Artificial Tears: Biological Role of Their Ingredients in the Management of Dry Eye Disease
Abstract
:1. Introduction
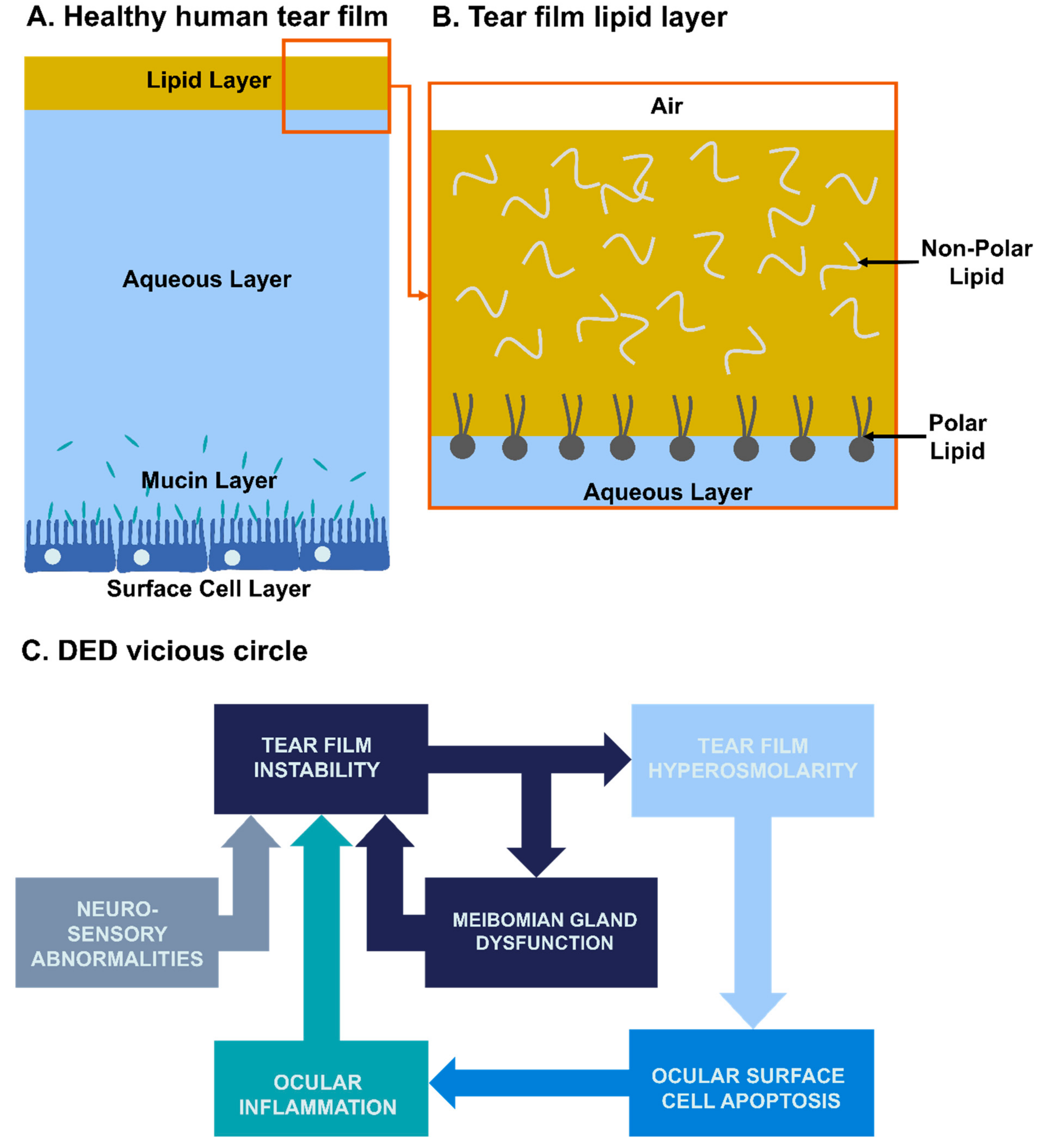
2. Physiopathology of Dry Eye Disease
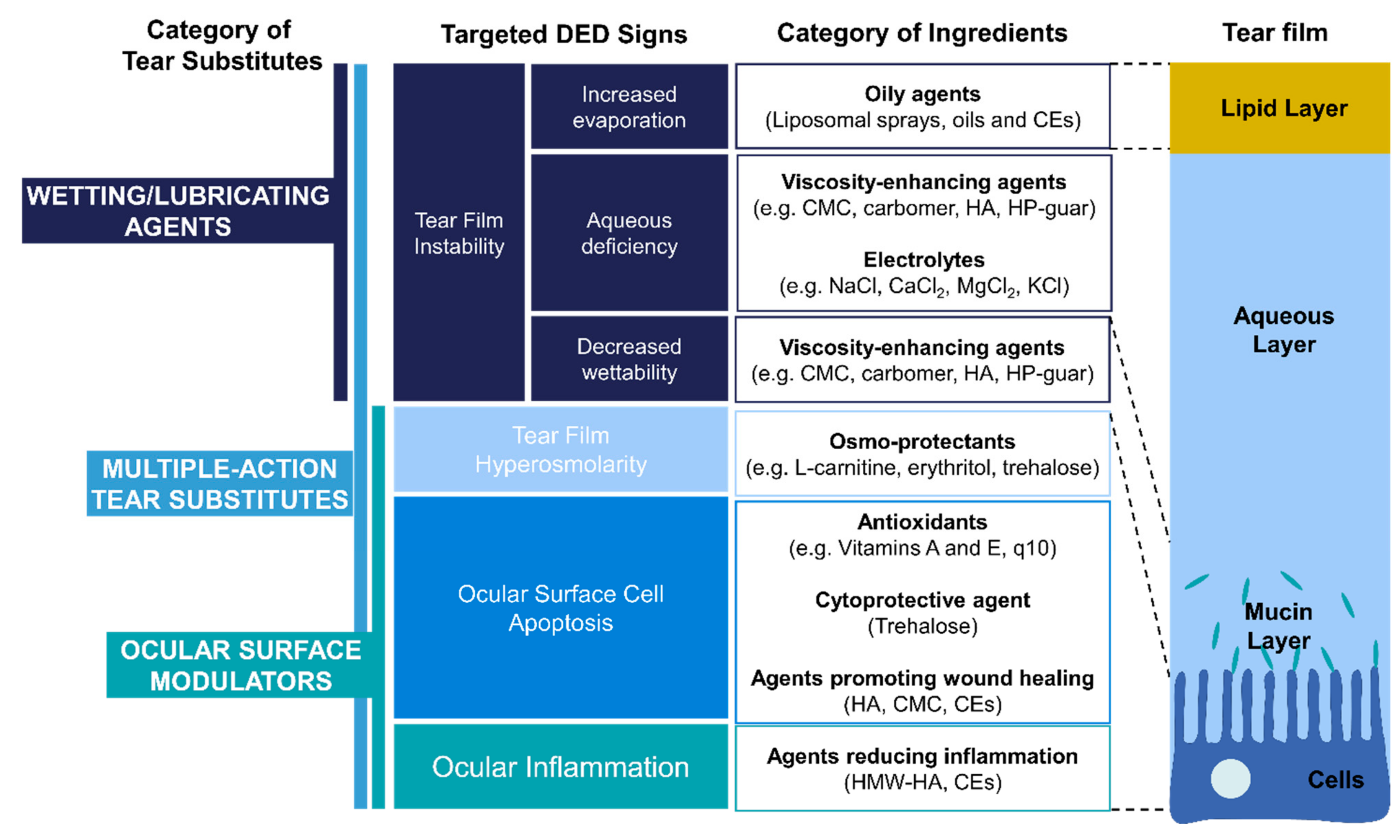
3. Types and Roles of Ingredients Used in Tear substitutes
3.1. Viscosity-Enhancing Agents
| Brand Name | Viscosity-Enhancing Agents | Electrolytes | Others | References of Clinical Studies |
|---|---|---|---|---|
| Refresh® Classic® (Allergan, Irvine, CA, USA) | Povidone, PVA | Sodium chloride | ||
| Bion® Tears (Alcon, Fort Worth, TX, USA) | Dextran 70, HPMC | Calcium chloride, magnesium chloride, potassium chloride, sodium bicarbonate, sodium chloride, zinc chloride | [27] | |
| Refresh® Plus® (Allergan, Irvine, CA, USA) | CMC | Calcium chloride, magnesium chloride, potassium chloride, sodium chloride, sodium lactate | [27] | |
| TheraTears® (Akorn Pharmaceutical, Lake Forest, IL, USA) | CMC | Calcium chloride, magnesium chloride, potassium chloride, sodium bicarbonate, sodium chloride, sodium phosphate, boric acid, sodium borate. | NCT02014922 (data not published) | |
| Systane® (Alcon, Fort Worth, TX, USA) | PEG 400, Propylene Glycol, HP-guar | Calcium chloride, magnesium chloride, potassium chloride, sodium chloride, zinc chloride, boric acid | Preservative: PQ (POLYQUAD®) | [28] |
| Blink® Tears (Johnson & Johnson Vision, Santa Ana, CA, USA) | PEG 400 HA | Calcium chloride, magnesium chloride, potassium chloride, sodium chloride, boric acid, sodium borate | Preservative: Sodium chlorite (OcuPure®) | [29] |
| Name | Type (Source) | Molecular Weight Range |
|---|---|---|
| Carbomer® (polyacrylic acid) | Synthetic polymer | From ~1 kDa to ~3 MDa |
| Carboxymethyl cellulose | Natural PS (cellulose derivative, from plants) | From ~90 to ~250 kDa |
| Dextran | Natural PS (glucose derivative) | From ~3 to ~2000 kDa |
| Hyaluronic acid | Natural exo-PS (from bacterial fermentation) | From ~8 kDa to ~1.8 MDa |
| Hydroxypropyl methylcellulose | Natural PS (cellulose derivative from plants) | From ~10 to ~120 kDa |
| Hydroxypropyl guar | Natural PS (guar gum derivative, from plants) | |
| Polyethylene glycol | Synthetic polymer | From ~1 to ~8 kDa |
| Polyvinyl alcohol | Synthetic polymer | From ~9 to ~200 kDa |
| Povidone (polyvinylpyrrolidone) | Synthetic polymer | From ~10 kDa to ~1 MDa |
| Propylene glycol | Synthetic polymer | From ~1 to ~15 kDa |
| Tamarind seed PS | Natural PS (from tamarind kernel powder) | From ~400 kDa to ~6 MDa |
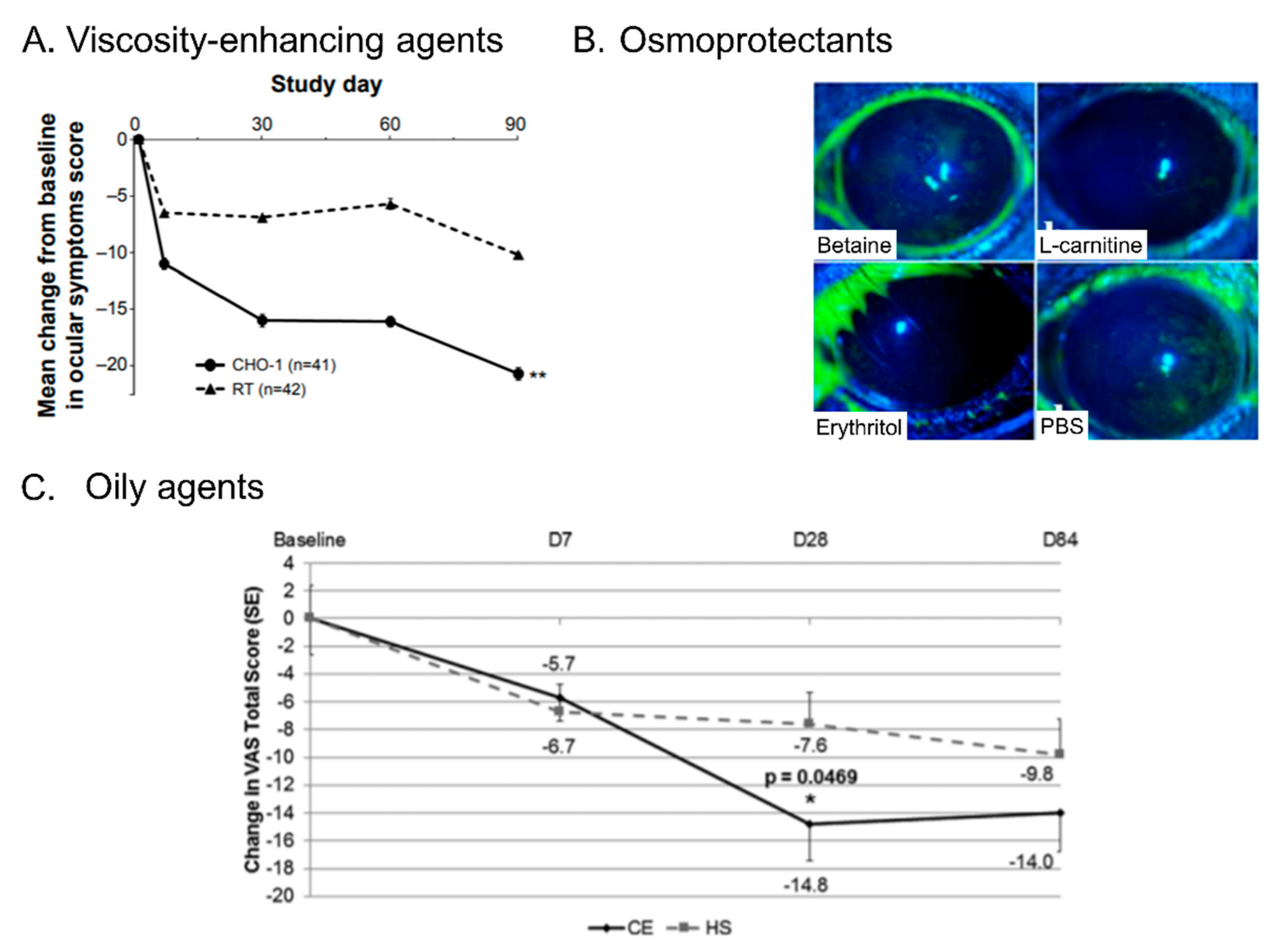
3.2. Electrolytes
3.3. Osmoprotectants
| Brand Name | Osmo-Protectants | Others | References of Clinical Studies |
|---|---|---|---|
| Thealoz® (Thea Laboratories, Clermont-Ferrand, France) | Trehalose |
| [48] |
| Systane® Ultra (Alcon, Fort Worth, TX, USA) | Sorbitol |
| [49] |
| Refresh® Optive® (Allergan, Irvine, CA, USA) | Erythritol, L-carnitine, glycerin |
| |
| Optive Fusion® (Allergan, Irvine, CA, USA) | Erythritol, glycerin |
| [23] |
3.4. Oily Agents and Surfactants
| Brand Name | Lipids (np: Non-Polar/p: Polar) | Surfactants | Others | References of Clinical Studies |
|---|---|---|---|---|
| Soothe® XP (Bausch & Lomb, Rochester, NY, USA) | Mineral oils (np) | Octoxynol 40, polysorbate 80 |
| [63] |
| Systane® Balance® (Alcon, Fort Worth, TX, USA) | Mineral oil (np) Dimyristoyl phosphatidylglycerol (p) | Polyoxyl 40 stearate |
| [60] |
| Cationorm® (Santen, Osaka, Japan) | Mineral oil (np), cetalkonium chloride (p) | Tyloxapol, poloxamer 188, |
| [61] |
| Cationorm® Pro (Santen, Osaka, Japan) | Medium chain triglycerides (np), cetalkonium chloride (p) | Tyloxapol, poloxamer 188, |
| |
| Refresh® Digital® (Allergan, Irvine, CA, USA)) | Castor oil (p) | Polysorbate 80 |
|
| Brand Name | Antioxidants | Others | References of Clinical Studies |
|---|---|---|---|
| VisuXL® (Visufarma, Roma, Italy) | Co-enzyme q10, vitamin E |
| [72] |
| Optrex® Actimist® (Optima Pharmazeutische GmbH, Moosburg an der Isar, Germany) | vitamin A palmitate, vitamin E |
| [74] |
| Neovis Total Multi (Horus Pharma, Saint Laurent du Var, France) | Lipoic acid |
| |
| Lion smile 40 EX a (Lion, Tokyo, Japan) | Vitamin A palminate, Vitamin E Vitamin B6 |
| |
| Sante 12 (Santen, Osaka, Japan) | Vitamin B12 Vitamin B6 |
| [73,75] |
| Rohto Cool 40α (Rohto, Osaka, Japan) | Vitamine E Vitamin B6 |
|
3.5. Agents Promoting Wound Healing and Reducing Inflammation
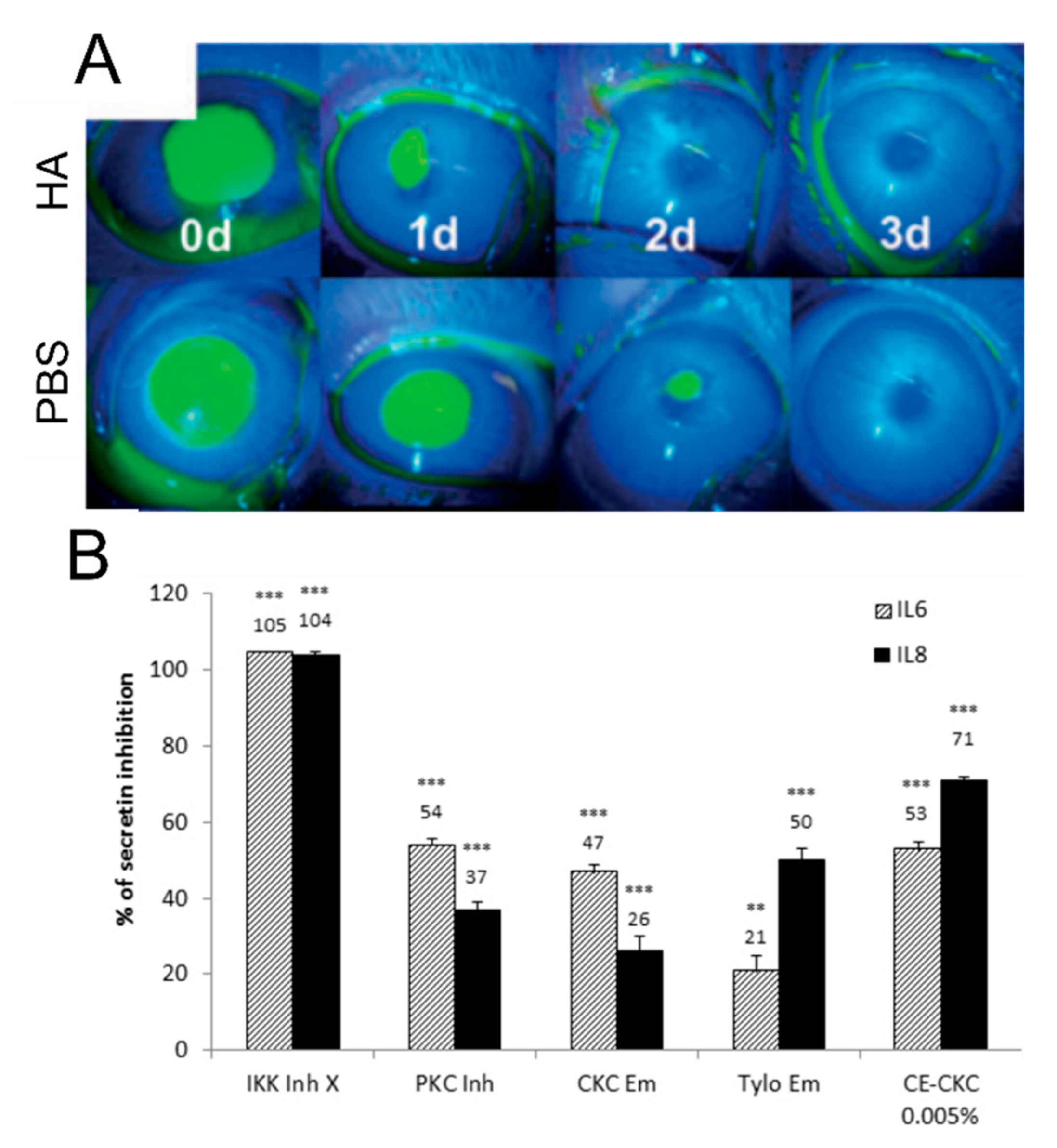
4. Considerations of Ingredients for the Management of DED
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Pucker, A.D.; Ng, S.M.; Nichols, J.J. Over the counter (OTC) artificial tear drops for dry eye syndrome. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EUR-Lex-32017R0745-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2017/745/oj (accessed on 1 July 2021).
- Barabino, S.; Benitez-Del-Castillo, J.M.; Fuchsluger, T.; Labetoulle, M.; Malachkova, N.; Meloni, M.; Utheim, T.P.; Rolando, M. Dry eye disease treatment: The role of tear substitutes, their future, and an updated classification. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8642–8652. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, G.A.; Eftimov, P.; Yokoi, N. Structure-function relationship of tear film lipid layer: A contemporary perspective. Exp. Eye Res. 2017, 163, 17–28. [Google Scholar] [CrossRef]
- Cwiklik, L. Tear film lipid layer: A molecular level view. Biochim. Biophys. Acta BBA-Biomembr. 2016, 1858, 2421–2430. [Google Scholar] [CrossRef]
- Willcox, M.D.P.; Argüeso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef] [Green Version]
- Rolando, M.; Zierhut, M. The ocular surface and tear film and their dysfunction in dry eye disease. Surv. Ophthalmol. 2001, 45 (Suppl. S2), S203–S210. [Google Scholar] [CrossRef]
- Baudouin, C. A new approach for better comprehension of diseases of the ocular surface. J. Fr. Ophtalmol. 2007, 30, 239–246. [Google Scholar] [CrossRef]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Lemp, M.A.; Crews, L.A.; Bron, A.J.; Foulks, G.N.; Sullivan, B.D. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: A retrospective study. Cornea 2012, 31, 472–478. [Google Scholar] [CrossRef]
- Teo, C.H.Y.; Ong, H.S.; Liu, Y.-C.; Tong, L. Meibomian gland dysfunction is the primary determinant of dry eye symptoms: Analysis of 2346 patients. Ocul. Surf. 2020, 18, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Yokoi, N.; Watanabe, H.; Dogru, M.; Kojima, T.; Yamada, M.; Kinoshita, S.; Kim, H.-M.; Tchah, H.-W.; Hyon, J.Y.; et al. A New Perspective on Dry Eye Classification: Proposal by the Asia Dry Eye Society. Eye Contact Lens 2020, 46, S2–S13. [Google Scholar] [CrossRef] [PubMed]
- CFR-Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=349&showFR=1 (accessed on 23 April 2021).
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-Del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II Management and Therapy Report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef] [PubMed]
- Bruix, A.; Adán, A.; Casaroli-Marano, R.P. Efficacy of sodium carboxymethylcellulose in the treatment of dry eye syndrome. Arch. Soc. Espanola Oftalmol. 2006, 81, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, P.; Craig, J.P.; Rupenthal, I.D. Formulation Considerations for the Management of Dry Eye Disease. Pharmaceutics 2021, 13, 207. [Google Scholar] [CrossRef] [PubMed]
- Eftimov, P.; Yokoi, N.; Melo, A.M.; Daull, P.; Georgiev, G.A. Interactions of Meibum and Tears with Mucomimetic Polymers: A Hint towards the Interplay between the Layers of the Tear Film. Int. J. Mol. Sci. 2021, 22, 2747. [Google Scholar] [CrossRef]
- Dhar, P.; McAuley, J. The Role of the Cell Surface Mucin MUC1 as a Barrier to Infection and Regulator of Inflammation. Front. Cell. Infect. Microbiol. 2019, 9, 117. [Google Scholar] [CrossRef]
- Jumelle, C.; Gholizadeh, S.; Annabi, N.; Dana, R. Advances and limitations of drug delivery systems formulated as eye drops. J. Control. Release Off. J. Control. Release Soc. 2020, 321, 1–22. [Google Scholar] [CrossRef]
- Rolando, M.; Valente, C. Establishing the tolerability and performance of tamarind seed polysaccharide (TSP) in treating dry eye syndrome: Results of a clinical study. BMC Ophthalmol. 2007, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Doughty, M.J.; Glavin, S. Efficacy of different dry eye treatments with artificial tears or ocular lubricants: A systematic review. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. Optom. 2009, 29, 573–583. [Google Scholar] [CrossRef]
- Simmons, P.A.; Liu, H.; Carlisle-Wilcox, C.; Vehige, J.G. Efficacy and safety of two new formulations of artificial tears in subjects with dry eye disease: A 3-month, multicenter, active-controlled, randomized trial. Clin. Ophthalmol. Auckl. NZ 2015, 9, 665–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, E.; Kao, W.W.Y.; Ogundele, A. Impact of Hyaluronic Acid-Containing Artificial Tear Products on Reepithelialization in an In Vivo Corneal Wound Model. J. Ocul. Pharmacol. Ther. 2018, 34, 360–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangarajan, R.; Kraybill, B.; Ogundele, A.; Ketelson, H.A. Effects of a Hyaluronic Acid/Hydroxypropyl Guar Artificial Tear Solution on Protection, Recovery, and Lubricity in Models of Corneal Epithelium. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2015, 31, 491–497. [Google Scholar] [CrossRef]
- Ng, A.; Keech, A.; Jones, L. Tear osmolarity changes after use of hydroxypropyl-guar-based lubricating eye drops. Clin. Ophthalmol. Auckl. NZ 2018, 12, 695. [Google Scholar] [CrossRef] [Green Version]
- Donshik, P.C.; Nelson, J.D.; Abelson, M.; McCulley, J.P.; Beasley, C.; Laibovitz, R.A. Effectiveness of BION tears, Cellufresh, Aquasite, and Refresh Plus for moderate to severe dry eye. Adv. Exp. Med. Biol. 1998, 438, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, A.; Berra, M.; Trédicce, J.; Berra, A. Efficacy of polyethylene glycol–propylene glycol-based lubricant eye drops in reducing squamous metaplasia in patients with dry eye disease. Clin. Ophthalmol. Auckl. NZ 2018, 12, 1237–1243. [Google Scholar] [CrossRef] [Green Version]
- Huth, S.; Tran, D.; Skotnitsky, C.; Lasswell, L.; Mahmud, P.; Kim, T. Wavelength-Dependent Optical Interferometry Measurements of Change in Thickness of Apical Corneal Tear Film Following Eye Drop Instillation. Investig. Ophthalmol. Vis. Sci. 2008, 49, 108. [Google Scholar]
- Chen, W.; Zhang, X.; Li, J.; Wang, Y.; Chen, Q.; Hou, C.; Garrett, Q. Efficacy of osmoprotectants on prevention and treatment of murine dry eye. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6287–6297. [Google Scholar] [CrossRef] [Green Version]
- Robert, P.-Y.; Cochener, B.; Amrane, M.; Ismail, D.; Garrigue, J.-S.; Pisella, P.-J.; Baudouin, C. Efficacy and safety of a cationic emulsion in the treatment of moderate to severe dry eye disease: A randomized controlled study. Eur. J. Ophthalmol. 2016, 26, 546–555. [Google Scholar] [CrossRef]
- Li, Y.; Cui, L.; Lee, H.S.; Kang, Y.S.; Choi, W.; Yoon, K.C. Comparison of 0.3% Hypotonic and Isotonic Sodium Hyaluronate Eye Drops in the Treatment of Experimental Dry Eye. Curr. Eye Res. 2017, 42, 1108–1114. [Google Scholar] [CrossRef]
- Gilbard, J.P.; Rossi, S.R. An electrolyte-based solution that increases corneal glycogen and conjunctival goblet-cell density in a rabbit model for keratoconjunctivitis sicca. Ophthalmology 1992, 99, 600–604. [Google Scholar] [CrossRef]
- Troiano, P.; Monaco, G. Effect of hypotonic 0.4% hyaluronic acid drops in dry eye patients: A cross-over study. Cornea 2008, 27, 1126–1130. [Google Scholar] [CrossRef] [PubMed]
- Ryan, G.; Fain, J.M.; Lovelace, C.; Gelotte, K.M. Effectiveness of ophthalmic solution preservatives: A comparison of latanoprost with 0.02% benzalkonium chloride and travoprost with the sofZia preservative system. BMC Ophthalmol. 2011, 11, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novartis Pharmaceuticals Corporation TRAVATAN Z Product Information. 2020.
- Bachman, W.G.; Wilson, G. Essential ions for maintenance of the corneal epithelial surface. Investig. Ophthalmol. Vis. Sci. 1985, 26, 1484–1488. [Google Scholar] [PubMed]
- Baudouin, C.; Aragona, P.; Messmer, E.M.; Tomlinson, A.; Calonge, M.; Boboridis, K.G.; Akova, Y.A.; Geerling, G.; Labetoulle, M.; Rolando, M. Role of Hyperosmolarity in the Pathogenesis and Management of Dry Eye Disease: Proceedings of the OCEAN Group Meeting. Ocul. Surf. 2013, 11, 246–258. [Google Scholar] [CrossRef] [Green Version]
- Corrales, R.M.; Luo, L.; Chang, E.Y.; Pflugfelder, S.C. Effects of osmoprotectants on hyperosmolar stress in cultured human corneal epithelial cells. Cornea 2008, 27, 574–579. [Google Scholar] [CrossRef]
- López-Cano, J.J.; González-Cela-Casamayor, M.A.; Andrés-Guerrero, V.; Herrero-Vanrell, R.; Benítez-Del-Castillo, J.M.; Molina-Martínez, I.T. Combined hyperosmolarity and inflammatory conditions in stressed human corneal epithelial cells and macrophages to evaluate osmoprotective agents as potential DED treatments. Exp. Eye Res. 2021, 211, 108723. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, X.; Liu, M.; Zhang, J.; Ye, Y.; Lin, Y.; Luyckx, J.; Qu, J. Trehalose protects against ocular surface disorders in experimental murine dry eye through suppression of apoptosis. Exp. Eye Res. 2009, 89, 311–318. [Google Scholar] [CrossRef]
- Matsuo, T.; Tsuchida, Y.; Morimoto, N. Trehalose eye drops in the treatment of dry eye syndrome. Ophthalmology 2002, 109, 2024–2029. [Google Scholar] [CrossRef]
- Laihia, J.; Kaarniranta, K. Trehalose for Ocular Surface Health. Biomolecules 2020, 10, 809. [Google Scholar] [CrossRef]
- Hernandez, E.; Taisne, C.; Lussignol, M.; Esclatine, A.; Labetoulle, M. Commercially Available Eye Drops Containing Trehalose Protect Against Dry Conditions via Autophagy Induction. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2021, 37, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, D.; Chen, X.; Bian, F.; Qin, W.; Gao, N.; Xiao, Y.; Li, J.; Pflugfelder, S.C.; Li, D.-Q. Trehalose Induces Autophagy Against Inflammation by Activating TFEB Signaling Pathway in Human Corneal Epithelial Cells Exposed to Hyperosmotic Stress. Investig. Ophthalmol. Vis. Sci. 2020, 61, 26. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, J.; Baudouin, C. Trehalose: An intriguing disaccharide with potential for medical application in ophthalmology. Clin. Ophthalmol. Auckl. NZ 2011, 5, 577–581. [Google Scholar] [CrossRef] [Green Version]
- Deng, R.; Su, Z.; Hua, X.; Zhang, Z.; Li, D.-Q.; Pflugfelder, S.C. Osmoprotectants suppress the production and activity of matrix metalloproteinases induced by hyperosmolarity in primary human corneal epithelial cells. Mol. Vis. 2014, 20, 1243–1252. [Google Scholar] [PubMed]
- Fondi, K.; Wozniak, P.A.; Schmidl, D.; Bata, A.M.; Witkowska, K.J.; Popa-Cherecheanu, A.; Schmetterer, L.; Garhöfer, G. Effect of Hyaluronic Acid/Trehalose in Two Different Formulations on Signs and Symptoms in Patients with Moderate to Severe Dry Eye Disease. J. Ophthalmol. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- McDonald, M.; Schachet, J.L.; Lievens, C.W.; Kern, J.R. Systane® ultra lubricant eye drops for treatment of contact lens-related dryness. Eye Contact Lens 2014, 40, 106–110. [Google Scholar] [CrossRef]
- Rabinovich-Guilatt, L.; Couvreur, P.; Lambert, G.; Dubernet, C. Cationic vectors in ocular drug delivery. J. Drug Target. 2004, 12, 623–633. [Google Scholar] [CrossRef]
- López-Cano, J.J.; González-Cela-Casamayor, M.A.; Andrés-Guerrero, V.; Herrero-Vanrell, R.; Molina-Martínez, I.T. Liposomes as vehicles for topical ophthalmic drug delivery and ocular surface protection. Expert Opin. Drug Deliv. 2021, 18, 819–847. [Google Scholar] [CrossRef]
- Craig, J.P.; Purslow, C.; Murphy, P.J.; Wolffsohn, J.S.W. Effect of a liposomal spray on the pre-ocular tear film. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2010, 33, 83–87. [Google Scholar] [CrossRef] [Green Version]
- Acar, D.; Molina-Martínez, I.T.; Gómez-Ballesteros, M.; Guzmán-Navarro, M.; Benítez-Del-Castillo, J.M.; Herrero-Vanrell, R. Novel liposome-based and in situ gelling artificial tear formulation for dry eye disease treatment. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2018, 41, 93–96. [Google Scholar] [CrossRef]
- Gokul, A.; Wang, M.T.M.; Craig, J.P. Tear lipid supplement prophylaxis against dry eye in adverse environments. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2018, 41, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.; Tomlinson, A.; Pearce, E.I.; Simmons, P.A. Effect of an oil-in-water emulsion on the tear physiology of patients with mild to moderate dry eye. Cornea 2007, 26, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Lallemand, F.; Daull, P.; Benita, S.; Buggage, R.; Garrigue, J.S. Successfully improving ocular drug delivery using the cationic nanoemulsion, novasorb. J. Drug Deliv. 2012, 26, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Daull, P.; Amrane, M.; Ismail, D.; Georgiev, G.; Cwiklik, L.; Baudouin, C.; Leonardi, A.; Garhofer, G.; Garrigue, J.-S. Cationic Emulsion-Based Artificial Tears as a Mimic of Functional Healthy Tear Film for Restoration of Ocular Surface Homeostasis in Dry Eye Disease. J. Ocul. Pharmacol. Ther. 2020, 36, 355–365. [Google Scholar] [CrossRef]
- Georgiev, G.A.; Yokoi, N.; Nencheva, Y.; Peev, N.; Daull, P. Surface Chemistry Interactions of Cationorm with Films by Human Meibum and Tear Film Compounds. Int. J. Mol. Sci. 2017, 18, 1558. [Google Scholar] [CrossRef] [Green Version]
- Pucker, A.D.; Haworth, K.M. The presence and significance of polar meibum and tear lipids. Ocul. Surf. 2015, 13, 26–42. [Google Scholar] [CrossRef]
- Benelli, U. Systane® lubricant eye drops in the management of ocular dryness. Clin. Ophthalmol. Auckl. NZ 2011, 5, 783–790. [Google Scholar] [CrossRef] [Green Version]
- Amrane, M.; Creuzot-Garcher, C.; Robert, P.-Y.; Ismail, D.; Garrigue, J.-S.; Pisella, P.-J.; Baudouin, C. Ocular tolerability and efficacy of a cationic emulsion in patients with mild to moderate dry eye disease—A randomised comparative study. J. Fr. Ophtalmol. 2014, 37, 589–598. [Google Scholar] [CrossRef]
- Garrigue, J.-S.; Amrane, M.; Faure, M.-O.; Holopainen, J.M.; Tong, L. Relevance of Lipid-Based Products in the Management of Dry Eye Disease. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2017, 33, 647–661. [Google Scholar] [CrossRef]
- Korb, D.R.; Scaffidi, R.C.; Greiner, J.V.; Kenyon, K.R.; Herman, J.P.; Blackie, C.A.; Glonek, T.; Case, C.L.; Finnemore, V.M.; Douglass, T. The effect of two novel lubricant eye drops on tear film lipid layer thickness in subjects with dry eye symptoms. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2005, 82, 594–601. [Google Scholar] [CrossRef] [Green Version]
- Augustin, A.J.; Spitznas, M.; Kaviani, N.; Meller, D.; Koch, F.H.; Grus, F.; Göbbels, M.J. Oxidative reactions in the tear fluid of patients suffering from dry eyes. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 1995, 233, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Su, Z.; Deng, R.; Lin, J.; Li, D.-Q.; Pflugfelder, S.C. Effects of L-carnitine, Erythritol and Betaine on Pro-inflammatory Markers in Primary Human Corneal Epithelial Cells Exposed to Hyperosmotic Stress. Curr. Eye Res. 2015, 40, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Fidilio, A.; Platania, C.B.M.; Geraci, F.; Lazzara, F.; Drago, F. Antioxidant and Osmoprotecting Activity of Taurine in Dry Eye Models. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2018, 34, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.C.; Choi, J.-S.; Joo, C.-K. A comparison of vitamin a and cyclosporine a 0.05% eye drops for treatment of dry eye syndrome. Am. J. Ophthalmol. 2009, 147, 206–213.e3. [Google Scholar] [CrossRef]
- Gomes, J.A.P.; Azar, D.T.; Baudouin, C.; Efron, N.; Hirayama, M.; Horwath-Winter, J.; Kim, T.; Mehta, J.S.; Messmer, E.M.; Pepose, J.S.; et al. TFOS DEWS II iatrogenic report. Ocul. Surf. 2017, 15, 511–538. [Google Scholar] [CrossRef]
- Stopyra, W. The impact of alpha lipoic acid eyedrops on tear break-up time in patients with dry eye disease. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2090. [Google Scholar]
- Ajith, T.A. Alpha-lipoic acid: A possible pharmacological agent for treating dry eye disease and retinopathy in diabetes. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1883–1890. [Google Scholar] [CrossRef]
- Fogagnolo, P.; De Cilla’, S.; Alkabes, M.; Sabella, P.; Rossetti, L. A Review of Topical and Systemic Vitamin Supplementation in Ocular Surface Diseases. Nutrients 2021, 13, 1998. [Google Scholar] [CrossRef]
- Postorino, E.I.; Rania, L.; Aragona, E.; Mannucci, C.; Alibrandi, A.; Calapai, G.; Puzzolo, D.; Aragona, P. Efficacy of eyedrops containing cross-linked hyaluronic acid and coenzyme Q10 in treating patients with mild to moderate dry eye. Eur. J. Ophthalmol. 2018, 28, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Macri, A.; Scanarotti, C.; Bassi, A.M.; Giuffrida, S.; Sangalli, G.; Traverso, C.E.; Iester, M. Evaluation of oxidative stress levels in the conjunctival epithelium of patients with or without dry eye, and dry eye patients treated with preservative-free hyaluronic acid 0.15% and vitamin B12 eye drops. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 2015, 253, 425–430. [Google Scholar] [CrossRef]
- Pult, H.; Gill, F.; Riede-Pult, B.H. Effect of three different liposomal eye sprays on ocular comfort and tear film. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2012, 35, 203–207; quiz 243–244. [Google Scholar] [CrossRef] [PubMed]
- Fogagnolo, P.; Melardi, E.; Tranchina, L.; Rossetti, L. Topical citicoline and vitamin B12 versus placebo in the treatment of diabetes-related corneal nerve damage: A randomized double-blind controlled trial. BMC Ophthalmol. 2020, 20, 315. [Google Scholar] [CrossRef] [PubMed]
- Brignole-Baudouin, F.; Riancho, L.; Liang, H.; Baudouin, C. Comparative In Vitro Toxicology Study of Travoprost Polyquad-preserved, Travoprost BAK-preserved, and Latanoprost BAK-preserved Ophthalmic Solutions on Human Conjunctival Epithelial Cells. Curr. Eye Res. 2011, 36, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Schrage, N.; Frentz, M.; Spoeler, F. The Ex Vivo Eye Irritation Test (EVEIT) in evaluation of artificial tears: Purite-preserved versus unpreserved eye drops. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 2012, 250, 1333–1340. [Google Scholar] [CrossRef]
- Nakamura, M.; Hikida, M.; Nakano, T. Concentration and molecular weight dependency of rabbit corneal epithelial wound healing on hyaluronan. Curr. Eye Res. 1992, 11, 981–986. [Google Scholar] [CrossRef]
- Yang, G.; Espandar, L.; Mamalis, N.; Prestwich, G.D. A cross-linked hyaluronan gel accelerates healing of corneal epithelial abrasion and alkali burn injuries in rabbits. Vet. Ophthalmol. 2010, 13, 144–150. [Google Scholar] [CrossRef]
- Peach, R.J.; Hollenbaugh, D.; Stamenkovic, I.; Aruffo, A. Identification of hyaluronic acid binding sites in the extracellular domain of CD44. J. Cell Biol. 1993, 122, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Garrett, Q.; Simmons, P.A.; Xu, S.; Vehige, J.; Zhao, Z.; Ehrmann, K.; Willcox, M. Carboxymethylcellulose binds to human corneal epithelial cells and is a modulator of corneal epithelial wound healing. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1559–1567. [Google Scholar] [CrossRef] [Green Version]
- Campo, G.M.; Avenoso, A.; Nastasi, G.; Micali, A.; Prestipino, V.; Vaccaro, M.; D’Ascola, A.; Calatroni, A.; Campo, S. Hyaluronan reduces inflammation in experimental arthritis by modulating TLR-2 and TLR-4 cartilage expression. Biochim. Biophys. Acta BBA - Mol. Basis Dis. 2011, 1812, 1170–1181. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.; Liang, J.; Fan, J.; Yu, S.; Chen, S.; Luo, Y.; Prestwich, G.D.; Mascarenhas, M.M.; Garg, H.G.; Quinn, D.A.; et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat. Med. 2005, 11, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Nagata, T.; Kudo, H.; Müller-Lierheim, W.G.K.; van Setten, G.-B.; Dogru, M.; Tsubota, K. The Effects of High Molecular Weight Hyaluronic Acid Eye Drop Application in Environmental Dry Eye Stress Model Mice. Int. J. Mol. Sci. 2020, 21, 3516. [Google Scholar] [CrossRef] [PubMed]
- Barabino, S.; De Servi, B.; Aragona, S.; Manenti, D.; Meloni, M. Efficacy of a New Ocular Surface Modulator in Restoring Epithelial Changes in an In Vitro Model of Dry Eye Syndrome. Curr. Eye Res. 2017, 42, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Meloni, M.; Carriero, F.; Ceriotti, L.; Barabino, S. Development of a Novel In Vitro Immuno-Competent Model of Dry Eye Disease and Its Use to Evaluate the Efficacy of an Ocular Surface Modulator. Ocul. Immunol. Inflamm. 2021, 1–9. [Google Scholar] [CrossRef]
- Liang, H.; Baudouin, C.; Daull, P.; Garrigue, J.-S.; Buggage, R.; Brignole-Baudouin, F. In vitro and in vivo evaluation of a preservative-free cationic emulsion of latanoprost in corneal wound healing models. Cornea 2012, 31, 1319–1329. [Google Scholar] [CrossRef]
- Daull, P.; Guenin, S.; Hamon de Almeida, V.; Garrigue, J.-S. Anti-inflammatory activity of CKC-containing cationic emulsion eye drop vehicles. Mol. Vis. 2018, 24, 459–470. [Google Scholar]
- Yokoi, N.; Georgiev, G.A. Tear Film–Oriented Diagnosis and Tear Film–Oriented Therapy for Dry Eye Based on Tear Film Dynamics. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES13–DES22. [Google Scholar] [CrossRef]
- Dubey, A.; Patel, V.D.; Walt, J.G.; Fox, K.M.; Schwartz, M. Treatment Patterns and Satisfaction in Dry Eye Patients in Real-World Practice Setting. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6258. [Google Scholar]
- Kathuria, A.; Shamloo, K.; Jhanji, V.; Sharma, A. Categorization of Marketed Artificial Tear Formulations Based on Their Ingredients: A Rational Approach for Their Use. J. Clin. Med. 2021, 10, 1289. [Google Scholar] [CrossRef]
- Baudouin, C.; Figueiredo, F.C.; Messmer, E.M.; Ismail, D.; Amrane, M.; Garrigue, J.-S.; Bonini, S.; Leonardi, A. A randomized study of the efficacy and safety of 0.1% cyclosporine A cationic emulsion in treatment of moderate to severe dry eye. Eur. J. Ophthalmol. 2017, 27, 520–530. [Google Scholar] [CrossRef] [Green Version]
- Bucolo, C.; Fidilio, A.; Fresta, C.G.; Lazzara, F.; Platania, C.B.M.; Cantarella, G.; Di Benedetto, G.; Burgaletto, C.; Bernardini, R.; Piazza, C.; et al. Ocular Pharmacological Profile of Hydrocortisone in Dry Eye Disease. Front. Pharmacol. 2019, 10, 1240. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labetoulle, M.; Benitez-del-Castillo, J.M.; Barabino, S.; Herrero Vanrell, R.; Daull, P.; Garrigue, J.-S.; Rolando, M. Artificial Tears: Biological Role of Their Ingredients in the Management of Dry Eye Disease. Int. J. Mol. Sci. 2022, 23, 2434. https://doi.org/10.3390/ijms23052434
Labetoulle M, Benitez-del-Castillo JM, Barabino S, Herrero Vanrell R, Daull P, Garrigue J-S, Rolando M. Artificial Tears: Biological Role of Their Ingredients in the Management of Dry Eye Disease. International Journal of Molecular Sciences. 2022; 23(5):2434. https://doi.org/10.3390/ijms23052434
Chicago/Turabian StyleLabetoulle, Marc, Jose Manuel Benitez-del-Castillo, Stefano Barabino, Rocio Herrero Vanrell, Philippe Daull, Jean-Sebastien Garrigue, and Maurizio Rolando. 2022. "Artificial Tears: Biological Role of Their Ingredients in the Management of Dry Eye Disease" International Journal of Molecular Sciences 23, no. 5: 2434. https://doi.org/10.3390/ijms23052434
APA StyleLabetoulle, M., Benitez-del-Castillo, J. M., Barabino, S., Herrero Vanrell, R., Daull, P., Garrigue, J.-S., & Rolando, M. (2022). Artificial Tears: Biological Role of Their Ingredients in the Management of Dry Eye Disease. International Journal of Molecular Sciences, 23(5), 2434. https://doi.org/10.3390/ijms23052434






