An Engineered Nanocomplex with Photodynamic and Photothermal Synergistic Properties for Cancer Treatment
Abstract
1. Introduction
2. Results
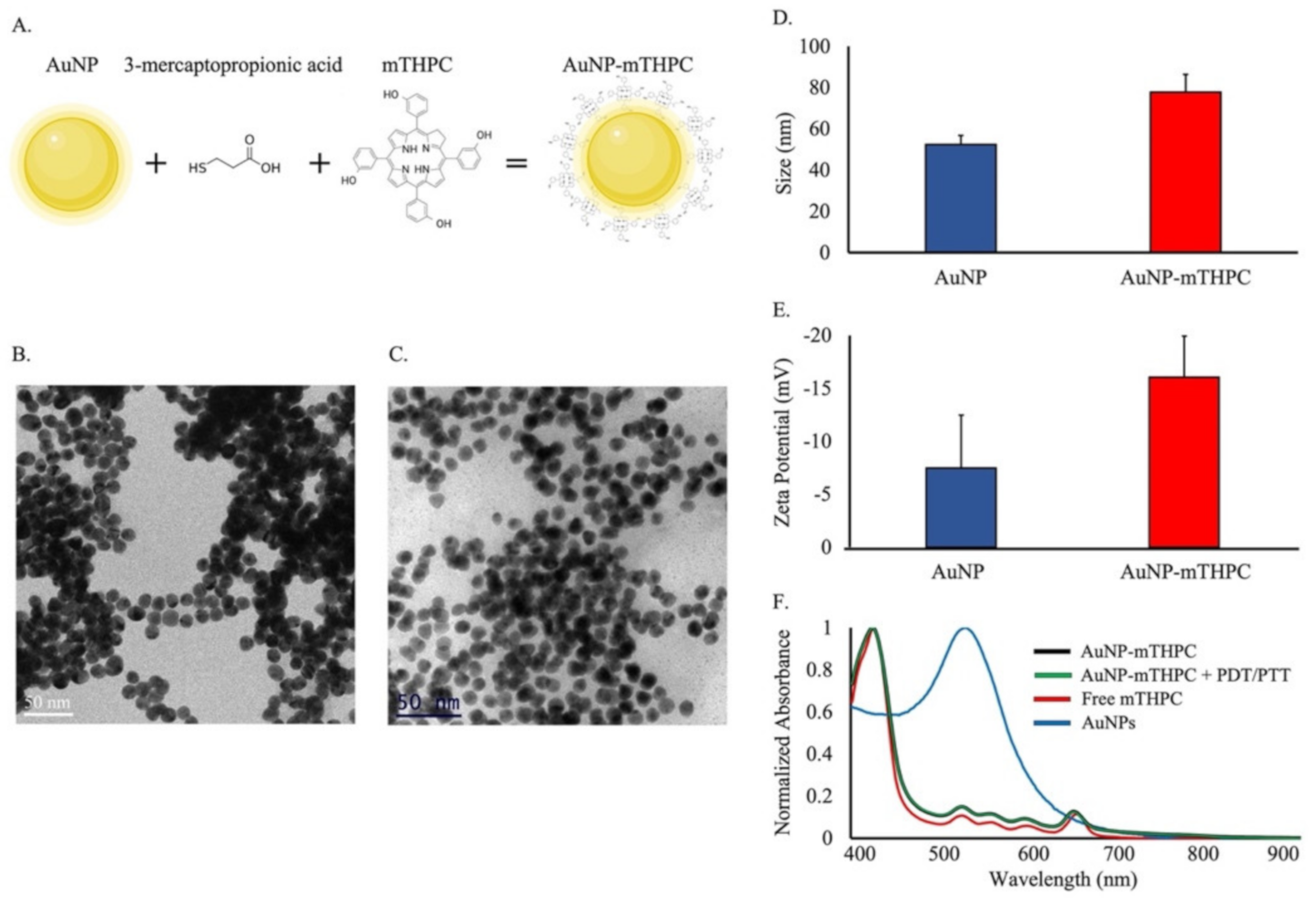
2.1. Synthesis and Characterization of AuNP–mTHPC Complexes
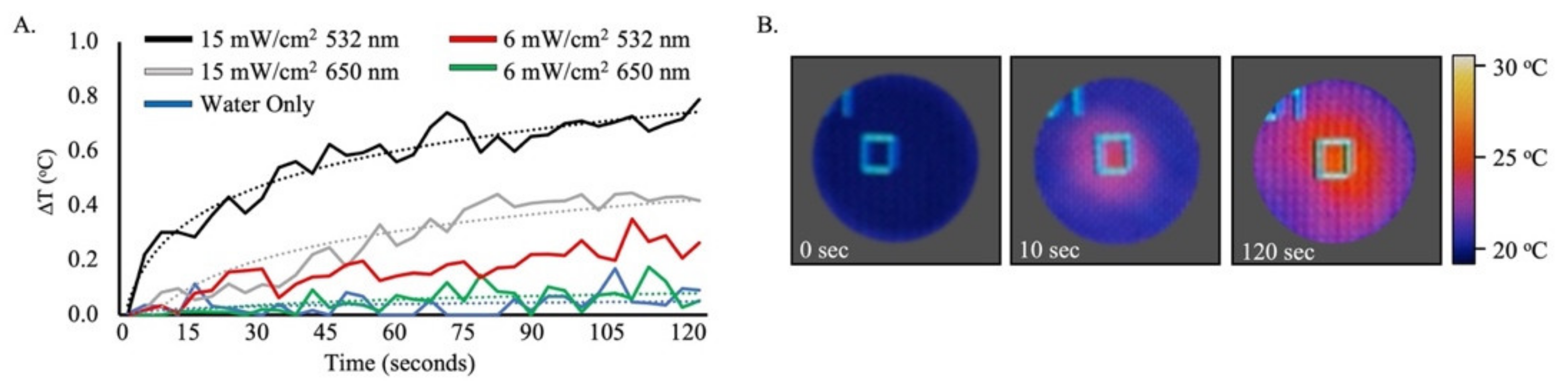
2.2. Photothermal Imaging of AuNP–mTHPC Complexes
2.3. Internalization of AuNP–mTHPC Complexes by SH-SY5Y Cells
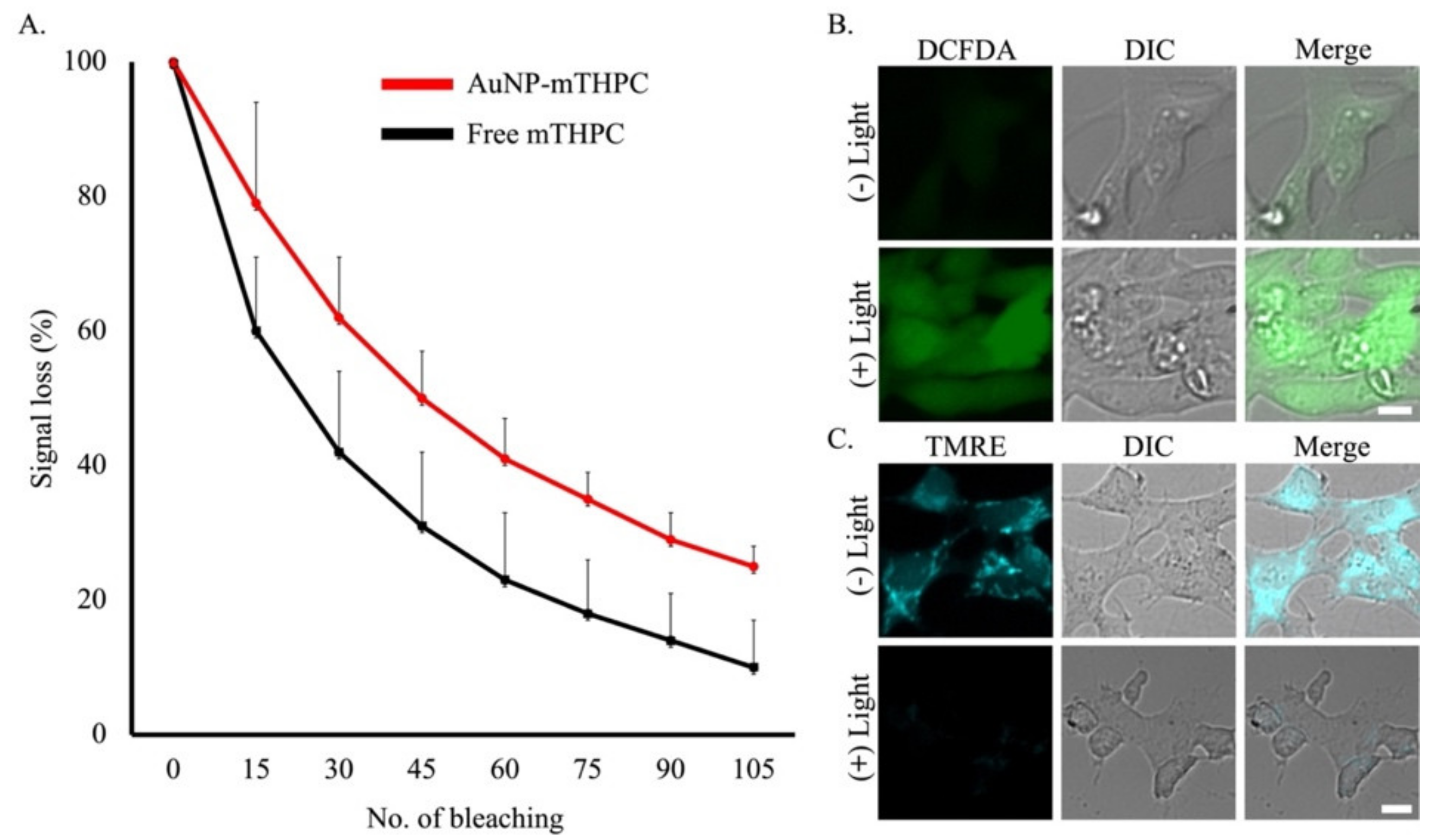
2.4. The mTHPC Stability Properties and Reactive Oxygen Species’ (ROS) Generation Disturbs Mitochondrial Membrane Potential (∆ψm)
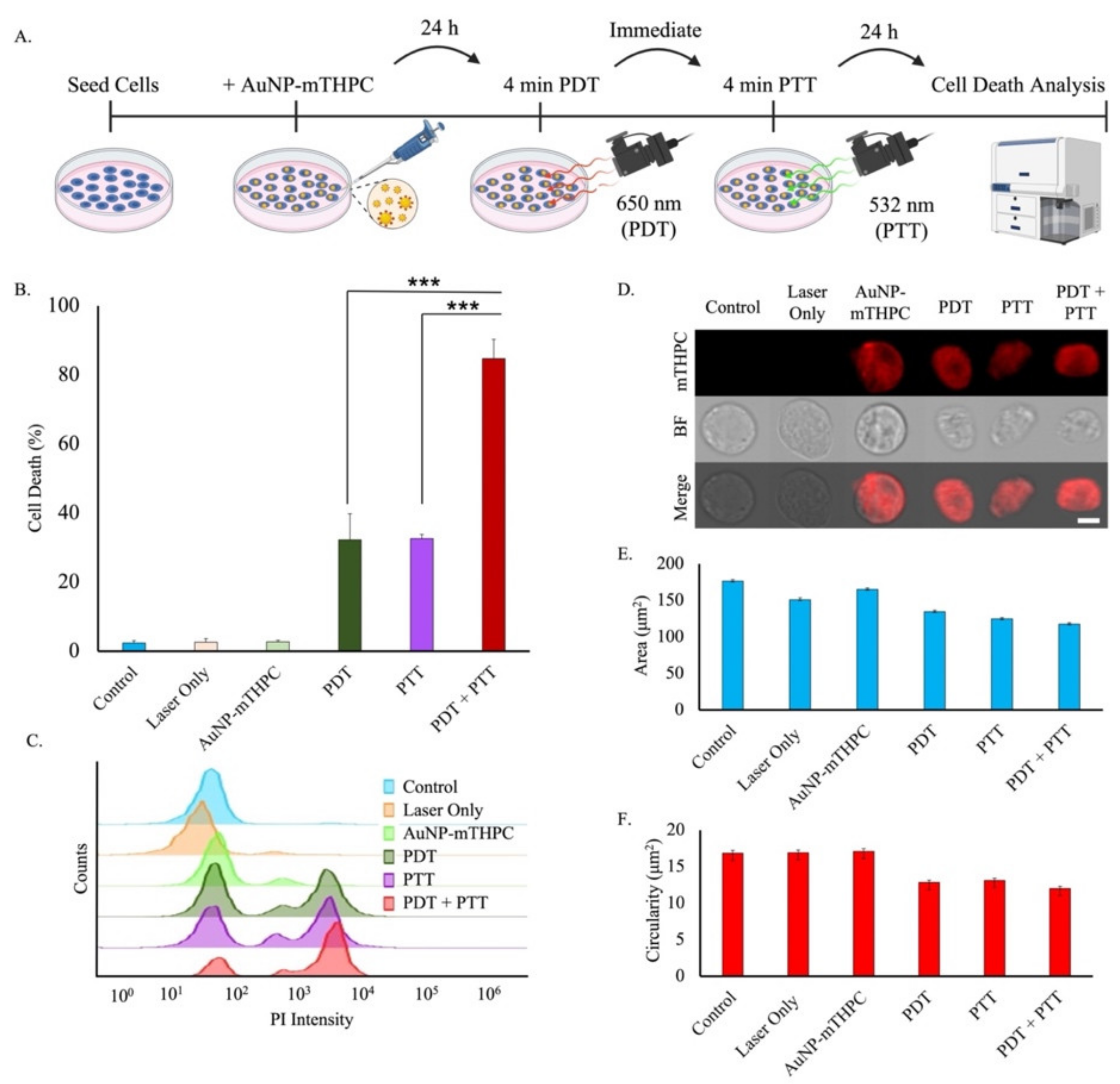
2.5. Phototoxicity of AuNP–mTHPC
2.6. Dual PDT/PTT Treatment Results in a Synergetic Effect
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation and Characterization of AuNP−mTHPC Conjugates
4.3. Cell Culture
4.4. TEM and HR-SEM
4.5. Optical Characterization of AuNP–mTHPC
4.6. Confocal and Wide-Field Microscopy
4.7. Flow Cytometry Studies
4.8. Modeling and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sawyers, C. Targeted cancer therapy. Nature 2004, 432, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Amreddy, N.; Babu, A.; Muralidharan, R.; Panneerselvam, J.; Srivastava, A.; Ahmed, R.; Mehta, M.; Munshi, A.; Ramesh, R. Recent Advances in Nanoparticle-Based Cancer Drug and Gene Delivery. Adv. Cancer Res. 2018, 137, 115–170. [Google Scholar] [CrossRef] [PubMed]
- Zilony, N.; Tzur-Balter, A.; Segal, E.; Shefi, O. Bombarding Cancer: Biolistic Delivery of therapeutics using Porous Si Carriers. Sci. Rep. 2013, 3, 2499. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Carter, K.A.; Miranda, D.; Lovell, J.F. Chemophototherapy: An Emerging Treatment Option for Solid Tumors. Adv. Sci. 2017, 4, 1600106. [Google Scholar] [CrossRef]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef]
- Min, C.; Zou, X.; Yang, Q.; Liao, L.; Zhou, G.; Liu, L. Near-infrared Light Responsive Polymeric Nanocomposites for Cancer Therapy. Curr. Top. Med. Chem. 2017, 17, 1805–1814. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic Therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Ozog, D.M.; Rkein, A.M.; Fabi, S.G.; Gold, M.H.; Goldman, M.P.; Lowe, N.J.; Martin, G.M.; Munavalli, G.S. Photodynamic Therapy: A Clinical Consensus Guide. Dermatol. Surg. 2016, 42, 804–827. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.W.; Dougherty, T.J. How does photodynamic therapy work? Photochem. Photobiol. 1992, 55, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Shafirstein, G.; Bellnier, D.; Oakley, E.; Hamilton, S.; Potasek, M.; Beeson, K.; Parilov, E. Interstitial Photodynamic Therapy—A Focused Review. Cancers 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Wagnieres, G.A.; Star, W.M.; Wilson, B.C. In Vivo Fluorescence Spectroscopy and Imaging for Oncological Applications. Photochem. Photobiol. 1998, 68, 603. [Google Scholar] [CrossRef]
- Foote, C.S. Definition of type I and type II photosensitized oxidation. Photochem. Photobiol. 1991, 54, 659. [Google Scholar] [CrossRef]
- Li, M.; Gao, Y.; Yuan, Y.; Wu, Y.; Song, Z.; Tang, B.Z.; Liu, B.; Zheng, Q.C. One-Step Formulation of Targeted Aggregation-Induced Emission Dots for Image-Guided Photodynamic Therapy of Cholangiocarcinoma. ACS Nano 2017, 11, 3922–3932. [Google Scholar] [CrossRef]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef]
- Huang, Z. A Review of Progress in Clinical Photodynamic Therapy. Technol. Cancer Res. Treat. 2005, 4, 283–293. [Google Scholar] [CrossRef]
- Senge, M.O.; Brandt, J.C. Temoporfin (Foscan®, 5,10,15,20-Tetra(m-hydroxyphenyl)chlorin)—A Second-generation Photosensitizer. Photochem. Photobiol. 2011, 87, 1240–1296. [Google Scholar] [CrossRef]
- Horlings, R.K.; Terra, J.B.; Witjes, M.J. mTHPC mediated, systemic photodynamic therapy (PDT) for nonmelanoma skin cancers: Case and literature review. Lasers Surg. Med. 2015, 47, 779–787. [Google Scholar] [CrossRef]
- Senge, M.O. mTHPC—A drug on its way from second to third generation photosensitizer? Photodiagn. Photodyn. Ther. 2012, 9, 170–179. [Google Scholar] [CrossRef]
- Haimov, E.; Weitman, H.; Polani, S.; Schori, H.; Zitoun, D.; Shefi, O. meso-Tetrahydroxyphenylchlorin-Conjugated Gold Nanoparticles as a Tool to Improve Photodynamic Therapy. ACS Appl. Mater. Interfaces 2018, 10, 2319–2327. [Google Scholar] [CrossRef]
- D’Cruz, A.K.; Robinson, M.H.; Biel, M.A. mTHPC-mediated photodynamic therapy in patients with advanced, incurable head and neck cancer: A multicenter study of 128 patients. Head Neck 2004, 26, 232–240. [Google Scholar] [CrossRef]
- Zimmermann, A.; Ritsch-Marte, M.; Kostron, H. mTHPC-mediated photodynamic diagnosis of malignant brain tumors. Photochem. Photobiol. 2001, 74, 611–616. [Google Scholar] [CrossRef]
- Ell, C.; Gossner, L.; May, A.; Schneider, H.T.; Hahn, E.G.; Stolte, M.; Sroka, R. Photodynamic ablation of early cancers of the stomach by means of mTHPC and laser irradiation: Preliminary clinical experience. Gut 1998, 43, 345–349. [Google Scholar] [CrossRef][Green Version]
- Bonnett, R.; Martínez, G. Photobleaching of Compounds of the 5,10,15,20-Tetrakis(m-hydroxyphenyl)porphyrin Series (m-THPP, m-THPC, and m-THPBC). Org. Lett. 2002, 4, 2013–2016. [Google Scholar] [CrossRef]
- Veenhuizen, R.B.; Ruevekamp, M.C.; Oppelaar, H.; Helmerhorst, T.J.; Kenemans, P.; Stewart, F.A. Foscan-mediated photodynamic therapy for a peritoneal-cancer model: Drug distribution and efficacy studies. Int. J. Cancer 1997, 73, 230–235. [Google Scholar] [CrossRef]
- Mitra, S.; Foster, T.H. Photophysical Parameters, Photosensitizer Retention and Tissue Optical Properties Completely Account for the Higher Photodynamic Efficacy of meso-Tetra-Hydroxyphenyl-Chlorin vs Photofrin. Photochem. Photobiol. 2007, 81, 849–859. [Google Scholar] [CrossRef]
- Ma, L.; Moan, J.; Berg, K. Evaluation of a new photosensitizer, meso-tetra-hydroxyphenyl-chlorin, for use in photodynamic therapy: A comparison of its photobiological properties with those of two other photosensitizers. Int. J. Cancer 1994, 57, 883–888. [Google Scholar] [CrossRef]
- Bonnett, R.; White, R.D.; Winfield, U.J.; Berenbaum, M.C. Hydroporphyrins of the meso-tetra(hydroxyphenyl)porphyrin series as tumour photosensitizers. Biochem. J. 1989, 261, 277–280. [Google Scholar] [CrossRef]
- Radu, A.; Wagnières, G.; Bergh, H.V.D.; Monnier, P. Photodynamic Therapy of Early Squamous Cell Cancers of the Esophagus. Gastrointest. Endosc. Clin. N. Am. 2000, 10, 439–460. [Google Scholar] [CrossRef]
- Glanzmann, T.; Hadjur, C.; Zellweger, M.; Grosiean, P.; Forrer, M.; Ballini, J.P.; Monnier, P.; van den Bergh, H.; Lim, C.K.; Wagnières, G. Pharmacokinetics of tetra(m-hydroxyphenyl)chlorin in human plasma and individualized light dosimetry in photodynamic therapy. Photochem. Photobiol. 1998, 67, 596–602. [Google Scholar] [PubMed]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2004, 56, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Pitsillides, C.M.; Joe, E.K.; Wei, X.; Anderson, R.R.; Lin, C.P. Selective Cell Targeting with Light-Absorbing Microparticles and Nanoparticles. Biophys. J. 2003, 84, 4023–4032. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.; El-Sayed, I.H.; El-Sayed, M.A. Determination of the Minimum Temperature Required for Selective Photothermal Destruction of Cancer Cells with the Use of Immunotargeted Gold Nanoparticles. Photochem. Photobiol. 2006, 82, 412–417. [Google Scholar] [CrossRef]
- Baranes, K.; Shevach, M.; Shefi, O.; Dvir, T. Gold Nanoparticle-Decorated Scaffolds Promote Neuronal Differentiation and Maturation. Nano Lett. 2016, 16, 2916–2920. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.; Fixler, D.; Popovtzer, R.; Shefi, O. Fluorescence life-time imaging and steady state polarization for examining binding of fluorophores to gold nanoparticles. J. Biophotonics 2015, 8, 944–951. [Google Scholar] [CrossRef]
- Polak, P.; Zalevsky, Z.; Shefi, O. Gold nanoparticles-based biosensing of single nucleotide DNA mutations. Int. J. Biol. Macromol. 2013, 59, 134–137. [Google Scholar] [CrossRef]
- Alon, N.; Duadi, H.; Cohen, O.; Samet, T.; Zilony, N.; Schori, H.; Shefi, O.; Zalevsky, Z. Promotion of neural sprouting using low-level green light-emitting diode phototherapy. J. Biomed. Opt. 2015, 20, 20502. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Marago, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef]
- Richardson, H.H.; Carlson, M.T.; Tandler, P.J.; Hernandez, P.; Govorov, A.O. Experimental and Theoretical Studies of Light-to-Heat Conversion and Collective Heating Effects in Metal Nanoparticle Solutions. Nano Lett. 2009, 9, 1139–1146. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef]
- Popovtzer, R.; Sinvani, M.; Jakoboshn, K.; Motiei, M. Towards real-time detection of tumor margins using photothermal imaging of immune-targeted gold nanoparticles. Int. J. Nanomed. 2012, 7, 4707–4713. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Pal, T. Interparticle Coupling Effect on the Surface Plasmon Resonance of Gold Nanoparticles: From Theory to Applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef]
- Waldow, S.M.; Morrison, P.R.; Grossweiner, L.I. Nd:YAG laser-induced hyperthermia in a mouse tumor model. Lasers Surg. Med. 1988, 8, 510–514. [Google Scholar] [CrossRef]
- Zou, L.; Wang, H.; He, B.; Zeng, L.; Tan, T.; Cao, H.; He, X.; Zhang, Z.; Guo, S.; Li, Y. Current Approaches of Photothermal Therapy in Treating Cancer Metastasis with Nanotherapeutics. Theranostics 2016, 6, 762–772. [Google Scholar] [CrossRef]
- Haimov-Talmoud, E.; Harel, Y.; Schori, H.; Motiei, M.; Atkins, A.; Popovtzer, R.; Lellouche, J.-P.; Shefi, O. Magnetic Targeting of mTHPC To Improve the Selectivity and Efficiency of Photodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 45368–45380. [Google Scholar] [CrossRef]
- Navalon, S.; Sempere, D.; Alvaro, M.; Garcia, H. Influence of Hydrogen Annealing on the Photocatalytic Activity of Diamond-Supported Gold Catalysts. ACS Appl. Mater. Interfaces 2013, 5, 7160–7169. [Google Scholar] [CrossRef]
- Gouterman, M. Spectra of porphyrins. J. Mol. Spectrosc. 1961, 6, 138–163. [Google Scholar] [CrossRef]
- Danan, Y.; Yariv, I.; Zalevsky, Z.; Sinvani, M. Improved Margins Detection of Regions Enriched with Gold Nanoparticles inside Biological Phantom. Materials 2017, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Karni, M.; Zidon, D.; Polak, P.; Zalevsky, Z.; Shefi, O. Thermal Degradation of DNA. DNA Cell Biol. 2013, 32, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Crawford, B.M.; Vo-Dinh, T. Gold nanoparticles-mediated photothermal therapy and immunotherapy. Immunotherapy 2018, 10, 1175–1188. [Google Scholar] [CrossRef]
- Marcus, M.; Karni, M.; Baranes, K.; Levy, I.; Alon, N.; Margel, S.; Shefi, O. Iron oxide nanoparticles for neuronal cell applications: Uptake study and magnetic manipulations. J. Nanobiotechnol. 2016, 14, 37. [Google Scholar] [CrossRef]
- Hilf, R. Mitochondria are targets of photodynamic therapy. J. Bioenerg. Biomembr. 2007, 39, 85–89. [Google Scholar] [CrossRef]
- Kessel, D.; Luo, Y. Mitochondrial photodamage and PDT-induced apoptosis. J. Photochem. Photobiol. B Biol. 1998, 42, 89–95. [Google Scholar] [CrossRef]
- Martins, W.K.; Santos, N.F.; Rocha, C.D.S.; Bacellar, I.O.L.; Tsubone, T.M.; Viotto, A.C.; Matsukuma, A.Y.; de P. Abrantes, A.B.; Siani, P.; Dias, L.; et al. Parallel damage in mitochondria and lysosomes is an efficient way to photoinduce cell death. Autophagy 2019, 15, 259–279. [Google Scholar] [CrossRef]
- Taba, F.; Onoda, A.; Hasegawa, U.; Enoki, T.; Ooyama, Y.; Ohshita, J.; Hayashi, T. Mitochondria-Targeting Polyamine-Protoporphyrin Conjugates for Photodynamic Therapy. ChemMedChem 2018, 13, 15–19. [Google Scholar] [CrossRef]
- Wilson, B.C.; Olivo, M.; Singh, G. Subcellular Localization of Photofrin and Aminolevulinic Acid and Photodynamic Cross-Resistance in Vitro in Radiation-Induced Fibrosarcoma Cells Sensitive or Resistant to Photofrin-Mediated Photodynamic Therapy. Photochem. Photobiol. 1997, 65, 166–176. [Google Scholar] [CrossRef]
- Cranfill, P.J.; Sell, B.R.; Baird, M.A.; Allen, J.R.; Lavagnino, Z.; de Gruiter, H.M.; Kremers, G.J.; Davidson, M.W.; Ustione, A.; Piston, D.W. Quantitative assessment of fluorescent proteins. Nat. Methods 2016, 13, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Bacellar, I.O.L.; Tsubone, T.M.; Pavani, C.; Baptista, M.D.S. Photodynamic Efficiency: From Molecular Photochemistry to Cell Death. Int. J. Mol. Sci. 2015, 16, 20523–20559. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Guo, C.; Li, D.; Liu, R.; Xu, X.; Guo, J.W.; Ding, J.; Li, J.; Chen, W.; Zhang, J. Hydrogen Peroxide-Activatable Nanoparticles for Luminescence Imaging and In Situ Triggerable Photodynamic Therapy of Cancer. ACS Appl. Mater. Interfaces 2020, 12, 17230–17243. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.-M.; Xue, L.-Y.; Usuda, J.; Azizuddin, K.; Oleinick, N.L. Bax is essential for mitochondrion-mediated apoptosis but not for cell death caused by photodynamic therapy. Br. J. Cancer 2003, 89, 1590–1597. [Google Scholar] [CrossRef]
- Crowley, L.C.; Christensen, M.E.; Waterhouse, N.J. Measuring Mitochondrial Transmembrane Potential by TMRE Staining. Cold Spring Harb. Protoc. 2016. [Google Scholar] [CrossRef]
- George, T.C.; Basiji, D.A.; Hall, B.E.; Lynch, D.H.; Ortyn, W.E.; Perry, D.J.; Seo, M.J.; Zimmerman, C.A.; Morrissey, P.J. Distinguishing modes of cell death using the ImageStream® multispectral imaging flow cytometer. Cytom. Part A 2004, 59, 237–245. [Google Scholar] [CrossRef]
- Bortner, C.D.; Cidlowski, J. Uncoupling Cell Shrinkage from Apoptosis Reveals That Na+ Influx Is Required for Volume Loss during Programmed Cell Death. J. Biol. Chem. 2003, 278, 39176–39184. [Google Scholar] [CrossRef]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell Death Pathways in Photodynamic Therapy of Cancer. Cancers 2011, 3, 2516–2539. [Google Scholar] [CrossRef]
- Vanneman, M.; Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 2012, 12, 237–251. [Google Scholar] [CrossRef]
- Chou, T.-C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Tallarida, R. Drug synergism: Its detection and applications. J. Pharmacol. Exp. Ther. 2001, 298, 865–872. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varon, E.; Blumrosen, G.; Sinvani, M.; Haimov, E.; Polani, S.; Natan, M.; Shoval, I.; Jacob, A.; Atkins, A.; Zitoun, D.; et al. An Engineered Nanocomplex with Photodynamic and Photothermal Synergistic Properties for Cancer Treatment. Int. J. Mol. Sci. 2022, 23, 2286. https://doi.org/10.3390/ijms23042286
Varon E, Blumrosen G, Sinvani M, Haimov E, Polani S, Natan M, Shoval I, Jacob A, Atkins A, Zitoun D, et al. An Engineered Nanocomplex with Photodynamic and Photothermal Synergistic Properties for Cancer Treatment. International Journal of Molecular Sciences. 2022; 23(4):2286. https://doi.org/10.3390/ijms23042286
Chicago/Turabian StyleVaron, Eli, Gaddi Blumrosen, Moshe Sinvani, Elina Haimov, Shlomi Polani, Michal Natan, Irit Shoval, Avi Jacob, Ayelet Atkins, David Zitoun, and et al. 2022. "An Engineered Nanocomplex with Photodynamic and Photothermal Synergistic Properties for Cancer Treatment" International Journal of Molecular Sciences 23, no. 4: 2286. https://doi.org/10.3390/ijms23042286
APA StyleVaron, E., Blumrosen, G., Sinvani, M., Haimov, E., Polani, S., Natan, M., Shoval, I., Jacob, A., Atkins, A., Zitoun, D., & Shefi, O. (2022). An Engineered Nanocomplex with Photodynamic and Photothermal Synergistic Properties for Cancer Treatment. International Journal of Molecular Sciences, 23(4), 2286. https://doi.org/10.3390/ijms23042286









