Generation and Characterization of a Zebrafish IL-2Rγc SCID Model
Abstract
:1. Introduction
2. Results
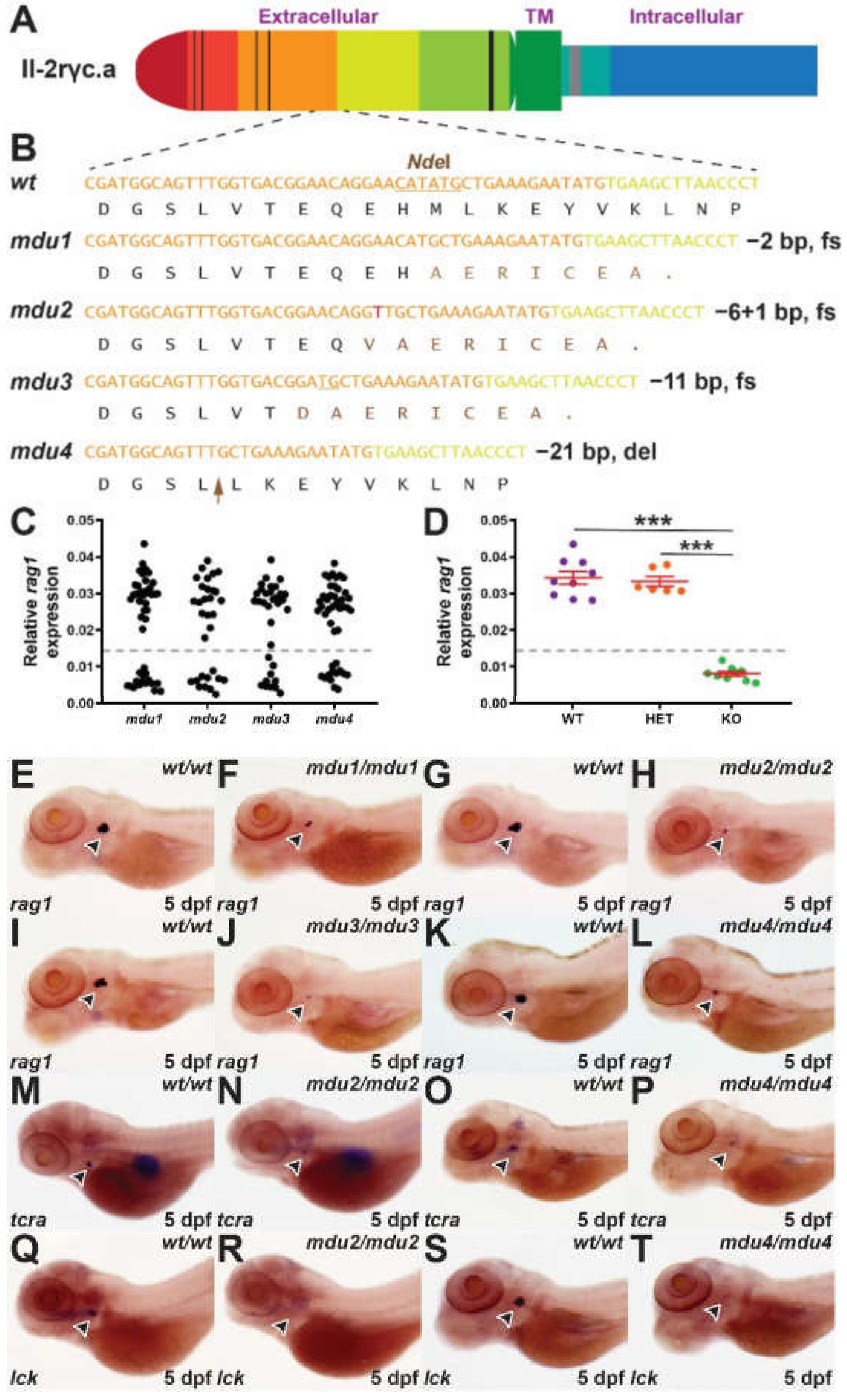
2.1. Generation of Il-2rγc.a Mutants
2.2. Impact on Embryonic Lymphopoiesis
2.3. Impact on Later Lymphopoiesis
2.4. Impact on Intestinal Microbiota
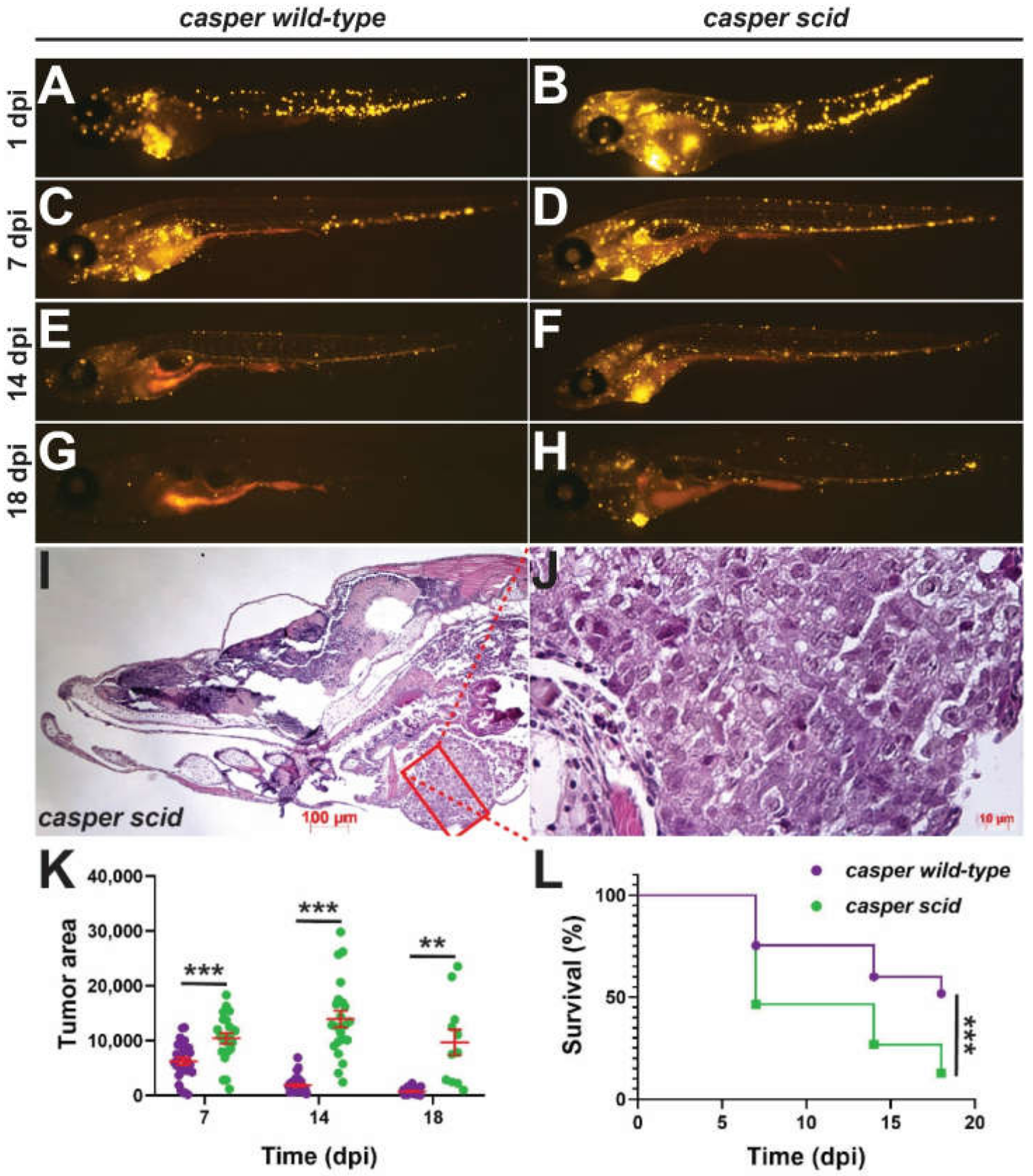
2.5. Impact on Anti-Tumor Immunity
3. Discussion
4. Materials and Methods
4.1. Zebrafish Husbandry and Manipulation
4.2. Reverse-Transcription Polymerase Chain Reaction
4.3. Whole-Mount in Situ Hybridization (WISH)
4.4. Ex Vivo Analysis
4.5. Tumor Xenotransplantation
4.6. Imaging
4.7. Statistical Analysis
4.8. Microbiome Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alves, N.L.; Arosa, F.A.; van Lier, R.A. Common gamma chain cytokines: Dissidence in the details. Immunol. Lett. 2007, 108, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Meazza, R.; Azzarone, B.; Orengo, A.M.; Ferrini, S. Role of common-gamma chain cytokines in NK cell development and function: Perspectives for immunotherapy. J. Biomed. Biotechnol. 2011, 2011, 861920. [Google Scholar] [CrossRef] [PubMed]
- Rochman, Y.; Spolski, R.; Leonard, W.J. New insights into the regulation of T cells by gamma(c) family cytokines. Nat. Rev. Immunol. 2009, 9, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Yi, H.; Rosenblatt, H.M.; Filipovich, A.H.; Adelstein, S.; Modi, W.S.; McBride, O.W.; Leonard, W.J. Interleukin-2 receptor γ chain mutation results in X-linked severe combined immunodeficiency in humans. Cell 1993, 73, 147–157. [Google Scholar] [CrossRef]
- Bosma, G.C.; Custer, R.P.; Bosma, M.J. A severe combined immunodeficiency mutation in the mouse. Nature 1983, 301, 527–530. [Google Scholar] [CrossRef]
- Yoder, J.A.; Nielsen, M.E.; Amemiya, C.T.; Litman, G.W. Zebrafish as an immunological model system. Microbes Infect. 2002, 4, 1469–1478. [Google Scholar] [CrossRef]
- Traver, D.; Herbomel, P.; Patton, E.E.; Murphey, R.D.; Yoder, J.A.; Litman, G.W.; Catic, A.; Amemiya, C.T.; Zon, L.I.; Trede, N.S. The zebrafish as a model organism to study development of the immune system. Adv. Immunol. 2003, 81, 253–330. [Google Scholar]
- Chen, A.T.; Zon, L.I. Zebrafish blood stem cells. J. Cell. Biochem. 2009, 108, 35–42. [Google Scholar] [CrossRef]
- Liongue, C.; Ward, A.C. Evolution of class I cytokine receptors. BMC Evol. Biol. 2007, 7, 120. [Google Scholar] [CrossRef] [Green Version]
- Liongue, C.; O’Sullivan, L.A.; Trengove, M.C.; Ward, A.C. Evolution of JAK-STAT pathway components: Mechanisms and role in immune system development. PLoS ONE 2012, 7, e32777. [Google Scholar] [CrossRef]
- Liongue, C.; Hall, C.; O’Connell, B.; Crozier, P.; Ward, A.C. Zebrafish granulocyte colony-stimulating factor receptor signalling promotes myelopoiesis and myeloid cell migration. Blood 2009, 113, 2535–2546. [Google Scholar] [CrossRef]
- Iwanami, N.; Mateos, F.; Hess, I.; Riffel, N.; Soza-Ried, C.; Schorpp, M.; Boehm, T. Genetic evidence for an evolutionarily conserved role of IL-7 signaling in T cell development of zebrafish. J. Immunol. 2011, 186, 7060–7066. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.Y.; Pan, P.P.; Fang, W.; Shao, J.Z.; Xiang, L.X. Essential role of IL-4 and IL-4Ralpha interaction in adaptive immunity of zebrafish: Insight into the origin of Th2-like regulatory mechanism in ancient vertebrates. J. Immunol. 2012, 188, 5571–5584. [Google Scholar] [CrossRef]
- Paffett-Lugassy, N.; Hsia, N.; Fraenkel, P.G.; Paw, B.; Leshinsky, I.; Barut, B.; Bahary, N.; Caro, J.; Handin, R.; Zon, L.I. Functional conservation of erythropoietin signaling in zebrafish. Blood 2007, 110, 2718–2726. [Google Scholar] [CrossRef] [Green Version]
- Aggad, D.; Stein, C.; Sieger, D.; Mazel, M.; Boudinot, P.; Herbomel, P.; Levraud, J.P.; Lutfalla, G.; Leptin, M. In vivo analysis of Ifn-γ1 and Ifn-γ2 signaling in zebrafish. J. Immunol. 2010, 185, 6774–6782. [Google Scholar] [CrossRef] [Green Version]
- Sertori, R.; Liongue, C.; Basheer, F.; Lewis, K.L.; Rasighaemi, P.; de Coninck, D.; Traver, D.; Ward, A.C. Conserved IL-2Rγc signaling mediates lymphopoiesis in zebrafish. J. Immunol. 2016, 196, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Kossack, M.E.; Draper, B.W. Genetic regulation of sex determination and maintenance in zebrafish (Danio rerio). Curr. Top. Dev. Biol. 2019, 134, 119–149. [Google Scholar] [CrossRef]
- Niemela, J.E.; Puck, J.M.; Fischer, R.E.; Fleisher, T.A.; Hsu, A.P. Efficient detection of thirty-seven new IL2RG mutations in human X-linked severe combined immunodeficiency. Clin. Immunol. 2000, 95, 33–38. [Google Scholar] [CrossRef]
- Langenau, D.M.; Zon, L.I. The zebrafish: A new model of T-cell and thymic development. Nat. Rev. Immunol. 2005, 5, 307–317. [Google Scholar] [CrossRef]
- Willett, C.E.; Cherry, J.J.; Steiner, L. Characterization and expression of the recombination activating genes (rag1 and rag2) of zebrafish. Immunogenetics 1997, 45, 394–404. [Google Scholar] [CrossRef]
- Danilova, N.; Hohman, V.S.; Sacher, F.; Ota, T.; Willett, C.E.; Steiner, L.A. T cells and the thymus in developing zebrafish. Dev. Comp. Immunol. 2004, 28, 755–767. [Google Scholar] [CrossRef]
- Langenau, D.M.; Ferrando, A.A.; Traver, D.; Kutok, J.L.; Hezel, J.P.; Kanki, J.P.; Zon, L.I.; Look, A.T.; Trede, N.S. In vivo tracking of T cell development, ablation, and engraftment in transgenic zebrafish. Proc. Natl. Acad. Sci. USA 2004, 101, 7369–7374. [Google Scholar] [CrossRef] [Green Version]
- Page, D.M.; Wittamer, V.; Bertrand, J.Y.; Lewis, K.L.; Pratt, D.N.; Delgado, N.; Schale, S.E.; McGue, C.; Jacobsen, B.H.; Doty, A.; et al. An evolutionarily conserved program of B-cell development and activation in zebrafish. Blood 2013, 122, e1–e11. [Google Scholar] [CrossRef] [Green Version]
- Moore, F.E.; Garcia, E.G.; Lobbardi, R.; Jain, E.; Tang, Q.; Moore, J.C.; Cortes, M.; Molodtsov, A.; Kasheta, M.; Luo, C.C.; et al. Single-cell transcriptional analysis of normal, aberrant, and malignant hematopoiesis in zebrafish. J. Exp. Med. 2016, 213, 979–992. [Google Scholar] [CrossRef] [Green Version]
- Schorpp, M.; Bialecki, M.; Diekhoff, D.; Walderich, B.; Odenthal, J.; Maischein, H.M.; Zapata, A.G.; Boehm, T. Conserved functions of Ikaros in vertebrate development: Genetic evidence for distinct larval and adult phases of T cell development and two lineages of B cells in zebrafish. J. Immunol. 2006, 177, 2463–2476. [Google Scholar] [CrossRef] [Green Version]
- Petrie-Hanson, L.; Hohn, C.; Hanson, L. Characterization of rag1 mutant zebrafish leukocytes. BMC Immunol. 2009, 10, 8. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, J.Y.; Kim, A.D.; Teng, S.; Traver, D. CD41+ cmyb+ precursors colonize the zebrafish pronephros by a novel migration route to initiate adult hematopoiesis. Development 2008, 135, 1853–1862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berbers, R.M.; Franken, I.A.; Leavis, H.L. Immunoglobulin A and microbiota in primary immunodeficiency diseases. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Gereige, J.D.; Maglione, P.J. Current understanding and recent developments in common variable immunodeficiency associated autoimmunity. Front. Immunol. 2019, 10, 2753. [Google Scholar] [CrossRef]
- Lane, J.P.; Stewart, C.J.; Cummings, S.P.; Gennery, A.R. Gut microbiome variations during hematopoietic stem cell transplant in severe combined immunodeficiency. J. Allergy Clin. Immunol. 2015, 135, 1654–1656. [Google Scholar] [CrossRef] [PubMed]
- White, R.M.; Sessa, A.; Burke, C.; Bowman, T.; LeBlanc, J.; Ceol, C.; Bourque, C.; Dovey, M.; Goessling, W.; Burns, C.E. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2008, 2, 183–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashikawa, Y.; Hayashi, R.; Tajima, M.; Okubo, T.; Azuma, S.; Kuwamura, M.; Takai, N.; Osada, Y.; Kunihiro, Y.; Mashimo, T.; et al. Generation of knockout rabbits with X-linked severe combined immunodeficiency (X-SCID) using CRISPR/Cas9. Sci. Rep. 2020, 10, 9957. [Google Scholar] [CrossRef]
- Suzuki, S.; Iwamoto, M.; Saito, Y.; Fuchimoto, D.; Sembon, S.; Suzuki, M.; Mikawa, S.; Hashimoto, M.; Aoki, Y.; Najima, Y.; et al. Il2rg gene-targeted severe combined immunodeficiency pigs. Cell Stem Cell 2012, 10, 753–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, I.H.; Chung, Y.Y.; Jung, D.E.; Kim, Y.J.; Kim, D.H.; Kim, K.S.; Park, S.W. Impaired lymphocytes development and xenotransplantation of gastrointestinal tumor cells in prkdc-null SCID zebrafish model. Neoplasia 2016, 18, 468–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Q.; Abdelfattah, N.S.; Blackburn, J.S.; Moore, J.C.; Martinez, S.A.; Moore, F.E.; Lobbardi, R.; Tenente, I.M.; Ignatius, M.S.; Berman, J.N.; et al. Optimized cell transplantation using adult rag2 mutant zebrafish. Nat. Methods 2014, 11, 821–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, I.; Iwanami, N.; Schorpp, M.; Boehm, T. Zebrafish model for allogeneic hematopoietic cell transplantation not requiring preconditioning. Proc. Natl. Acad. Sci. USA 2013, 110, 4327–4332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, A.; Notarangelo, L.D.; Neven, B.; Cavazzana, M.; Puck, J.M. Severe combined immunodeficiencies and related disorders. Nat. Rev. Dis. Primers 2015, 1, 15061. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zhao, T.; Yuan, S.; Yang, L.; Ding, J.; Cui, L.; Xu, M. Immunodeficiency promotes adaptive alterations of host gut microbiome: An observational metagenomic study in mice. Front. Microbiol. 2019, 10, 2415. [Google Scholar] [CrossRef]
- Rebelo de Almeida, C.; Mendes, R.V.; Pezzarossa, A.; Gago, J.; Carvalho, C.; Alves, A.; Nunes, V.; Brito, M.J.; Cardoso, M.J.; Ribeiro, J.; et al. Zebrafish xenografts as a fast screening platform for bevacizumab cancer therapy. Commun. Biol. 2020, 3, 299. [Google Scholar] [CrossRef]
- Lawrence, C. The husbandry of zebrafish (Danio rerio): A review. Aquaculture 2007, 269, 1–20. [Google Scholar] [CrossRef]
- Dahlem, T.J.; Hoshijima, K.; Jurynec, M.J.; Gunther, D.; Starker, C.G.; Locke, A.S.; Weis, A.M.; Voytas, D.F.; Grunwald, D.J. Simple methods for generating and detecting locus-specific mutations induced with TALENs in the zebrafish genome. PLoS Genet. 2012, 8, e1002861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garritano, S.; Gemignani, F.; Voegele, C.; Nguyen-Dumont, T.; Le Calvez-Kelm, F.; De Silva, D.; Lesueur, F.; Landi, S.; Tavtigian, S.V. Determining the effectiveness of high resolution melting analysis for SNP genotyping and mutation scanning at the TP53 locus. BMC Genet. 2009, 10, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thisse, C.; Thisse, B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 2008, 3, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [Green Version]
- Callahan, B.J.; Sankaran, K.; Fukuyama, J.A.; McMurdie, P.J.; Holmes, S.P. Bioconductor workflow for microbiome data analysis: From raw reads to community analyses. F1000Research 2016, 5, 1492. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sertori, R.; Jones, R.; Basheer, F.; Rivera, L.; Dawson, S.; Loke, S.; Heidary, S.; Dhillon, A.; Liongue, C.; Ward, A.C. Generation and Characterization of a Zebrafish IL-2Rγc SCID Model. Int. J. Mol. Sci. 2022, 23, 2385. https://doi.org/10.3390/ijms23042385
Sertori R, Jones R, Basheer F, Rivera L, Dawson S, Loke S, Heidary S, Dhillon A, Liongue C, Ward AC. Generation and Characterization of a Zebrafish IL-2Rγc SCID Model. International Journal of Molecular Sciences. 2022; 23(4):2385. https://doi.org/10.3390/ijms23042385
Chicago/Turabian StyleSertori, Robert, Realla Jones, Faiza Basheer, Leni Rivera, Samantha Dawson, Stella Loke, Somayyeh Heidary, Amardeep Dhillon, Clifford Liongue, and Alister C. Ward. 2022. "Generation and Characterization of a Zebrafish IL-2Rγc SCID Model" International Journal of Molecular Sciences 23, no. 4: 2385. https://doi.org/10.3390/ijms23042385
APA StyleSertori, R., Jones, R., Basheer, F., Rivera, L., Dawson, S., Loke, S., Heidary, S., Dhillon, A., Liongue, C., & Ward, A. C. (2022). Generation and Characterization of a Zebrafish IL-2Rγc SCID Model. International Journal of Molecular Sciences, 23(4), 2385. https://doi.org/10.3390/ijms23042385






