Low Temperature Affects Fatty Acids Profiling and Key Synthesis Genes Expression Patterns in Zanthoxylum bungeanum Maxim
Abstract
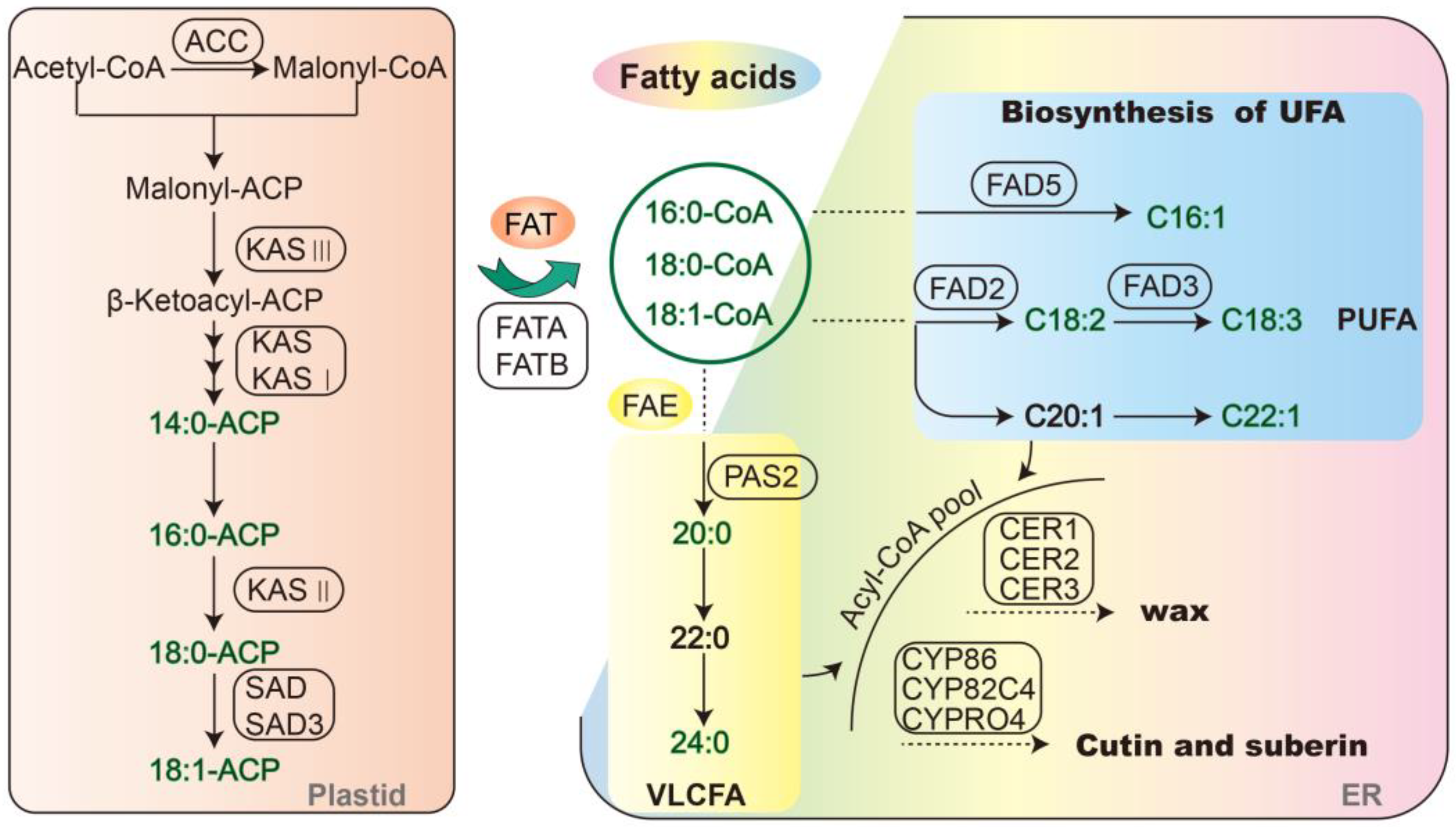
:1. Introduction
2. Results
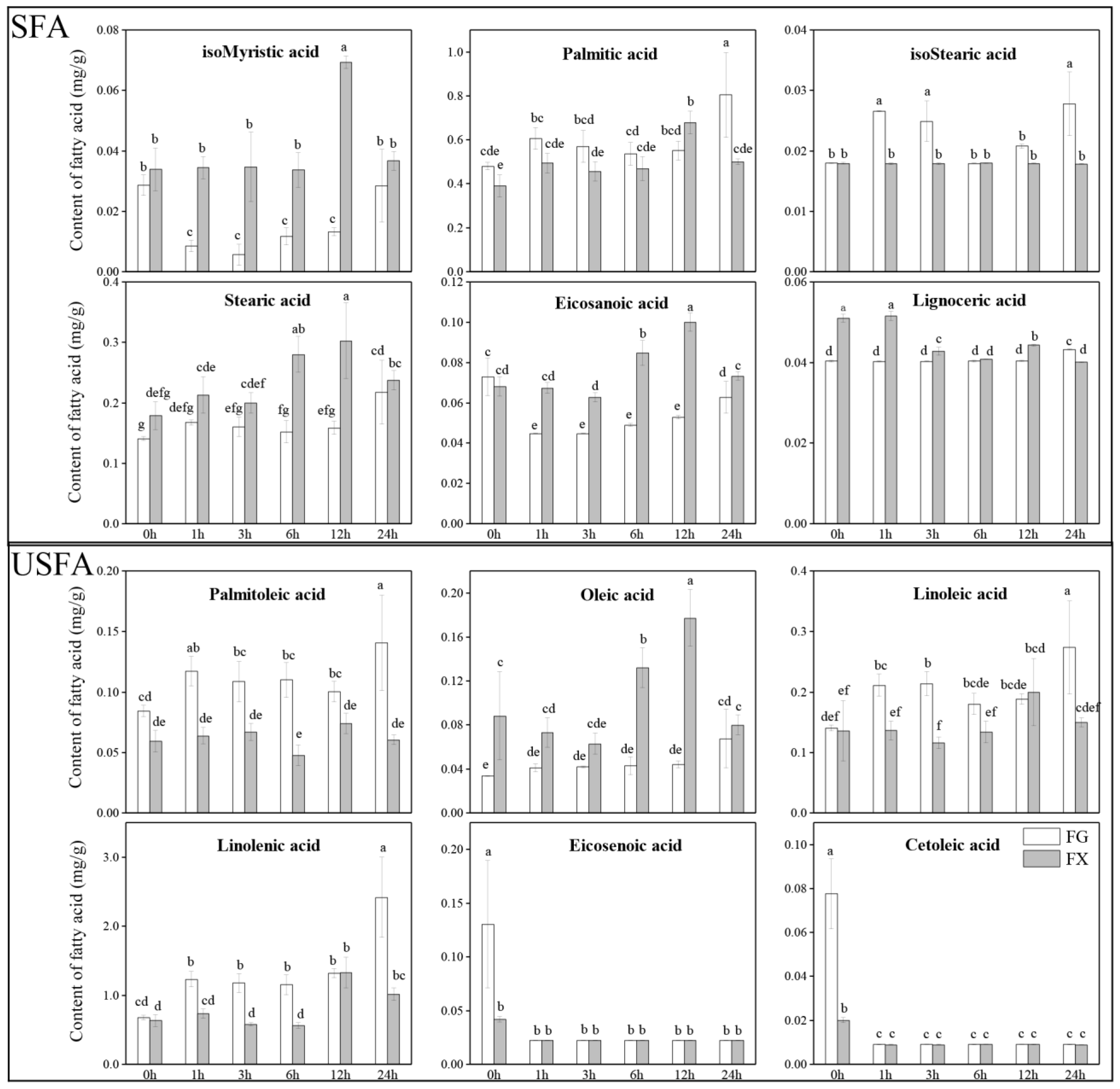
2.1. Fatty Acid Profiling in Leaves under 4 °C Cold Stress for Different Time Treatments among Two Z. bungeanum Varieties
2.2. Chemometric Analysis of Fatty Acid Components in Z. bungeanum
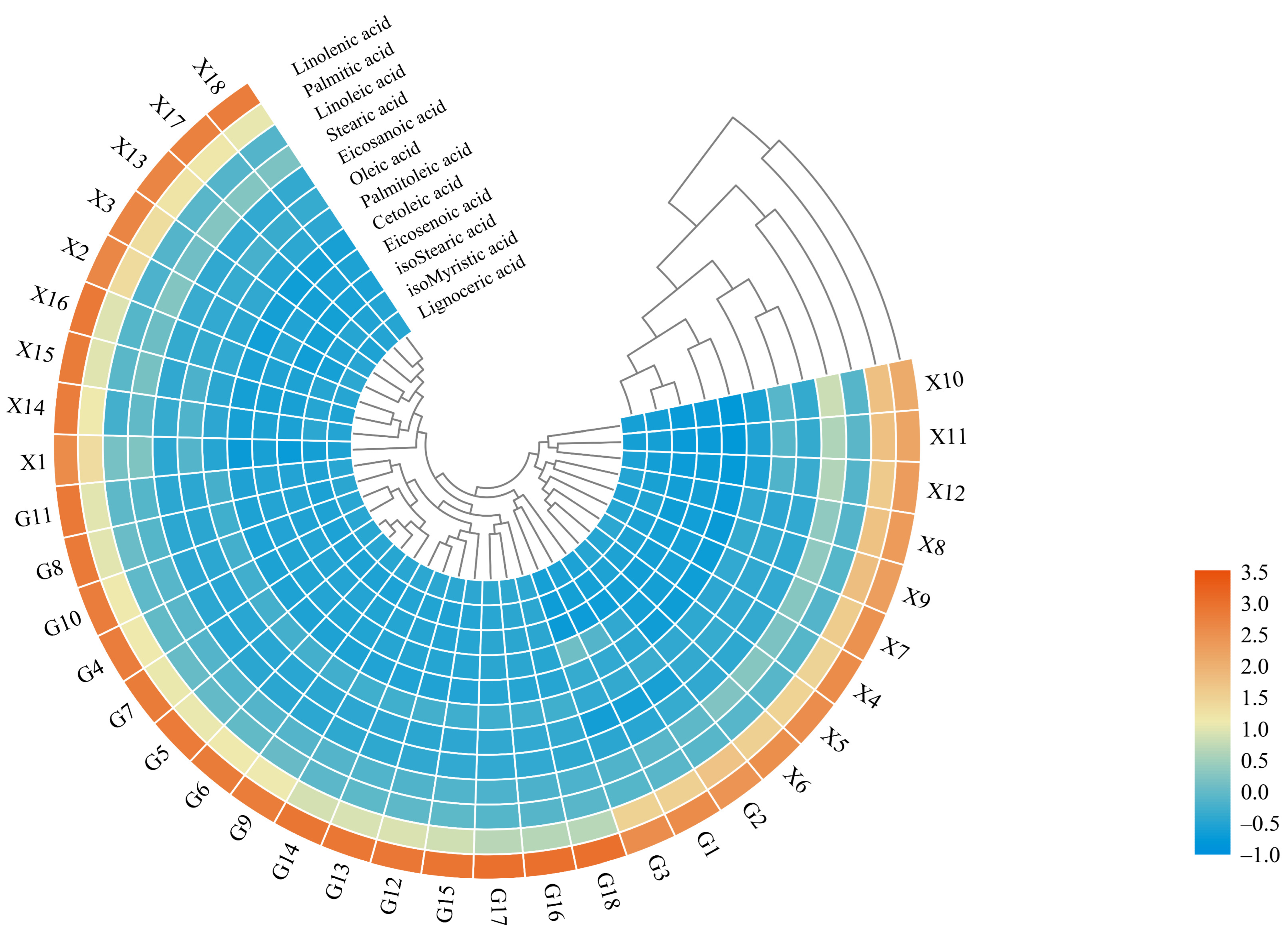
2.2.1. Cluster Heat Map Analysis
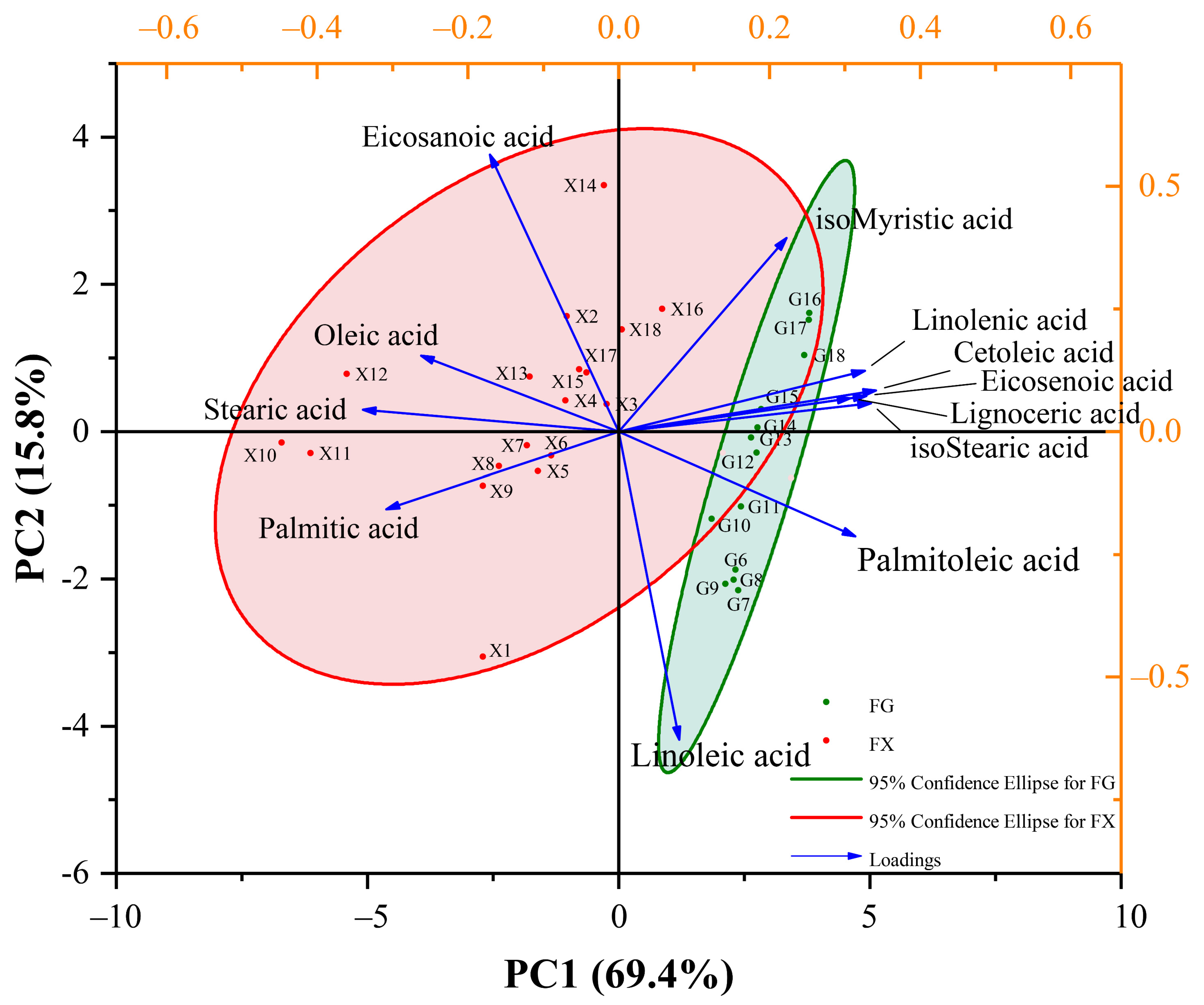
2.2.2. Principal Component Analysis
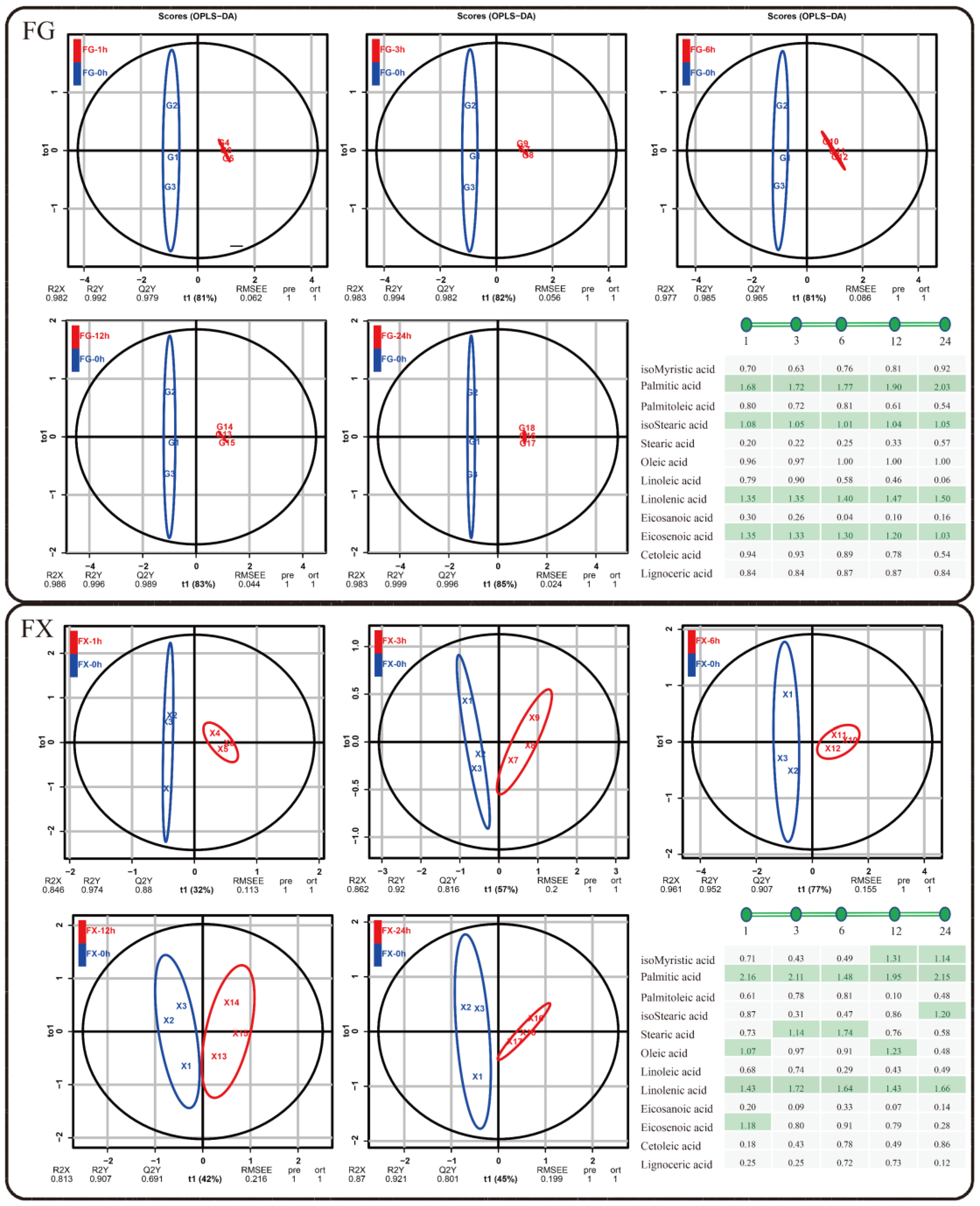
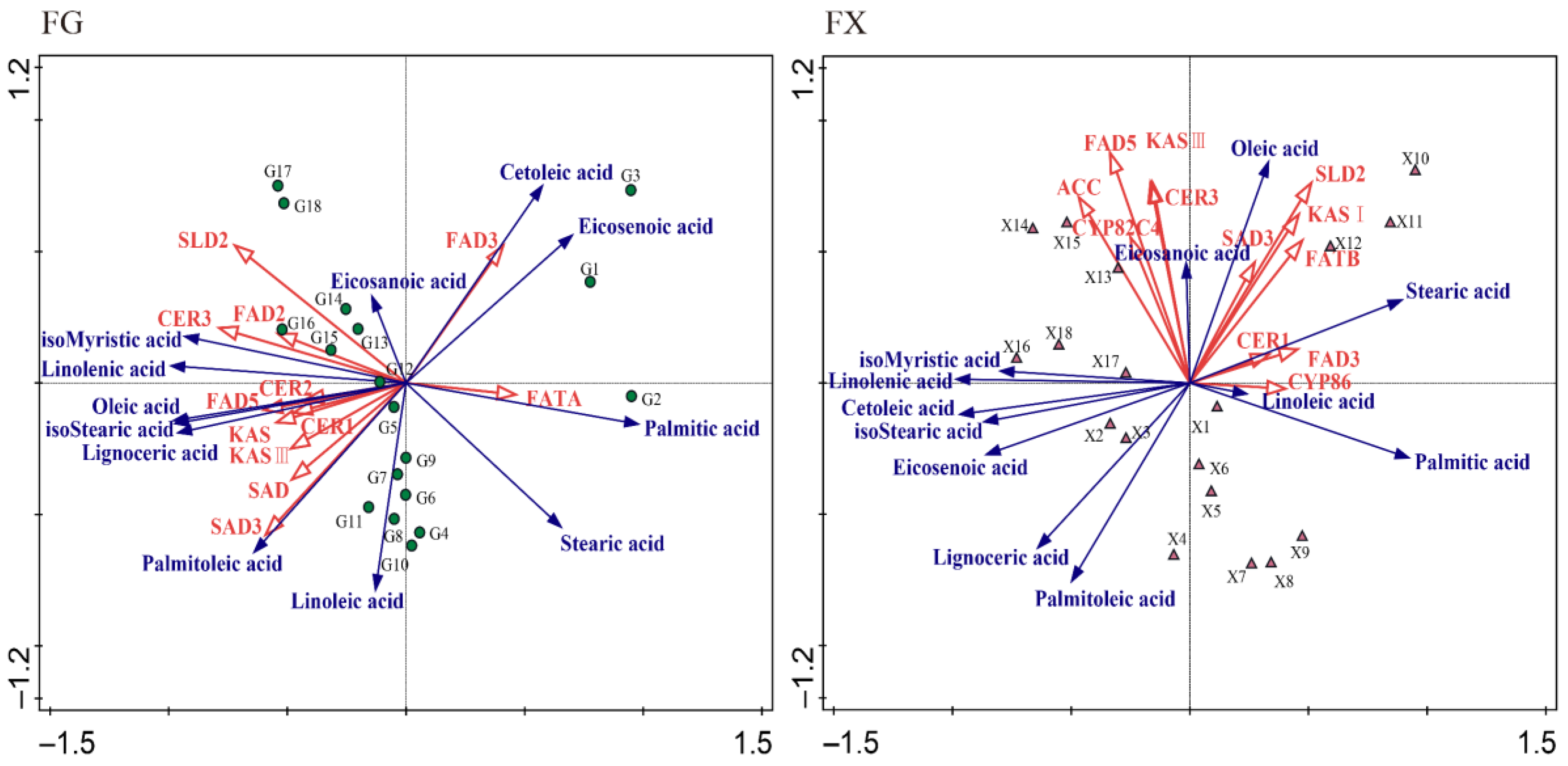
2.2.3. Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA)
2.3. Correlation and Redundancy (RDA) Analysis of Fatty Acid Content and Synthesis-Related Genes
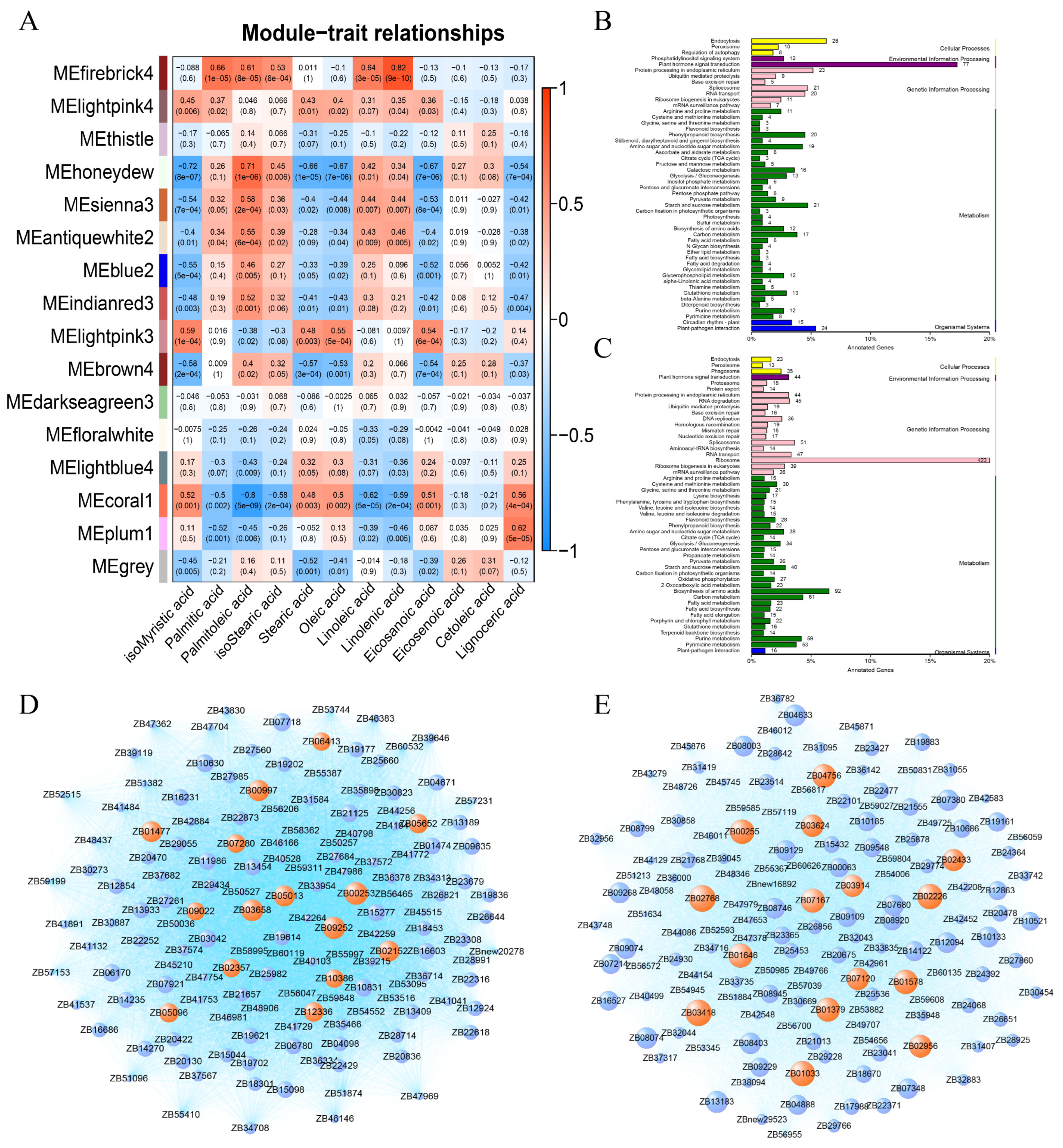
2.4. Weighted Gene Co-Expression Network Analysis (WGCNA) of Fatty Acids and Transcriptome in Response to Low Temperature in Z. bungeanum
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Cold Treatment
4.2. Fatty Acid Extraction and Composition Analysis by Gas Chromatography–Mass Spectrometry (GC-MS)
4.3. RNA Extraction and Sequencing
4.4. Quantitative Real-Time PCR Analysis of Fatty Acid Related Genes
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Feng, S.; Liu, Z.; Hu, Y.; Tian, J.; Yang, T.; Wei, A. Genomic analysis reveals the genetic diversity, population structure, evolutionary history and relationships of Chinese pepper. Hortic. Res. 2020, 7, 158. [Google Scholar] [CrossRef] [PubMed]
- Bader, M.; Stark, T.D.; Dawid, C.; Lösch, S.; Hofmann, T. All-trans-configuration in Zanthoxylum alkylamides swaps the tingling with a numbing sensation and diminishes salivation. J. Agric. Food Chem. 2014, 62, 2479–2488. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Deng, Y.-X.; Xie, H.; Yao, C.-Y.; Cai, C.-C.; Pan, S.-L.; Wang, Y.-L. Antinociceptive and anti-inflammatory activities of ethyl acetate fraction from Zanthoxylum armatum in mice. Fitoterapia 2011, 82, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Ibrar, M.; Muhammad, N.; Khan, A.; Khan, S.A.; Zafar, S.; Jan, S.; Riaz, N.; Ullah, Z.; Farooq, U.; Hussain, J. Pharmacognostic and phytochemical studies of Zanthoxylum armatum DC. Pak. J. Pharm. Sci. 2017, 30, 429–438. [Google Scholar]
- Zhang, M.; Wang, J.; Zhu, L.; Li, T.; Jiang, W.; Zhou, J.; Peng, W.; Wu, C. Zanthoxylum bungeanum Maxim. (Rutaceae): A systematic review of its traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics, and toxicology. Int. J. Mol. Sci. 2017, 18, 2172. [Google Scholar] [CrossRef]
- Ding, J. Genome-wide analysis of three fatty acids biosynthesis gene family and in Gossypium raimondii and the expression pattern of their corresponding orthologs in Tetraploid cultivated cotton (G. hirsutum). Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2015. [Google Scholar]
- Lee, C.-H.; Olson, P.; Evans, R. Minireview: Lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology 2003, 144, 2201–2207. [Google Scholar] [CrossRef] [Green Version]
- Pérez, M.D.L.C.M.; Sánchez-Calvo, B.; Padilla, M.N.; Begara-Morales, J.C.; Valderrama, R.; Corpas, F.J.; Barroso, J.B. Nitro-fatty acids in plant signaling: New key mediators of nitric oxide metabolism. Redox Biol. 2017, 11, 554–561. [Google Scholar] [CrossRef]
- Okazaki, Y.; Saito, K. Roles of lipids as signaling molecules and mitigators during stress response in plants. Plant J. 2014, 79, 584–596. [Google Scholar] [CrossRef]
- Miquel, M.; James, D.; Dooner, H.; Browse, J. Arabidopsis requires polyunsaturated lipids for low-temperature survival. Proc. Natl. Acad. Sci. USA 1993, 90, 6208–6212. [Google Scholar] [CrossRef] [Green Version]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef]
- Routaboul, J.-M.; Fischer, S.F.; Browse, J. Trienoic fatty acids are required to maintain chloroplast function at low temperatures. Plant Physiol. 2000, 124, 1697–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Yu, C.; Liu, Y.; Yang, L.; Li, Y.; Yao, W.; Cai, Y.; Yan, X.; Li, S.; Cai, Y.; et al. GmFAD3A, A ω-3 fatty acid desaturase gene, enhances cold tolerance and seed germination rate under low temperature in rice. Int. J. Mol. Sci. 2019, 20, 3796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horiguchi, G.; Fuse, T.; Kawakami, N.; Kodama, H.; Iba, K. Temperature-dependent translational regulation of the ER omega-3 fatty acid desaturase gene in wheat root tips. Plant J. 2000, 24, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Falcone, D.L.; Ogas, J.P.; Somerville, C.R. Regulation of membrane fatty acid composition by temperature in mutants of Arabidopsis with alterations in membrane lipid composition. BMC Plant Biol. 2004, 4, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodama, H.; Horiguchi, G.; Nishiuchi, T.; Nishimura, M.; Iba, K. Fatty acid desaturation during chilling acclimation is one of the factors involved in conferring low-temperature tolerance to young tobacco leaves. Plant Physiol. 1995, 107, 1177–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Cao, Y.; Fan, X.; Li, M.; Wang, Y.; Ming, F. A rice microsomal delta-12 fatty acid desaturase can enhance resistance to cold stress in yeast and Oryza sativa. Mol. Breed. 2011, 29, 743–757. [Google Scholar] [CrossRef]
- Ménard, R.; Verdier, G.; Ors, M.; Erhardt, M.; Beisson, F.; Shen, W.-H. Histone H2B monoubiquitination is involved in the regulation of cutin and wax composition in Arabidopsis thaliana. Plant Cell Physiol. 2014, 55, 455–466. [Google Scholar] [CrossRef]
- Ni, Y.; Song, C.; Wang, X. Investigation on response mechanism of epicuticular wax on Arabidopsis thaliana under cold stress. Sci. Agric. Sin. 2014, 47, 252–261. [Google Scholar]
- Thomas, D.; Barber, H. Studies on leaf characteristics of a cline of Eucalyptus urnigera from mount wellington, tasmania. I. water repellency and the freezing of leaves. Aust. J. Bot. 1974, 22, 501–512. [Google Scholar] [CrossRef]
- Jenks, M.A.; Eigenbrode, S.D.; Lemieux, B. Cuticular waxes of Arabidopsis. Arab. Book 2002, 1, e0016. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, Z.; Liu, H.; Peng, D.; Zhang, J.; Chen, M. Linum usitatissimum FAD2A and FAD3A enhance seed polyunsaturated fatty acid accumulation and seedling cold tolerance in Arabidopsis thaliana. Plant Sci. 2021, 311, 111014. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Yang, Y.; Yang, T.; Ba, L.; Zhang, H.; Han, Y.; Xiao, Y.; Shan, W.; Kuang, J.; Chen, J.; et al. MaMYB4 recruits histone deacetylase mahda2 and modulates the expression of ω-3 fatty acid desaturase genes during cold stress response in banana fruit. Plant Cell Physiol. 2019, 60, 2410–2422. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, X.; He, J.; Xie, Y.; Xu, Y.; Ma, F.; Guan, Q. Apple time for coffee contributes to freezing tolerance by promoting unsaturation of fatty acids. Plant Sci. 2021, 302, 110695. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.C.; Coelho, N.; Olsson, M.E.; Brodelius, P.E.; Carvalho, I.S.; Brodelius, M. Molecular cloning and expression analysis of three omega-6 desaturase genes from purslane (Portulaca oleracea L.). Biotechnol. Lett. 2009, 31, 1089–1101. [Google Scholar] [CrossRef]
- Shahandashti, S.S.K.; Amiri, R.M.; Zeinali, H.; Ramezanpour, S.S. Change in membrane fatty acid compositions and cold-induced responses in chickpea. Mol. Biol. Rep. 2012, 40, 893–903. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Ding, N.-Z. Plant unsaturated fatty acids: Multiple roles in stress response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef]
- Lyons, J.M. Chilling injury in plants. Annu. Rev. Plant Physiol. 1973, 24, 445–466. [Google Scholar] [CrossRef]
- Ma, N.; Wang, Y.; Zhou, K.; Fu, C.; Ding, Z. Determination of fatty acid component of tea leaves with the nature temperature decrease. J. Qingdao Agric. Univ. 2012, 29, 101–105. [Google Scholar]
- Da Cruz, R.P.; Golombieski, J.I.; Bazana, M.T.; Cabreira, C.; Silveira, T.F.; da Silva, L.P. Alterations in fatty acid composition due to cold exposure at the vegetative stage in rice. Braz. J. Plant Physiol. 2010, 22, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Lim, G.; Singhal, R.; Kachroo, A.; Kachroo, P. Fatty acid– and lipid-mediated signaling in plant defense. Annu. Rev. Phytopathol. 2017, 55, 505–536. [Google Scholar] [CrossRef]
- Tehlivets, O.; Scheuringer, K.; Kohlwein, S.D. Fatty acid synthesis and elongation in yeast. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2007, 1771, 255–270. [Google Scholar] [CrossRef]
- Harwood, J. Recent advances in the biosynthesis of plant fatty acids. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1996, 1301, 7–56. [Google Scholar] [CrossRef]
- Salas, J.J.; Ohlrogge, J.B. Characterization of substrate specificity of plant FatA and FatB acyl-ACP thioesterases. Arch. Biochem. Biophys. 2002, 403, 25–34. [Google Scholar] [CrossRef]
- Zhang, M.; Barg, R.; Yin, M.; Gueta-Dahan, Y.; Leikin-Frenkel, A.; Salts, Y.; Shabtai, S.; Ben-Hayyim, G. Modulated fatty acid desaturation via overexpression of two distinct omega-3 desaturases differentially alters tolerance to various abiotic stresses in transgenic tobacco cells and plants. Plant J. 2005, 44, 361–371. [Google Scholar] [CrossRef]
- Domínguez, T.; Hernández, M.L.; Pennycooke, J.C.; Jiménez, P.; Martínez-Rivas, J.M.; Sanz, C.; Stockinger, E.J.; Sánchez-Serrano, J.J.; Sanmartín, M. Increasing omega-3 desaturase expression in tomato results in altered aroma profile and enhanced resistance to cold stress. Plant Physiol. 2010, 153, 655–665. [Google Scholar] [CrossRef] [Green Version]
- Saucedo-García, M.; Gavilanes-Ruíz, M.; Arce-Cervantes, O. Long-chain bases, phosphatidic acid, MAPKs, and reactive oxygen species as nodal signal transducers in stress responses in Arabidopsis. Front. Plant Sci. 2015, 6, 55. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.-J.; Zhang, T.; Liu, Z.-H.; Chen, X.; Guo, H.-S.; Ju, B.-H.; Zhang, Y.-Y.; Li, G.-Z.; Zhou, Q.-H.; Qin, Y.-M.; et al. Phytosphinganine affects plasmodesmata permeability via facilitating pdlp5-stimulated callose accumulation in Arabidopsis. Mol. Plant 2020, 13, 128–143. [Google Scholar] [CrossRef]
- Chen, M.; Markham, J.E.; Cahoon, E.B. Sphingolipid Δ8 unsaturation is important for glucosylceramide biosynthesis and low-temperature performance in Arabidopsis. Plant J. 2011, 69, 769–781. [Google Scholar] [CrossRef]
- Samuels, L.; Kunst, L.; Jetter, R. Sealing plant surfaces: Cuticular wax formation by epidermal cells. Annu. Rev. Plant Biol. 2008, 59, 683–707. [Google Scholar] [CrossRef] [Green Version]
- Rahman, T.; Shao, M.; Pahari, S.; Venglat, P.; Soolanayakanahally, R.; Qiu, X.; Rahman, A.; Tanino, K. Dissecting the roles of cuticular wax in plant resistance to shoot dehydration and low-temperature stress in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 1554. [Google Scholar] [CrossRef]
- Bernard, A.; Domergue, F.; Pascal, S.; Jetter, R.; Renne, C.; Faure, J.-D.; Haslam, R.; Napier, J.; Lessire, R.; Joubès, J. Reconstitution of plant alkane biosynthesis in yeast demonstrates that Arabidopsis eceriferum1 and eceriferum3 are core components of a very-long-chain alkane synthesis complex. Plant Cell 2012, 24, 3106–3118. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. String Tie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, S.; Liu, Z.; Cheng, J.; Li, Z.; Tian, L.; Liu, M.; Yang, T.; Liu, Y.; Liu, Y.; Dai, H.; et al. Zanthoxylum-specific whole genome duplication and recent activity of transposable elements in the highly repetitive paleotetraploid Z. bungeanum genome. Hortic. Res. 2021, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Ma, Y.; Tian, L.; Huang, C.; Chen, M.; Wei, A. Comparative physiology and transcriptome response patterns in cold-tolerant and cold-sensitive varieties of Zanthoxylum bungeanum Maxim. Ind. Crop. Prod. 2021, 167, 113562. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
| Fatty Acid (mg·g−1) | TFA | USFA | SFA | IUFA |
|---|---|---|---|---|
| FG-0 h | 1.92 ± 0.10 cde | 1.14 ± 0.11 cde | 0.78 ± 0.03 b | 2.63 ± 0.19 cd |
| FG-1 h | 2.52 ± 0.20 bc | 1.63 ± 0.14 b | 0.89 ± 0.06 b | 4.30 ± 0.38 b |
| FG-3 h | 2.41 ± 0.27 bcd | 1.57 ± 0.18 bc | 0.84 ± 0.09 b | 4.13 ± 0.47 b |
| FG-6 h | 2.32 ± 0.24 cde | 1.51 ± 0.17 bc | 0.81 ± 0.07 b | 3.99 ± 0.47 b |
| FG-12 h | 2.51 ± 0.14 bc | 1.68 ± 0.08 b | 0.84 ± 0.06 b | 4.50 ±0.22 b |
| FG-24 h | 4.11 ± 0.99 a | 2.93 ± 0.72 a | 1.18 ± 0.27 a | 8.03 ± 1.96 a |
| FX-0 h | 1.72 ± 0.24 e | 0.98 ± 0.16 de | 0.74 ± 0.08 b | 2.38 ± 0.36 cd |
| FX-1 h | 1.91 ± 0.18 cde | 1.04 ± 0.10 de | 0.88 ± 0.08 b | 2.64 ± 0.25 cd |
| FX-3 h | 1.67 ± 0.12 e | 0.85 ± 0.05 e | 0.81 ± 0.08 b | 2.13 ± 0.11 d |
| FX-6 h | 1.83 ± 0.17 de | 0.91 ± 0.09 de | 0.93 ± 0.09 b | 2.16 ± 0.19 d |
| FX-12 h | 3.02 ± 0.38 b | 1.81 ± 0.29 b | 1.21 ± 0.10 a | 4.67 ± 0.76 b |
| FX-24 h | 2.24 ± 0.09 cde | 1.34 ± 0.09 bcd | 0.90 ± 0.02 b | 3.52 ± 0.28 bc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Tian, L.; Chen, M.; Chen, Y.; Wei, A. Low Temperature Affects Fatty Acids Profiling and Key Synthesis Genes Expression Patterns in Zanthoxylum bungeanum Maxim. Int. J. Mol. Sci. 2022, 23, 2319. https://doi.org/10.3390/ijms23042319
Tian J, Tian L, Chen M, Chen Y, Wei A. Low Temperature Affects Fatty Acids Profiling and Key Synthesis Genes Expression Patterns in Zanthoxylum bungeanum Maxim. International Journal of Molecular Sciences. 2022; 23(4):2319. https://doi.org/10.3390/ijms23042319
Chicago/Turabian StyleTian, Jieyun, Lu Tian, Ming Chen, Yabing Chen, and Anzhi Wei. 2022. "Low Temperature Affects Fatty Acids Profiling and Key Synthesis Genes Expression Patterns in Zanthoxylum bungeanum Maxim" International Journal of Molecular Sciences 23, no. 4: 2319. https://doi.org/10.3390/ijms23042319
APA StyleTian, J., Tian, L., Chen, M., Chen, Y., & Wei, A. (2022). Low Temperature Affects Fatty Acids Profiling and Key Synthesis Genes Expression Patterns in Zanthoxylum bungeanum Maxim. International Journal of Molecular Sciences, 23(4), 2319. https://doi.org/10.3390/ijms23042319





