Blood Vessels: The Pathway Used by Schwann Cells to Colonize Nerve Conduits
Abstract
:1. Introduction
2. Results
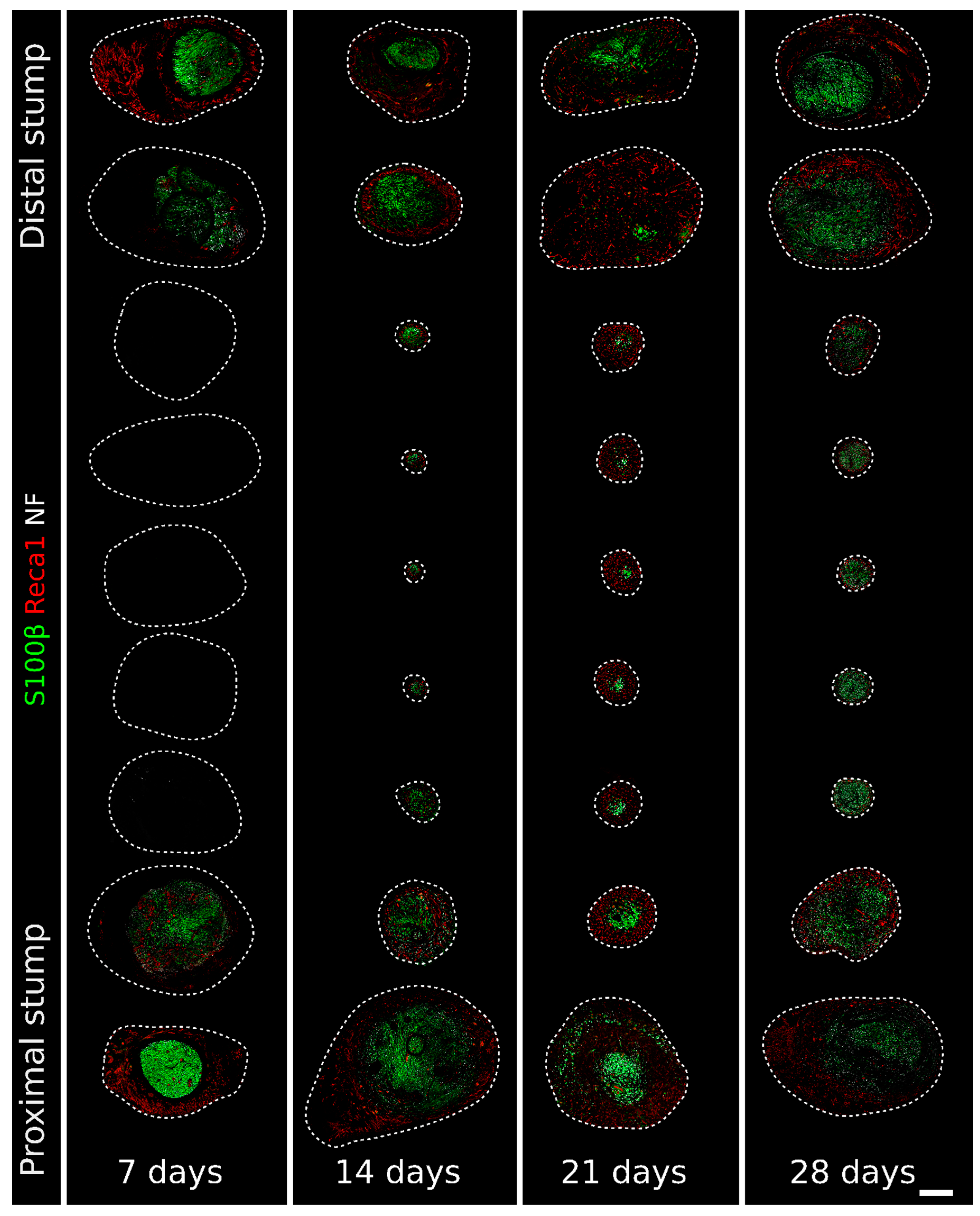
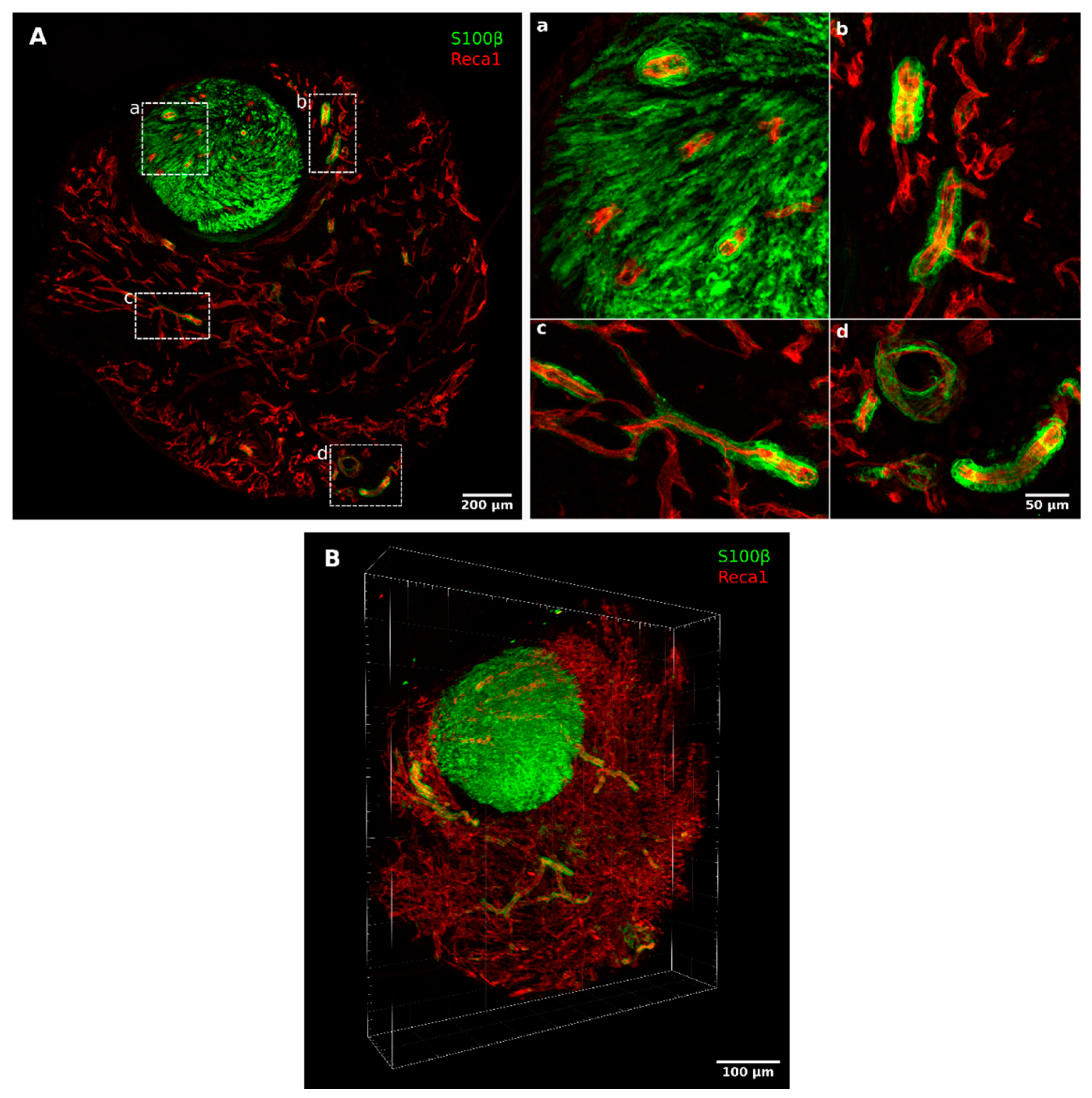
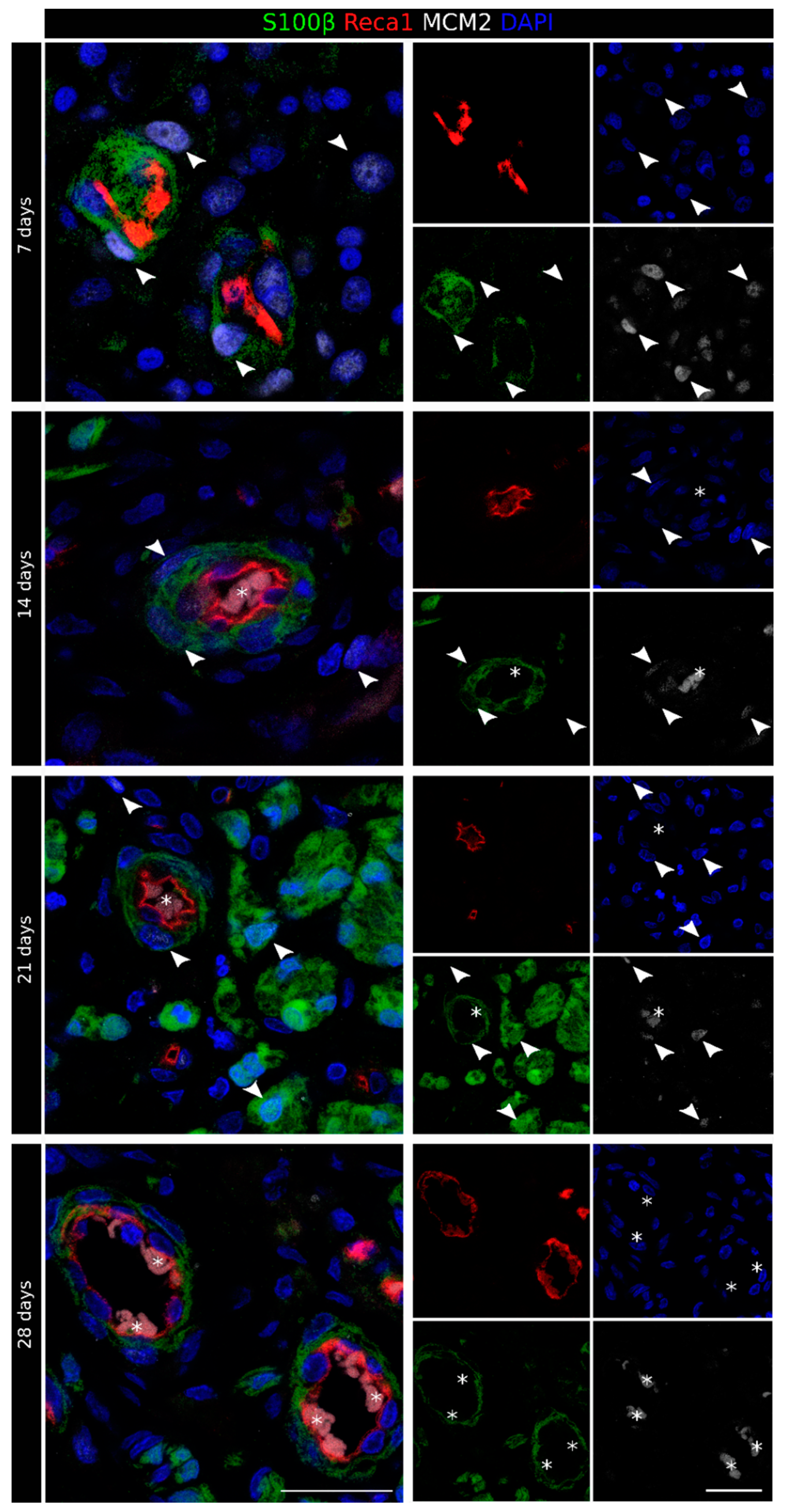
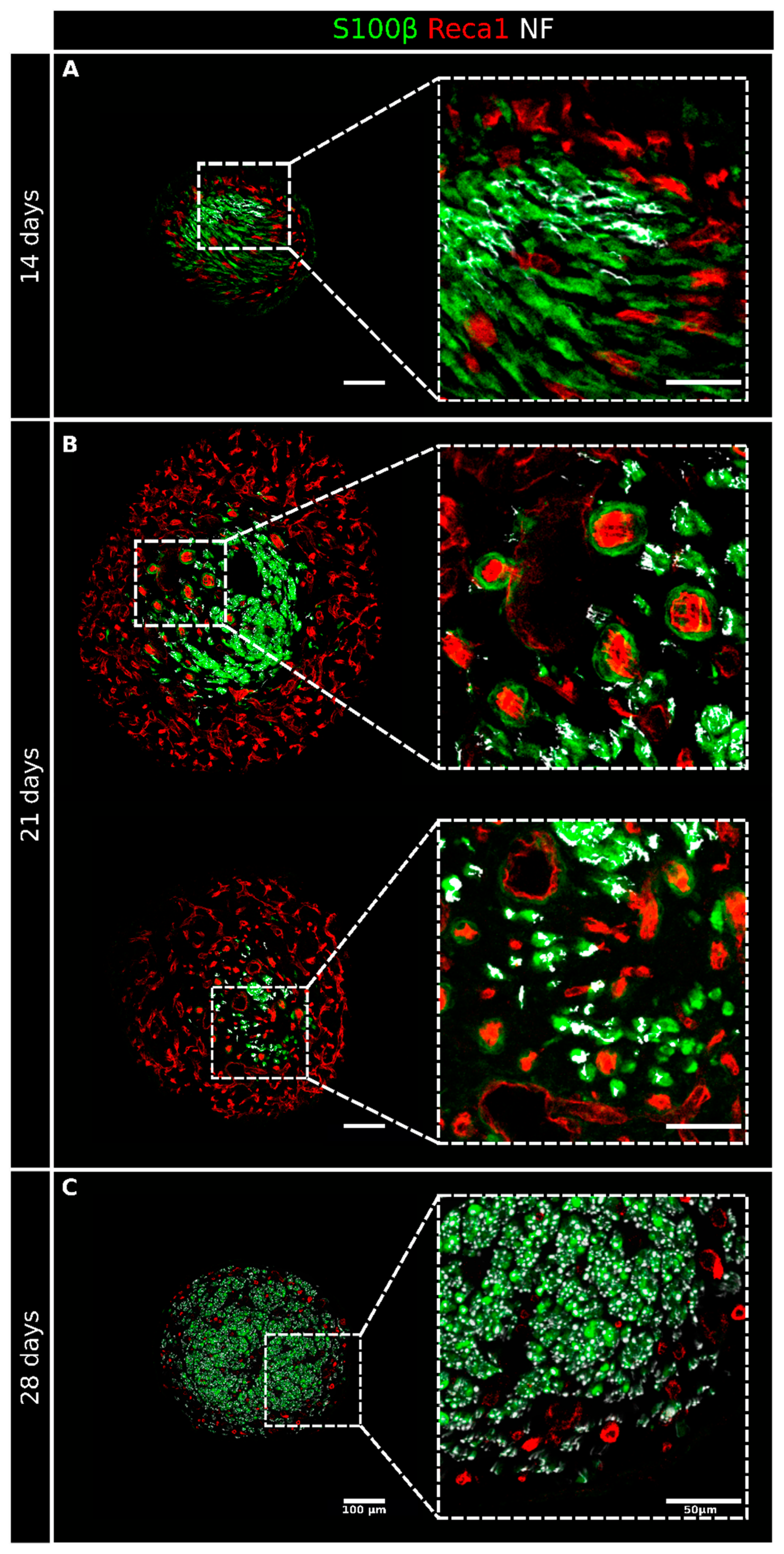
2.1. Migrating Schwann Cells Use Newly Formed Blood Vessels as a Substrate for Migration within the Conduit
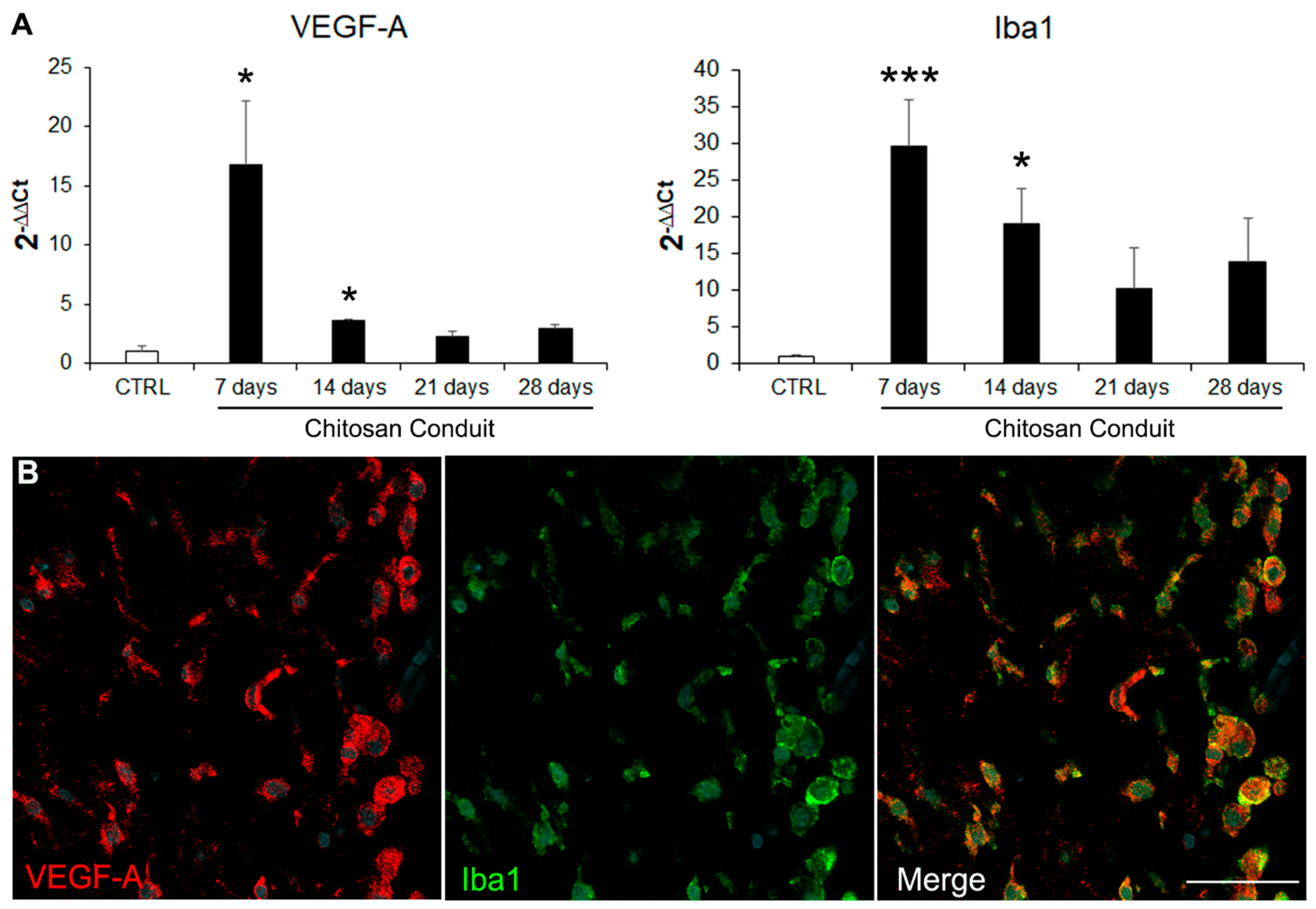
2.2. VEGF-A Is Highly Expressed inside the Chitosan Conduit 7 Days after the Nerve Injury and Repair
3. Discussion
4. Materials and Methods
4.1. In Vivo Surgical Procedure
4.2. Immunofluorescence Analysis
4.3. Three-Dimensional Reconstructions
4.4. RNA Extraction, cDNA Preparation, and Quantitative Real-Time PCR (qRT-PCR) Analysis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Modrak, M.; Talukder, M.A.H.; Gurgenashvili, K.; Noble, M.; Elfar, J.C. Peripheral nerve injury and myelination: Potential therapeutic strategies. J. Neurosci. Res. 2020, 98, 780–795. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.W. Evaluation and management of peripheral nerve injury. Clin. Neurophysiol. 2008, 119, 1951–1965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhandari, P. Management of peripheral nerve injury. J. Clin. Orthop. Trauma 2019, 10, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Jurecka, W.; Ammerer, H.; Lassmann, H. Regeneration of a transected peripheral nerve. An autoradiographic and electron microscopic study. Acta Neuropathol. 1975, 32, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.; Kucenas, S. Perineurial glia are essential for motor axon regrowth following nerve injury. J. Neurosci. 2014, 34, 12762–12777. [Google Scholar] [CrossRef] [Green Version]
- Cattin, A.L.; Burden, J.J.; Van Emmenis, L.; MacKenzie, F.E.; Hoving, J.J.A.; Garcia Calavia, N.; Guo, Y.; McLaughlin, M.; Rosenberg, L.H.; Quereda, V.; et al. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef] [Green Version]
- Parrinello, S.; Napoli, I.; Ribeiro, S.; Wingfield Digby, P.; Fedorova, M.; Parkinson, D.B.; Doddrell, R.D.S.; Nakayama, M.; Adams, R.H.; Lloyd, A.C. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell 2010, 143, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Deumens, R.; Bozkurt, A.; Meek, M.F.; Marcus, M.A.E.; Joosten, E.A.J.; Weis, J.; Brook, G.A. Repairing injured peripheral nerves: Bridging the gap. Prog. Neurobiol. 2010, 92, 245–276. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef] [Green Version]
- Kataria, H.; Alizadeh, A.; Karimi-Abdolrezaee, S. Neuregulin-1/ErbB network: An emerging modulator of nervous system injury and repair. Prog. Neurobiol. 2019, 180, 101643. [Google Scholar] [CrossRef]
- Fornasari, B.E.; El Soury, M.; Nato, G.; Fucini, A.; Carta, G.; Ronchi, G.; Crosio, A.; Perroteau, I.; Geuna, S.; Raimondo, S.; et al. Fibroblasts Colonizing Nerve Conduits Express High Levels of Soluble Neuregulin1, a Factor Promoting Schwann Cell Dedifferentiation. Cells 2020, 9, 1366. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Sant, D.; Andersen, N.; Silvera, R.; Camarena, V.; Piñero, G.; Graham, R.; Khan, A.; Xu, X.M.; Wang, G.; et al. Magnetic separation of peripheral nerve-resident cells underscores key molecular features of human Schwann cells and fibroblasts: An immunochemical and transcriptomics approach. Sci. Rep. 2020, 10, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Li, R.H.; Chen, J.; Hammonds, G.; Phillips, H.; Armanini, M.; Wood, P.; Bunge, R.; Godowski, P.J.; Sliwkowski, M.X.; Mather, J.P. Identification of Gas6 as a growth factor for human Schwann cells. J. Neurosci. 1996, 16, 2012–2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.; Hu, R.; Min, Q.; Li, Y.; Parkinson, D.B.; Dun, X. FGF5 Regulates Schwann Cell Migration and Adhesion. Front. Cell. Neurosci. 2020, 14, 237. [Google Scholar] [CrossRef]
- Min, Q.; Parkinson, D.B.; Dun, X.P. Migrating Schwann cells direct axon regeneration within the peripheral nerve bridge. Glia 2021, 69, 235–254. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, M.; Razavi, S.; Bakhtiari, A. The advances in nerve tissue engineering: From fabrication of nerve conduit to in vivo nerve regeneration assays. J. Tissue Eng. Regen. Med. 2019, 13, 2077–2100. [Google Scholar] [CrossRef]
- Belanger, K.; Dinis, T.M.; Taourirt, S.; Vidal, G.; Kaplan, D.L.; Egles, C. Recent Strategies in Tissue Engineering for Guided Peripheral Nerve Regeneration. Macromol. Biosci. 2016, 16, 472–481. [Google Scholar] [CrossRef]
- Boni, R.; Ali, A.; Shavandi, A.; Clarkson, A.N. Current and novel polymeric biomaterials for neural tissue engineering. J. Biomed. Sci. 2018, 25, 90. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, C.R.; Oliveira, J.M.; Reis, R.L. Modern Trends for Peripheral Nerve Repair and Regeneration: Beyond the Hollow Nerve Guidance Conduit. Front. Bioeng. Biotechnol. 2019, 7, 337. [Google Scholar] [CrossRef] [Green Version]
- Williams, L.R.; Longo, F.M.; Powell, H.C.; Lundborg, G.; Varon, S. Spatial-Temporal progress of peripheral nerve regeneration within a silicone chamber: Parameters for a bioassay. J. Comp. Neurol. 1983, 218, 460–470. [Google Scholar] [CrossRef]
- Schröder, J.M.; May, R.; Weis, J. Perineurial cells are the first to traverse gaps of peripheral nerves in silicone tubes. Clin. Neurol. Neurosurg. 1993, 95, 78–83. [Google Scholar] [CrossRef]
- Weis, J.; May, R.; Schröder, J.M. Fine structural and immunohistochemical identification of perineurial cells connecting proximal and distal stumps of transected peripheral nerves at early stages of regeneration in silicone tubes. Acta Neuropathol. 1994, 88, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Lundborg, G.; Gelberman, R.H.; Longo, F.M.; Powell, H.C.; Varon, S. In vivo regeneration of cut nerves encased in silicone tubes growth across a six-millimeter gap. J. Neuropathol. Exp. Neurol. 1982, 41, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, J.G.; Ai, X.; Hu, S.; Gu, Y.D.; Matloub, H.S.; Sanger, J.R. Ultrastructural analysis of peripheral-nerve regeneration within a nerve conduit. J. Reconstr. Microsurg. 2004, 20, 565–569. [Google Scholar] [CrossRef]
- Yousef, E.M.; Furrer, D.; Laperriere, D.L.; Tahir, M.R.; Mader, S.; DIorio, C.; Gaboury, L.A. MCM2: An alternative to Ki-67 for measuring breast cancer cell proliferation. Mod. Pathol. 2017, 30, 682–697. [Google Scholar] [CrossRef]
- Bovetti, S.; Hsieh, Y.C.; Bovolin, P.; Perroteau, I.; Kazunori, T.; Puche, A.C. Blood vessels form a scaffold for neuroblast migration in the adult olfactory bulb. J. Neurosci. 2007, 27, 5976–5980. [Google Scholar] [CrossRef]
- Tsai, H.H.; Niu, J.; Munji, R.; Davalos, D.; Chang, J.; Zhang, H.; Tien, A.C.; Kuo, C.J.; Chan, J.R.; Daneman, R.; et al. Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science 2016, 351, 379–384. [Google Scholar] [CrossRef] [Green Version]
- Fujioka, T.; Kaneko, N.; Ajioka, I.; Nakaguchi, K.; Omata, T.; Ohba, H.; Fässler, R.; García-Verdugo, J.M.; Sekiguchi, K.; Matsukawa, N.; et al. β1 integrin signaling promotes neuronal migration along vascular scaffolds in the post-stroke brain. EBioMedicine 2017, 16, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Diaz, B.; Bachelin, C.; Coulpier, F.; Gerschenfeld, G.; Deboux, C.; Zujovic, V.; Charnay, P.; Topilko, P.; Baron-Van Evercooren, A. Blood vessels guide Schwann cell migration in the adult demyelinated CNS through Eph/ephrin signaling. Acta Neuropathol. 2019, 138, 457–476. [Google Scholar] [CrossRef] [Green Version]
- Cattin, A.-L.; Lloyd, A.C. The multicellular complexity of peripheral nerve regeneration. Curr. Opin. Neurobiol. 2016, 39, 38–46. [Google Scholar] [CrossRef]
- Fornasari, B.E.; Carta, G.; Gambarotta, G.; Raimondo, S. Natural-Based Biomaterials for Peripheral Nerve Injury Repair. Front. Bioeng. Biotechnol. 2020, 8, 4257. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, G.; Fornasari, B.E.; Crosio, A.; Budau, C.A.; Tos, P.; Perroteau, I.; Battiston, B.; Geuna, S.; Raimondo, S.; Gambarotta, G. Chitosan Tubes Enriched with Fresh Skeletal Muscle Fibers for Primary Nerve Repair. Biomed. Res. Int. 2018, 2018, 9175248. [Google Scholar] [CrossRef] [PubMed]
- Crosio, A.; Fornasari, B.; Gambarotta, G.; Geuna, S.; Raimondo, S.; Battiston, B.; Tos, P.; Ronchi, G. Chitosan tubes enriched with fresh skeletal muscle fibers for delayed repair of peripheral nerve defects. Neural Regen. Res. 2019, 14, 1079. [Google Scholar] [CrossRef] [PubMed]
- Haastert-Talini, K.; Geuna, S.; Dahlin, L.B.; Meyer, C.; Stenberg, L.; Freier, T.; Heimann, C.; Barwig, C.; Pinto, L.F.V.; Raimondo, S.; et al. Chitosan tubes of varying degrees of acetylation for bridging peripheral nerve defects. Biomaterials 2013, 34, 9886–9904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapira, Y.; Tolmasov, M.; Nissan, M.; Reider, E.; Koren, A.; Biron, T.; Bitan, Y.; Livnat, M.; Ronchi, G.; Geuna, S.; et al. Comparison of results between chitosan hollow tube and autologous nerve graft in reconstruction of peripheral nerve defect: An experimental study. Microsurgery 2016, 36, 664–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Perez, F.; Cobianchi, S.; Geuna, S.; Barwig, C.; Freier, T.; Udina, E.; Navarro, X. Tubulization with chitosan guides for the repair of long gap peripheral nerve injury in the rat. Microsurgery 2015, 35, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Kornfeld, T.; Vogt, P.M.; Radtke, C. Nerve grafting for peripheral nerve injuries with extended defect sizes. Wien. Med. Wochenschr. 2018, 169, 240–251. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097. [Google Scholar] [CrossRef]
- Podhajsky, R.J.; Myers, R.R. The vascular response to nerve crush: Relationship to Wallerian degeneration and regeneration. Brain Res. 1993, 623, 117–123. [Google Scholar] [CrossRef]
- Hobson, M.I.; Brown, R.; Green, C.J.; Terenghi, G. Inter-relationships between angiogenesis and nerve regeneration: A histochemical study. Br. J. Plast. Surg. 1997, 50, 125–131. [Google Scholar] [CrossRef]
- Muangsanit, P.; Shipley, R.J.; Phillips, J.B. Vascularization Strategies for Peripheral Nerve Tissue Engineering. Anat. Rec. 2018, 301, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Pola, R.; Aprahamian, T.R.; Bosch-Marcé, M.; Curry, C.; Gaetani, E.; Flex, A.; Smith, R.C.; Isner, J.M.; Losordo, D.W. Age-dependent VEGF expression and intraneural neovascularization during regeneration of peripheral nerves. Neurobiol. Aging 2004, 25, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Farber, S.J.; Hoben, G.M.; Hunter, D.A.; Yan, Y.; Johnson, P.J.; Mackinnon, S.E.; Wood, M.D. Vascularization is delayed in long nerve constructs compared with nerve grafts. Muscle Nerve 2016, 54, 319–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nato, G.; Caramello, A.; Trova, S.; Avataneo, V.; Rolando, C.; Taylor, V.; Buffo, A.; Peretto, P.; Luzzati, F. Striatal astrocytes produce neuroblasts in an excitotoxic model of Huntington’s disease. Development 2015, 142, 840–845. [Google Scholar] [CrossRef] [Green Version]
- Luzzati, F. Combining Multichannel Confocal Laser Scanning Microscopy with Serial Section Reconstruction to Analyze Large Tissue Volumes at Cellular Resolution. Neuromethods 2014, 87, 83–103. [Google Scholar] [CrossRef]
- Preibisch, S.; Saalfeld, S.; Tomancak, P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 2009, 25, 1463. [Google Scholar] [CrossRef]
- Cardona, A.; Hartenstein, V.; Saalfeld, S.; Preibisch, S.; Schmid, B.; Cheng, A.; Pulokas, J.; Tomancak, P. An Integrated Micro- and Macroarchitectural Analysis of the Drosophila Brain by Computer-Assisted Serial Section Electron Microscopy. PLoS Biol. 2010, 8, e1000502. [Google Scholar] [CrossRef] [Green Version]
- Ronchi, G.; Haastert-Talini, K.; Fornasari, B.E.; Perroteau, I.; Geuna, S.; Gambarotta, G. The Neuregulin1/ErbB system is selectively regulated during peripheral nerve degeneration and regeneration. Eur. J. Neurosci. 2016, 43, 351–364. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gambarotta, G.; Ronchi, G.; Friard, O.; Galletta, P.; Perroteau, I.; Geuna, S. Identification and validation of suitable housekeeping genes for normalizing quantitative real-time PCR assays in injured peripheral nerves. PLoS ONE 2014, 9, e105601. [Google Scholar] [CrossRef]

| Antibodies for Immunofluorescence Analysis | ||||
|---|---|---|---|---|
| Primary Antibodies | ||||
| Code | Dilution | Host | Source | |
| Neurofilament/NF | Ab4680 | 1:10,000 | Chicken | Abcam |
| S100β | HPA015768 | 1:200 | Rabbit | Sigma-Merck |
| Reca1 | MCA970R | 1:500 | Mouse | Bio-Rad (AbD Serotec) |
| Iba1 | 019-19741 | 1:1000 | Rabbit | FUJIFILM Wako Chemicals U.S.A. Corporation |
| VEGF-A | ab1316 | 1:200 | Mouse | Abcam |
| MCM2 | sc-9839 | 1:500 | Goat | Santa Cruz Biotechnology |
| Secondary Antibodies | ||||
| Code | Dilution | Host | Source | |
| AlexaFluor 488 Anti-rabbit | 711-545-152 | 1:400 | Donkey | Jackson ImmunoResearch |
| AlexaFluor 488 Anti-goat | 705-545-147 | 1:400 | Donkey | Jackson ImmunoResearch |
| Cy3 Anti-Mouse | 715-165-151 | 1:800 | Donkey | Jackson ImmunoResearch |
| Cy3 Anti-rabbit | 711-165-152 | 1:800 | Donkey | Jackson ImmunoResearch |
| AlexaFluor 647 Anti-chicken | 703-605-155 | 1:800 | Donkey | Jackson ImmunoResearch |
| AlexaFluor 647 Anti-mouse | 715-605-151 | 1:800 | Donkey | Jackson ImmunoResearch |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fornasari, B.E.; Zen, F.; Nato, G.; Fogli, M.; Luzzati, F.; Ronchi, G.; Raimondo, S.; Gambarotta, G. Blood Vessels: The Pathway Used by Schwann Cells to Colonize Nerve Conduits. Int. J. Mol. Sci. 2022, 23, 2254. https://doi.org/10.3390/ijms23042254
Fornasari BE, Zen F, Nato G, Fogli M, Luzzati F, Ronchi G, Raimondo S, Gambarotta G. Blood Vessels: The Pathway Used by Schwann Cells to Colonize Nerve Conduits. International Journal of Molecular Sciences. 2022; 23(4):2254. https://doi.org/10.3390/ijms23042254
Chicago/Turabian StyleFornasari, Benedetta Elena, Federica Zen, Giulia Nato, Marco Fogli, Federico Luzzati, Giulia Ronchi, Stefania Raimondo, and Giovanna Gambarotta. 2022. "Blood Vessels: The Pathway Used by Schwann Cells to Colonize Nerve Conduits" International Journal of Molecular Sciences 23, no. 4: 2254. https://doi.org/10.3390/ijms23042254
APA StyleFornasari, B. E., Zen, F., Nato, G., Fogli, M., Luzzati, F., Ronchi, G., Raimondo, S., & Gambarotta, G. (2022). Blood Vessels: The Pathway Used by Schwann Cells to Colonize Nerve Conduits. International Journal of Molecular Sciences, 23(4), 2254. https://doi.org/10.3390/ijms23042254








