Chitosan Functionalized with Carboxyl Groups as a Recyclable Biomaterial for the Adsorption of Cu (II) and Zn (II) Ions in Aqueous Media
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Chitosan Modified by Monochloroacetic Acid (CTS-CAA)
- 1.
- The cross-linking of chitosan by glutaraldehyde to obtain (CTS-GL). To this purpose, 4 g of chitosan were breaking down into acetic acid solution (200 mL; 5 M). The solution was magnetically stirred until it became homogenous. Then, we added 2 mL of glutaraldehyde solution (50%). The mixture was kept at gentle heat (40 °C) for 2 h. The obtained material was filtered and was washed using ethanol and allowed to dry for using in the second step. The obtained material was reported as (CTS-GL) [37,38].
- 2.
- The reaction of (CTS-GL) with epichlorohydrin to yield (CTS-EC). 15 mL of epichlorohydrin were mixed with 200 mL of (acetone/water; volume ratio 1:1). Then, 4 g of (CTS-GL) were added, which was previously mixed in 150 mL of isopropanol for 30 min under stirring conditions. The obtained mixture allowed for refluxing for 24 h at 65 °C. The obtained material was filtered and was washed using ethanol and allowed to dry for using in the third step. This material was reported as (CTS-EC) [39,40].
- 3.
- The reaction of (CTS-EC) with diethylene triamine to obtain (CTS-DET). For this purpose, 4 g of (CTS-EC) were mixed in 200 mL of (ethanol/water; volume ratio 1:1) under stirring at 25 °C. Then, 10 mL of diethylene triamine were introduced to this reaction media, and the mixture was allowed to react at 65 °C for 12 h under stirring. The obtained material was filtered and was washed using ethanol and allowed to dry for using in the fourth step. The resulting material was reported as (CTS-DET).
- 4.
- The reaction of (CTS-DET) with monochloroacetic acid to synthesize (CTS-CAA). In this step, 4 g of (CTS-DET) were mixed in 55 mL of solvent (44 mL of isopropyl alcohol and 11 mL of H2O). Then, 6 g of monochloroacetic acid (previously dissolved in 8 mL of isopropanol) were slowly mixed with the media components, and the reaction was continuously run in a water bath under stirring at 60 °C for 5 h. The reaction was stopped by adding 150 mL of diluted ethanol (60%). The obtained material was filtered and was washed using ethanol and allowed to dry for using as an effective sorbent. The product was reported as (CTS-CAA).
2.3. Characterization
2.4. Sorption and Desorption Experiments
3. Results and Discussion
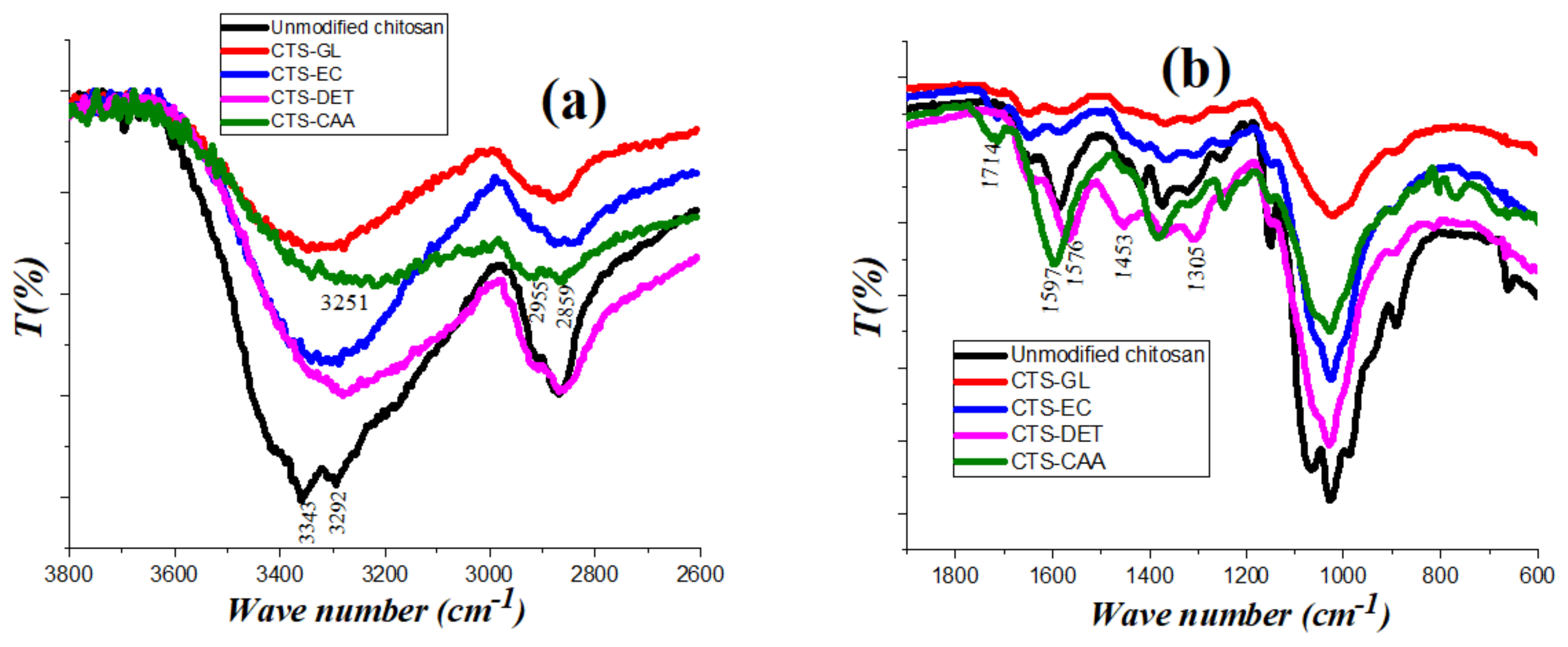
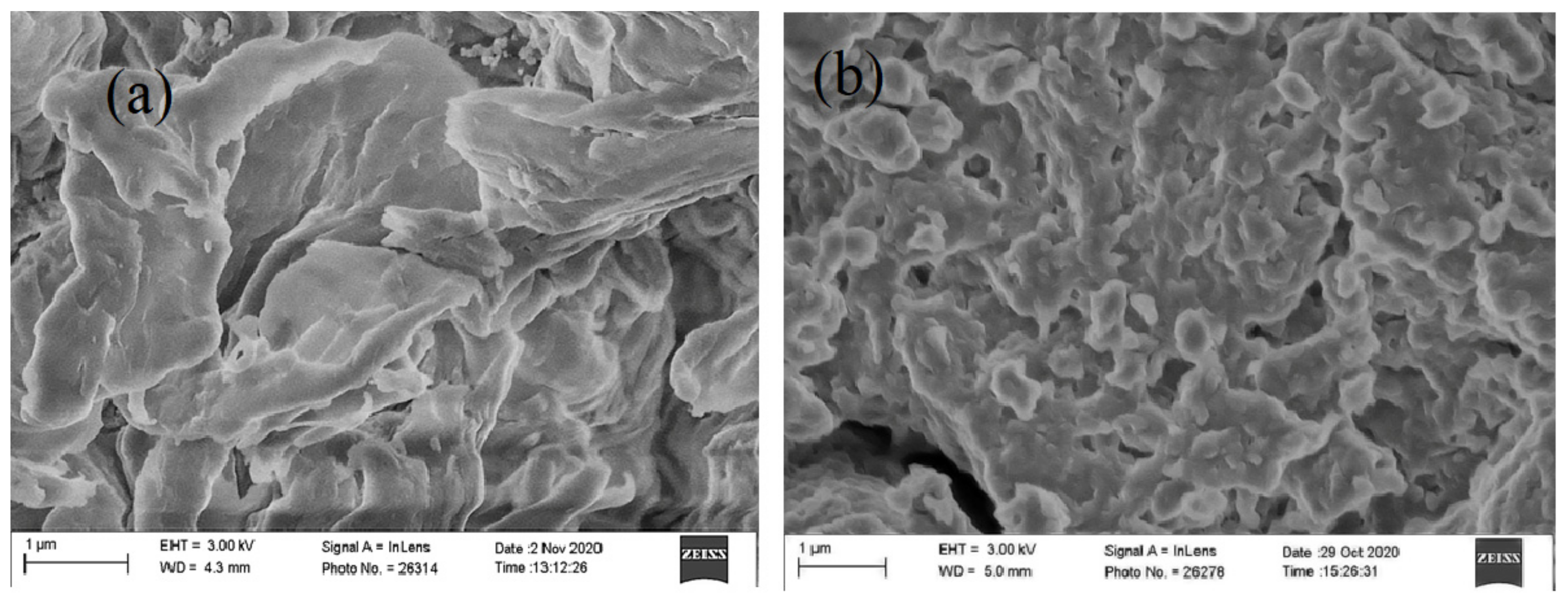
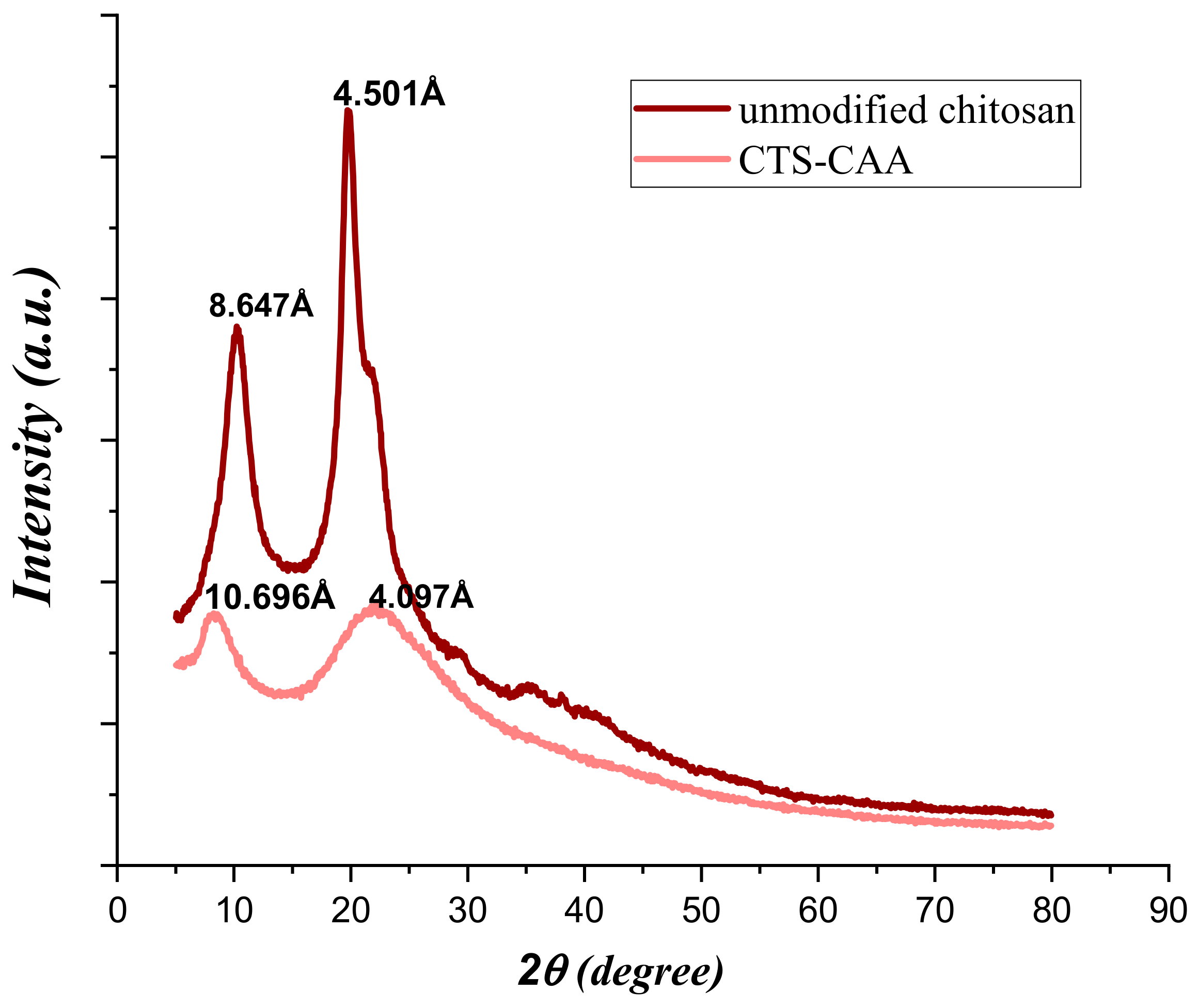
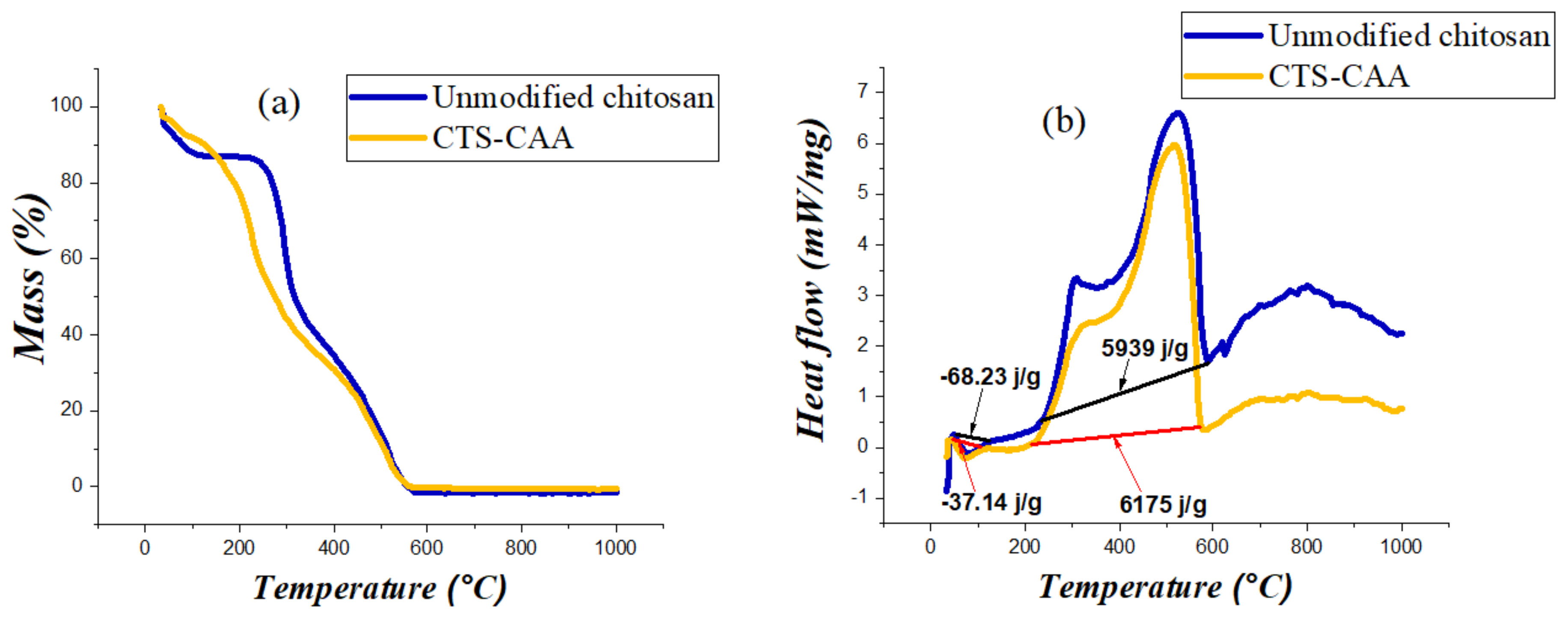
3.1. Characterization of Chitosan Modified by Monochloroacetic Acid
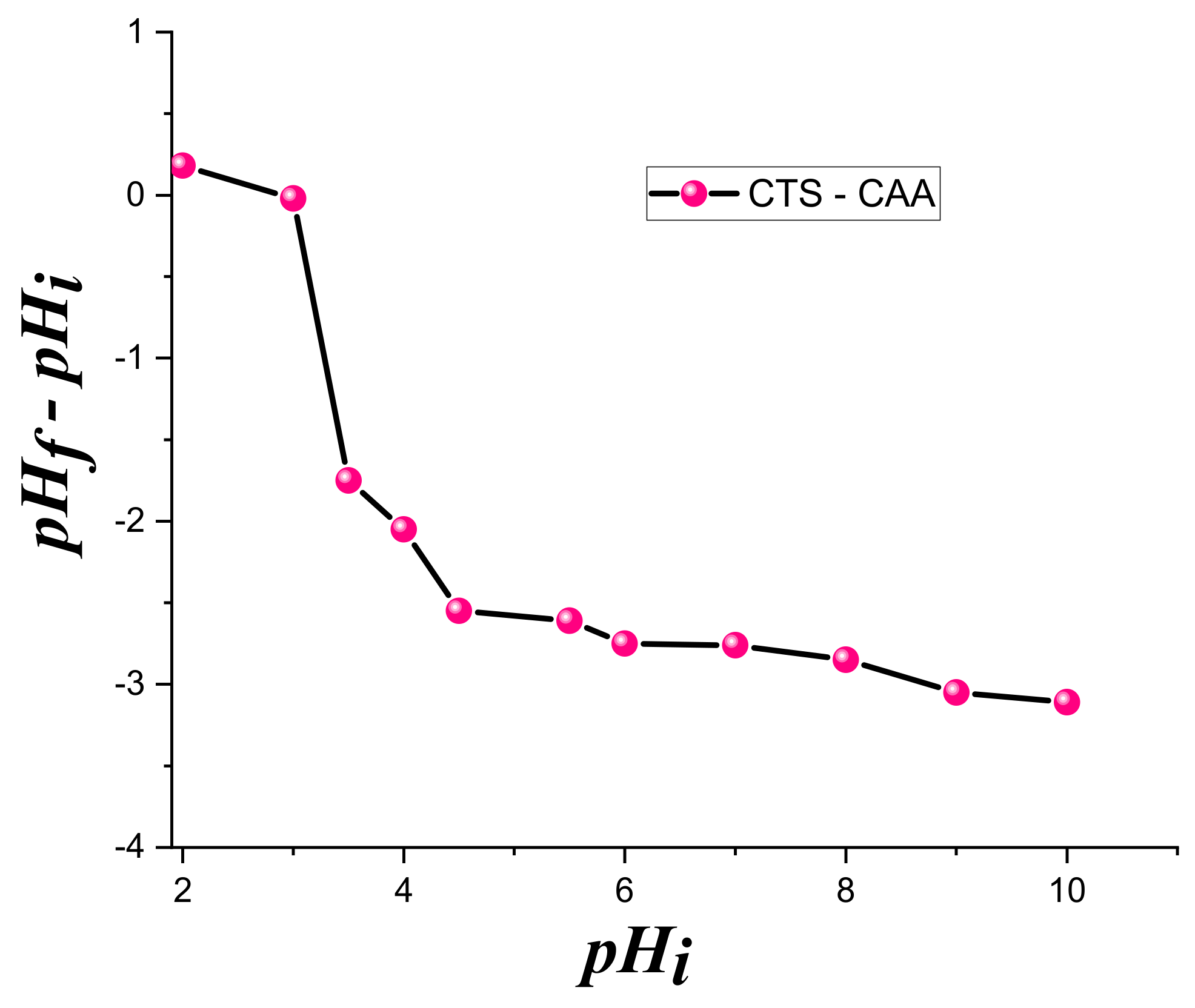
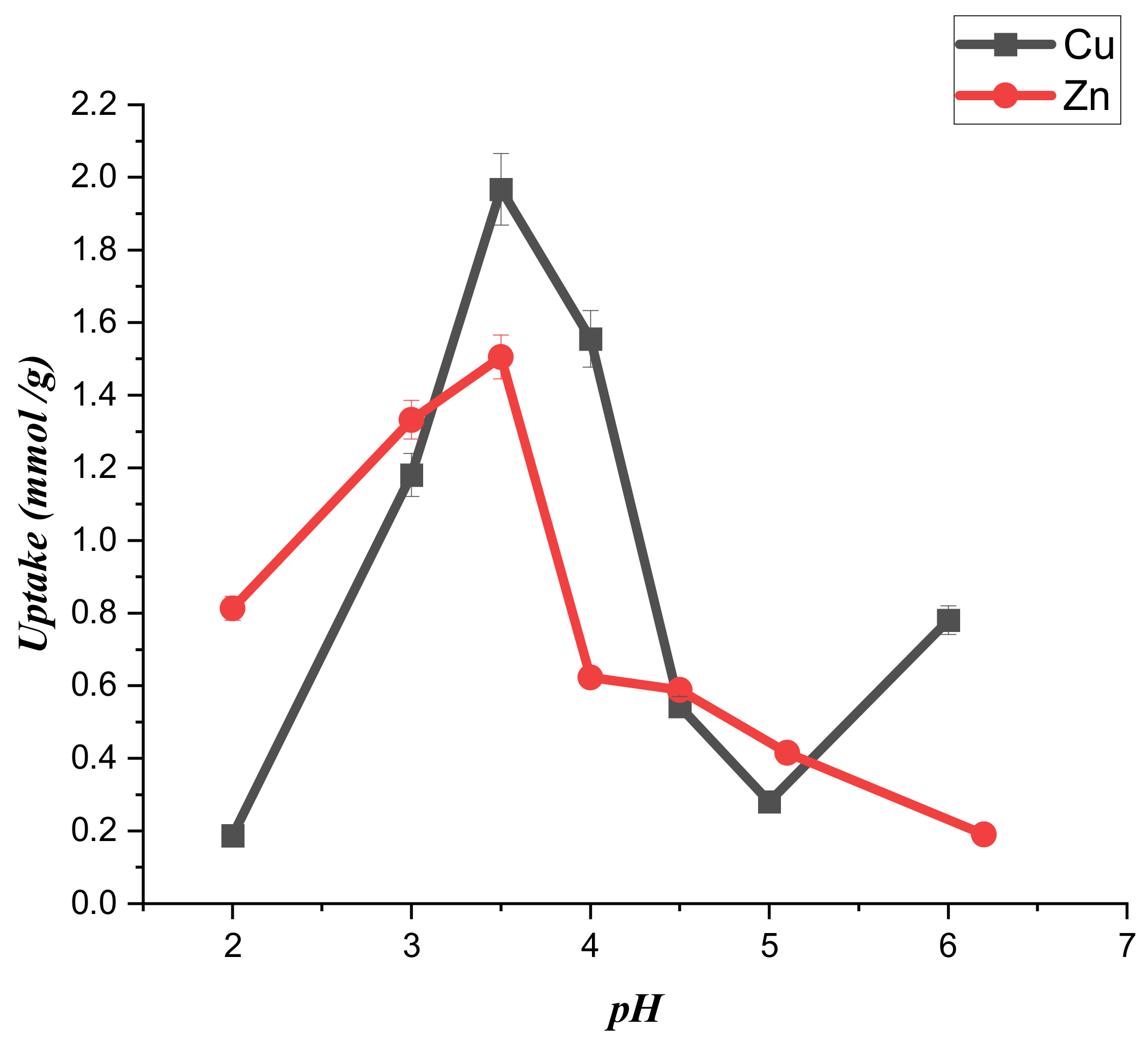
3.2. Mechanism of Adsorption of Copper and Zinc Ions on CTS-CAA
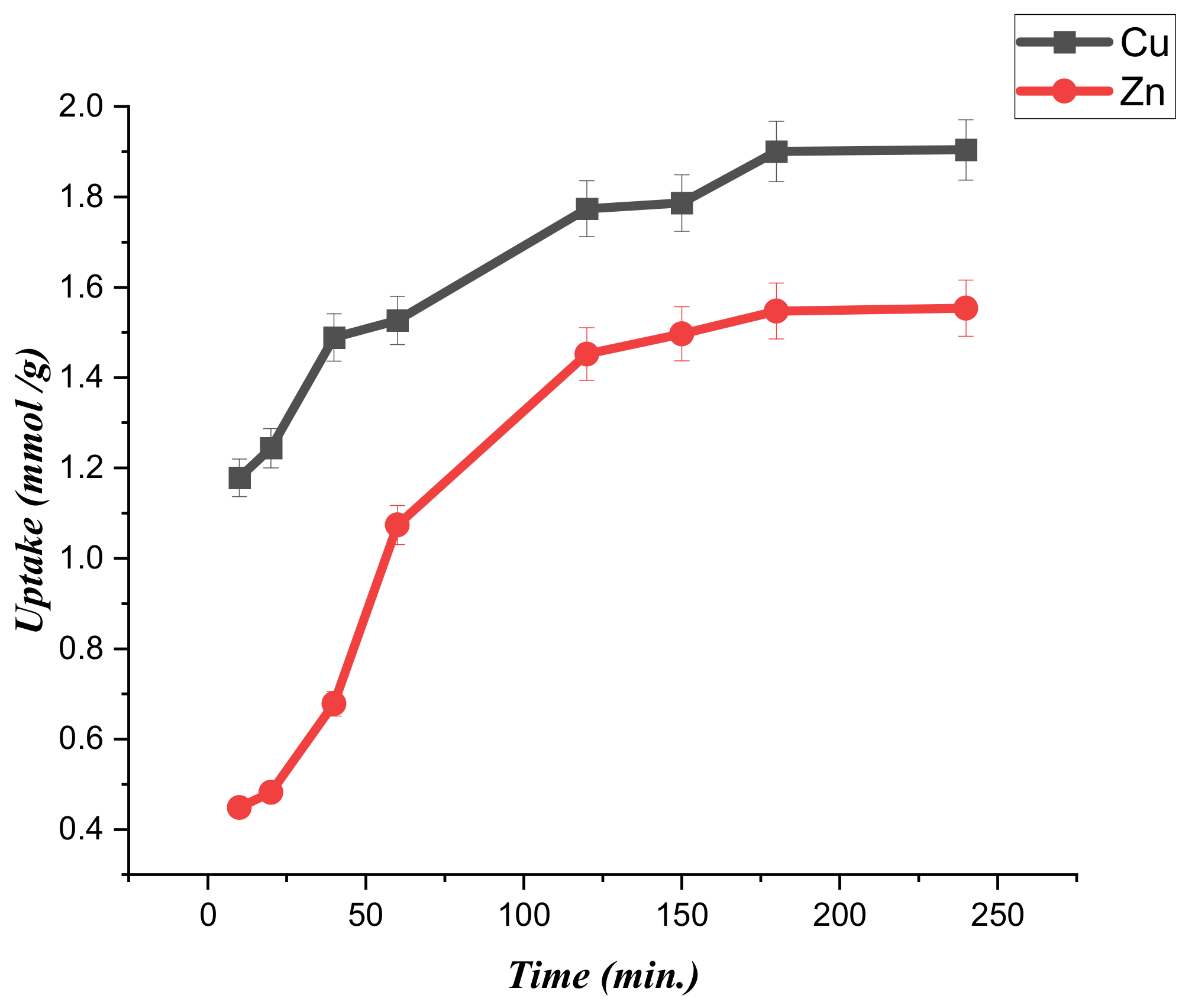
3.3. Kinetics of the Metal Ions Adsorption on Modified Chitosan
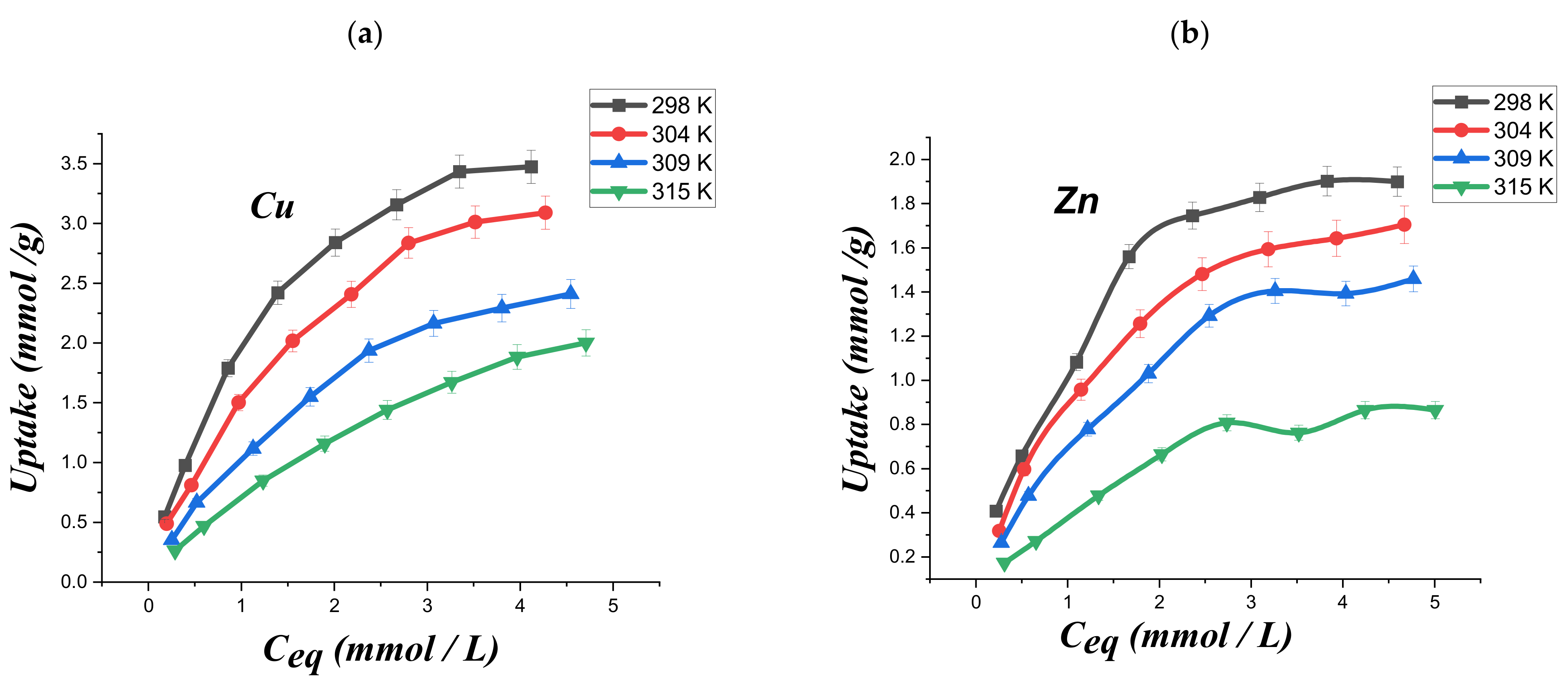
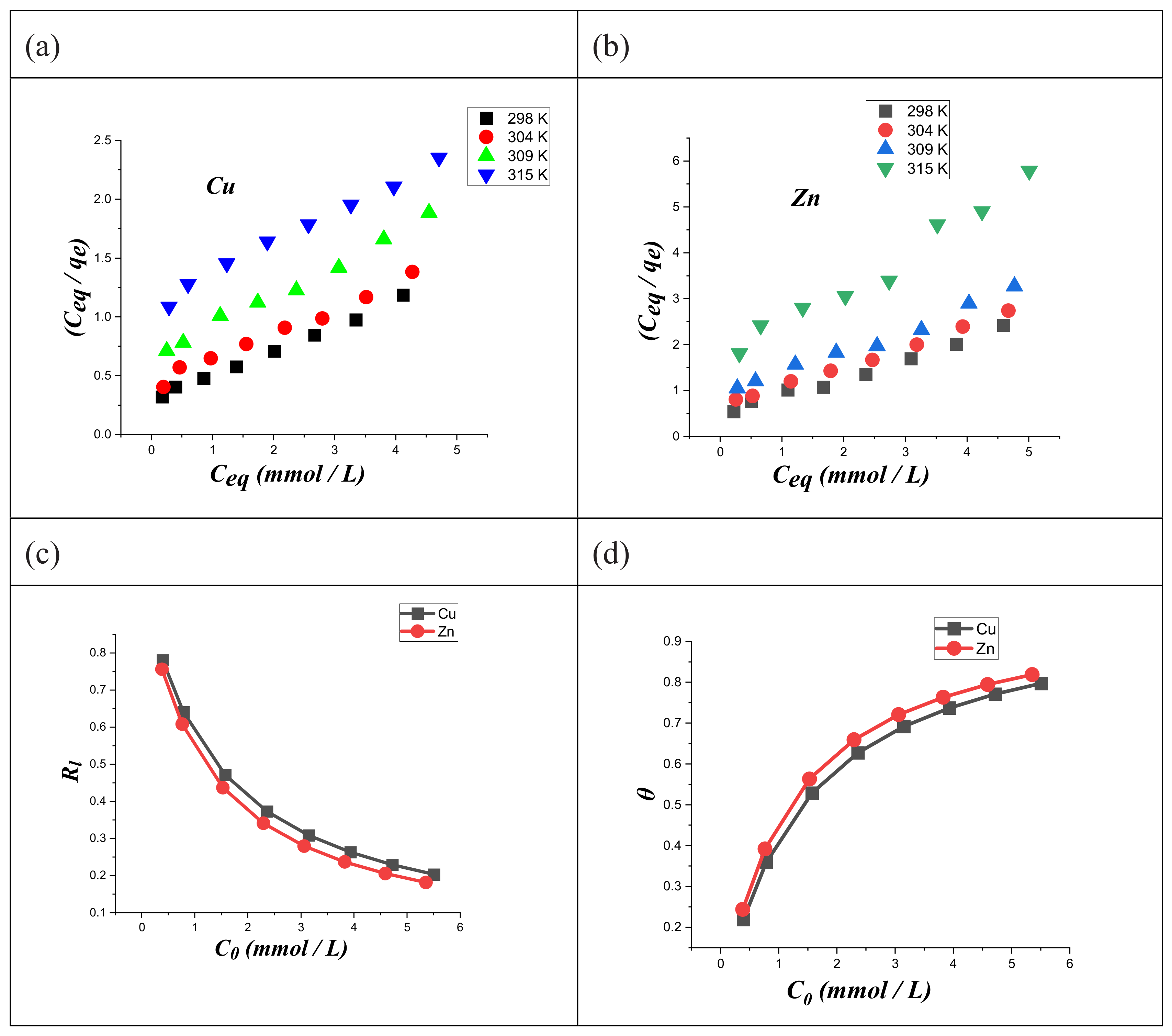
3.4. Adsorption Isotherms of the Metal Ions Adsorption on Modified Chitosan
3.5. Desorption and Regeneration Processes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ayub, A.; Raza, Z.A. Arsenic Removal Approaches: A Focus on Chitosan Biosorption to Conserve the Water Sources. Int. J. Biol. Macromol. 2021, 192, 1196–1216. [Google Scholar] [CrossRef]
- Bertinato, J.; L’Abbe, M.R. Maintaining Copper Homeostasis: Regulation of Copper-Trafficking Proteins in Response to Copper Deficiency or Overload. J. Nutr. Biochem. 2004, 15, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.H.; Chen, J.; Liu, Q.; Wark, S.E.; Son, D.H.; Batteas, J.D. Ultrasensitive Copper (II) Detection Using Plasmon-Enhanced and Photo-Brightened Luminescence of CdSe Quantum Dots. Anal. Chem. 2010, 82, 3671–3678. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Davis, M.L.; Cornwell, D.A. Introduction to Environmental Engineering, 2nd ed.; McGraw-Hill: New York, NY, USA, 1991. [Google Scholar]
- Fergusson, J.E. The Heavy Elements: Chemistry, Environmental Impact and Health Effects; Pergamon: Oxford, UK, 1990. [Google Scholar]
- Ghaedi, M.; Asadpour, E.; Vafaie, A. Simultaneous Preconcentration and Determination of Copper, Nickel, Cobalt, Lead and Iron Content Using a Surfactant Coated Alumina. Bull. Chem. Soc. Jpn. 2006, 79, 432–436. [Google Scholar] [CrossRef]
- Li, Y.H.; Di, Z.; Ding, J.; Wu, D.; Luan, Z.; Zhu, Y. Adsorption Thermodynamics, Kinetic and Desorption Studies of Pb2+ on Carbon Nanotubes. Water Res. 2005, 39, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Dermibas, E.; Dizge, N.; Sulak, M.T.; Kobya, M. Adsorption Kinetics and Equilibrium of Copper from Aqueous Solutions Using Hazelnut Shell Activated Carbon. Chem. Eng. J. 2009, 148, 480–487. [Google Scholar]
- Musso, T.B.; Parolo, M.E.; Pettinari, G.; Francisca, F.M. Cu (II) and Zn (II) Adsorption Capacity of Three Different Clay Liner Materials. J. Environ. Manag. 2014, 146, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Ngo, H.H.; Guo, W.S.; Setiadi, T. Adsorption and Desorption of Copper (II) Ions onto Garden Grass. Bioresour. Tech. 2012, 121, 386–395. [Google Scholar] [CrossRef]
- Norton, L.; Baskaran, K.; McKenzie, T. Biosorption of Zinc from Aqueous Solutions Using Biosolids. Adv. Environ. Res. 2004, 8, 629–635. [Google Scholar] [CrossRef]
- Krishnani, K.K.; Meng, X.; Christodoulatos, C.; Bodduc, V.M. Biosorption Mechanism of Nine Different Heavy Metals onto Biomatrix from Rice Husk. J. Hazard. Mater. 2008, 153, 1222–1234. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Mall, I.D.; Mishra, I.M. Characterization of Mesoporous Rice Husk Ash (RHA) and Adsorption Kinetics of Metal Ions from Aqueous Solution onto RHA. J. Hazard. Mater. 2006, 134, 257–267. [Google Scholar] [CrossRef]
- Pentari, D.; Perdikatsis, V.; Katsimicha, D.; Kanaki, A. Sorption Properties of Low Calorific Value Greek Lignites: Removal of Lead, Cadmium, Zinc and Copper Ions from Aqueous Solutions. J. Hazard. Mater. 2009, 168, 1017–1021. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Saber-Samandari, S.; Gazi, M. Cellulose-Graft-Polyacrylamide/Hydroxyapatite Composite Hydrogel with Possible Application in Removal of Cu (II) Ions. React. Funct. Polym. 2013, 73, 1523–1530. [Google Scholar] [CrossRef]
- Wang, W.; Tian, G.; Zhang, Z.; Wang, A. A Simple Hydrothermal Approach to Modify Palygorskite for High-Efficient Adsorption of Methylene Blue and Cu (II) Ions. Chem. Eng. J. 2015, 265, 228–238. [Google Scholar] [CrossRef]
- Gunasundari, E.; Kumar, P.S. Adsorption Isotherm, Kinetics and Thermodynamic Analysis of Cu (II) Ions onto the Dried Algal Biomass (Spirulina Platensis). J. Ind. Eng. Chem. 2017, 56, 129–144. [Google Scholar]
- Safa, M.; Larouci, M.; Meddah, B.; Valemens, P. The Sorption of Lead, Cadmium, Copper and Zinc Ions from Aqueous Solutions on a Raw Diatomite from Algeria. Water Sci. Technol. 2012, 65, 1729–1737. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Stafiej, A. Sorption Behavior of Cu (II), Pb (II), and Zn (II) onto Carbon Nanotubes. Solvent Extr. Ion. Exch. 2012, 30, 41–53. [Google Scholar] [CrossRef]
- El-Shafey, E.I.; Cox, M.; Pichugin, A.A.; Appleton, Q. Application of a Carbon Sorbent for the Removal of Cadmium and Other Heavy Metal Ions from Aqueous Solution. J. Chem. Technol. Biotechnol. 2002, 77, 429–436. [Google Scholar] [CrossRef]
- Mishra, S.P.; Tiwari, D.; Dubey, R.S. The Uptake Behavior of Rice (Jaya) Husk in the Removal of Zn (II) Ions: A Radiotracer Study. Appl. Radiat. Lsot. 1997, 48, 877–882. [Google Scholar] [CrossRef]
- Feng, S.; Liu, F.; Guo, Y.; Ye, M.; He, J.; Zhou, H.; Liu, L.; Cai, L.; Zhang, Y.; Li, R. Exploring the Role of Chitosan in Affecting the Adhesive, Rheological and Antimicrobial Properties of Carboxymethyl Cellulose Composite Hydrogels. Int. J. Biol. Macromol. 2021, 190, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Labidi, A.; Salaberria, A.M.; Fernandes, S.C.M.; Labidi, J.; Abderrabba, M. Adsorption of Copper on Chitin-Based Materials: Kinetic and Thermodynamic Studies. J. Taiwan Inst. Chem. Eng. 2016, 65, 140–148. [Google Scholar] [CrossRef]
- Modrzejewska, Z. Sorption Mechanism of Copper in Chitosan Hydrogel. React. Funct. Polym. 2013, 73, 719–729. [Google Scholar] [CrossRef]
- Taketa, T.B.; Mahl, C.R.A.; Calais, G.B.; Beppu, M.M. Amino Acid-Functionalized Chitosan Beads for in Vitro Copper Ions Uptake in the Presence of Histidine. Int. J. Biol. Macromol. 2021, 188, 421–431. [Google Scholar] [CrossRef]
- Cavallaro, G.; Micciulla, S.; Chiappisi, L.; Lazzara, G. Chitosan-Based Smart Hybrid Materials: A Physico-Chemical Perspective. J. Mater. Chem. B 2020, 9, 594–611. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Hueckel, T.; Cavallaro, G.; Sacanna, S.; Lazzara, G. Pickering Emulsions Based on Wax and Halloysite Nanotubes: AnEcofriendly Protocol for the Treatment of Archeological Woods. ACS Appl. Mater. Interfaces 2020, 13, 1651–1661. [Google Scholar] [CrossRef]
- Azmana, M.; Mahmood, S.; Hilles, A.R.; Rahman, A.; Bin Arifin, M.A.; Ahmed, S. A Review on Chitosan and Chitosan-Based Bionanocomposites: Promising Material for Combatting Global Issues and Its Applications. Int. J. Biol. Macromol. 2021, 185, 832–848. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Parisi, F.; Milioto, S.; Fakhrullin, R.; Lazzara, G. Core/Shell Gel Beads with Embedded Halloysite Nanotubes for Controlled Drug Release. Coatings 2019, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Chang, Y.; Yang, J.; You, Y.; He, R.; Chen, T.; Zhou, C. Functionalized Halloysite Nanotube by Chitosan Grafting for Drug Delivery of Curcumin to Achieve Enhanced Anticancer Efficacy. J. Mater. Chem. B 2016, 4, 2253–2263. [Google Scholar] [CrossRef]
- Othman, S.I.; Alturki, A.M.; Abu-Taweel, G.M.; Altoom, N.G.; Allam, A.A.; Abdelmonem, R. Chitosan for Biomedical Applications, Promising Antidiabetic Drug Delivery System, and New Diabetes Mellitus Treatment Based on Stem Cell. Int. J. Biol. Macromol. 2021, 190, 417–432. [Google Scholar] [CrossRef]
- Bui, T.V.; Umbarila, S.J.; Wang, B.; Sooknoi, T.; Li, G.; Chen, B.; Resasco, D.E. High-Temperature Grafting Silylation for Minimizing Leaching of Acid Functionality from Hydrophobic Mesoporous Silicas Used as Catalysts in the Liquid Phase. Langmuir 2019, 35, 6838–6852. [Google Scholar] [CrossRef]
- Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite Nanotubes: Interfacial Properties and Applications in Cultural Heritage. Langmuir 2020, 36, 3677–3689. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Layered Composite Based on Halloysite and Natural Polymers: A Carrier for the PH Controlled Release of Drugs. New J. Chem. 2019, 43, 10887–10893. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Wu, C.; Jiao, Y.; Xiong, S.; Zhou, C. Chitosan–Halloysite Nanotubes Nanocomposite Scaffolds for Tissue Engineering. J. Mater. Chem. B 2013, 1, 2078–2089. [Google Scholar] [CrossRef]
- Abd El-Magied, M.O.; Mansour, A.; Alsayed, F.A.A.G.; Atrees, M.S.; Abd Eldayem, S. Biosorption of Beryllium from Aqueous Solutions onto Modified Chitosan Resin: Equilibrium, Kinetic and Thermodynamic Study. J. Dispers. Sci. Technol. 2018, 39, 1597–1605. [Google Scholar] [CrossRef]
- Khapre, M.A.; Pandey, S.; Jugade, R.M. Glutaraldehyde-Cross-Linked Chitosan—Alginate Composite for Organic Dyes Removal from Aqueous Solutions. Int. J. Biol. Macromol. 2021, 190, 862–875. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Atia, A.A.; Guibal, E. Fast Removal of Uranium from Aqueous Solutions Using Tetraethylenepentamine Modified Magnetic Chitosan Resin. Bioresour. Technol. 2014, 160, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Elwakeel, K.Z. Removal of Cr (VI) from Alkaline Aqueous Solutions Using Chemically Modified Magnetic Chitosan Resins. Desalination 2010, 250, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Ramon, M.V.; Stoeckli, F.; Moreno-Castilla, C.; Carrasco-Marin, F. On the Characterization of Acidic and Basic Surface Sites on Carbons by Various Techniques. Carbon 1999, 37, 1215–1221. [Google Scholar] [CrossRef]
- Marczenko, Z.; Balcerzak, M. Separation, Preconcentration and Spectrophotometry in Inorganic Analysis, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 10, ISBN 0 444 50524 5. [Google Scholar]
- Abd El-Magied, M.O.; Hassan, A.M.A.; Gad, H.M.H.; Mohammaden, T.F.; Youssef, M.A.M. Removal of Nickel (II) Ions from Aqueous Solutions Using Modified Activated Carbon: A Kinetic and Equilibrium Study. J. Dispers. Sci. Technol. 2018, 39, 862–873. [Google Scholar] [CrossRef]
- Abd El-magied, M.O.; Galhoum, A.A.; Atia, A.A.; Tolba, A.A.; Maize, M.; Vincent, T.; Guibal, E. Cellulose and Chitosan Derivatives for Enhanced Sorption of Erbium (III). Colloids Surf. A 2017, 529, 580–593. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica, and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1368. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, G.L.; Amy, M.; Prevos, S.; Nour, M.; Jekel, P.M.; Blumenschein, C.D. Kinetic and Thermodynamic Aspects of Adsorption of Arsenic onto Granular Ferric Hydroxide. Water Res. 2008, 42, 3371–3378. [Google Scholar] [CrossRef] [PubMed]
- Abu El-Soad, A.M.; Abd El-Magied, M.O.; Atrees, M.S.; Kovaleva, E.G.; Lazzara, G. Synthesis and Characterization of Modified Sulfonated Chitosan for Beryllium Recovery. Int. J. Biol. Macromol. 2019, 139, 153–160. [Google Scholar] [CrossRef]
- Freundlich, H. Uber Die Adsorption in Lusungen. Z. Phys. Chem. 1906, 57, 384–470. [Google Scholar]
- Akkaya, R.; Akkaya, B. Adsorption Isotherms, Kinetics, Thermodynamics and Desorption Studies for Uranium and Thorium Ions from Aqueous Solution by Novel Microporous Composite P(HEMA-EP). J. Nucl. Mater. 2013, 434, 328–333. [Google Scholar] [CrossRef]
- Aharoni, C.; Ungarish, M. Kinetics of Activated Chemisorption. Part 2.-Theoretical Models. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1977, 73, 456–464. [Google Scholar] [CrossRef]
- Reddad, Z.; Gerente, C.; Andres, Y.; Le Cloirec, P. Comparison of the Fixation of Several Metal Ions onto a Low Cost Biopolymer. Water Sci. Technol. Water Supply 2002, 2, 217–224. [Google Scholar] [CrossRef]
- Reddad, Z.; Zerente, C.; Andres, Y.; Le Cloirec, P. Adsorption of Several Metal Ions onto a Low Cost Biosorbent: Kinetic and Equilibrium Studies. Environ. Sci. Technol. 2002, 36, 2067–2073. [Google Scholar] [CrossRef]
- Reddad, Z.; Gerente, C.; Andres, Y.; Marie-Christine, R.; Thibault, J.-F.; Le Cloirec, P. Ni(II) and Cu(II) Binding Properties of Native and Modified Sugar Beet Pulp. Carbohydr. Polym. 2002, 49, 23–31. [Google Scholar] [CrossRef]
- Tsai, W.-C.; de Luna, M.D.G.; Bermillo-Arriesgado, H.L.P.; Futalan, C.M.; Colades, J.I.; Wan, M.-W. Competitive Fixed-Bed Adsorption of Pb (II), Cu (II), and Ni (II) from Aqueous Solution Using Chitosan-Coated Bentonite. Int. J. Polym. Sci. 2016, 2016, 11. [Google Scholar] [CrossRef] [Green Version]
- Xia, L.; Huang, Z.; Zhong, L.; Xie, F.; Tang, C.Y.; Tsui, C.P. Bagasse Cellulose Grafted with an Amino-TerminatedHyperbranched Polymer for the Removal of Cr (VI)from Aqueous Solution. Polymers 2018, 10, 931. [Google Scholar] [CrossRef] [Green Version]
- Igberase, E.; Osifo, P.; Ofomaja, A. The Adsorption of Copper (II) Ions by Polyaniline Graft Chitosan Beads from Aqueous Solution: Equilibrium, Kinetic and Desorption Studies. Chem. Eng. J. 2014, 2, 362–369. [Google Scholar] [CrossRef]
- Zhan, W.; Xu, C.; Qian, G.; Huang, G.; Tang, X.; Lin, B. Adsorption of Cu (II), Zn (II), and Pb (II) from Aqueous Single and Binary Metal Solutions by Regenerated Cellulose and Sodium Alginate Chemically Modified with Polyethyleneimine. RSC Adv. 2018, 8, 18723. [Google Scholar] [CrossRef] [Green Version]
- Hubicki, Z.; Geca, M.; Kołodynska, D. The Effect of the Presence of Metatartaric Acid on Removal Effectiveness of Heavy Metal Ions on Chelating Ion Exchangers. Environ. Technol. 2011, 32, 805–816. [Google Scholar] [CrossRef]
- Morcali, M.H.; Zeytuncu, B.; Baysal, A.; Akman, S.; Yucel, O. Adsorption of Copper and Zinc from Sulfate Media on a Commercial Sorbent. J. Environ. Chem. Eng. 2014, 2, 1655–1662. [Google Scholar] [CrossRef]
| Sample | Content (%) | ||
|---|---|---|---|
| C | H | N | |
| Chitosan | 40.27 | 7.91 | 6.22 |
| (CTS-GL) | 43.01 | 7.23 | 5.81 |
| (CTS-EC) | 37.18 | 6.17 | 5.61 |
| (CTS-DET) | 43.19 | 7.34 | 8.94 |
| (CTS-CAA) | 42.54 | 6.98 | 7.38 |
| T (K) | Langmuir Parameters | Thermodynamic Parameters | ||||
|---|---|---|---|---|---|---|
| qx | Kl | R2 | ∆S° J/K mol | ∆H° KJ/mol | ∆G° J/mol | |
| 298 ± 1 | 4.77 ± 0.05 | 0.713 ± 0.01 | 0.996 | −164.44 ± 0.2 | −48.28 ± 0.2 | 719.51 ± 0.2 |
| 304 ± 1 | 4.56 ± 0.05 | 0.525 ± 0.01 | 0.987 | −164.44 ± 0.2 | −48.28 ± 0.2 | 1706.16 ± 0.2 |
| 309 ± 1 | 3.79 ± 0.05 | 0.404 ± 0.01 | 0.992 | −164.44 ± 0.2 | −48.28 ± 0.2 | 2528.37 ± 0.2 |
| 315 ± 1 | 3.75 ± 0.05 | 0.245 ± 0.01 | 0.991 | −164.44 ± 0.2 | −48.28 ± 0.2 | 3515.03 ± 0.2 |
| T (K) | Langmuir Parameters | Thermodynamic Parameters | ||||
|---|---|---|---|---|---|---|
| qx | Kl | R2 | ∆S° J/K mol | ∆H° KJ/mol | ∆G° J/mol | |
| 298 ± 1 | 2.47 ± 0.04 | 0.843 ± 0.015 | 0.987 | −88.327 ± 0.15 | −25.82 ± 0.15 | 493.45 ± 0.15 |
| 304 ± 1 | 2.30 ± 0.04 | 0.66 ± 0.015 | 0.996 | −88.327 ± 0.15 | −25.82 ± 0.15 | 1023.42 ± 0.15 |
| 309 ± 1 | 2.09 ± 0.04 | 0.528 ± 0.015 | 0.986 | −88.327 ± 0.15 | −25.82 ± 0.15 | 1465.06 ± 0.15 |
| 315 ± 1 | 1.26 ± 0.04 | 0.489 ± 0.015 | 0.972 | −88.327 ± 0.15 | −25.82 ± 0.15 | 1995.03 ± 0.15 |
| Adsorbent | Adsorption Capacity (mg/g) | ||
|---|---|---|---|
| Cu (II) | Zn (II) | References | |
| Sugar beet pulp | 21.1 | 17.8 | [51,52,53] |
| A biomatrix derived from rice husk | 10.8 | 7.47 | [13] |
| Chitosan–cellulose beads | 53.2 | - | [54] |
| Pyromellitic dianhydride modified SCB | 77.4 | 65.0 | [55] |
| Polyaniline graft chitosan | 83.30 | - | [56] |
| PEI-RCSA | 177.1 | 110.2 | [57] |
| Waste activated sludge biosolid | - | 36.88 | [12] |
| Lewatit SP 112 | 40.32 | 64.10 | [58] |
| Lewatit TP 207 | 68.50 | 73.00 | [59] |
| CTS-CAA | 220.5 | 124.3 | The current study |
| Cycle | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| Metal | Adsorption (%) | Desorption (%) | Adsorption (%) | Desorption (%) | Adsorption (%) | Desorption (%) |
| copper | 100 | 80.23 | 91.56 | 76.54 | 88.47 | 74.36 |
| Zinc | 100 | 84.21 | 93.42 | 79.89 | 89.74 | 78.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu El-Soad, A.M.; Lazzara, G.; Abd El-Magied, M.O.; Cavallaro, G.; Al-Otaibi, J.S.; Sayyed, M.I.; Kovaleva, E.G. Chitosan Functionalized with Carboxyl Groups as a Recyclable Biomaterial for the Adsorption of Cu (II) and Zn (II) Ions in Aqueous Media. Int. J. Mol. Sci. 2022, 23, 2396. https://doi.org/10.3390/ijms23042396
Abu El-Soad AM, Lazzara G, Abd El-Magied MO, Cavallaro G, Al-Otaibi JS, Sayyed MI, Kovaleva EG. Chitosan Functionalized with Carboxyl Groups as a Recyclable Biomaterial for the Adsorption of Cu (II) and Zn (II) Ions in Aqueous Media. International Journal of Molecular Sciences. 2022; 23(4):2396. https://doi.org/10.3390/ijms23042396
Chicago/Turabian StyleAbu El-Soad, Asmaa M., Giuseppe Lazzara, Mahmoud O. Abd El-Magied, Giuseppe Cavallaro, Jamelah S. Al-Otaibi, M. I. Sayyed, and Elena G. Kovaleva. 2022. "Chitosan Functionalized with Carboxyl Groups as a Recyclable Biomaterial for the Adsorption of Cu (II) and Zn (II) Ions in Aqueous Media" International Journal of Molecular Sciences 23, no. 4: 2396. https://doi.org/10.3390/ijms23042396
APA StyleAbu El-Soad, A. M., Lazzara, G., Abd El-Magied, M. O., Cavallaro, G., Al-Otaibi, J. S., Sayyed, M. I., & Kovaleva, E. G. (2022). Chitosan Functionalized with Carboxyl Groups as a Recyclable Biomaterial for the Adsorption of Cu (II) and Zn (II) Ions in Aqueous Media. International Journal of Molecular Sciences, 23(4), 2396. https://doi.org/10.3390/ijms23042396








