused by SARS-CoV-2 Spike and for Inhibition by Competitive Binding
Competitive Inhibition of SARS-CoV-2 Spike Protein Binding by Ivermectin (IVM)
5. Discussion
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 7nAChr | alpha-7 nicotinic acetylcholine receptor |
| CEC | circulating endothelial cell |
| COVID-19 | coronavirus disease 2019 |
| GPA | glycophorin A |
| HE | hemagglutinin esterase |
| IVM | ivermectin |
| Neu5Ac | α5-N-acetylneuraminic acid |
| NTD | N-terminal domain |
| RBC | red blood cell |
| RBD | receptor-binding domain |
| RCT | randomized clinical trial |
| SA | sialic acid |
Appendix A. Calculations and Notes on the Proposed Hemagglutination Experiment
Appendix A.1. Calculation 1
Appendix A.2. Calculation 2
Appendix A.3. Calculation 3
Appendix A.4. Notes on the Proposed Hemagglutination Experiment
References
- Valyaeva, A.A.; Zharikova, A.A.; Kasianov, A.S.; Vassetzky, Y.S.; Sheval, E.V. Expression of SARS-CoV-2 entry factors in lung epithelial stem cells and its potential implications for COVID-19. Sci. Rep. 2020, 10, 17772. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Z.; Ge, J. Scientific research progress of COVID-19/SARS-CoV-2 in the first five months. J. Cell. Mol. Med. 2020, 24, 6558–6570. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Couzin-Frankel, J. The mystery of the pandemic’s ‘happy hypoxia’. Science 2020, 368, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Rapkiewicz, A.V.; Mai, X.; Carsons, S.E.; Pittaluga, S.; Kleiner, D.E.; Berger, J.S.; Thomas, S.; Adler, N.M.; Charytan, D.M.; Gasmi, B.; et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine 2020, 24, 100434. [Google Scholar] [CrossRef]
- Negri, E.M.; Piloto, B.M.; Morinaga, L.K.; Jardim, C.V.P.; Lamy, S.A.E.-D.; Ferreira, M.A.; D’Amico, E.A.; Deheinzelin, D. Heparin Therapy Improving Hypoxia in COVID-19 Patients—A Case Series. Front. Physiol. 2020, 11, 1341. [Google Scholar] [CrossRef]
- Lodigiani, C.; Lapichino, G.; Carenzo, L.; Cecconi, M.; Ferrazzi, P.; Sebastian, T.; Kucher, N.; Studt, J.D.; Sacco, C.; Alexia, B.; et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020, 191, 9–14. [Google Scholar] [CrossRef]
- Price, L.C.; McCabe, C.; Garfield, B.; Wort, S.J. Thrombosis and COVID-19 pneumonia: The clot thickens! Eur. Respir. J. 2020, 56, 2001608. [Google Scholar] [CrossRef]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Fagot Gandet, F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef]
- Huertas, A.; Montani, D.; Savale, L.; Pichon, J.; Tu, L.; Parent, F.; Guignabert, C.; Humbert, M. Endothelial cell dysfunction: A major player in SARS-CoV-2 infection (COVID-19)? Eur. Respir. J. 2020, 56. [Google Scholar] [CrossRef]
- Sardu, C.; Gambardella, J.; Morelli, M.B.; Wang, X.; Marfella, R.; Santulli, G. Hypertension, thrombosis, kidney failure, and diabetes: Is COVID-19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J. Clin. Med. 2020, 9, 1417. [Google Scholar] [CrossRef] [PubMed]
- Jung, F.; Krüger-Genge, A.; Franke, R.P.; Hufert, F.; Küpper, J.H. COVID-19 and the endothelium. Clin. Hemorheol. Microcirc. 2020, 75, 7–11. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Lüscher, T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020, 41, 3038–3044. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.C. COVID-19 update: Covid-19-associated coagulopathy. J. Thromb Thrombolysis 2020, 50, 54–67. [Google Scholar] [CrossRef]
- Mondal, R.; Lahiri, D.; Deb, S.; Bandyopadhyay, D.; Shome, G.; Sarkar, S.; Paria, S.R.; Thakurta, T.G.; Singla, P.; Biswas, S.C. COVID-19: Are we dealing with a multisystem vasculopathy in disguise of a viral infection? J. Thromb Thrombolysis 2020, 50, 567–579. [Google Scholar] [CrossRef]
- Magro, C.; Mulvey, J.J.; Berlin, D.; Nuovo, G.; Salvatore, S.; Harp, J.; Baxter-Stoltzfus, A.; Laurence, J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl. Res. 2020, 220, 1–13. [Google Scholar] [CrossRef]
- Menter, T.; Haslbauer, J.D.; Nienhold, R.; Savic, S.; Hopfer, H.; Deigendesch, N.; Frank, S.; Turek, D.; Willi, N.; Pargger, H.; et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 2020, 77, 198–209. [Google Scholar] [CrossRef]
- Gattinoni, L.; Coppola, S.; Cressoni, M.; Busana, M.; Rossi, S.; Chiumello, D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2020, 201, 1299–1300. [Google Scholar] [CrossRef]
- Marini, J.J.; Gattinoni, L. Management of COVID-19 respiratory distress. JAMA 2020, 323, 2329–2330. [Google Scholar] [CrossRef] [PubMed]
- Scheim, D.E. From Cold to Killer: How SARS-CoV-2 Evolved without Hemagglutinin Esterase to Agglutinate, then Clot Blood Cells in Pulmonary and Systemic Microvasculature. Available online: http://ssrn.com/abstract=3706347 (accessed on 24 November 2021).
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.; et al. Angiotensin-converting enzyme-2 (ACE2), SARS-CoV-2 and pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 25, 228–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.-Y.; et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell 2020, 181, 894–904.e899. [Google Scholar] [CrossRef]
- Ströh, L.J.; Stehle, T. Glycan engagement by viruses: Receptor switches and specificity. Annu Rev. Virol 2014, 1, 285–306. [Google Scholar] [CrossRef] [PubMed]
- Milanetti, E.; Miotto, M.; Di Rienzo, L.; Nagaraj, M.; Monti, M.; Golbek, T.W.; Gosti, G.; Roeters, S.J.; Weidner, T.; Otzen, D.E.; et al. In-Silico Evidence for a Two Receptor Based Strategy of SARS-CoV-2. Front Mol Biosci 2021, 8, 690655. [Google Scholar] [CrossRef]
- Morniroli, D.; Giannì, M.L.; Consales, A.; Pietrasanta, C.; Mosca, F. Human sialome and coronavirus disease-2019 (COVID-19) pandemic: An understated correlation? Front. Immunol. 2020, 11, 1480. [Google Scholar] [CrossRef]
- Fantini, J.; Di Scala, C.; Chahinian, H.; Yahi, N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int. J. Antimicrob. Agents 2020, 55, 105960. [Google Scholar] [CrossRef]
- Roe, K. High COVID-19 virus replication rates, the creation of antigen–antibody immune complexes and indirect haemagglutination resulting in thrombosis. Transbound. Emerg. Dis. 2020, 67, 1418–1421. [Google Scholar] [CrossRef]
- Koehler, M.; Delguste, M.; Sieben, C.; Gillet, L.; Alsteens, D. Initial step of virus entry: Virion binding to cell-surface glycans. Annu. Rev. Virol. 2020, 7, 143–165. [Google Scholar] [CrossRef]
- Baum, J.; Ward, R.H.; Conway, D.J. Natural selection on the erythrocyte surface. Mol. Biol. Evol. 2002, 19, 223–229. [Google Scholar] [CrossRef]
- Storry, J.R. Review: The function of blood group-specific RBC membrane components. Immunohematology 2004, 20, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.; Levine, M.; Sharp, K.A.; Brooks, D.E. Theory of the electrokinetic behavior of human erythrocytes. Biophys. J. 1983, 42, 127–135. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, W.; Ma, L.T.; Jiang, J.L.; Chen, Z.N. Importance of N-glycosylation on CD147 for its biological functions. Int. J. Mol. Sci. 2014, 15, 6356–6377. [Google Scholar] [CrossRef] [PubMed]
- Modrof, J.; Kerschbaum, A.; Farcet, M.R.; Niemeyer, D.; Corman, V.M.; Kreil, T.R. SARS-CoV-2 and the safety margins of cell-based biological medicinal products. Biologicals 2020, 68, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.M.; Murphy, S.J.; Kuri-Cervantes, L.; Weisman, A.R.; Ittner, C.A.G.; Reilly, J.P.; Pampena, M.B.; Betts, M.R.; Wherry, E.J.; Song, W.-C.; et al. Erythrocytes reveal complement activation in patients with COVID-19. medRxiv 2020, 05.20.20104398. [Google Scholar] [CrossRef]
- Howe, C.; Lee, L.T. Virus-erythrocyte interactions. In Advances in Virus Research; Smith, K.M., Lauffer, M.A., Bang, F.B., Eds.; Academic Press: New York, NY, USA, 1972; Volume 17, pp. 1–50. [Google Scholar]
- Matrosovich, M.; Herrler, G.; Klenk, H.D. Sialic acid receptors of viruses. In SialoGlyco Chemistry and Biology II: Tools and Techniques to Identify and Capture Sialoglycans; Gerardy-Schahn, R., Delannoy, P., von Itzstein, M., Eds.; Springer International Publishing: New York, NY, USA, 2015; pp. 1–28. [Google Scholar]
- Kapikian, A.Z.; James, H.D., Jr.; Kelly, S.J.; King, L.M.; Vaughn, A.L.; Chanock, R.M. Hemadsorption by coronavirus strain OC43. Proc. Soc. Exp. Biol. Med. 1972, 139, 179–186. [Google Scholar] [CrossRef]
- Agafonov, A.P.; Gus’kov, A.A.; Ternovoi, V.A.; Ryabchikova, E.I.; Durymanov, A.G.; Vinogradov, I.V.; Maksimov, N.L.; Ignat’ev, G.M.; Nechaeva, E.A.; Netesov, S.V. Primary characterization of SARS coronavirus strain Frankfurt 1. Dokl. Biol. Sci. 2004, 394, 58–60. [Google Scholar] [CrossRef]
- Vlasak, R.; Luytjes, W.; Spaan, W.; Palese, P. Human and bovine coronaviruses recognize sialic acid-containing receptors similar to those of influenza C viruses. Proc. Natl. Acad. Sci. USA 1988, 85, 4526–4529. [Google Scholar] [CrossRef]
- Storz, J.; Zhang, X.M.; Rott, R. Comparison of hemagglutinating, receptor-destroying, and acetylesterase activities of avirulent and virulent bovine coronavirus strains. Arch. Virol. 1992, 125, 193–204. [Google Scholar] [CrossRef]
- Brian, D.A.; Hogue, B.G.; Kienzle, T.E. The coronavirus hemagglutinin esterase glycoprotein. In The Coronaviridae. The Viruses; Siddell, S.G., Ed.; Springer: Boston, MA, USA, 1995. [Google Scholar]
- Qing, E.; Hantak, M.; Perlman, S.; Gallagher, T. Distinct Roles for sialoside and protein receptors in coronavirus infection. mBio 2020, 11, e02764–e02819. [Google Scholar] [CrossRef]
- Schultze, B.; Cavanagh, D.; Herrler, G. Neuraminidase treatment of avian infectious bronchitis coronavirus reveals a hemagglutinating activity that is dependent on sialic acid-containing receptors on erythrocytes. Virology 1992, 189, 792–794. [Google Scholar] [CrossRef]
- Schultze, B.; Enjuanes, L.; Herrler, G. Analysis of the sialic acid-binding activity of transmissible gastroenteritis virus. In Corona- and Related Viruses: Current Concepts in Molecular Biology and Pathogenesis; Talbot, P.J., Levy, G.A., Eds.; Springer: Boston, MA, USA, 1995; pp. 367–370. [Google Scholar] [CrossRef]
- Hulswit, R.J.G.; de Haan, C.A.M.; Bosch, B.J. Chapter two—Coronavirus spike protein and tropism changes. In Advances in Virus Research; Ziebuhr, J., Ed.; Academic Press: New York, NY, USA, 2016; Volume 96, pp. 29–57. [Google Scholar]
- Li, W.; Hulswit, R.J.G.; Widjaja, I.; Raj, V.S.; McBride, R.; Peng, W.; Widagdo, W.; Tortorici, M.A.; van Dieren, B.; Lang, Y.; et al. Identification of sialic acid-binding function for the Middle East respiratory syndrome coronavirus spike glycoprotein. Proc. Natl. Acad. Sci. USA 2017, 114, E8508–E8517. [Google Scholar] [CrossRef] [PubMed]
- Hirst, G.K. The agglutination of red cells by allantoic fluid of chick embryos infected with influenza virus. Science 1941, 94, 22–23. [Google Scholar] [CrossRef] [PubMed]
- Hirst, G.K. Adsorption of influenza hemagglutinins and virus by red blood cells. J. Exp. Med. 1942, 76, 195–209. [Google Scholar] [CrossRef] [PubMed]
- McClelland, L.; Hare, R. The adsorption of influenza virus by red cells and a new in vitro method of measuring antibodies for influenza virus. Can. Public Health J. 1941, 32, 530–538. [Google Scholar]
- Salk, J.E. A Simplified procedure for titrating hemagglutinating capacity of influenza-virus and the corresponding antibody. J. Immunol. 1944, 49, 87–98. [Google Scholar]
- Salk, J.E. A plastic plate for use in tests involving virus hemagglutination and other similar reactions. Science 1948, 108, 749. [Google Scholar] [CrossRef]
- Hierholzer, J.C.; Suggs, M.T.; Hall, E.C. Standardized viral hemagglutination and hemagglutination-inhibition tests. II. Description and statistical evaluation. Appl Microbiol 1969, 18, 824–833. [Google Scholar] [CrossRef]
- Defang, G.N.; Martin, N.J.; Burgess, T.H.; Millar, E.V.; Pecenka, L.A.; Danko, J.R.; Arnold, J.C.; Kochel, T.J.; Luke, T.C. Comparative analysis of hemagglutination inhibition titers generated using temporally matched serum and plasma samples. PLoS ONE 2012, 7, e48229. [Google Scholar] [CrossRef]
- Nguyen, M.; Fries, K.; Khoury, R.; Zheng, L.; Hu, B.; Hildreth, S.W.; Parkhill, R.; Warren, W. Automated imaging and analysis of the hemagglutination inhibition assay. J. Lab. Autom. 2015, 21, 287–296. [Google Scholar] [CrossRef]
- Killian, M.L. Hemagglutination assay for influenza virus. In Animal Influenza Virus; Spackman, E., Ed.; Springer: New York, NY, USA, 2014; pp. 3–9. [Google Scholar]
- Barrett, T.; Inglis, S. Growth, purification and titration of influenza viruses. In Virology: A Practical Approach; Mahy, Ed.; Oxford IRL Press Ltd.: Oxford, UK, 1985; pp. 119–150. [Google Scholar]
- Ryu, W.-S. Chapter 4—Diagnosis and methods. In Molecular Virology of Human Pathogenic Viruses; Ryu, W.-S., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 47–62. [Google Scholar]
- Pedersen, J.C. Hemagglutination-inhibition assay for influenza virus subtype identification and the detection and quantitation of serum antibodies to influenza Virus. In Animal Influenza Virus; Spackman, E., Ed.; Springer: New York, NY, USA, 2014; pp. 11–25. [Google Scholar]
- Bakkers, M.J.G.; Lang, Y.; Feitsma, L.J.; Hulswit, R.J.G.; de Poot, S.A.H.; van Vliet, A.L.W.; Margine, I.; de Groot-Mijnes, J.D.F.; van Kuppeveld, F.J.M.; Langereis, M.A.; et al. Betacoronavirus adaptation to humans involved progressive loss of hemagglutinin-esterase lectin activity. Cell Host Microbe 2017, 21, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Hulswit, R.J.G.; Lang, Y.; Bakkers, M.J.G.; Li, W.; Li, Z.; Schouten, A.; Ophorst, B.; van Kuppeveld, F.J.M.; Boons, G.J.; Bosch, B.J.; et al. Human coronaviruses OC43 and HKU1 bind to 9-O-acetylated sialic acids via a conserved receptor-binding site in spike protein domain A. Proc. Natl. Acad. Sci. USA 2019, 116, 2681–2690. [Google Scholar] [CrossRef] [PubMed]
- Neu, U.; Bauer, J.; Stehle, T. Viruses and sialic acids: Rules of engagement. Curr. Opin. Struct. Biol. 2011, 21, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Dong, W.; Milewska, A.; Golda, A.; Qi, Y.; Zhu, Q.K.; Marasco, W.A.; Baric, R.S.; Sims, A.C.; Pyrc, K.; et al. Human Coronavirus HKU1 spike protein uses o-acetylated sialic acid as an attachment receptor determinant and employs hemagglutinin-esterase protein as a receptor-destroying enzyme. J. Virol. 2015, 89, 7202–7213. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, X.; Ostrikov, K.; Abrahamyan, L. Host receptors: The key to establishing cells with broad viral tropism for vaccine production. Crit. Rev. Microbiol. 2020, 46, 147–168. [Google Scholar] [CrossRef]
- Newton, R.; Delguste, M.; Koehler, M.; Dumitru, A.C.; Laskowski, P.R.; Müller, D.J.; Alsteens, D. Combining confocal and atomic force microscopy to quantify single-virus binding to mammalian cell surfaces. Nat. Protoc. 2017, 12, 2275–2292. [Google Scholar] [CrossRef]
- Park, Y.-J.; Walls, A.C.; Wang, Z.; Sauer, M.M.; Li, W.; Tortorici, M.A.; Bosch, B.-J.; DiMaio, F.; Veesler, D. Structures of MERS-CoV spike glycoprotein in complex with sialoside attachment receptors. Nat. Struct. Mol. Biol. 2019, 26, 1151–1157. [Google Scholar] [CrossRef]
- Sieben, C.; Kappel, C.; Zhu, R.; Wozniak, A.; Rankl, C.; Hinterdorfer, P.; Grubmüller, H.; Herrmann, A. Influenza virus binds its host cell using multiple dynamic interactions. Proc. Natl. Acad. Sci. USA 2012, 109, 13626–13631. [Google Scholar] [CrossRef]
- Tiralongo, J. Chapter 29—Sialic acid-specific microbial lectins. In Microbial Glycobiology; Holst, O., Brennan, P.J., Itzstein, M.V., Moran, A.P., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 585–598. [Google Scholar]
- Wielgat, P.; Rogowski, K.; Godlewska, K.; Car, H. Coronaviruses: Is Sialic acid a gate to the eye of cytokine storm? From the entry to the effects. Cells 2020, 9, 1963. [Google Scholar] [CrossRef]
- Nelson, D.S. Immune adherence. In Advances in Immunology; Dixon, F.J., Humphrey, J.H., Eds.; Academic Press: Cambridge, MA, USA, 1963; Volume 3, pp. 131–180. [Google Scholar]
- Anderson, H.L.; Brodsky, I.E.; Mangalmurti, N.S. The evolving erythrocyte: Red blood cells as modulators of innate immunity. J. Immunol. 2018, 201, 1343–1351. [Google Scholar] [CrossRef]
- Varki, A.; Gagneux, P. Multifarious roles of sialic acids in immunity. Ann. N. Y. Acad. Sci. 2012, 1253, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T. A comprehensive review of our current understanding of red blood cell (RBC) glycoproteins. Membranes 2017, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Viitala, J.; Järnefelt, J. The red cell surface revisited. Trends Biochem. Sci. 1985, 10, 392–395. [Google Scholar] [CrossRef]
- De Back, D.Z.; Kostova, E.; Klei, T.; Beuger, B.; van Zwieten, R.; Kuijpers, T.; Juffermans, N.; van den Berg, T.; Korte, D.; van Kraaij, M.; et al. RBC Adhesive Capacity Is Essential for Efficient ‘Immune Adherence Clearance’ and Provide a Generic Target to Deplete Pathogens from Septic Patients. Blood 2016, 128, 1031. [Google Scholar] [CrossRef]
- Nelson, R.A. The Immune-Adherence Phenomenon: An Immunologically Specific Reaction Between Microorganisms and Erythrocytes Leading to Enhanced Phagocytosis. Science 1953, 118, 733–737. [Google Scholar] [CrossRef]
- Nelson, R.A., Jr. The immune-adherence phenomenon; a hypothetical role of erythrocytes in defence against bacteria and viruses. Proc. R Soc. Med. 1956, 49, 55–58. [Google Scholar]
- de Groot, R.J.; Baker, S.C.; Baric, R.; Enjuanes, L. Family—Coronaviridae. In Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier: San Diego, CA, USA, 2012; pp. 806–828. [Google Scholar]
- Sriwilaijaroen, N.; Suzuki, Y. Sialoglycovirology of lectins: Sialyl Glycan binding of enveloped and non-enveloped viruses. In Lectin Purification and Analysis: Methods and Protocols; Hirabayashi, J., Ed.; Springer: New York, NY, USA, 2020; pp. 483–545. [Google Scholar]
- Lang, Y.; Li, W.; Li, Z.; Koerhuis, D.; van den Burg, A.C.S.; Rozemuller, E.; Bosch, B.-J.; van Kuppeveld, F.J.M.; Boons, G.-J.; Huizinga, E.G.; et al. Coronavirus hemagglutinin-esterase and spike proteins coevolve for functional balance and optimal virion avidity. Proc. Natl. Acad. Sci. USA 2020, 117, 25759–25770. [Google Scholar] [CrossRef]
- Belouzard, S.; Millet, J.K.; Licitra, B.N.; Whittaker, G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses 2012, 4, 1011–1033. [Google Scholar] [CrossRef]
- Awasthi, M.; Gulati, S.; Sarkar, D.; Tiwari, S.; Kateriya, S.; Ranjan, P.; Verma, S.K. The Sialoside-Binding Pocket of SARS-CoV-2 Spike Glycoprotein Structurally Resembles MERS-CoV. Viruses 2020, 12, 909. [Google Scholar] [CrossRef]
- Chen, W.; Hui, Z.; Ren, X.; Luo, Y.; Shu, J.; Yu, H.; Li, Z. The N-glycosylation sites and Glycan-binding ability of S-protein in SARS-CoV-2 Coronavirus. bioRxiv 2020, 12.01.406025. [Google Scholar] [CrossRef]
- Guo, W.; Lakshminarayanan, H.; Rodriguez-Palacios, A.; Salata, R.A.; Xu, K.; Draz, M.S. Glycan Nanostructures of Human Coronaviruses. Int. J. Nanomed. 2021, 16, 4813–4830. [Google Scholar] [CrossRef] [PubMed]
- Shajahan, A.; Supekar, N.T.; Gleinich, A.S.; Azadi, P. Deducing the N- and O-glycosylation profile of the spike protein of novel coronavirus SARS-CoV-2. Glycobiology 2020, 30, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zeng, J.; Jia, N.; Stavenhagen, K.; Matsumoto, Y.; Zhang, H.; Li, J.; Hume, A.J.; Mühlberger, E.; van Die, I.; et al. SARS-CoV-2 Spike Protein Interacts with Multiple Innate Immune Receptors. bioRxiv 2020, 07.29.227462. [Google Scholar] [CrossRef]
- Chan, J.F.-W.; Kok, K.-H.; Zhu, Z.; Chu, H.; To, K.K.-W.; Yuan, S.; Yuen, K.-Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Nyodu, R.; Maurya, V.K.; Saxena, S.K. Morphology, Genome Organization, Replication, and Pathogenesis of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). In Coronavirus Disease 2019 (COVID-19): Epidemiology, Pathogenesis, Diagnosis, and Therapeutics; Saxena, S.K., Ed.; Springer: Singapore, 2020; pp. 23–31. [Google Scholar]
- Yoshimoto, F.K. The Proteins of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS CoV-2 or n-COV19), the Cause of COVID-19. Protein J. 2020, 39, 198–216. [Google Scholar] [CrossRef] [PubMed]
- Bakkers, M.J.G.; Zeng, Q.; Feitsma, L.J.; Hulswit, R.J.G.; Li, Z.; Westerbeke, A.; van Kuppeveld, F.J.M.; Boons, G.-J.; Langereis, M.A.; Huizinga, E.G.; et al. Coronavirus receptor switch explained from the stereochemistry of protein–carbohydrate interactions and a single mutation. Proc. Natl. Acad. Sci. USA 2016, 113, E3111–E3119. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.N.; Richards, S.-J.; Guy, C.S.; Congdon, T.R.; Hasan, M.; Zwetsloot, A.J.; Gallo, A.; Lewandowski, J.R.; Stansfeld, P.J.; Straube, A.; et al. The SARS-COV-2 Spike Protein Binds Sialic Acids and Enables Rapid Detection in a Lateral Flow Point of Care Diagnostic Device. ACS Cent. Sci. 2020, 6, 2046–2052. [Google Scholar] [CrossRef]
- Collins, B.E.; Paulson, J.C. Cell surface biology mediated by low affinity multivalent protein–glycan interactions. Curr. Opin. Chem. Biol. 2004, 8, 617–625. [Google Scholar] [CrossRef]
- Cohen, M.; Varki, A. Chapter Three—Modulation of Glycan Recognition by Clustered Saccharide Patches. In International Review of Cell and Molecular Biology; Jeon, K.W., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 308, pp. 75–125. [Google Scholar]
- Xiong, X.; Coombs, P.J.; Martin, S.R.; Liu, J.; Xiao, H.; McCauley, J.W.; Locher, K.; Walker, P.A.; Collins, P.J.; Kawaoka, Y.; et al. Receptor binding by a ferret-transmissible H5 avian influenza virus. Nature 2013, 497, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Shaw, D.E. A simple model of multivalent adhesion and its application to influenza infection. Biophys. J. 2016, 110, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Sauter, N.K.; Hanson, J.E.; Glick, G.D.; Brown, J.H.; Crowther, R.L.; Park, S.J.; Skehel, J.J.; Wiley, D.C. Binding of influenza virus hemagglutinin to analogs of its cell-surface receptor, sialic acid: Analysis by proton nuclear magnetic resonance spectroscopy and X-ray crystallography. Biochemistry 1992, 31, 9609–9621. [Google Scholar] [CrossRef]
- Mitnaul, L.J.; Matrosovich, M.N.; Castrucci, M.R.; Tuzikov, A.B.; Bovin, N.V.; Kobasa, D.; Kawaoka, Y. Balanced Hemagglutinin and Neuraminidase Activities Are Critical for Efficient Replication of Influenza A Virus. J. Virol. 2000, 74, 6015–6020. [Google Scholar] [CrossRef] [PubMed]
- Pourrajab, F.; Zare-Khormizi, M.R.; Sheikhha, M.H. Molecular basis for pathogenicity of human coronaviruses. Infect. Drug Resist. 2020, 13, 2385–2405. [Google Scholar] [CrossRef] [PubMed]
- Radzikowska, U.; Ding, M.; Tan, G.; Zhakparov, D.; Peng, Y.; Wawrzyniak, P.; Wang, M.; Li, S.; Morita, H.; Altunbulakli, C.; et al. Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy 2020, 75, 2829–2845. [Google Scholar] [CrossRef] [PubMed]
- Silva-Filho, J.C.; Melo, C.G.F.d.; Oliveira, J.L.d. The influence of ABO blood groups on COVID-19 susceptibility and severity: A molecular hypothesis based on carbohydrate-carbohydrate interactions. Med. Hypotheses 2020, 144, 110155. [Google Scholar] [CrossRef]
- Wang, K.; Chen, W.; Zhang, Z.; Deng, Y.; Lian, J.-Q.; Du, P.; Wei, D.; Zhang, Y.; Sun, X.-X.; Gong, L.; et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal. Transduct. Target. Ther. 2020, 5, 283. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.; Zheng, Z.-H.; Wei, D.; Wen, A.; Zhang, Z.; Lian, J.-Q.; Kang, W.-Z.; Hao, C.-Q.; Wang, J.; Xie, R.-H.; et al. Safety and efficacy of meplazumab in healthy volunteers and COVID-19 patients: A randomized phase 1 and an exploratory phase 2 trial. Signal. Transduct. Target. Ther. 2021, 6, 194. [Google Scholar] [CrossRef]
- Shelokov, A.; Vogel, J.E.; Chi, L. Hemadsorption (Adsorption-Hemagglutination) Test for Viral Agents in Tissue Culture with Special Reference to Influenza. Proc. Soc. Exp. Biol. Med. 1958, 97, 802–809. [Google Scholar] [CrossRef]
- Gagneten, S.; Gout, O.; Dubois-Dalcq, M.; Rottier, P.; Rossen, J.; Holmes, K.V. Interaction of mouse hepatitis virus (MHV) spike glycoprotein with receptor glycoprotein MHVR is required for infection with an MHV strain that expresses the hemagglutinin-esterase glycoprotein. J. Virol. 1995, 69, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.H.; Sim, B.K.; Dolan, S.A.; Fang, X.; Kaslow, D.C.; Miller, L.H. A family of erythrocyte binding proteins of malaria parasites. Proc. Natl. Acad. Sci. USA 1992, 89, 7085–7089. [Google Scholar] [CrossRef] [PubMed]
- Persson, K.E.; McCallum, F.J.; Reiling, L.; Lister, N.A.; Stubbs, J.; Cowman, A.F.; Marsh, K.; Beeson, J.G. Variation in use of erythrocyte invasion pathways by Plasmodium falciparum mediates evasion of human inhibitory antibodies. J. Clin. Invest. 2008, 118, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Crosnier, C.; Bustamante, L.Y.; Bartholdson, S.J.; Bei, A.K.; Theron, M.; Uchikawa, M.; Mboup, S.; Ndir, O.; Kwiatkowski, D.P.; Duraisingh, M.T.; et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature 2011, 480, 534–537. [Google Scholar] [CrossRef]
- Zenonos, Z.A.; Dummler, S.K.; Müller-Sienerth, N.; Chen, J.; Preiser, P.R.; Rayner, J.C.; Wright, G.J. Basigin is a druggable target for host-oriented antimalarial interventions. J. Exp. Med. 2015, 212, 1145–1151. [Google Scholar] [CrossRef]
- Berzuini, A.; Bianco, C.; Migliorini, A.C.; Maggioni, M.; Valenti, L.; Prati, D. Red blood cell morphology in patients with COVID-19-related anaemia. Blood Transfus. 2021, 19, 34–36. [Google Scholar]
- Lakhdari, N.; Tabet, B.; Boudraham, L.; Laoussati, M.; Aissanou, S.; Beddou, L.; Bensalem, S.; Bellik, Y.; Bournine, L.; Fatmi, S.; et al. Red blood cells injuries and hypersegmented neutrophils in COVID-19 peripheral blood film. medRxiv 2020, 07.24.20160101. [Google Scholar] [CrossRef]
- Melkumyants, A.; Buryachkovskaya, L.; Lomakin, N.; Antonova, O.; Serebruany, V. Mild COVID-19 and impaired blood cell–endothelial crosstalk: Considering long-term use of antithrombotics? Thromb. Haemost. 2022, 122, 123–130. [Google Scholar] [CrossRef]
- Diez-Silva, M.; Dao, M.; Han, J.; Lim, C.-T.; Suresh, S. Shape and Biomechanical Characteristics of Human Red Blood Cells in Health and Disease. MRS Bull. 2010, 35, 382–388. [Google Scholar] [CrossRef]
- Guest, M.M.; Bond, T.P.; Cooper, R.G.; Derrick, J.R. Red Blood Cells: Change in Shape in Capillaries. Science 1963, 142, 1319–1321. [Google Scholar] [CrossRef]
- Shahid, M.; Nunhuck, A. Physiology; Elsevier Health Sciences: Philadelphia, PA, USA, 2008; p. 273. [Google Scholar]
- Kuchel, P.W.; Shishmarev, D. Accelerating metabolism and transmembrane cation flux by distorting red blood cells. Sci. Adv. 2017, 3, EAAO1016. [Google Scholar] [CrossRef] [PubMed]
- Ocak, I.; Kara, A.; Ince, C. Monitoring microcirculation. Best Pract. Res. Clin. Anaesthesiol. 2016, 30, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Chasis, J.A.; Mohandas, N. Red blood cell glycophorins. Blood 1992, 80, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Barshtein, G.; Wajnblum, D.; Yedgar, S. Kinetics of linear rouleaux formation studied by visual monitoring of red cell dynamic organization. Biophys. J. 2000, 78, 2470–2474. [Google Scholar] [CrossRef]
- Fung, Y.-C. The flow properties of blood. In Biomechanics: Mechanical Properties of Living Tissues; Fung, Y.-C., Ed.; Springer New York: New York, NY, USA, 1993; pp. 66–108. [Google Scholar] [CrossRef]
- Maeda, N.; Seike, M.; Kon, K.; Shiga, T. Erythrocyte Aggregation as a Determinant of Blood Flow: Effect of pH, Temperature and Osmotic Pressure. In Oxygen Transport to Tissue X; Mochizuki, M., Honig, C.R., Koyama, T., Goldstick, T.K., Bruley, D.F., Eds.; Springer: New York, NY, USA, 1988; pp. 563–570. [Google Scholar] [CrossRef]
- Sakariassen, K.S.; Orning, L.; Turitto, V.T. The impact of blood shear rate on arterial thrombus formation. Future Sci. OA 2015, 1, FSO30. [Google Scholar] [CrossRef] [PubMed]
- Ahmetaj-Shala, B.; Vaja, R.; Atanur, S.; George, P.; Kirkby, N.; Mitchell, J. Systemic analysis of putative SARS-CoV-2 entry and processing genes in cardiovascular tissues identifies a positive correlation of BSG with age in endothelial cells. bioRxiv 2020, 06.23.165324. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Siddiqi, H.; Libby, P.; Ridker, P. COVID-19 and vascular disease. EBioMedicine 2020, 58, 102966. [Google Scholar] [CrossRef]
- Lee, M.-H.; Perl, D.P.; Nair, G.; Li, W.; Maric, D.; Murray, H.; Dodd, S.J.; Koretsky, A.P.; Watts, J.A.; Cheung, V.; et al. Microvascular Injury in the Brains of Patients with Covid-19. N. Engl. J. Med. 2020, 384, 481–483. [Google Scholar] [CrossRef]
- Colunga Biancatelli, R.M.L.; Solopov, P.A.; Sharlow, E.R.; Lazo, J.S.; Marik, P.E.; Catravas, J.D. The SARS-CoV-2 spike protein subunit S1 induces COVID-19-like acute lung injury in Κ18-hACE2 transgenic mice and barrier dysfunction in human endothelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 321, L477–L484. [Google Scholar] [CrossRef]
- Nuovo, G.J.; Magro, C.; Shaffer, T.; Awad, H.; Suster, D.; Mikhail, S.; He, B.; Michaille, J.-J.; Liechty, B.; Tili, E. Endothelial cell damage is the central part of COVID-19 and a mouse model induced by injection of the S1 subunit of the spike protein. Ann. Diagn. Pathol. 2021, 51, 151682. [Google Scholar] [CrossRef] [PubMed]
- Perico, L.; Morigi, M.; Galbusera, M.; Pezzotta, A.; Gastoldi, S.; Imberti, B.; Ruggenenti, P.; Benigni, A.; Remuzzi, G. SARS-CoV-2 Spike Protein 1 Activates Microvascular Endothelial Cells and Complement System Leading to Thrombus Formation. Available online: https://ssrn.com/abstract=3864027 (accessed on 23 November 2021).
- Magro, C.; Mulvey, J.J.; Laurence, J.; Sanders, S.; Crowson, N.; Grossman, M.; Harp, J.; Nuovo, G. The differing pathophysiologies that underlie COVID-19 associated perniosis and thrombotic retiform purpura: A case series. Br. J. Derm. 2020, 184, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Magro, C.M.; Mulvey, J.J.; Laurence, J.; Seshan, S.; Crowson, A.N.; Dannenberg, A.J.; Salvatore, S.; Harp, J.; Nuovo, G.J. Docked severe acute respiratory syndrome coronavirus 2 proteins within the cutaneous and subcutaneous microvasculature and their role in the pathogenesis of severe coronavirus disease 2019. Hum. Pathol. 2020, 106, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.J.; Harigopal, M.; Gehlhausen, J.R.; Bosenberg, M.; McNiff, J.M.; Damsky, W. Discordant anti-SARS-CoV-2 spike protein and RNA staining in cutaneous perniotic lesions suggests endothelial deposition of cleaved spike protein. J. Cutan Pathol 2021, 48, 47–52. [Google Scholar] [CrossRef]
- Bernard, I.; Limonta, D.; Mahal, L.K.; Hobman, T.C. Endothelium Infection and Dysregulation by SARS-CoV-2: Evidence and Caveats in COVID-19. Viruses 2021, 13, 29. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef] [PubMed]
- Benton, D.J.; Wrobel, A.G.; Xu, P.; Roustan, C.; Martin, S.R.; Rosenthal, P.B.; Skehel, J.J.; Gamblin, S.J. Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion. Nature 2020, 588, 327–330. [Google Scholar] [CrossRef]
- Li, F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu. Rev. Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef]
- Weiss, A.; Jellingsø, M.; Sommer, M.O.A. Spatial and temporal dynamics of SARS-CoV-2 in COVID-19 patients: A systematic review and meta-analysis. EBioMedicine 2020, 58, 102916. [Google Scholar] [CrossRef]
- Sikora, M.; von Bülow, S.; Blanc, F.E.C.; Gecht, M.; Covino, R.; Hummer, G. Computational epitope map of SARS-CoV-2 spike protein, Supplementary Information, Movie S1. PLoS Comput. Biol. 2021, 17, e1008790. [Google Scholar] [CrossRef]
- Casalino, L.; Gaieb, Z.; Goldsmith, J.A.; Hjorth, C.K.; Dommer, A.C.; Harbison, A.M.; Fogarty, C.A.; Barros, E.P.; Taylor, B.C.; McLellan, J.S.; et al. Beyond Shielding: The Roles of Glycans in the SARS-CoV-2 Spike Protein; supplementary Information. ACS Cent. Sci. 2020, 6, 1722–1734. [Google Scholar] [CrossRef]
- Beniac, D.R.; Andonov, A.; Grudeski, E.; Booth, T.F. Architecture of the SARS coronavirus prefusion spike. Nat. Struct. Mol. Biol. 2006, 13, 751–752. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.; Oton, J.; Qu, K.; Cortese, M.; Zila, V.; McKeane, L.; Nakane, T.; Zivanov, J.; Neufeldt, C.J.; Cerikan, B.; et al. Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature 2020, 588, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Kiss, B.; Kis, Z.; Pályi, B.; Kellermayer, M.S.Z. Topography, Spike Dynamics, and Nanomechanics of Individual Native SARS-CoV-2 Virions. Nano Lett. 2021, 21, 2675–2680. [Google Scholar] [CrossRef]
- Laue, M.; Kauter, A.; Hoffmann, T.; Möller, L.; Michel, J.; Nitsche, A. Morphometry of SARS-CoV and SARS-CoV-2 particles in ultrathin plastic sections of infected Vero cell cultures. Sci. Rep. 2021, 11, 3515. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Xu, X.-f.; Xu, W.; Liu, S.-w. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Chabert, A.; Hamzeh-Cognasse, H.; Pozzetto, B.; Cognasse, F.; Schattner, M.; Gomez, R.M.; Garraud, O. Human platelets and their capacity of binding viruses: Meaning and challenges? BMC Immunol. 2015, 16, 26. [Google Scholar] [CrossRef]
- Pryzdial, E.L.G.; Lin, B.H.; Sutherland, M.R. Virus–Platelet Associations. In Platelets in Thrombotic and Non-Thrombotic Disorders: Pathophysiology, Pharmacology and Therapeutics: An Update; Gresele, P., Kleiman, N.S., Lopez, J.A., Page, C.P., Eds.; Springer International Publishing: New York, NY, USA, 2017; pp. 1085–1102. [Google Scholar]
- Stocker, T.J.; Ishikawa-Ankerhold, H.; Massberg, S.; Schulz, C. Small but mighty: Platelets as central effectors of host defense. Thromb. Haemost. 2017, 117, 651–661. [Google Scholar]
- Hyvärinen, S.; Meri, S.; Jokiranta, T.S. Disturbed sialic acid recognition on endothelial cells and platelets in complement attack causes atypical hemolytic uremic syndrome. Blood 2016, 127, 2701–2710. [Google Scholar] [CrossRef]
- Nissilä, E.; Hakala, P.; Leskinen, K.; Roig, A.; Syed, S.; Van Kessel, K.P.M.; Metso, J.; De Haas, C.J.C.; Saavalainen, P.; Meri, S.; et al. Complement Factor H and Apolipoprotein E Participate in Regulation of Inflammation in THP-1 Macrophages. Front. Immunol. 2018, 9, 2701. [Google Scholar] [CrossRef] [PubMed]
- Soerensen, A.L.; Wandall, H.H.; Patel, S.; Richardson, J.; Italiano, J.; Clausen, H.; Stossel, T.P.; Hartwig, J.H.; Hoffmeister, K.M. Platelets Lacking Sialic Acid Clear Rapidly from the Circulation Due to Ingestion by Asialoglycoprotein Receptor-Expressing Liver Macrophages and Hepatocytes. Blood 2006, 108, 1521. [Google Scholar] [CrossRef]
- Assinger, A. Platelets and Infection—An Emerging Role of Platelets in Viral Infection. Front. Immunol. 2014, 5, 649. [Google Scholar] [CrossRef] [PubMed]
- Kasinrerk, W.; Tokrasinwit, N.; Phunpae, P. CD147 monoclonal antibodies induce homotypic cell aggregation of monocytic cell line U937 via LFA-1/ICAM-1 pathway. Immunology 1999, 96, 184–192. [Google Scholar] [CrossRef]
- Pennings, G.J.; Kritharides, L. CD147 in cardiovascular disease and thrombosis. Semin. Thromb. Hemost. 2014, 40, 747–755. [Google Scholar]
- Loh, D. The potential of melatonin in the prevention and attenuation of oxidative hemolysis and myocardial injury from cd147 SARS-CoV-2 spike protein receptor binding. Melatonin Res. 2020, 3, 380–416. [Google Scholar] [CrossRef]
- Pushkarsky, T.; Zybarth, G.; Dubrovsky, L.; Yurchenko, V.; Tang, H.; Guo, H.; Toole, B.; Sherry, B.; Bukrinsky, M. CD147 facilitates HIV-1 infection by interacting with virus-associated cyclophilin A. Proc. Natl. Acad. Sci. USA 2001, 98, 6360–6365. [Google Scholar] [CrossRef]
- Rowe, J.A.; Claessens, A.; Corrigan, R.A.; Arman, M. Adhesion of Plasmodium falciparum-infected erythrocytes to human cells: Molecular mechanisms and therapeutic implications. Expert Rev. Mol. Med. 2009, 11, e16. [Google Scholar] [CrossRef]
- Adams, Y.; Kuhnrae, P.; Higgins, M.K.; Ghumra, A.; Rowe, J.A. Rosetting Plasmodium falciparum-infected erythrocytes bind to human brain microvascular endothelial cells in vitro, demonstrating a dual adhesion phenotype mediated by distinct P. falciparum erythrocyte membrane protein 1 domains. Infect. Immun. 2014, 82, 949–959. [Google Scholar] [CrossRef]
- Arman, M.; Rowe, J.A. Experimental conditions affect the outcome of Plasmodium falciparum platelet-mediated clumping assays. Malar. J. 2008, 7, 243. [Google Scholar] [CrossRef][Green Version]
- Deroost, K.; Pham, T.T.; Opdenakker, G.; Van den Steen, P.E. The immunological balance between host and parasite in malaria. FEMS Microbiol. Rev. 2016, 40, 208–257. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Cao, Y.; Frank, M.; Woo, H.; Park, S.-J.; Yeom, M.S.; Croll, T.I.; Seok, C.; Im, W. Structure, Dynamics, Receptor Binding, and Antibody Binding of the Fully Glycosylated Full-Length SARS-CoV-2 Spike Protein in a Viral Membrane. J. Chem. Theory Comput. 2021, 17, 2479–2487. [Google Scholar] [CrossRef]
- Lardone, R.D.; Garay, Y.C.; Parodi, P.; de la Fuente, S.; Angeloni, G.; Bravo, E.O.; Schmider, A.K.; Irazoqui, F.J. How glycobiology can help us treat and beat the COVID-19 pandemic. J. Biol. Chem. 2021, 296, 100375. [Google Scholar] [CrossRef]
- Bharara, R.; Singh, S.; Pattnaik, P.; Chitnis, C.E.; Sharma, A. Structural analogs of sialic acid interfere with the binding of erythrocyte binding antigen-175 to glycophorin A, an interaction crucial for erythrocyte invasion by Plasmodium falciparum. Mol. Biochem. Parasitol. 2004, 138, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Jaskiewicz, E.; Jodłowska, M.; Kaczmarek, R.; Zerka, A. Erythrocyte glycophorins as receptors for Plasmodium merozoites. Parasites Vectors 2019, 12, 317. [Google Scholar] [CrossRef] [PubMed]
- Halbhuber, K.J.; Gliesing, M.; Stibenz, D.; Makovitzky, J. Topo-optical investigations of the human erythrocyte glycocalyx-age related changes. Histochemistry 1984, 81, 187–193. [Google Scholar] [CrossRef]
- van Oss, C.J.; Absolom, D.R. Zeta potentials, van der Waals forces and hemagglutination. Vox Sang. 1983, 44, 183–190. [Google Scholar] [CrossRef]
- Pretini, V.; Koenen, M.H.; Kaestner, L.; Fens, M.H.A.M.; Schiffelers, R.M.; Bartels, M.; Van Wijk, R. Red Blood Cells: Chasing Interactions. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, H.P.; Cesar, C.L.; Barjas-Castro Mde, L. Electrical properties of the red blood cell membrane and immunohematological investigation. Rev. Bras. Hematol Hemoter 2011, 33, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Rajah, M.M.; Bernier, A.; Buchrieser, J.; Schwartz, O. The Mechanism and Consequences of SARS-CoV-2 Spike-Mediated Fusion and Syncytia Formation. J. Mol. Biol. 2021, 167280. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Zheng, Q.; Zhang, H.; Niu, Y.; Lou, Y.; Wang, H. The SARS-CoV-2 Spike Glycoprotein Biosynthesis, Structure, Function, and Antigenicity: Implications for the Design of Spike-Based Vaccine Immunogens. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Bar-On, Y.M.; Gleizer, S.; Bernshtein, B.; Flamholz, A.; Phillips, R.; Milo, R. The total number and mass of SARS-CoV-2 virions. Proc. Natl. Acad. Sci. USA 2021, 118, e2024815118. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Moreno, I.; Sanjuán, R.; Racaniello Vincent, R. Collective Viral Spread Mediated by Virion Aggregates Promotes the Evolution of Defective Interfering Particles. mBio 2020, 11, e02156–e02219. [Google Scholar] [CrossRef] [PubMed]
- Acosta Saltos, F.; Acosta Saltos, A.D. Entry of SARS-CoV2 through the Basal Surface of Alveolar Endothelial Cells—A Proposed Mechanism Mediated by CD147 in COVID-19. Available online: https://www.preprints.org/manuscript/202005.0359/v1 (accessed on 25 January 2022).
- Chen, L.; Wang, G.; Long, X.; Hou, H.; Wei, J.; Cao, Y.; Tan, J.; Liu, W.; Huang, L.; Meng, F.; et al. Dynamics of Blood Viral Load Is Strongly Associated with Clinical Outcomes in Coronavirus Disease 2019 (COVID-19) Patients: A Prospective Cohort Study. J. Mol. Diagn. 2021, 23, 10–18. [Google Scholar] [CrossRef]
- Cooling, L. Blood Groups in Infection and Host Susceptibility. Clin. Microbiol. Rev. 2015, 28, 801–870. [Google Scholar] [CrossRef]
- Watanabe, Y.; Allen, J.D.; Wrapp, D.; McLellan, J.S.; Crispin, M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science 2020, 369, 330–333. [Google Scholar] [CrossRef]
- Tai, L.; Zhu, G.; Yang, M.; Cao, L.; Xing, X.; Yin, G.; Chan, C.; Qin, C.; Rao, Z.; Wang, X.; et al. Nanometer-resolution in situ structure of the SARS-CoV-2 postfusion spike protein. Proc. Natl. Acad. Sci. USA 2021, 118, e2112703118. [Google Scholar] [CrossRef]
- Chen, J.; Gao, K.; Wang, R.; Nguyen, D.D.; Wei, G.-W. Review of COVID-19 Antibody Therapies. Annu. Rev. Biophys 2021, 50, 1–30. [Google Scholar] [CrossRef]
- Sharma, D.; Rawat, P.; Janakiraman, V.; Gromiha, M.M. Elucidating important structural features for the binding affinity of spike—SARS-CoV-2 neutralizing antibody complexes. Proteins Struct. Funct. Bioinform. 2021. [Google Scholar] [CrossRef]
- Klein, J.S.; Gnanapragasam, P.N.P.; Galimidi, R.P.; Foglesong, C.P.; West, A.P.; Bjorkman, P.J. Examination of the contributions of size and avidity to the neutralization mechanisms of the anti-HIV antibodies b12 and 4E10. Proc. Natl. Acad. Sci. USA 2009, 106, 7385. [Google Scholar] [CrossRef]
- Kruse, R.L.; Huang, Y.; Smetana, H.; Gehrie, E.A.; Amukele, T.K.; Tobian, A.A.R.; Mostafa, H.H.; Wang, Z.Z. A rapid, point-of-care red blood cell agglutination assay detecting antibodies against SARS-CoV-2. Biochem. Biophys. Res. Commun. 2021, 553, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Townsend, A.; Rijal, P.; Xiao, J.; Tan, T.K.; Huang, K.A.; Schimanski, L.; Huo, J.; Gupta, N.; Rahikainen, R.; Matthews, P.C.; et al. A haemagglutination test for rapid detection of antibodies to SARS-CoV-2. Nat. Commun. 2021, 12, 1951. [Google Scholar] [CrossRef] [PubMed]
- Semmler, G.; Traugott Marianna, T.; Graninger, M.; Hoepler, W.; Seitz, T.; Kelani, H.; Karolyi, M.; Pawelka, E.; Aragón de La Cruz, S.; Puchhammer-Stöckl, E.; et al. Assessment of S1-, S2-, and NCP-Specific IgM, IgA, and IgG Antibody Kinetics in Acute SARS-CoV-2 Infection by a Microarray and Twelve Other Immunoassays. J. Clin. Microbiol. 2021, 59, e02890–e02920. [Google Scholar] [CrossRef]
- Bläckberg, A.; Fernström, N.; Sarbrant, E.; Rasmussen, M.; Sunnerhagen, T. Antibody kinetics and clinical course of COVID-19 a prospective observational study. PLoS ONE 2021, 16, e0248918. [Google Scholar] [CrossRef] [PubMed]
- Kurano, M.; Morita, Y.; Nakano, Y.; Yokoyama, R.; Shimura, T.; Qian, C.; Xia, F.; He, F.; Zheng, L.; Ohmiya, H.; et al. Response kinetics of different classes of antibodies to SARS-CoV2 infection in the Japanese population: The IgA and IgG titers increased earlier than the IgM titers. Int. Immunopharmacol. 2022, 103, 108491. [Google Scholar] [CrossRef]
- Osman, A.M.; Farouk, S.; Osman, N.M.; Abdrabou, A.M. Longitudinal assessment of chest computerized tomography and oxygen saturation for patients with COVID-19. Egypt. J. Radiol. Nucl. Med. 2020, 51, 255. [Google Scholar] [CrossRef]
- Annunziata, A.; Coppola, A.; Carannante, N.; Simioli, F.; Lanza, M.; Di Micco, P.; Fiorentino, G. Home Management of Patients with Moderate or Severe Respiratory Failure Secondary to COVID-19, Using Remote Monitoring and Oxygen with or without HFNC. Pathogens 2021, 10, 413. [Google Scholar] [CrossRef]
- Stone, J.C.; Ndarukwa, P.; Scheim, D.E.; Dancis, B.M.; Dancis, J.; Gill, M.G.; Aldous, C. Rapid increase of SpO2 on room air for 34 severe COVID-19 patients after ivermectin-based combination treatment: 55–62% normalization within 12–24 h. Res. Sq. 2021. Preprint. [Google Scholar] [CrossRef]
- Schultze, B.; Gross, H.J.; Brossmer, R.; Herrler, G. The S protein of bovine coronavirus is a hemagglutinin recognizing 9-O-acetylated sialic acid as a receptor determinant. J. Virol. 1991, 65, 6232–6237. [Google Scholar] [CrossRef]
- Callebaut, P.E.; Pensaert, M.B. Characterization and isolation of structural polypeptides in haemagglutinating encephalomyelitis virus. J. Gen. Virol. 1980, 48, 193–204. [Google Scholar] [CrossRef]
- Dayer, M. Coronavirus (2019-nCoV) Deactivation via Spike Glycoprotein Shielding by Old Drugs, Bioinformatic Study. Preprints. Org. 2020. [Google Scholar] [CrossRef]
- Kalhor, H.; Sadeghi, S.; Abolhasani, H.; Kalhor, R.; Rahimi, H. Repurposing of the approved small molecule drugs in order to inhibit SARS-CoV-2 S protein and human ACE2 interaction through virtual screening approaches. J. Biomol. Struct. Dyn. 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nallusamy, S.; Mannu, J.; Ravikumar, C.; Angamuthu, K.; Nathan, B.; Nachimuthu, K.; Ramasamy, G.; Muthurajan, R.; Subbarayalu, M.; Neelakandan, K. Shortlisting Phytochemicals Exhibiting Inhibitory Activity against Major Proteins of SARS-CoV-2 through Virtual Screening. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Suravajhala, R.; Parashar, A.; Malik, B.; Nagaraj, V.A.; Padmanaban, G.; Kavi Kishor, P.B.; Polavarapu, R.; Suravajhala, P. Comparative Docking Studies on Curcumin with COVID-19 Proteins. Preprints.Org 2020. [Google Scholar] [CrossRef]
- Yagisawa, M.; Foster, P.J.; Hanaki, H.; Omura, S. Global Trends in Clinical Studies of Ivermectin in COVID-19. Jpn. J. Antibiot. 2021, 74, 1. [Google Scholar]
- Juarez, M.; Schcolnik-Cabrera, A.; Dueñas-Gonzalez, A. The multitargeted drug ivermectin: From an antiparasitic agent to a repositioned cancer drug. Am. J. Cancer Res. 2018, 8, 317–331. [Google Scholar]
- Campbell, W.C. History of avermectin and ivermectin, with notes on the history of other macrocyclic lactone antiparasitic agents. Curr Pharm Biotechnol 2012, 13, 853–865. [Google Scholar] [CrossRef]
- Lehrer, S.; Rheinstein, P.H. Ivermectin Docks to the SARS-CoV-2 Spike Receptor-binding Domain Attached to ACE2. In Vivo 2020, 34, 3023–3026. [Google Scholar] [CrossRef]
- Dasgupta, J.; Sen, U.; Bakashi, A.; Dasgupta, A. Nsp7 and Spike Glycoprotein of SARS-CoV-2 Are Envisaged as Potential Targets of Vitamin D and Ivermectin. Preprints.Org 2020. [Google Scholar] [CrossRef]
- Hussien, M.A.; Abdelaziz, A.E.M. Molecular docking suggests repurposing of brincidofovir as a potential drug targeting SARS-CoV-2 ACE2 receptor and main protease. Netw. Modeling Anal. Health Inform. Bioinform. 2020, 9, 56. [Google Scholar] [CrossRef]
- Kaur, H.; Shekhar, N.; Sharma, S.; Sarma, P.; Prakash, A.; Medhi, B. Ivermectin as a potential drug for treatment of COVID-19: An in-sync review with clinical and computational attributes. Pharmacol. Rep. 2021, 73, 736–749. [Google Scholar] [CrossRef]
- Maurya, D. A Combination of Ivermectin and Doxycycline Possibly Blocks the Viral Entry and Modulate the Innate Immune Response in COVID-19 Patients. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Saha, J.K.; Raihan, J. The Binding mechanism of Ivermectin and levosalbutamol with spike protein of SARS-CoV-2. Struct. Chem. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kory, P.; Kanne, J.P. SARS-CoV-2 organising pneumonia: ‘Has there been a widespread failure to identify and treat this prevalent condition in COVID-19?’. BMJ Open Respir. Res. 2020, 7, e000724. [Google Scholar] [CrossRef]
- Santin, A.D.; Scheim, D.E.; McCullough, P.A.; Yagisawa, M.; Borody, T.J. Ivermectin: A multifaceted drug of Nobel prize-honored distinction with indicated efficacy against a new global scourge, COVID-19. New Microbes New Infect. 2021, 43, 100924. [Google Scholar] [CrossRef]
- López-Medina, E.; López, P.; Hurtado, I.C.; Dávalos, D.M.; Ramirez, O.; Martínez, E.; Díazgranados, J.A.; Oñate, J.M.; Chavarriaga, H.; Herrera, S.; et al. Effect of Ivermectin on Time to Resolution of Symptoms Among Adults with Mild COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Scheim, D.E.; Hibberd, J.A.; Chamie, J.J. Protocol Violations in López-Medina et al. 38 Switched Ivermectin (IVM) and Placebo Doses, Failure of Blinding, Ubiquitous IVM use OTC in Cali, and Nearly Identical AEs for the IVM and Control Groups. Available online: https://doi.org/10.31219/osf.io/u7ewz (accessed on 21 December 2021).
- Elgazzar, A.; Hany, B.; Abo Youssef, S.; Hany, B. Efficacy and Safety of Ivermectin for Treatment and prophylaxis of COVID-19 Pandemic. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Crump, A.; Ōmura, S. Ivermectin, ‘wonder drug’ from Japan: The human use perspective. Proc. Jpn Acad Ser. B Phys. Biol. Sci. 2011, 87, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, C.A.; Furtek, C.I.; Porras, A.G.; Chen, C.; Tipping, R.; Clineschmidt, C.M.; Sciberras, D.G.; Hsieh, J.Y.; Lasseter, K.C. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J. Clin. Pharm. 2002, 42, 1122–1133. [Google Scholar] [CrossRef]
- Navarro, M.; Camprubí, D.; Requena-Méndez, A.; Buonfrate, D.; Giorli, G.; Kamgno, J.; Gardon, J.; Boussinesq, M.; Muñoz, J.; Krolewiecki, A. Safety of high-dose ivermectin: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2020, 75, 827–834. [Google Scholar] [CrossRef]
- The 2015 Nobel Prize in Physiology or Medicine—Press Release; The Nobel Assembly at Karolinska Institutet: Solna, Sweden, 5 October 2015. Available online: https://www.nobelprize.org/prizes/medicine/2015/press-release/ (accessed on 5 October 2015).
- Melo, G.D.; Lazarini, F.; Larrous, F.; Feige, L.; Kergoat, L.; Marchio, A.; Pineau, P.; Lecuit, M.; Lledo, P.-M.; Changeux, J.-P.; et al. Anti-COVID-19 efficacy of ivermectin in the golden hamster. bioRxiv 2020, 11.21.392639. [Google Scholar] [CrossRef]
- Arévalo, A.P.; Pagotto, R.; Pórfido, J.L.; Daghero, H.; Segovia, M.; Yamasaki, K.; Varela, B.; Hill, M.; Verdes, J.M.; Duhalde Vega, M.; et al. Ivermectin reduces in vivo coronavirus infection in a mouse experimental model. Sci. Rep. 2021, 11, 7132. [Google Scholar] [CrossRef] [PubMed]
- Krolewiecki, A.; Lifschitz, A.; Moragas, M.; Travacio, M.; Valentini, R.; Alonso, D.F.; Solari, R. Antiviral Effect of High-Dose Ivermectin in Adults with COVID-19: A Pilot Randomised, Controlled, Open Label, Multicentre Trial. Available online: http://ssrn.com/abstract=3714649 (accessed on 21 December 2021).
- Kirti, R.; Roy, R.; Pattadar, C.; Raj, R.; Agarwal, N.; Biswas, B.; Majhi, P.K.; Rai, D.K.; Shyama; Kumar, A.; et al. Ivermectin as a potential treatment for mild to moderate COVID-19—A double blind randomized placebo-controlled trial. medRxiv 2021, 01.05.21249310. [Google Scholar] [CrossRef]
- Chaccour, C.; Casellas, A.; Blanco-Di Matteo, A.; Pineda, I.; Fernandez-Montero, A.; Ruiz-Castillo, P.; Richardson, M.-A.; Rodríguez-Mateos, M.; Jordán-Iborra, C.; Brew, J.; et al. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial. EClinicalMedicine 2021, 32, 100720. [Google Scholar] [CrossRef] [PubMed]
- Seet, R.C.S.; Quek, A.M.L.; Ooi, D.S.Q.; Sengupta, S.; Lakshminarasappa, S.R.; Koo, C.Y.; So, J.B.Y.; Goh, B.C.; Loh, K.S.; Fisher, D.; et al. Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: An open-label randomized trial. Int. J. Infect. Dis. 2021, 106, 314–322. [Google Scholar] [CrossRef]
- Hazan, S.; Dave, S.; Gunaratne, A.W.; Dolai, S.; Clancy, R.L.; McCullough, P.A.; Borody, T.J. Effectiveness of Ivermectin-Based Multidrug Therapy in Severe Hypoxic Ambulatory COVID-19 Patients. medRxiv 2021, 07.06.21259924. [Google Scholar] [CrossRef]
- Chaccour, C.; Hammann, F.; Rabinovich, N.R. Ivermectin to reduce malaria transmission I. Pharmacokinetic and pharmacodynamic considerations regarding efficacy and safety. Malar. J. 2017, 16, 161. [Google Scholar] [CrossRef]
- Munoz, J.; Ballester, M.R.; Antonijoan, R.M.; Gich, I.; Rodriguez, M.; Colli, E.; Gold, S.; Krolewiecki, A.J. Safety and pharmacokinetic profile of fixed-dose ivermectin with an innovative 18mg tablet in healthy adult volunteers. PLoS Negl. Trop. Dis. 2018, 12, e0006020. [Google Scholar] [CrossRef]
- Aminpour, M.; Cannariato, M.; Safaeeardebili, M.E.; Moracchiato, A.; Doria, D.; Donato, F.; Zizzi, E.A.; Deriu, M.A.; Scheim, D.E.; Santin, A.D.; et al. Computational Investigations of the Multi-targeted Mode of Action of Ivermectin and Related Compounds. Computation 2021. Manuscript in Progress. [Google Scholar]
- Chang, M.W.; Lindstrom, W.; Olson, A.J.; Belew, R.K. Analysis of HIV Wild-Type and Mutant Structures via in Silico Docking against Diverse Ligand Libraries. J. Chem. Inf. Modeling 2007, 47, 1258–1262. [Google Scholar] [CrossRef]
- Audus, K.L.; Knaub, S.R.; Guillot, F.L.; Schaeffer, J.M. The effect of protein binding on ivermectin uptake by bovine brain microvessel endothelial cells. Vet. Res. Commun 1992, 16, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, S.P.; Evans, D.V. Binding characteristics of ivermectin in plasma from collie dogs. Vet. Res. Commun 1990, 14, 157–165. [Google Scholar] [PubMed]
- Alsmadi, M.M. Physiologically Based Pharmacokinetic (PBPK) Model of Ivermectin (IVM). Ph.D. Thesis, Univeristy of Iowa, Iowa City, IA, USA, 2014. [Google Scholar]
- Sugio, S.; Kashima, A.; Mochizuki, S.; Noda, M.; Kobayashi, K. Crystal structure of human serum albumin at 2.5 A resolution. Protein Eng. 1999, 12, 439–446. [Google Scholar] [CrossRef]
- Mol-Instincts, Structure of IVERMECTIN (C48H74O14), Interactive 3-Dimensional (3D) Visualization. Available online: https://www.molinstincts.com/structure/IVERMECTIN-cstr-CT1079779157.html (accessed on 1 December 2021).
- Stencel-Baerenwald, J.E.; Reiss, K.; Reiter, D.M.; Stehle, T.; Dermody, T.S. The sweet spot: Defining virus–sialic acid interactions. Nat. Rev. Microbiol. 2014, 12, 739–749. [Google Scholar] [CrossRef]
- Jeanloz, R.W.; Codington, J.F. Chapter 7. The Biological Role of Sialic Acid at the Surface of the Cell. In Biological Roles of Sialic Acid; Rosenberg, A., Schengrund, C.-L., Eds.; Springer: Boston, MA, USA, 1976; pp. 201–238. [Google Scholar] [CrossRef]
- Changeux, J.P.; Amoura, Z.; Rey, F.A.; Miyara, M. A nicotinic hypothesis for Covid-19 with preventive and therapeutic implications. C.R. Biol. 2020, 343, 33–39. [Google Scholar] [PubMed]
- Lagoumintzis, G.; Chasapis, C.T.; Alexandris, N.; Kouretas, D.; Tzartos, S.; Eliopoulos, E.; Farsalinos, K.; Poulas, K. Nicotinic cholinergic system and COVID-19: In silico identification of interactions between alpha7 nicotinic acetylcholine receptor and the cryptic epitopes of SARS-Co-V and SARS-CoV-2 Spike glycoproteins. Food Chem. Toxicol. 2021, 149, 112009. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Sharma, S. Physiology, blood volume. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Dean, L. Blood and the Cells it Contains; National Center for Biotechnology Information: Bethesda, MD, USA, 2005. [Google Scholar]
- Jaffe, E.A. Cell biology of endothelial cells. Hum. Pathol. 1987, 18, 234–239. [Google Scholar] [CrossRef]
- BioServUK Trimeric SARS CoV-2 Spike Antigen, aa 1-1273, Full-Length, 3 × 142 kDam Datasheet. Available online: https://bioservuk.com/trimeric-sars-cov-2-spike-protein-full-length/ (accessed on 20 January 2022).
- Fink, D.W.; Porras, A.G. Pharmacokinetics of Ivermectin in Animals and Humans. In Ivermectin and Abamectin; Campbell, W.C., Ed.; Springer: New York, NY, USA, 1989; pp. 113–130. [Google Scholar]
- Samsel, R.W.; Perelson, A.S. Kinetics of rouleau formation. II. Reversible reactions. Biophys J. 1984, 45, 805–824. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheim, D.E. A Deadly Embrace: Hemagglutination Mediated by SARS-CoV-2 Spike Protein at Its 22 N-Glycosylation Sites, Red Blood Cell Surface Sialoglycoproteins, and Antibody. Int. J. Mol. Sci. 2022, 23, 2558. https://doi.org/10.3390/ijms23052558
Scheim DE. A Deadly Embrace: Hemagglutination Mediated by SARS-CoV-2 Spike Protein at Its 22 N-Glycosylation Sites, Red Blood Cell Surface Sialoglycoproteins, and Antibody. International Journal of Molecular Sciences. 2022; 23(5):2558. https://doi.org/10.3390/ijms23052558
Chicago/Turabian StyleScheim, David E. 2022. "A Deadly Embrace: Hemagglutination Mediated by SARS-CoV-2 Spike Protein at Its 22 N-Glycosylation Sites, Red Blood Cell Surface Sialoglycoproteins, and Antibody" International Journal of Molecular Sciences 23, no. 5: 2558. https://doi.org/10.3390/ijms23052558
APA StyleScheim, D. E. (2022). A Deadly Embrace: Hemagglutination Mediated by SARS-CoV-2 Spike Protein at Its 22 N-Glycosylation Sites, Red Blood Cell Surface Sialoglycoproteins, and Antibody. International Journal of Molecular Sciences, 23(5), 2558. https://doi.org/10.3390/ijms23052558





