Micro-Current Stimulation Suppresses Inflammatory Responses in Peptidoglycan-Treated Raw 264.7 Macrophages and Propionibacterium acnes-Induced Skin Inflammation via TLR2/NF-κB Signaling Pathway
Abstract
1. Introduction
2. Results
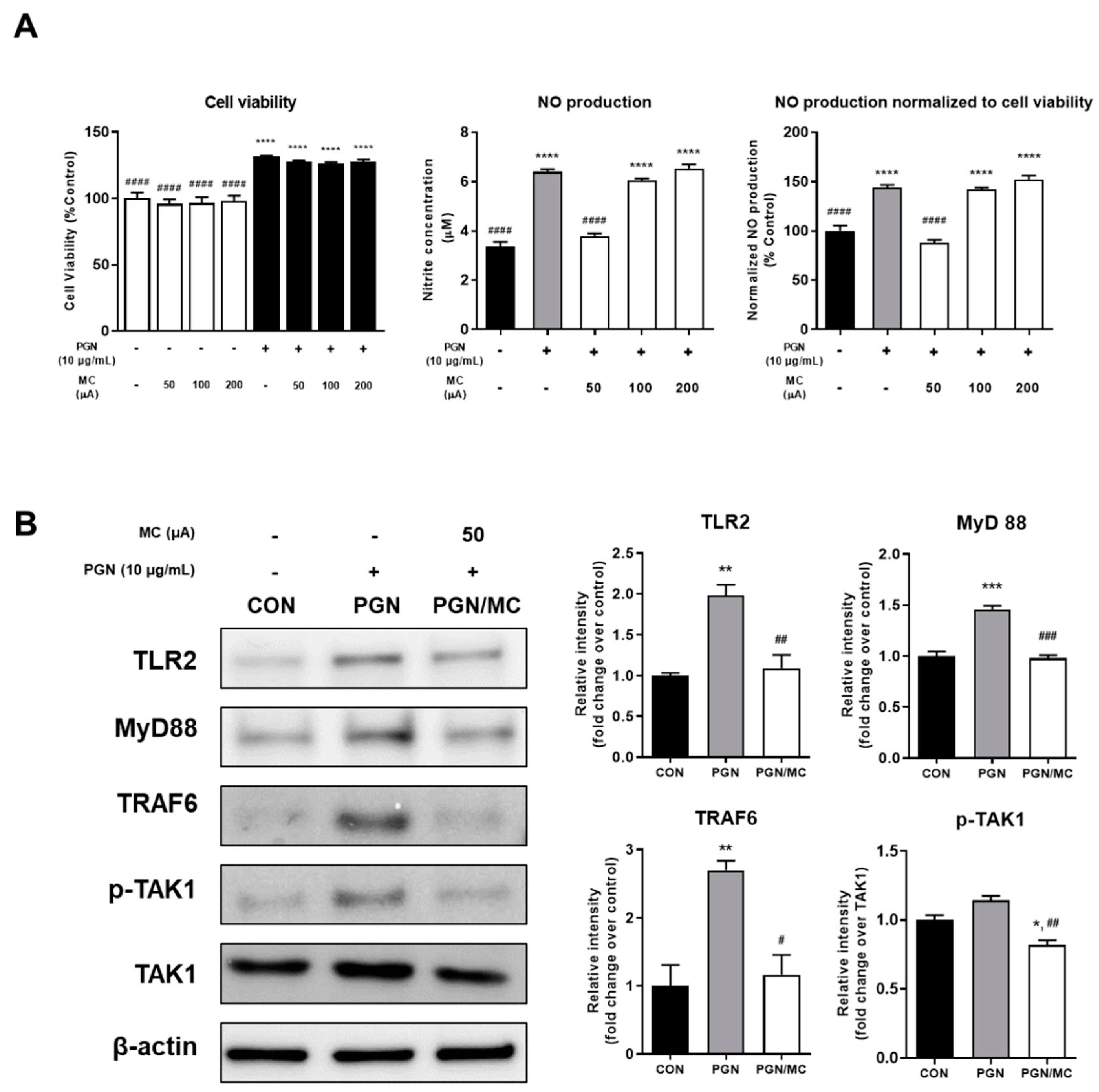
2.1. Micro-Current Stimulation Could Decrease the NO Production and TLR2 Related Protein Expression
2.2. MC Could Decrease Activity of NF-κB and Related Protein Expression
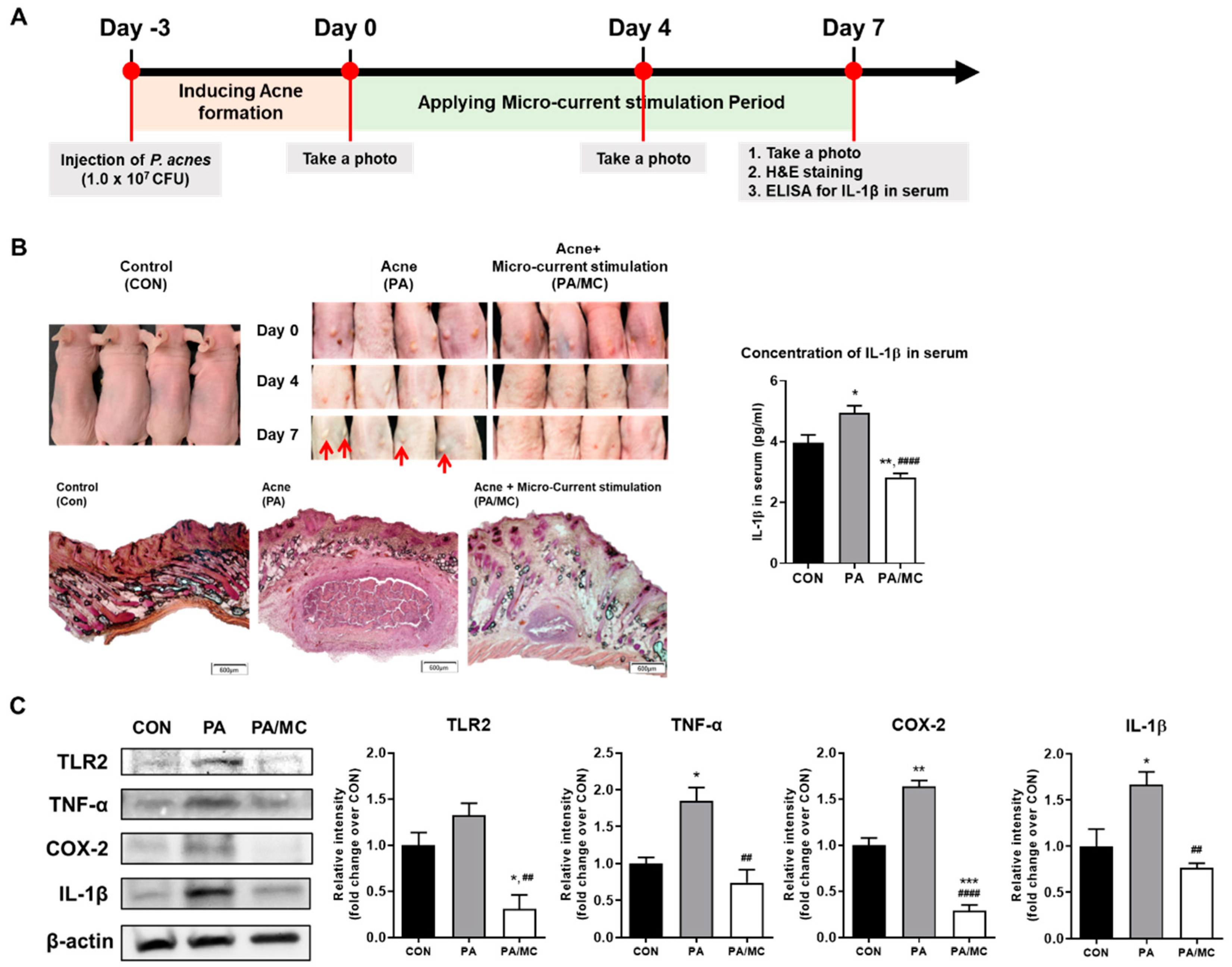
2.3. MC Suppressed P. acnes-Mediated Skin Inflammation in Mice
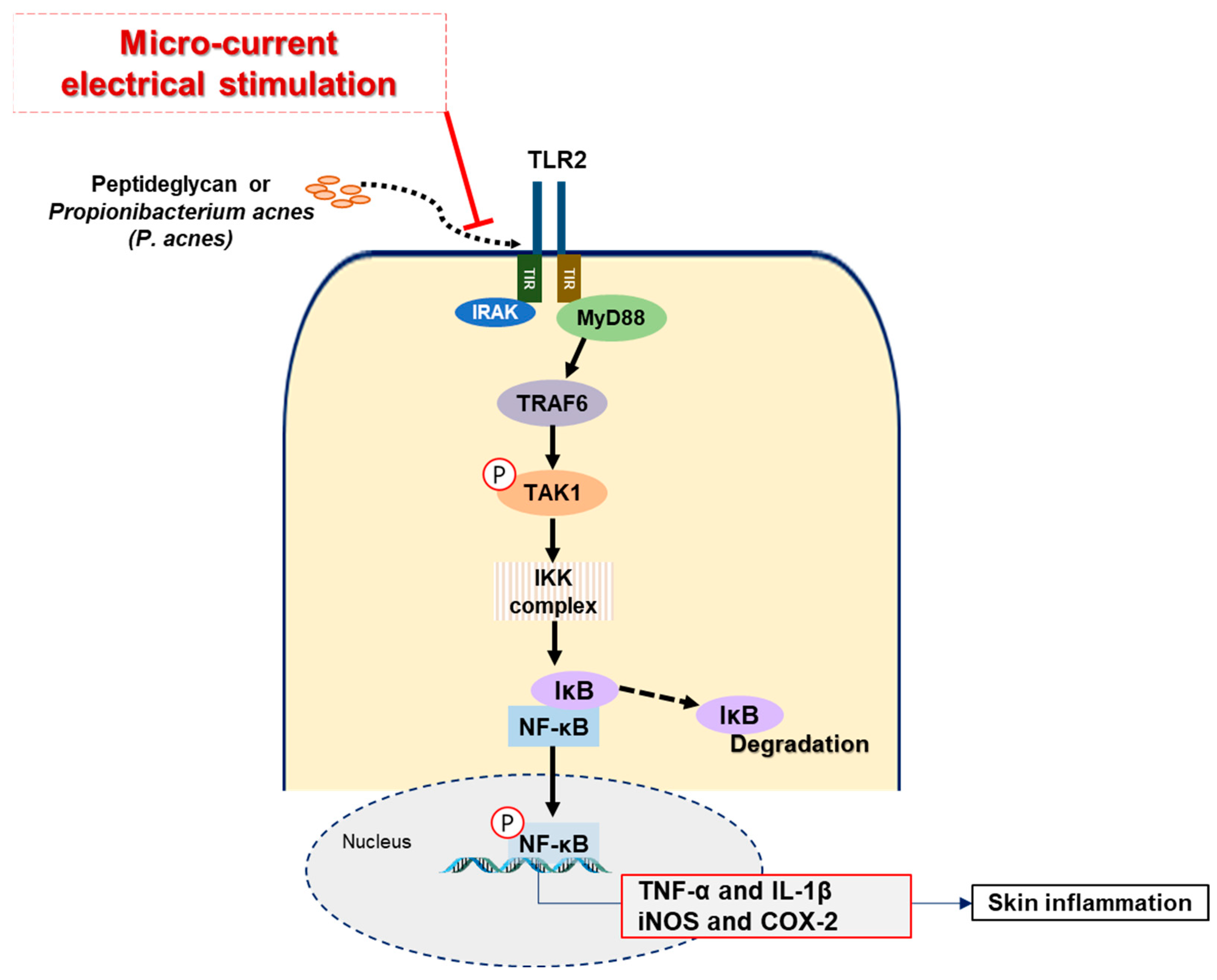
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Micro-Current Electrical Stimulation (MC)
4.3. Cell Culture and Preparation of Bacteria
4.4. Cell Viability
4.5. Determination of Nitric Oxide (NO) Production
4.6. Animals
4.7. Immunoblotting and Immunofluorescence Analysis
4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
4.9. Histological Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harder, J.; Tsuruta, D.; Murakami, M.; Kurokawa, I. What is the role of antimicrobial peptides (AMP) in acne vulgaris? Exp. Dermatol. 2013, 22, 386–391. [Google Scholar] [CrossRef]
- Jain, A.; Basal, E. Inhibition of propionibacterium acnes-induced mediators of inflammation by indian herbs. Phytomedicine 2003, 10, 34–38. [Google Scholar] [CrossRef]
- Williams, H.C.; Dellavalle, R.P.; Garner, S. Acne vulgaris. Lancet 2012, 379, 361–372. [Google Scholar] [CrossRef]
- Gamble, R.; Dunn, J.; Dawson, A.; Petersen, B.; McLaughlin, L.; Small, A.; Dellavalle, R.P. Topical antimicrobial treatment of acne vulgaris. Am. J. Clin. Dermatol. 2012, 13, 141–152. [Google Scholar] [CrossRef]
- Zuliani, T.; Khammari, A.; Chaussy, H.; Knol, A.C.; Dréno, B. Ex vivo demonstration of a synergistic effect of Adapalene and benzoyl peroxide on inflammatory acne lesions. Exp. Dermatol. 2011, 20, 850–853. [Google Scholar] [CrossRef]
- Eady, E.A.; Cove, J.H.; Holland, K.T.; Cunliffe, W.J. Erythromycin resistant propionibacteria in antibiotic treated acne patients: Association with therapeutic failure. Br. J. Dermatol. 1989, 121, 51–57. [Google Scholar] [CrossRef]
- Ochsendorf, F. Minocycline in acne vulgaris. Am. J. Clin. Dermatol. 2010, 11, 327–341. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Bosanac, S.S.; Sivamani, R.K.; Larsen, L.N. Emerging therapies for acne vulgaris. Am. J. Clin. Dermatol. 2018, 19, 505–516. [Google Scholar] [CrossRef]
- Alexiades, M. Laser and light-based treatments of acne and acne scarring. Clin. Dermatol. 2017, 35, 183–189. [Google Scholar]
- Gold, M.H. The utilization of ALA-PDT and a new photoclearing device for the treatment of severe inflammatory acne vulgaris—Results of an initial clinical trial. J. Lasers Surg. Med. 2003, 15, 46. [Google Scholar]
- Tzung, T.Y.; Wu, K.H.; Huang, M.L. Blue light phototherapy in the treatment of acne. Photodermatol. Photoimmunol. Photomed. 2004, 20, 266–269. [Google Scholar] [CrossRef]
- Goldman, M.P.; Boyce, S.M. A single-center study of aminolevulinic acid and 417 NM photodynamic therapy in the treatment of moderate to severe acne vulgaris. J. Drugs Dermatol. JDD 2003, 2, 393–396. [Google Scholar]
- Papageorgiou, P.; Katsambas, A.; Chu, A. Phototherapy with blue (415 nm) and red (660 nm) light in the treatment of acne vulgaris. Br. J. Dermatol. 2003, 142, 973–978. [Google Scholar] [CrossRef]
- Rouabhia, M.; Park, H.; Meng, S.; Derbali, H.; Zhang, Z. Electrical stimulation promotes wound healing by enhancing dermal fibroblast activity and promoting myofibroblast transdifferentiation. PLoS ONE 2013, 8, e71660. [Google Scholar] [CrossRef]
- Denda, M.; Kumazawa, N. Negative electric potential induces alteration of ion gradient and lamellar body secretion in the epidermis, and accelerates skin barrier recovery after barrier disruption. J. Investig. Dermatol. 2002, 118, 65–72. [Google Scholar] [CrossRef]
- Ngok Cheng, M.D.; Van Hoof, H.; Bockx, E.; Hoogmartens, M.J.; Mulier, J.C.; De Ducker, F.J.; Sansen, W.M.; De Loecker, W. The effects of electric currents on ATP generation, protein synthesis, and membrane transport in rat skin. Clin. Orthop. Relat. Res. 1982, 171, 264–272. [Google Scholar]
- Cho, S.; Kim, S.G.; Kim, Y.M.; Park, S.K.; Lee, C.H.; Kim, H. Clinical Test for Evaluation of Effectiveness of the Micro-current Stimulation in Facial Skin Care. J. Biomed. Eng. Res. 2016, 37, 195–207. [Google Scholar] [CrossRef][Green Version]
- Kaur, S.; Lyte, P.; Garay, M.; Liebel, F.; Sun, Y.; Liu, J.C.; Southall, M.D. Galvanic zinc–copper microparticles produce electrical stimulation that reduces the inflammatory and immune responses in skin. Arch. Dermatol. Res. 2011, 303, 551–562. [Google Scholar] [CrossRef]
- Lee, J.W.; Yoon, S.W.; Kim, T.H.; Park, S.J. The effects of microcurrents on inflammatory reaction induced by ultraviolet irradiation. J. Phys. Ther. Sci. 2011, 23, 693–696. [Google Scholar] [CrossRef][Green Version]
- Blount, A.L.; Foster, S.; Rapp, D.A.; Wilcox, R. The use of bioelectric dressings in skin graft harvest sites: A prospective case series. J. Burn Care Res. 2012, 33, 354–357. [Google Scholar] [CrossRef]
- Daeschlein, G.; Assadian, O.; Kloth, L.C.; Meinl, C.; Ney, F.; Kramer, A. Antibacterial activity of positive and negative polarity low-voltage pulsed current (LVPC) on six typical Gram-positive and Gram-negative bacterial pathogens of chronic wounds. Wound Repair Regen. 2007, 15, 399–403. [Google Scholar] [CrossRef]
- Hou, X.X.; Chen, G.; Hossini, A.M.; Hu, T.; Wang, L.; Pan, Z.; Lingyi, L.; Cao, K.; Ma, Y.; Zouboulis, C.C.; et al. Aryl hydrocarbon receptor modulates the expression of TNF-α and IL-8 in human sebocytes via the MyD88-p65NF-κB/p38MAPK signaling pathways. J. Innate Immun. 2019, 11, 41–51. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Sah, S.K.; Zouboulis, C.C.; Kim, T.Y. Inhibitory effects of superoxide dismutase 3 on Propionibacterium acnes-induced skin inflammation. Sci. Rep. 2018, 8, 4024. [Google Scholar] [CrossRef]
- Fernández, J.R.; Webb, C.; Rouzard, K.; Healy, J.; Tamura, M.; Voronkov, M.; Huber, K.L.; Stock, J.B.; Stock, M.; Gordon, J.S.; et al. SIG1459: A novel phytyl-cysteine derived TLR2 modulator with in vitro and clinical anti-acne activity. Exp. Dermatol. 2018, 27, 993–999. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Ryu, A.R.; Jin, S.; Jeon, Y.M.; Lee, M.Y. Chlorin e6-mediated photodynamic therapy suppresses P. acnes-induced inflammatory response via NFκB and MAPKs signaling pathway. PLoS ONE 2017, 12, e0170599. [Google Scholar] [CrossRef]
- Suvanprakorn, P.; Tongyen, T.; Prakhongcheep, O.; Laoratthaphong, P.; Chanvorachote, P. Establishment of an Anti-acne Vulgaris Evaluation Method Based on TLR2 and TLR4-mediated Interleukin-8 Production. In Vivo 2019, 33, 1929–1934. [Google Scholar] [CrossRef]
- Takeuchi, O.; Hoshino, K.; Kawai, T.; Sanjo, H.; Takada, H.; Ogawa, T.; Takeda, K.; Akira, S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 1999, 11, 443–451. [Google Scholar] [CrossRef]
- Lee, W.R.; Kim, K.H.; An, H.J.; Kim, J.Y.; Chang, Y.C.; Chung, H.; Park, Y.Y.; Lee, M.L.; Park, K.K. The protective effects of Melittin on Propionibacterium acnes–induced inflammatory responses in vitro and in vivo. J. Investig. Dermatol. 2014, 134, 1922–1930. [Google Scholar] [CrossRef]
- Dispenza, M.C.; Wolpert, E.B.; Gilliland, K.L.; Dai, J.P.; Cong, Z.; Nelson, A.M.; Thiboutot, D.M. Systemic isotretinoin therapy normalizes exaggerated TLR-2-mediated innate immune responses in acne patients. J. Investig. Dermatol. 2012, 132, 2198–2205. [Google Scholar] [CrossRef]
- Kumar, B.; Pathak, R.; Mary, P.B.; Jha, D.; Sardana, K.; Gautam, H.K. New insights into acne pathogenesis: Exploring the role of acne-associated microbial populations. Dermatol. Sin. 2016, 34, 67–73. [Google Scholar] [CrossRef]
- Yoshimura, A.; Lien, E.; Ingalls, R.R.; Tuomanen, E.; Dziarski, R.; Golenbock, D. Cutting edge: Recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 1999, 163, 1–5. [Google Scholar]
- Kim, H.; Han, T.H.; Lee, S.G. Anti-inflammatory activity of a water extract of Acorus calamus L. leaves on keratinocyte HaCaT cells. J. Ethnopharmacol. 2009, 122, 149–156. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y. The therapeutic effect of tanshinone IIA on Propionibacterium acnes-induced inflammation in vitro. Dermatol. Ther. 2018, 31, e12716. [Google Scholar] [CrossRef]
- Tanghetti, E.A. The role of inflammation in the pathology of acne. J. Clin. Aesthetic Dermatol. 2013, 6, 27. [Google Scholar]
- Kim, J. Review of the innate immune response in acne vulgaris: Activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology 2005, 211, 193–198. [Google Scholar] [CrossRef]
- Zouboulis, C.C. Is acne vulgaris a genuine inflammatory disease? Dermatology 2001, 203, 277–279. [Google Scholar] [CrossRef]
- Jefferies, C.; O’Neill, L.A.J. Signal transduction pathway activated by Toll-like receptors. Mod. Asp. Immunobiol. 2002, 2, 169–175. [Google Scholar]
- Vowels, B.R.; Yang, S.; Leyden, J.J. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: Implications for chronic inflammatory acne. Infect. Immun. 1995, 63, 3158–3165. [Google Scholar] [CrossRef]
- Hoeffler, U. Enzymatic and hemolytic properties of Propionibacterium acnes and related bacteria. J. Clin. Microbiol. 1997, 6, 555–558. [Google Scholar] [CrossRef]
- Ingham, E.; Holland, K.T.; Gowland, G.; Cunliffe, W.J. Purification and partial characterization of an acid phosphatase (EC 3.1. 3.2) produced by Propionibacterium acnes. Microbiology 1980, 118, 59–65. [Google Scholar] [CrossRef]
- Puhvel, S.M.; Reisner, R.M. The production of hyaluronidase (hyaluronate lyase) by Corynebacterium acnes. J. Investig. Dermatol. 1972, 58, 66–70. [Google Scholar] [CrossRef]
- Eady, E.A.; Gloor, M.; Leyden, J.J. Propionibacterium acnes resistance: A worldwide problem. Dermatology 2003, 206, 54–56. [Google Scholar] [CrossRef]
- Zaenglein, A.L.; Schlosser, B.J.; Alikhan, A. Guidelines of care for acne vulgaris management. J. Am. Acad. Derm. 2016, 74, 945–973. [Google Scholar] [CrossRef]
- Lazarenko, N.N.; Supova, M.V.; Trunova, O.V.; Smirnova, S.N.; Prikuls, V.F. The influence of electrical stimulation on the peripheral immune system under the experimental and clinical conditions. Vopr. Kurortol. Fizioter. I Lech. Fiz. Kult. 2015, 92, 41–47. [Google Scholar] [CrossRef]
- Ye, Y.S.; Pan, A.Z.; Zhen, Y.; Kang, M.R.; Zhang, B.; Yi, W.M. Antipruritic effects of electroacupuncture on morphine-induced pruritus model mice through the TLR2/4-MyD88-NF-κB pathway. Neuroreport 2019, 30, 331. [Google Scholar] [CrossRef]
- Hwang, D.; Lee, H.; Lee, J.; Lee, M.; Cho, S.; Kim, T.; Kim, H. Micro-Current Stimulation Has Potential Effects of Hair Growth-Promotion on Human Hair Follicle-Derived Papilla Cells and Animal Model. Int. J. Mol. Sci. 2021, 22, 4361. [Google Scholar] [CrossRef]
- Lee, H.; Kim, S.; Hwang, D.; Yoo, L.; Yu, J.; Kim, M.; Cho, S.K.; Kim, H.S. The Effect of Micro-current Electrical Therapy on Muscle Atrophy and Delayed Wound Healing Process Induced Rat Caused by Traumatic Peripheral Nerve Injury. J. Biomed. Eng. Res. 2018, 39, 1–9. [Google Scholar]
- Rosas-Martínez, M.; Gutiérrez-Venegas, G. Myricetin Inhibition of Peptidoglycan-Induced COX-2 Expression in H9c2 Cardiomyocytes. Prev. Nutr. Food Sci. 2019, 24, 202. [Google Scholar] [CrossRef]
- Tsai, H.H.; Lee, W.R.; Wang, P.H.; Cheng, K.T.; Chen, Y.C.; Shen, S.C. Propionibacterium acnes-induced iNOS and COX-2 protein expression via ROS-dependent NF-κB and AP-1 activation in macrophages. J. Dermatol. Sci. 2013, 69, 122–131. [Google Scholar] [CrossRef]
- Lee, W.R.; Kim, K.H.; An, H.J.; Kim, J.Y.; Han, S.M.; Lee, K.G.; Park, K.K. Protective effect of melittin against inflammation and apoptosis on Propionibacterium acnes-induced human THP-1 monocytic cell. Eur. J. Pharmacol. 2014, 740, 218–226. [Google Scholar] [CrossRef]
- Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001, 1, 135–145. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C., Jr. Innate immunity. N. Engl. J. Med. 2000, 343, 338–344. [Google Scholar] [CrossRef]
- Jin, M.S.; Lee, J.O. Structures of the toll-like receptor family and its ligand complexes. Immunity 2008, 29, 182–191. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef]
- Jenkins, K.A.; Mansell, A. TIR-containing adaptors in Toll-like receptor signalling. Cytokine 2010, 49, 237–244. [Google Scholar] [CrossRef]
- Chen, Z.J. Ubiquitin signalling in the NF-κB pathway. Nat. Cell Biol. 2005, 7, 758–765. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef]
- Miller, L.S. Toll-like receptors in skin. Adv. Dermatol. 2008, 24, 71. [Google Scholar] [CrossRef]
- Huang, Y.C.; Yang, C.H.; Li, T.T.; Zouboulis, C.C.; Hsu, H.C. Cell-free extracts of Propionibacterium acnes stimulate cytokine production through activation of p38 MAPK and Toll-like receptor in SZ95 sebocytes. Life Sci. 2015, 139, 123–131. [Google Scholar] [CrossRef]
- Murphy, T.L.; Cleveland, M.G.; Kulesza, P.; Magram, J.; Murphy, K.M. Regulation of interleukin 12 p40 expression through an NF-kappa B half-site. Mol. Cell. Biol. 1995, 15, 5258–5267. [Google Scholar] [CrossRef]
- Kunsch, C.; Rosen, C.A. NF-kappa B subunit-specific regulation of the interleukin-8 promoter. Mol. Cell. Biol. 1993, 13, 6137–6146. [Google Scholar]
- Agrawal, V.; Jaiswal, M.K.; Mallers, T.; Katara, G.K.; Gilman-Sachs, A.; Beaman, K.D.; Hirsch, E. Altered autophagic flux enhances inflammatory responses during inflammation-induced preterm labor. Sci. Rep. 2015, 5, 9410. [Google Scholar] [CrossRef]
- Park, J.Y.; Chung, T.W.; Jeong, Y.J.; Kwak, C.H.; Ha, S.H.; Kwon, K.M.; Abekura, F.; Cho, S.H.; Lee, Y.C.; Ha, K.T.; et al. Ascofuranone inhibits lipopolysaccharide–induced inflammatory response via NF-kappaB and AP-1, p-ERK, TNF-α, IL-6 and IL-1β in RAW 264.7 macrophages. PLoS ONE 2017, 12, e0171322. [Google Scholar] [CrossRef]
- Cho, B.O.; So, Y.; Jin, C.H.; Nam, B.M.; Yee, S.T.; Jeong, I.Y. 3-deoxysilybin exerts anti-inflammatory effects by suppressing NF-κB activation in lipopolysaccharide-stimulated RAW264. 7 macrophages. Biosci. Biotechnol. Biochem. 2014, 78, 2051–2058. [Google Scholar] [CrossRef]
- Maurya, A.K.; Mohanty, S.; Pal, A.; Chanotiya, C.S.; Bawankule, D.U. The essential oil from Citrus limetta Risso peels alleviates skin inflammation: In-vitro and in-vivo study. J. Ethnopharmacol. 2018, 212, 86–94. [Google Scholar] [CrossRef]
- Kurokawa, I.; Danby, F.W.; Ju, Q.; Wang, X.; Xiang, L.F.; Xia, L.; Chen, W.; Nagy, I.; Picardo, M.; Suh, D.H.; et al. New developments in our understanding of acne pathogenesis and treatment. Exp. Dermatol. 2009, 18, 821–832. [Google Scholar] [CrossRef]
- An, H.J.; Lee, W.R.; Kim, K.H.; Kim, J.Y.; Lee, S.J.; Han, S.M.; Lee, K.G.; Lee, C.K.; Park, K.K. Inhibitory effects of bee venom on Propionibacterium acnes-induced inflammatory skin disease in an animal model. Int. J. Mol. Med. 2014, 34, 1341–1348. [Google Scholar] [CrossRef]
- Bergler-Czop, B. The aetiopathogenesis of acne vulgaris–what’s new? Int. J. Cosmet. Sci. 2014, 36, 187–194. [Google Scholar] [CrossRef]
- Szabó, K.; Tax, G.; Teodorescu-Brinzeu, D.; Koreck, A.; Kemény, L. TNFα gene polymorphisms in the pathogenesis of acne vulgaris. Arch. Dermatol. Res. 2011, 303, 19–27. [Google Scholar] [CrossRef]
- Kim, J.B.; Han, A.R.; Park, E.Y.; Kim, J.Y.; Cho, W.; Lee, J.; Seo, E.K.; Lee, K.T. Inhibition of LPS-induced iNOS, COX-2 and cytokines expression by poncirin through the NF-κB inactivation in RAW 264.7 macrophage cells. Biol. Pharm. Bull. 2007, 30, 2345–2351. [Google Scholar] [CrossRef]
- Zelová, H.; Hošek, J. TNF-α signalling and inflammation: Interactions between old acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef]
- Kistowska, M.; Gehrke, S.; Jankovic, D.; Kerl, K.; Fettelschoss, A.; Feldmeyer, L.; Fenini, G.; Kolios, A.; Navarini, A.; Ganceviciene, R.; et al. IL-1β drives inflammatory responses to propionibacterium acnes in vitro and in vivo. J. Investig. Dermatol. 2014, 134, 677–685. [Google Scholar] [CrossRef]
- Satoh, T.; Otsuka, A.; Contassot, E.; French, L.E. The inflammasome and IL-1β: Implications for the treatment of inflammatory diseases. Immunotherapy 2015, 7, 243–254. [Google Scholar] [CrossRef]
- Weissman, B.A.; Gross, S.S. Measurement of NO and NO synthase. Curr. Protoc. Neurosci. 1998, 5, 7–13. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Hwang, D.; Lee, M.; Lee, J.; Cho, S.; Kim, T.-J.; Kim, H.S. Micro-Current Stimulation Suppresses Inflammatory Responses in Peptidoglycan-Treated Raw 264.7 Macrophages and Propionibacterium acnes-Induced Skin Inflammation via TLR2/NF-κB Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 2508. https://doi.org/10.3390/ijms23052508
Lee H, Hwang D, Lee M, Lee J, Cho S, Kim T-J, Kim HS. Micro-Current Stimulation Suppresses Inflammatory Responses in Peptidoglycan-Treated Raw 264.7 Macrophages and Propionibacterium acnes-Induced Skin Inflammation via TLR2/NF-κB Signaling Pathway. International Journal of Molecular Sciences. 2022; 23(5):2508. https://doi.org/10.3390/ijms23052508
Chicago/Turabian StyleLee, Hana, Donghyun Hwang, Minjoo Lee, Jinho Lee, Seungkwan Cho, Tack-Joong Kim, and Han Sung Kim. 2022. "Micro-Current Stimulation Suppresses Inflammatory Responses in Peptidoglycan-Treated Raw 264.7 Macrophages and Propionibacterium acnes-Induced Skin Inflammation via TLR2/NF-κB Signaling Pathway" International Journal of Molecular Sciences 23, no. 5: 2508. https://doi.org/10.3390/ijms23052508
APA StyleLee, H., Hwang, D., Lee, M., Lee, J., Cho, S., Kim, T.-J., & Kim, H. S. (2022). Micro-Current Stimulation Suppresses Inflammatory Responses in Peptidoglycan-Treated Raw 264.7 Macrophages and Propionibacterium acnes-Induced Skin Inflammation via TLR2/NF-κB Signaling Pathway. International Journal of Molecular Sciences, 23(5), 2508. https://doi.org/10.3390/ijms23052508





