Abstract
Analysis of the function, structure, and intracellular organization of mitochondria is important for elucidating energy metabolism and intracellular energy transfer. In addition, basic and clinically oriented studies that investigate organ/tissue/cell dysfunction in various human diseases, including myopathies, cardiac/brain ischemia-reperfusion injuries, neurodegenerative diseases, cancer, and aging, require precise estimation of mitochondrial function. It should be noted that the main metabolic and functional characteristics of mitochondria obtained in situ (in permeabilized cells and tissue samples) and in vitro (in isolated organelles) are quite different, thereby compromising interpretations of experimental and clinical data. These differences are explained by the existence of the mitochondrial network, which possesses multiple interactions between the cytoplasm and other subcellular organelles. Metabolic and functional crosstalk between mitochondria and extra-mitochondrial cellular environments plays a crucial role in the regulation of mitochondrial metabolism and physiology. Therefore, it is important to analyze mitochondria in vivo or in situ without their isolation from the natural cellular environment. This review summarizes previous studies and discusses existing approaches and methods for the analysis of mitochondrial function, structure, and intracellular organization in situ.
1. Introduction
Mitochondria are involved in numerous metabolic pathways [1,2,3,4,5,6,7] and produce a major portion of intracellular ATP through the electron transport chain (ETC) in some cells, coupled with oxidative phosphorylation; they provide over 90% of cellular ATP in high oxygen consuming organs such as the heart, liver, and brain. Mitochondria are composed of two separate and functionally different membranes, the outer mitochondrial membrane (OMM) and the inner mitochondrial membrane (IMM), with the intermembrane space between them and the internal space known as the matrix enclosed by the IMM [6]. These organelles contain the circular genome, mitochondrial DNA (mtDNA), which was reduced in size during evolution through deletions or point mutations and its transfer to the nucleus. The size, shape, and number of mitochondria vary in different cell types. Cells contain independent and/or interconnected mitochondria, which create the subcellular mitochondrial network. In addition to ATP production, mitochondria also participate in other aspects of cell metabolism and physiology, such as the regulation of ion homeostasis, particularly intracellular Ca2+, fatty acid and cholesterol metabolism, redox status, cell survival, and cell death mechanisms [2,4,8,9,10,11,12,13,14]. Mitochondria bioenergetics and intracellular energy transfer are involved in the pathogenesis of numerous metabolic and hereditary diseases, including cardiovascular and neurodegenerative diseases, diabetes, cancer, and aging [15,16,17,18,19,20,21,22,23,24,25,26,27]. Mitochondria are the major source of reactive oxygen species (ROS) that, at low concentrations, participate in cellular signalling mechanisms whereas, at high concentrations, cause oxidative stress and cell death [22,23,24,25,26,27,28]. Notably, energy metabolism and crosstalk between cytoplasmic and mitochondrial ATP production are markedly different in cancer cells compared to non-cancer cells in terms of adaptation to environmental conditions. Therefore, a multifaceted and comprehensive analysis of mitochondrial function and morphology is important for the precise estimation of cell physiology and pathophysiology. In the following sections, we will discuss the biochemical and biophysical approaches that are currently applied to analyze the function and structural organization of mitochondria in situ or in vivo using techniques that do not require isolation of the organelles.
2. Advantages of Using Intact Cells or In Situ Approaches for Mitochondria Research
Mitochondrial oxygen consumption rates can be determined using a traditional Clark electrode in isolated mitochondria [29,30,31,32,33] in vitro and non-isolated mitochondria of intact permeabilized cells or muscle fibers in situ [6,34,35,36,37]). In addition, cytometry, capillary electrophoresis, patch-clamping, and optical trapping were used for the analysis of mitochondria [32]. Analysis of mitochondria in muscle biopsies remains an important initial screening procedure for the potential presence of metabolic diseases in humans. The changes in the mitochondrial structure, dynamics, organization, interaction with other cell systems, and several functional properties, such as redox state, mitochondrial ROS (mtROS), biogenesis, etc., can be analyzed with a confocal fluorescent imaging approach using TMRM or flavoproteins fluorescence (Figure 1) in situ or in vivo [6,38,39,40].
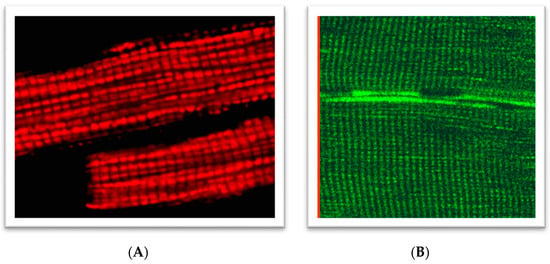
Figure 1.
Regular arrangements of mitochondria in: (A) rat cardiomyocytes loaded with 0.1 µM tetramethylrhodamine methyl ester (TMRM), 30 min incubation of cells at room temperature (543 nm laser excitation); (B) rat skeletal (quadriceps) muscles (flavoproteins autofluorescence, 488 nm laser excitation). A portion of the figure was reprinted with permission from ref [38,39]. Copyright 2006 Elsevier.
Figure 2 compares imaging of mitochondria in skeletal muscles performed by ultra-high-resolution scanning electron microscopy [41] to the specific fluorescent mitochondrial Ca2+ probe Rhod-2 [39]. These two images bear several similarities in terms of mitochondrial shapes, intracellular positions, and organization of these organelles in muscles, including numerous inter-mitochondrial contacts and clusters of the subsarcolemmal mitochondrial subpopulation. The major difference is that for electron microscopy, muscles were always fixed. In contrast, muscles for fluorescent imaging were not fixed and mitochondria were functional, thereby preserving their capacity for dynamics and accessibility for various experimental manipulations.
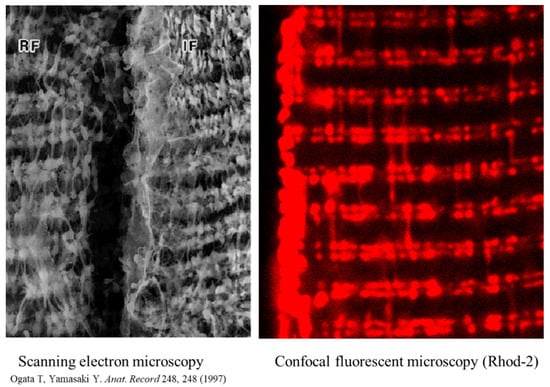
Figure 2.
Mitochondrial intracellular arrangement in skeletal muscles, visualized by ultra-high-resolution scanning electron microscopy and in muscles loaded (60 min, room temperature) with 5 µM of the specific fluorescent mitochondrial Ca2+ probe Rhod-2AM. Reprinted with permission from ref [41,42]. Copyright 2006 Elsevier. Rhod-2 has a net positive charge, thereby allowing for its specific accumulation in mitochondria. Reprinted with permission from ref [41]. Copyright 2006 Elsevier. Fluorescence was measured using 543 nm for excitation (helium-neon laser) and greater than 580 nm for emission. Note: A portion of the figure was reprinted with permission from ref [39,43]. Copyright 2006 Elsevier.
A specific protocol was developed for the analysis of functional mitochondria in situ, without isolation of organelles, in selectively permeabilized cells or muscle fibers using digitonin or saponin, which only permeabilize the plasma membrane, thereby leaving mitochondrial membranes intact [6,33,34,35,36]. Digitonin and saponin specifically interact and solubilize cholesterol in the plasma membrane and thus permeabilize the membrane, leading to the formation of non-selective pores. The plasma membrane contains high cholesterol amounts, whereas the membranes of the sarco/endoplasmic reticulum or mitochondria have considerably lower cholesterol contents. Based on these differences, chemical permeabilizing agents, such as saponin, digitonin, filipin, solanine, tomatine, or alamethicin, selectively permeabilize the cells or muscle fibers [6,44,45,46,47,48,49,50,51], leaving the intracellular membranes of the mitochondria, sarco/endoplasmic reticulum, myofilaments, and cytoskeleton intact, and equilibrating the intracellular spaces with incubation medium. Therefore, the cell permeabilization technique provides a unique possibility to analyze human biopsies or genetically modified animals and can be used in basic research as well as for the diagnosis of various human diseases in clinically oriented studies.
Enzymatic assays of individual respiratory (ETC) complexes or other mitochondrial enzymes are widely used for the estimation of mitochondrial function under physiological and pathological conditions [52,53,54]. However, this method has been shown to be insufficient for the accomplished analysis of mitochondrial function and injury since all enzymes and their complexes interact with each other. Hence, oxidative phosphorylation should be investigated in intact mitochondria [32,55,56] through the measurement of oxygen consumption [57,58]. Standard procedures of organelle isolation based on differential centrifugation of tissue or cell homogenates allow for a precise characterization of functional properties of mitochondria in vitro [32]. Although remarkable knowledge has been gained using isolated mitochondria, this technique has several serious limitations: (i) it requires relatively large amounts of tissues or cells; (ii) mitochondria are frequently damaged by the isolation (several centrifugations and washings) procedure [59,60]; and (iii) mitochondria isolated from cells and tissues lack their essential intracellular environment. The isolation procedure has been shown to result in changes to mitochondrial morphology, sensitization to permeability transition pore (PTP) opening, changes in respiratory mitochondrial function, and increased mitochondrial ROS production [6,59,60]. Moreover, mitochondrial networks and all contacts of mitochondria within the sarco/endoplasmic reticulum and cytoskeleton are disrupted during isolation, which significantly affects their structural organization, metabolism, and physiology.
Cells are highly organized units with multiple and multifaceted functional and structural interactions between various subcellular systems. A large number of studies provide strong evidence that elucidating individual organelles alone is not sufficient, and systemic approaches must be applied for a better understanding of cell physiology, crosstalk between organelles, and cellular signaling pathways. Since mitochondria actively interact with the cytoskeleton and sarco/endoplasmic reticulum [8,12,61,62,63,64,65], the interaction between mitochondria and cytoskeletal proteins (plectin and tubulin beta II) and their connections beyond the voltage-dependent anion channel (VDAC) can be studied only in vivo or in situ. These interconnections are ultimately involved in the regulation of mitochondrial function and can be studied using combinations of several modern techniques. Several cytoskeletal elements play a vital role in the structural and functional organization of mitochondria, including mitochondrial morphology, dynamics, motility, and mitosis. In the heart, mitochondrial bioenergetics and oxygen consumption are linearly dependent on the cardiac contractile activity [66,67] and rather stable concentration of ADP, a regulator of mitochondrial respiration. The apparent Michaelis constant (appKm) for ADP is an important parameter for mitochondrial respiration; it indicates the affinity of mitochondrial respiration to ADP (response to ADP), thereby reflecting the permeability state of the OMM [63,68] that is calculated from the respiratory ADP kinetics. A high response to ADP (low appKm, about 10–30 µM) has been obtained for isolated mitochondria in vitro [58,59]. Interestingly, measurements of mitochondrial respiration in situ (e.g., in permeabilized cells) showed more than 10-fold higher (200–350 µM) appKm ADP values, demonstrating that their interaction with cytoskeletal proteins (plectin, tubulin beta II) could be essential for the permeability of the OMM and, therefore, for the regulation of mitochondrial respiratory function since the absence of certain cytoskeletal proteins leads to low appKmADP [69,70]. To overcome the limitations associated with isolated mitochondria and simultaneously maintain a similar high scope for experimental manipulations, a new method for studying the function of intact or permeabilized cells, muscle fibers, or tissue homogenates has been established [6,34,35,36,37,44,45,46,47,48,49,50,51]. This approach uses the capacity of biological detergents (mostly digitonin and saponin) to specifically interact with the plasma membrane cholesterol of cells or muscle fibers. In permeabilized cells or muscle fibers, the organization, structure, and function of mitochondria and the cytoskeletal proteins remain mostly intact [35,36,37]. The respiratory control ratio (state 3, after ADP addition)/(state 2, before ADP addition) in permeabilized muscle (e.g., quadriceps muscles) fibers can be even higher than in carefully isolated mitochondria. Moreover, mitochondria become fully accessible to specific substrates or inhibitors in these preparations and can be used for the mitochondrial respiratory analysis while remaining connected to other cellular systems. This is especially important considering recent evidence of enzymatic/metabolic channeling enzyme redistribution and nucleotides (ADP/ATP) compartmentation in the cell [63,71,72].
3. Analysis of ETC Complexes
A specifically designed substrate/inhibitor/uncoupler titration protocol is applied for a step-by-step functional analysis of mitochondrial ETC complexes (I, II, III, IV) under in situ-like conditions. Notably, numerous studies using permeabilized muscle fibers or cells confirmed that mitochondria are capable of utilizing various substrates (e.g., glutamate, malate, pyruvate, succinate, TMPD/ascorbate) with a high sensitivity to specific inhibitors of mitochondrial ETC complexes (rotenone, antimycin A, etc.), thereby demonstrating functional intactness of well-coupled mitochondria [6,73,74,75]. In addition, the analysis of β-oxidation of fatty acids or glutamine oxidation, medium- and long-chain acylcarnitines may also be used for the measurements [6,76]. The uncoupled maximum respiration flux can be induced by the addition of frequently used uncouplers such as CCCP or FCCP to collapse the proton-motive force through the IMM and thus estimate the maximum mitochondrial capacity. Since CCCP and FCCP are membrane-permeable agents, they can be used in intact living cells without permeabilization to estimate cellular respiration under uncoupled conditions and maximal mitochondrial capacity. CCCP and FCCP must be used at optimal concentrations since they have inhibitory effects at extremely high concentrations. Usually, the uncoupled control ratio (UCR) [73,74,75,77] in intact cells is in a range of 4.3–4.5. Importantly, endogenous and uncoupled respirations were found to be linearly dependent on the cell density in a range of 0.2–6.0 × 106 cells/mL. This mitochondrial uncoupling has also been suggested for a cardioprotection strategy under oxidative stress, diabetes, and ischemia-reperfusion injury [77,78]. In addition to respiratory parameters, analysis of the mitochondrial membrane potential using different fluorescent potential-dependent dyes (e.g., TMRM, JC-1) by FACS analysis and other techniques provides useful additional information about mitochondrial function.
4. Cytochrome c Test for the Assessment of the Intactness of OMM
Cytochrome c is a soluble peripheral membrane protein localized in the intermembrane space, which is only loosely bound to the IMM; this membrane facilitates the transport of electrons to complex IV, whereas other cytochromes represent integral proteins. When the OMM is intact, cytochrome c remains in the intermembrane space and the addition of exogenous cytochrome c does not affect respiration. However, if the OMM is damaged, the endogenous cytochrome c can be released from the IMS at physiological ionic strength. In this case, the exogenous cytochrome c added to the experimental chamber will remarkably stimulate the respiratory rates in permeabilized cells and muscle fibers [77]. After the addition of a saturating concentration of cytochrome c, the maximal respiration downregulated by cytochrome c depletion can be restored. In particular, the cytochrome c test is recommended to be performed in cases of multiple respiratory chain defects. The level of cytochrome c in mitochondria and its release into the cytosol is estimated in studies that elucidate the role of mitochondria in apoptosis [79].
5. Mitochondrial Creatine Kinase Coupling and Energy Transfer
Several isoforms of creatine kinases (CKs) are involved in cell energy metabolism and intracellular energy transport, in particular, for the synthesis of phosphocreatine in the intermembrane space by means of the mitochondrial isoform of CK (mi-CK). In the heart and oxidative muscles, mi-CK is coupled with oxidative phosphorylation through the ATP-ADP carrier under normal conditions [63,71,72]. Therefore, the addition of creatine (creatine test) substantially increases mitochondrial respiration since mi-CK acts as an ADP-regenerating system. Similarly, mitochondrial AMP kinase or hexokinase can be the regenerating systems for ADP upon the addition of AMP or glucose [63,80]. Previous studies showed that coupling of mi-CK is significantly hampered in cardiac pathologies, such as ischemia-reperfusion injury or congestive heart failure [81]. Moreover, the mi-CK system is highly sensitive to oxidative stress due to the oxidation of essential SH groups [82]. Therefore, the mi-CK functional activity and coupling can be considered a sensitive parameter for evidencing alterations in mitochondrial physiology and cell bioenergetics and can be used as a diagnostic tool in cardiac injuries. The simple creatine test allows for the rapid evaluation of the functional (coupling) state of this mitochondrial enzyme in tissue biopsies. This test can be even more sensitive for the evaluation of the quality of permeabilized muscle fibers or cardiomyocyte preparations than simple measurements of fluxes.
More recently, a microplate-based assay (Seahorse assay [83]) of oxygen consumption rates was used for the assessment of cellular respiration (kinetics of oxygen concentration) in intact cells. In particular, this approach was successively applied to the measurement of respiration of non-permeabilized murine skeletal muscle cells [84,85,86,87]. The muscles were not permeabilized or mechanically dissected and were enzymatically dissociated to minimize manipulations that may affect the sample intactness prior to these measurements. The technologies allow for the measurement of cellular oxygen consumption in muscle fibers and various permeabilized or intact cells/tissues, thus estimating the cellular energy and ions (e.g., protons) in cells with intact physiology.
It should be noted that mitochondria in vivo are morphologically and functionally heterogeneous in cells [88,89], and this variety is thought to have important physiological consequences. It has been suggested that different mitochondrial subsets may perform diverse cellular functions depending on cellular demands [87]. Individual mitochondria may have different membrane potentials, mitoCa2+, mtROS, sensitivities to mitochondrial PTP induction, and even different motilities [90,91,92]. However, the precise mechanisms underlying the development of mitochondrial heterogeneity remain unknown.
6. Mitochondrial Swelling and Calcium Retention Capacity
Changes in the matrix volume induced by ions, particularly Ca2+ and K+, play a crucial role in the regulation of mitochondrial function and metabolism [93,94]. Modest increases in the matrix volume stimulate mitochondrial bioenergetics and ATP production [95,96], whereas excessive swelling of mitochondria due to sustained PTP opening impairs mitochondrial function, leading to cell death [97,98]. Mitochondrial Ca2+ overload accompanied by high mtROS levels is the main event that provokes PTP induction [99]. Due to the crucial role of mitochondria in human diseases [100], analysis of mitochondrial swelling is important for the estimation of mitochondrial damage induced by various pathological stimuli [101]. The Ca2+ retention capacity (CRC) represents the capability of mitochondria to uptake maximum Ca2+ and, therefore, is used to quantify the extent of PTP opening. Usually, the CRC is quantified in isolated mitochondria spectrophotometrically (light scattering) or using different Ca2+-sensitive dyes. For example, along with Ca2+-sensitive fluorescent dyes, arsenazo III, a non-membrane-permeant Ca2+-sensitive dye [101,102,103,104], and Ca2+-sensitive electrodes [104,105] have been used to measure Ca2+ release/PTP opening in isolated mitochondria. Notably, the estimation of the CRC/PTP opening in isolated mitochondria has several disadvantages [6,49,50].
The quantification of mitochondrial PTP/CRC in situ or in vivo was performed previously in cells and tissue samples. For example, mitochondrial PTP opening in the heart in vivo was quantified by the [3 H] 2-deoxyglucose (3 H-DOG) entrapment technique without isolation of mitochondria [106,107,108]. The isolated heart is perfused with 3 H-DOG, which is converted to 3 H-DOG-6-phosphate in the cytoplasm and enters the mitochondria through the PTP. The amount of 3 H-DOG-6-phosphate entrapped in mitochondria corresponds to the extent of PTP opening [109,110,111]. Calcein-AM, a cell-permeant fluorescent probe, is utilized to measure PTP/CRC in cells in situ, where Ca2+ release from mitochondria through the PTP indicates the extent of pore opening [112,113,114]. The technique for the measurement of PTP opening/CRC has certain disadvantages. In this approach, the fluorescence intensity of calcein in the cytosol is quenched by Co2+, a heavy metal, which exerts toxic effects on cells. Moreover, it is difficult to conclude whether calcein quenching is associated with its release through PTPs, or whether Co2+ enters through the PTP and quenches calcein in the matrix of mitochondria. In favor of this, cytosolic calcein has been shown to exit from normal mitochondria and enter back after PTP opening [115]. It should also be noted that calcein-AM is not cleaved in all cell types, such as hepatocytes [116].
In addition to mitochondrial bioenergetics, respiration rates, membrane potential, mtROS, and ions, permeabilized cells were previously used to elucidate the CRC using different Ca2+-sensitive fluorescent probes [117,118]. As mentioned above, two biological detergents (digitonin and saponin) are primarily used to permeabilize cells; digitonin disrupts the plasmalemma by targeting lipid rafts, while saponins permeabilize it by selectively removing cholesterol from the membranes without affecting membrane proteins [6,34]. Importantly, permeabilization does not affect the essential subcellular organization and the structural and functional integrity of cellular organelles. We have recently shown that the mitochondrial CRC measured by the Ca2+-sensitive fluorescence probe Calcium Green-5N in saponin-permeabilized cardiomyocytes was significantly higher than in isolated mitochondria [118] (Figure 3). Furthermore, a comparative analysis of the permeabilization capacity of saponin and digitonin revealed that saponin-permeabilized cardiomyocytes exhibited a higher CRC than digitonin-permeabilized cells. This study, along with studies from other groups, suggests that analysis of the CRC in saponin-permeabilized intact cells has more advantages compared to the isolated mitochondria. Analysis of the CRC in permeabilized cells can be used in basic research as well as to diagnose different human diseases.
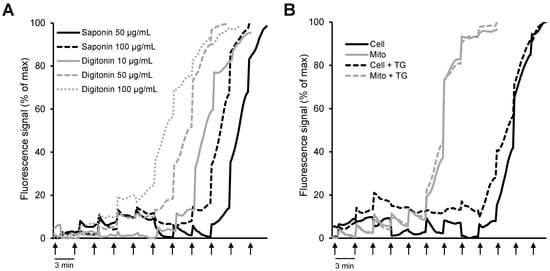
Figure 3.
Comparative analysis of mitochondrial CRC in isolated mitochondria in vitro and permeabilized cells in situ: (A) H9c2 cardiomyocytes permeabilized by saponin (50 and 100 μg/mL) or digitonin (10, 50, and 100 μg/mL); (B) permeabilized H9c2 cardiomyocytes vs. isolated mitochondria in the presence or absence of 1 μM thapsigargin (TG, a SERCA inhibitor). Ca2+ was added every 3 min (arrows) by increments of 1 nmol/injection. (Note: the figure was reprinted with permission from ref [118]. Copyright 2006 Elsevier.)
7. Mitochondrial Fluorescent Confocal Imaging
Rhodamine 123 was the first fluorescent dye used in flow cytometry [119] and in mitochondrial imaging. As it is specifically and potential-driven concentrated in mitochondria in living cells, rhodamine 123 was considered a useful probe for monitoring the abundance of mitochondria. However, due to the relatively low resolution, its application in imaging was limited. Applying techniques for autofluorescence and digital fluorescence imaging of mitochondrial NAD(P)H, endogenous flavoproteins, and specific mitochondrial fluorescent probes to permeabilized muscle fibers and cardiomyocytes (Figure 4) provided options for more precise imaging [38,39,42,63,65,87,120].
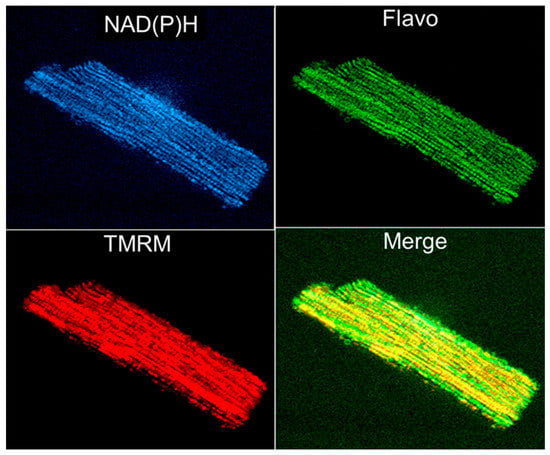
Figure 4.
Simultaneous imaging of endogenous autofluorescence of mitochondrial NAD(P)H (two-photon excitation), flavoproteins (488 nm laser excitation), and membrane potential sensitive probe TMRM (see Figure 1 legend) in an adult rat single cardiomyocyte.
It is noteworthy that the imaging approach enables not only the observation of mitochondrial structure/morphology but also the ability to obtain information about the general mitochondrial properties and functions. Since mitochondrial flavoproteins are fluorescent in an oxidized state and NAD(P)H in a reduced state, it has been possible to continuously monitor mitochondrial redox states. The addition of mitochondrial substrates, ADP, or potassium cyanide resulted in strong changes in the NAD redox system. The intensities of fluorescent flavoproteins and NAD(P)H demonstrated inverse fluorescence signal behavior (Figure 5).
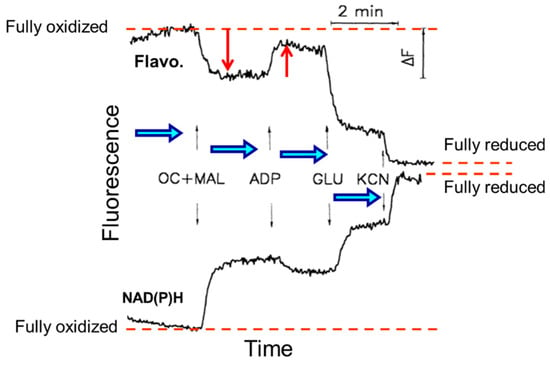
Figure 5.
Quantitative measurements of flavoproteins (Flavo.) and NAD(P)H fluorescence and their responses to substrates additions of octanoylcarnitine and malate (OC + MAL), ADP, glutamate (GLU), and inhibitor potassium cyanide (KCN) from fully oxidized to a fully reduced state in permeabilized skeletal rat skeletal muscle (quadriceps femoris).
Importantly, flavoproteins and NADH fluorescence were fully co-localized with MitoTrackerTM Green FM, an established fluorescent marker for mitochondria. It has been shown that the ratio of the intensities of fluorescent flavoproteins and NAD(P)H is practically non-sensitive to any other types of fluorescence, thereby eliminating the possible side effects of artificial fluorescent probes and can therefore be used as a sensitive indicator of mitochondrial redox states. Using the imaging approach, the phenomenon of mitochondrial heterogeneity has been established. For example, a much higher oxidative state of subsarcolemmal as compared to intermyofibrillar mitochondria has been found [39]. The flavoprotein autofluorescence signals of these mitochondrial subpopulations were four times different. The identification of the membrane potential and mtROS also revealed the heterogeneity of pathologically altered mitochondria (e.g., after cold ischemia-reperfusion and transplantation of rat hearts) [121]. Mitochondrial imaging, therefore, permits the assessment of mitochondrial defects topology, which provides information about the molecular mechanisms of cardiac cold ischemia-reperfusion injury. Similarly, flavoprotein redox states and mitochondrial membrane potential heterogeneity have been demonstrated in intact cardiomyocytes under conditions of substrate (glucose) deprivation [40]. This included metabolic transients, well-coordinated redox transitions, and wave-like metabolic propagation within one cell and even between cells. The mechanism may involve some diffusible cytosolic messengers. Therefore, in addition to respirometry, fluorescence imaging approaches can be used for the analysis of functional mitochondria in permeabilized muscles and intact cardiac cells. Altogether, analysis of the mitochondrial structural organization, bioenergetics, and redox status using the aforementioned in situ techniques is useful for the precise estimation of the cardioprotective and anti-aging effects of newly developed mitochondria-targeted compounds [122,123,124,125,126,127]. Mitochondria-targeted antioxidants such as SS-31, MitoQ, XJB-5-131, SkQ, CoQ10, SOD mimetics, mitochondria-targeting glutathione (mitoGSH), and polyphenols, among others, could be examined in future studies.
8. Conclusions
Respirometry and imaging of mitochondria in intact or permeabilized cardiomyocytes and muscle fibers and tissues are reliable tools for the functional analysis of mitochondria in situ or in vivo, associated with preserved essential interactions with other intracellular systems. These approaches can be used in basic research as well as various clinically oriented studies where the minimization of sample sizes is an important advantage for analyzing mitochondria in human biopsies. In addition, imaging of endogenous fluorescent flavoproteins, NADH or mitochondria-specific fluorescent dyes, and genetically encoded fluorescent proteins can also be applied in mitochondrial studies. We reviewed methods that are applied to elucidate mitochondrial morphology, mitochondrial membrane potential, ion homeostasis, mitochondrial pH regulation, redox state transitions, mtROS production, and PTP opening/CRC in situ. Biochemical and functional characteristics of mitochondria obtained using in situ methods are markedly different from those obtained in isolated mitochondria in vitro. In summary, we reviewed currently available methods to study mitochondrial function, structure, and intracellular organization in situ in cardiomyocytes and skeletal muscle fibers.
Author Contributions
Conceptualization, A.V.K., S.J. and M.J.A.; validation, S.J., J.H. and M.J.A.; formal analysis, S.J., J.H. and M.J.A.; resources, A.V.K. and M.J.A.; writing—original draft, A.V.K. and S.J.; writing—review and editing, A.V.K., S.J. and M.J.A.; supervision, M.J.A. and R.M.; project administration, M.J.A.; funding acquisition, M.J.A. and R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Science Foundation, USA (Grant 2006477 to S.J.), the Austrian Science Fund (FWF Projects I3089-B28 and FG15), the Austrian Research Promotion Agency (Project 880666), the “Tuba-Forschungsförderung”, and the Federal Ministry Republic of Austria for Education, Science and Research (Project “Replacement of animal experiments in science”).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Newmeyer, D.D.; Ferguson-Miller, S. Mitochondria: Releasing power for life and unleashing the machineries of death. Cell 2003, 112, 481–490. [Google Scholar] [CrossRef] [Green Version]
- McBride, H.M.; Neuspiel, M.; Wasiak, S. Mitochondria: More than Just a Powerhouse. Curr. Biol. 2006, 16, R551–R560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riedl, S.J.; Salvesen, G.S. The apoptosome: Signalling platform of cell death. Nat. Rev. Mol. Cell Biol. 2007, 8, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Reed, J.C. Mitochondrial control of cell death. Nat. Med. 2000, 6, 513–519. [Google Scholar] [CrossRef]
- Bernardi, P.; Petronilli, V.; Di Lisa, F.; Forte, M. A mitochondrial perspective on cell death. Trends Biochem. Sci. 2001, 26, 112–117. [Google Scholar] [CrossRef]
- Salabei, J.K.; Gibb, A.A.; Hill, B.G. Comprehensive measurement of respiratory activity in permeabilized cells using extracellular flux analysis. Nat. Protoc. 2014, 9, 421–438. [Google Scholar] [CrossRef] [Green Version]
- Jonathan, R.; Friedman, N.J. Mitochondrial form and function. Nature 2014, 505, 335–343. [Google Scholar]
- Csordás, G.; Weaver, D.; Hajnóczky, G. Endoplasmic Reticulum-Mitochondrial Contactology: Structure and Signaling Functions. Trends Cell Biol. 2018, 28, 523–540. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef] [Green Version]
- Pozzan, T.; Rudolf, R. Measurements of mitochondrial calcium in vivo. Biochim. Biophys. Acta 2009, 1787, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Bowser, D.N.; Minamikawa, T.; Nagley, P.; Williams, D.A. Role of Mitochondria in Calcium Regulation of Spontaneously Contracting Cardiac Muscle Cells. Biophys. J. 1998, 75, 2004–2014. [Google Scholar] [CrossRef] [Green Version]
- Rizzuto, R.; Pinton, P.; Carrington, W.; Fay, F.S.; Fogarty, K.E.; Lifshitz, L.M.; Tuft, R.A.; Pozzan, T. Close Contacts with the Endoplasmic Reticulum as Determinants of Mitochondrial Ca2+ Responses. Science 1998, 280, 1763–1766. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Jones, D.P. Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J. Biol. Chem. 1998, 273, 11401–11404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagenbuchner, J.; Scholl-Buergi, S.; Karall, D.; Ausserlechner, M.J. Very long-/ and long Chain-3-Hydroxy Acyl CoA Dehydrogenase Deficiency correlates with deregulation of the mitochondrial fusion/fission machinery. Sci. Rep. 2018, 8, 3254. [Google Scholar] [CrossRef] [Green Version]
- Hagenbuchner, J.; Oberacher, H.; Arnhard, K.; Kiechl-Kohlendorfer, U.; Ausserlechner, M.J. Modulation of Respiration and Mitochondrial Dynamics by SMAC-Mimetics for Combination Therapy in Chemoresistant Cancer. Theranostics 2019, 9, 4909–4922. [Google Scholar] [CrossRef]
- Hagenbuchner, J.; Kiechl-Kohlendorfer, U.; Obexer, P.; Ausserlechner, M.J. BIRC5/Survivin as a target for glycolysis inhibition in high-stage neuroblastoma. Oncogene 2016, 35, 2052–2061. [Google Scholar] [CrossRef]
- Griffiths, E.J.; Rutter, G.A. Mitochondrial calcium as a key regulator of mitochondrial ATP production in mammalian cells. Biochim. Biophys. Acta 2009, 1787, 1324–1333. [Google Scholar] [CrossRef] [Green Version]
- Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria in cancer cells: What is so special about them? Trends Cell Biol. 2008, 18, 165–173. [Google Scholar] [CrossRef]
- Cottrell, D.A.; Turnbull, D.M. Mitochondria and ageing. Curr. Opin. Clin. Nutr. Metab. Care 2000, 3, 473–478. [Google Scholar] [CrossRef]
- Nickel, A.; Kohlhaas, M.; Maack, C. Mitochondrial reactive oxygen species production and elimination. J. Mol. Cell. Cardiol. 2014, 73, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Sagara, Y.; Liu, Y.; Maher, P.; Schubert, D. The Regulation of Reactive Oxygen Species Production during Programmed Cell Death. J. Cell Biol. 1998, 141, 1423–1432. [Google Scholar] [CrossRef] [Green Version]
- Valente, A.J.; Fonseca, J.; Moradi, F.; Foran, G.; Necakov, A.; Stuart, J.A. Quantification of Mitochondrial Network Characteristics in Health and Disease. Adv. Exp. Med. Biol. 2019, 1158, 183–196. [Google Scholar]
- Kuznetsov, A.V.; Hagenbuchner, J.; Ausserlechner, M.J. ROS Flashes in Mitochondria Occur Concomitantly with Inner Mitochondrial Membrane Depolarization and Mitochondrial Calcium Sparks. New Front. Med. Res. 2021, 16, 83–104. [Google Scholar]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Graham, J.M. Isolation of Mitochondria from Tissues and Cells by Differential Centrifugation. Curr. Protoc. Cell Biol. 1999, 34, 3–15. [Google Scholar] [CrossRef]
- Frezza, C.; Cipolat, S.; Scorrano, L. Organelle isolation: Functional mitochondria from mouse liver, muscle and cultured filroblasts. Nat. Protoc. 2007, 2, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Graham, B.H. Measurement of Mitochondrial Oxygen Consumption Using a Clark Electrode. Methods Mol. Biol. 2011, 837, 63–72. [Google Scholar] [CrossRef]
- Lehr, S.; Hartwig, S.; Kotzka, J. Preparation of “functional” mitochondria: A challenging business. Methods Mol. Biol. 2015, 1264, 1–8. [Google Scholar] [PubMed]
- Fuller, K.M.; Arriaga, E. Advances in the analysis of single mitochondria. Curr. Opin. Biotechnol. 2003, 14, 35–41. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Veksler, V.; Gellerich, F.N.; Saks, V.; Margreiter, R.; Kunz, W.S. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat. Protoc. 2008, 3, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Wiedemann, F.R.; Winkler, K.; Kunz, W. Use of saponin-permeabilized muscle fibers for the diagnosis of mitochondrial diseases. BioFactors 1998, 7, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Kunz, W.; Kuznetsov, A.V.; Schulze, W.; Eichhorn, K.; Schild, L.; Striggow, F.; Bohnensack, R.; Neuhof, S.; Grasshoff, H.; Neumann, H.W. Functional characterization of mitochondrial oxidative phosphorylation in saponin-skinned human muscle fibers. Biochim. Biophys. Acta 1993, 1144, 46–53. [Google Scholar] [CrossRef]
- Saks, V.A.; Veksler, V.I.; Kuznetsov, A.V.; Kay, L.; Sikk, P.; Tiivel, T.; Tranqui, L.; Olivares, J.; Winkler, K.; Wiedemann, F.; et al. Permeabilized cell and skinned fiber techniques in studies of mitochondrial function in vivo. Mol. Cell Biochem. 1998, 184, 81–100. [Google Scholar] [CrossRef]
- Appaix, F.; Kuznetsov, A.V.; Usson, Y.; Kay, L.; Andrienko, T.; Olivares, J.; Kaambre, T.; Sikk, P.; Margreiter, R.; Saks, V. Possible Role of Cytoskeleton in Intracellular Arrangement and Regulation of Mitochondria. Exp. Physiol. 2003, 88, 175–190. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, A.V.; Mayboroda, O.; Kunz, D.; Winkler, K.; Schubert, W.; Kunz, W. Functional Imaging of Mitochondria in Saponin-permeabilized Mice Muscle Fibers. J. Cell Biol. 1998, 140, 1091–1099. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, A.V.; Troppmair, J.; Sucher, R.; Hermann, M.; Saks, V.; Margreiter, R. Mitochondrial subpopulations and heterogeneity revealed by confocal imaging: Possible physiological role? Biochim. Biophys. Acta 2006, 1757, 686–691. [Google Scholar] [CrossRef] [Green Version]
- Romashko, D.N.; Marban, E.; O’Rourke, B. Subcellular metabolic transients and mitochondrial redox waves in heart cells. Proc. Natl. Acad. Sci. USA 1998, 95, 1618–1623. [Google Scholar] [CrossRef] [Green Version]
- Paredes, R.M.; Etzler, J.C.; Watts, L.T.; Zheng, W.; Lechleiter, J.D. Chemical calcium indicators. Methods 2008, 46, 143–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuznetsov, A.V.; Javadov, S.; Saks, V.; Margreiter, R.; Grimm, M. Synchronism in mitochondrial ROS flashes, membrane depolarization and calcium sparks in human carcinoma cells. Biochim. Biophys. Acta Bioenergy 2017, 1858, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Ogata, T.; Yamasaki, Y. Ultra-high resolution scanning electron microscopic studies on the sarcoplasmic reticulum and mitochondria in various muscles: A review. Scanning Microsc. 1993, 7, 145–156. [Google Scholar] [PubMed]
- Dedkova, E.N.; Blatter, L.A. Measuring mitochondrial function in intact cardiac myocytes. J. Mol. Cell. Cardiol. 2012, 52, 48–61. [Google Scholar] [CrossRef] [Green Version]
- Hughey, C.C.; Hittel, D.S.; Johnsen, V.L.; Shearer, J. Respirometric Oxidative Phosphorylation Assessment in Saponin-permeabilized Cardiac Fibers. J. Vis. Exp. 2011, 48, e2431. [Google Scholar] [CrossRef] [Green Version]
- Rasmusson, A.G.; Møller, I.M.; Widell, S. Assessment of Respiratory Enzymes in Intact Cells by Permeabilization with Alamethicin. Methods Mol. Biol. 2022, 2363, 77–84. [Google Scholar] [PubMed]
- Adlakha, Y.K.; Swaroop, A. Determination of Mitochondrial Oxygen Consumption in the Retina Ex Vivo: Applications for Retinal Disease. Methods Mol. Biol. 2018, 1753, 167–177. [Google Scholar] [CrossRef]
- Villani, G.; Attardi, G. In vivo measurements of respiration control by cytochrome c oxidase and in situ analysis of oxidative phosphorylation. Methods Cell Biol. 2001, 65, 119–131. [Google Scholar]
- Barrientos, A. In vivo and in organello assessment of OXPHOS activities. Methods 2002, 26, 307–316. [Google Scholar] [CrossRef]
- Safiulina, D.; Kaasik, A.; Seppet, E.; Peet, N.; Zharkovsky, A.; Seppet, E. Method for in situ detection of the mitochondrial function in neurons. J. Neurosci. Methods 2004, 137, 87–95. [Google Scholar] [CrossRef]
- Jonckheere, A.I.; Huigsloot, M.; Janssen, A.J.; Kappen, A.J.; Smeitink, J.A.; Rodenburg, R.J. High-Throughput Assay to Measure Oxygen Consumption in Digitonin-Permeabilized Cells of Patients with Mitochondrial Disorders. Clin. Chem. 2010, 56, 424–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chance, B.; Williams, G.R. Respiratory enzymes in oxidative phosphorylation. II. Difference spectra. J. Biol. Chem. 1955, 217, 395–407. [Google Scholar] [CrossRef]
- Chance, B.; Williams, G.R. Respiratory enzymes in oxidative phosphorylation. VI. The effects of adenosine diphosphate on azide-treated mitochondria. J. Biol. Chem. 1956, 221, 477–489. [Google Scholar] [CrossRef]
- Chretien, D.; Rustin, P. Mitochondrial oxidative phosphorylation: Pitfalls and tips in measuring and interpreting enzyme activities. J. Inherit. Metab. Dis. 2003, 26, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Pallotti, F.; Lenaz, G. Isolation and Subfractionation of Mitochondria from Animal Cells and Tissue Culture Lines. Rev. Methods Cell Biol. 2007, 80, 3–44. [Google Scholar] [CrossRef]
- Lenaz, G.; Genova, M.L. Structural and functional organization of the mitochondrial respiratory chain: A dynamic super-assembly. Int. J. Biochem. Cell Biol. 2009, 41, 1750–1772. [Google Scholar] [CrossRef]
- Villani, G.; Attardi, G. Polarographic Assays of Respiratory Chain Complex Activity. Methods Cell Biol. 2007, 80, 121–133. [Google Scholar] [CrossRef]
- Lessler, M.A.; Brierley, G.P. Oxygen Electrode Measurements in Biochemical Analysis. Methods Biochem. Anals 1969, 17, 1–29. [Google Scholar] [CrossRef]
- Picard, M.; Ritchie, D.; Wright, K.J.; Romestaing, C.; Thomas, M.M.; Rowan, S.L.; Taivassalo, T.; Hepple, R.T. Mitochondrial functional impairment with aging is exaggerated in isolated mitochondria compared to permeabilized myofibers. Aging Cell 2010, 9, 1032–1046. [Google Scholar] [CrossRef] [Green Version]
- Picard, M.; Taivassalo, T.; Ritchie, D.; Wright, K.J.; Thomas, M.M.; Romestaing, C.; Hepple, R.T. Mitochondrial Structure and Function Are Disrupted by Standard Isolation Methods. PLoS ONE 2011, 6, e18317. [Google Scholar] [CrossRef]
- Rappaport, L.; Oliviero, P.; Samuel, J.L. Cytoskeleton and mitochondrial morphology and function. Mol. Cell. Biochem. 1998, 184, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Johannes, M.H.; Westermann, B. Analysis of protein-protein interactions in mitochondria. Methods Cell Biol. 2007, 80, 743–759. [Google Scholar]
- Guzun, R.; Gonzalez-Granillo, M.; Karu-Varikmaa, M.; Grichine, A.; Usson, Y.; Kaambre, T.; Guerrero-Roesch, K.; Kuznetsov, A.; Schlattner, U.; Saks, V. Regulation of respiration in muscle cells in vivo by VDAC through interaction with the cytoskeleton and Mt, CK within Mitochondrial Interactosome. Biochim. Biophys. Acta 2012, 1818, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Milner, D.J.; Mavroidis, M.; Weisleder, N.; Capetanaki, Y. Desmin Cytoskeleton Linked to Muscle Mitochondrial Distribution and Respiratory Function. J. Cell Biol. 2000, 150, 1283–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winter, L.; Kuznetsov, A.V.; Grimm, M.; Zeöld, A.; Fischer, I.; Wiche, G. Plectin isoform P1b and P1d deficiencies differentially affect mitochondrial morphology and function in skeletal muscle. Hum. Mol. Genet. 2015, 24, 4530–4544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, J.R. Mitochondrial Function in the Heart. Annu. Rev. Physiol. 1979, 41, 485–506. [Google Scholar] [CrossRef]
- Balaban, R.S. Regulation of oxidative phosphorylation in the mammalian cell. Am. J. Physiol. 1990, 258, C377–C389. [Google Scholar] [CrossRef]
- Brdiczka, D. Function of the outer mitochondrial compartment in regulation of energy metabolism. Biochim. Biophys. Acta 1994, 1187, 264–269. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Tiivel, T.; Sikk, P.; Kaambre, T.; Kay, L.; Daneshrad, Z.; Rossi, A.; Kadaja, L.; Peet, N.; Seppet, E.; et al. Striking difference between slow and fast twitch muscles in the kinetics of regulation of respiration by ADP in the cells in vivo. Eur. J. Biochem. 1996, 241, 909–915. [Google Scholar] [CrossRef]
- Clark, J.F.; Kuznetsov, A.V.; Radda, G.K. ADP-regenerating enzyme systems in mitochondria of guinea pig myometrium and heart. Am. J. Physiol. 1997, 272 Pt 1, C399–C404. [Google Scholar] [CrossRef]
- Saks, V.; Kuznetsov, A.V.; Gonzalez-Granillo, M.; Tepp, K.; Timohhina, N.; Karu-Varikmaa, M.; Kaambre, T.; Dos Santos, P.; Boucher, F.; Guzun, R. Intracellular Energetic Units regulate metabolism in cardiac cells. J. Mol. Cell. Cardiol. 2012, 52, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Kaasik, A.; Veksler, V.; Boehm, E.; Novotova, M.; Minajeva, A.; Ventura-Clapier, R. Energetic crosstalk between organelles: Architectural integration of energy production and utilization. Circ. Res. 2001, 89, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesta, D.; Gnaiger, E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol. Biol. 2012, 810, 25–58. [Google Scholar]
- Doerrier, C.; Garcia-Souza, L.F.; Krumschnabel, G.; Wohlfarter, Y.; Mészáros, A.T.; Gnaiger, E. High-Resolution Fluo, Respirometry and OXPHOS Protocols for Human Cells, Permeabilized Fibers from Small Biopsies of Muscle, and Isolated Mitochondria. Methods Mol. Biol. 2018, 1782, 31–70. [Google Scholar] [CrossRef] [PubMed]
- Duicu, O.; Gheorgheosu, D.; Mirica, N.; Trancotă, S.; Cristina, D.; Firă-Mladinescu, O.; Muntean, D.M. High-resolution respirometry with multiple substrates titration in permeabilized myocardial fibers. Rev. Med.-Chirurg. 2012, 116, 207–213. [Google Scholar]
- Marin-Garcia, J.; Goldenthal, M.J. Fatty acid metabolism in cardiac failure: Biochemical, genetic and cellular analysis. Cardiovasc. Res. 2002, 54, 516–527. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, A.V.; Schneeberger, S.; Seiler, R.; Brandacher, G.; Mark, W.; Steurer, W.; Saks, V.; Usson, Y.; Margreiter, R.; Gnaiger, E. Mitochondrial defects and heterogeneous cytochrome c release after cardiac cold ischemia and reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1633–H1641. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, A.V.; Javadov, S.; Margreiter, R.; Grimm, M.; Hagenbuchner, J.; Ausserlechner, M.J. The Role of Mitochondria in the Mechanisms of Cardiac Ischemia-Reperfusion Injury. Antioxidants 2019, 8, 454. [Google Scholar] [CrossRef] [Green Version]
- Krippner, A.; Matsuno-Yagi, A.; Gottlieb, R.A.; Babior, B.M. Loss of Function of Cytochrome c in Jurkat Cells Undergoing Fas-mediated Apoptosis. J. Biol. Chem. 1996, 271, 21629–21636. [Google Scholar] [CrossRef] [Green Version]
- Eimre, M.; Paju, K.; Pelloux, S.; Beraud, N.; Roosimaa, M.; Kadaja, L.; Gruno, M.; Peet, N.; Orlova, E.; Remmelkoor, R.; et al. Distinct organization of energy metabolism in HL-1 cardiac cell line and cardiomyocytes. Biochim. Biophys. Acta 2008, 1777, 514–524. [Google Scholar] [CrossRef] [Green Version]
- Saks, V.A.; Kupriyanov, V.V.; Kuznetsov, A.V.; Kapelko, V.I.; Sharov, V.G.; Veksler, V.I.; Javadov, S.A. Quantitative evaluation of relationship between cardiac energy metabolism and post-ischemic recovery of contractile function. J. Mol. Cell. Cardiol. 1989, 21, 67–78. [Google Scholar] [CrossRef]
- Font, B.; Vial, C.; Goldschmidt, D.; Eichenberger, D.; Gautheron, D. Effects of SH group reagents on creatine kinase interaction with the mitochondrial membrane. Arch. Biochem. Biophys. 1983, 220, 541–548. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q. Using Seahorse Machine to Measure OCR and ECAR in Cancer Cells. Methods Mol. Biol. 2019, 1928, 353–363. [Google Scholar] [PubMed]
- Clerc, P.; Polster, B.M. Investigation of Mitochondrial Dysfunction by Sequential Microplate-Based Respiration Measurements from Intact and Permeabilized Neurons. PLoS ONE 2012, 7, e34465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shintaku, J.; Guttridge, D.C. Analysis of Aerobic Respiration in Intact Skeletal Muscle Tissue by Microplate-Based Respirometry. Methods Mol. Biol. 2016, 1460, 337–343. [Google Scholar]
- Divakaruni, A.S.; Paradyse, A.; Ferrick, D.A.; Murphy, A.N.; Jastroch, M. Analysis and Interpretation of Microplate-Based Oxygen Consumption and p, H Data. Methods Enzymol. 2014, 547, 309–354. [Google Scholar] [CrossRef]
- Hynes, J.; Carey, C.; Will, Y. Fluorescence-Based Microplate Assays for In Vitro Assessment of Mitochondrial Toxicity, Metabolic Perturbation, and Cellular Oxygenation. Curr. Protoc. Toxicol. 2016, 70, 16–30. [Google Scholar] [CrossRef]
- Collins, T.J.; Berridge, M.J.; Lipp, P.; Bootman, M. Mitochondria are morphologically and functionally heterogeneous within cells. EMBO J. 2002, 21, 1616–1627. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Margreiter, R. Heterogeneity of Mitochondria and Mitochondrial Function within Cells as Another Level of Mitochondrial Complexity. Int. J. Mol. Sci. 2009, 10, 1911–1929. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, A.F.; Mariotti, F.R.; Máximo, V.; Campello, S. Mitochondria dynamism: Of shape, transport and cell migration. Cell Mol. Life Sci. 2014, 71, 2313–2324. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Hermann, M.; Saks, V.; Hengster, P.; Margreiter, R. The cell-type specificity of mitochondrial dynamics. Int. J. Biochem. Cell Biol. 2009, 41, 1928–1939. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Hermann, M.; Troppmair, J.; Margreiter, R.; Hengster, P. Complex patterns of mitochondrial dynamics in human pancreatic cells revealed by fluorescent confocal imaging. J. Cell. Mol. Med. 2010, 14, 417–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garlid, K.D.; Paucek, P. Mitochondrial potassium transport: The K+ cycle. Biochim. Biophys. Acta 2003, 1606, 23–41. [Google Scholar] [CrossRef] [Green Version]
- Szabo, I.; Zoratti, M. Mitochondrial Channels: Ion Fluxes and More. Physiol. Rev. 2014, 94, 519–608. [Google Scholar] [CrossRef]
- Tarasov, A.I.; Griffiths, E.J.; Rutter, G.A. Regulation of ATP production by mitondrial Ca(2+). Cell Calcium 2012, 52, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Halestrap, A.P. Regulation of mitochondrial metabolism through changes in matrix volume. Biochem. Soc. Trans. 1994, 22, 522–529. [Google Scholar] [CrossRef] [Green Version]
- Halestrap, A.P.; Kerr, P.M.; Javadov, S.; Woodfield, K.-Y. Elucidating the molecular mechanism of the permeability transition pore and its role in reperfusion injury of the heart. Biochim. Biophys. Acta 1998, 1366, 79–94. [Google Scholar] [CrossRef] [Green Version]
- Javadov, S.; Karmazyn, M.; Escobales, N. Mitochondrial Permeability Transition Pore Opening as a Promising Therapeutic Target in Cardiac Diseases. J. Pharmacol. Exp. Ther. 2009, 330, 670–678. [Google Scholar] [CrossRef] [Green Version]
- Haworth, R.A.; Hunter, D.R. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch. Biochem. Biophys. 1979, 195, 460–467. [Google Scholar] [CrossRef]
- Javadov, S.; Kozlov, A.V.; Camara, A.K.S. Mitochondria in Health and Diseases. Cells 2020, 9, 1177. [Google Scholar] [CrossRef]
- Javadov, S.; Chapa-Dubocq, X.; Makarov, V. Different approaches to modeling analysis of mitochondrial swelling. Mitochondrion 2018, 38, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Brookes, P.; Salinas, E.P.; Darley-Usmar, K.; Eiserich, J.P.; Freeman, B.A.; Darley-Usmar, V.; Anderson, P.G. Concentration-dependent Effects of Nitric Oxide on Mitochondrial Permeability Transition and Cytochrome cRelease. J. Biol. Chem. 2000, 275, 20474–20479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubinin, M.V.; Starinets, V.S.; Talanov, E.Y.; Mikheeva, I.B.; Belosludtseva, N.V.; Belosludtsev, K.N. Alisporivir Improves Mitochondrial Function in Skeletal Muscle of mdx Mice but Suppresses Mitochondrial Dynamics and Biogenesis. Int. J. Mol. Sci. 2021, 22, 9780. [Google Scholar] [CrossRef] [PubMed]
- Akopova, O.; Kotsiuruba, A.; Korkach, Y.; Kolchinskaya, L.; Nosar, V.; Gavenauskas, B.; Serebrovska, Z.; Mankovska, I.; Sagach, V. The Effect of NO Donor on Calcium Uptake and Reactive Nitrogen Species Production in Mitochondria. Cell. Physiol. Biochem. 2016, 39, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.J.M.; Vicente, J.A. Use of a Calcium-Sensitive Electrode for Studies on Mitochondrial Calcium Transport. Methods Mol. Biol. 2011, 810, 207–217. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Talanov, E.Y.; Starinets, V.S.; Agafonov, A.V.; Dubinin, M.V.; Belosludtseva, N.V. Transport of Ca2+ and Ca2+-Dependent Permeability Transition in Rat Liver Mitochondria under the Streptozotocin-Induced Type I Diabetes. Cells 2019, 8, 1014. [Google Scholar] [CrossRef] [Green Version]
- Javadov, S.; Huang, C.; Kirshenbaum, L.; Karmazyn, M. NHE-1 inhibition improves impaired mitochondrial permeability transition and respiratory function during postinfarction remodelling in the rat. J. Mol. Cell Cardiol. 2005, 38, 135–143. [Google Scholar] [CrossRef]
- Ciminelli, M.; Ascah, A.; Bourduas, K.; Burelle, Y. Short Term Training Attenuates Opening of the Mitochondrial Permeability Transition Pore Without Affecting Myocardial Function Following Ischemia-Reperfusion. Mol. Cell. Biochem. 2006, 291, 39–47. [Google Scholar] [CrossRef]
- Griffiths, E.J.; Halestrap, A. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem. J. 1995, 307, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Javadov, S.A.; Clarke, S.; Das, M.; Griffiths, E.J.; Lim, K.H.H.; Halestrap, A.P. Ischaemic Preconditioning Inhibits Opening of Mitochondrial Permeability Transition Pores in the Reperfused Rat Heart. J. Physiol. 2003, 549, 513–524. [Google Scholar] [CrossRef]
- Javadov, S.A.; Lim, K.H.; Kerr, P.M.; Suleiman, M.-S.; Angelini, G.; Halestrap, A.P. Protection of hearts from reperfusion injury by propofol is associated with inhibition of the mitochondrial permeability transition. Cardiovasc. Res. 2000, 45, 360–369. [Google Scholar] [CrossRef] [Green Version]
- Petronilli, V.; Miotto, G.; Canton, M.; Brini, M.; Colonna, R.; Bernardi, P.; Di Lisa, F. Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys. J. 1999, 76, 725–734. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, J.S.; Barreto-Torres, G.; Kuznetsov, A.V.; Khuchua, Z.; Javadov, S. Crosstalk between AMPK activation and angiotensin II-induced hypertrophy in cardiomyocytes: The role of mitochondria. J. Cell Mol. Med. 2014, 18, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, G.; Chiari, P.; Fauconnier, J.; Abrial, M.; Couture-Lepetit, E.; Harisseh, R.; Pillot, B.; Lacampagne, A.; Tourneur, Y.; Gharib, A.; et al. Involvement of Cyclophilin D and Calcium in Isoflurane-induced Preconditioning. Anesthesiology 2015, 123, 1374–1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, R.A.; Smail, A.; Wilson, M. Detecting mitochondrial permeability transition by confocal imaging of intact cells pinocytically loaded with calcein. Eur. J. Biol. Chem. 2002, 269, 3990–3997. [Google Scholar] [CrossRef]
- Lemasters, J.J.; Nieminen, A.-L.; Qian, T.; Trost, L.C.; Elmore, S.P.; Nishimura, Y.; Crowe, R.A.; Cascio, W.E.; Bradham, C.A.; Brenner, D.A.; et al. The mitochondrial permeability transition in cell death: A common mechanism in necrosis, apoptosis and autophagy. Biochim. Biophys. Acta 1998, 1366, 177–196. [Google Scholar] [CrossRef] [Green Version]
- Harisseh, R.; Abrial, M.; Chiari, P.; Al-Mawla, R.; Villedieu, C.; Tessier, N.; Bidaux, G.; Ovize, M.; Gharib, A. A modified calcium retention capacity assay clarifies the roles of extra- and intracellular calcium pools in mitochondrial permeability transition pore opening. J. Biol. Chem. 2019, 294, 15282–15292. [Google Scholar] [CrossRef]
- Jang, S.; Chapa-Dubocq, X.R.; Fossati, S.; Javadov, S. Analysis of Mitochondrial Calcium Retention Capacity in Cultured Cells: Permeabilized Cells Versus Isolated Mitochondria. Front. Physiol. 2021, 12, 839. [Google Scholar] [CrossRef]
- Ronot, X.; Benel, L.; Adolphe, M.; Mounolou, J.C. Mitochondrial analysis in living cells: The use of rhodamine 123 and flow cytometry. Biol. Cell 1986, 57, 1–7. [Google Scholar] [CrossRef]
- Chen, L.B. Fluorescent Labeling of Mitochondria. Methods Cell Biol. 1988, 29, 103–123. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Schneeberger, S.; Renz, O.; Meusburger, H.; Saks, V.; Usson, Y.; Margreiter, R. Functional heterogeneity of mitochondria after cardiac cold ischemia and reperfusion revealed by confocal imaging. Transplantation 2004, 77, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Siegel, M.P.; Kruse, S.E.; Percival, J.M.; Goh, J.; White, C.C.; Hopkins, H.C.; Kavanagh, T.J.; Szeto, H.H.; Rabinovitch, P.S.; Marcinek, D.J. Mitochondrial-targeted peptide rapidly improves mitochondrial energetics and skeletal muscle performance in aged mice. Aging Cell 2013, 12, 763–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelso, G.F.; Porteous, C.M.; Coulter, C.V.; Hughes, G.; Porteous, W.K.; Ledgerwood, E.C.; Smith, A.J.; Murphy, M.P. Selective targeting of a redox-active ubiquinone to mitochondria within cells: Antioxidant and antiapoptotic properties. J. Biol. Chem. 2001, 276, 4588–4596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javadov, S.; Jang, S.; Rodriguez-Reyes, N.; Rodriguez-Zayas, A.E.; Soto Hernandez, J.; Krainz, T.; Wipf, P.; Frontera, W. Mitochondria-targeted antioxidant preserves contractile properties and mitochondrial function of skeletal muscle in aged rats. Oncotarget 2015, 6, 39469–39481. [Google Scholar] [CrossRef] [Green Version]
- Anisimov, V.N.; Egorov, M.V.; Krasilshchikova, M.S.; Lyamzaev, K.G.; Manskikh, V.N.; Moshkin, M.P.; Novikov, E.A.; Popovich, I.G.; Rogovin, K.A.; Shabalina, I.G.; et al. Effects of the mitochondria-targeted antioxidant SkQ1 on lifespan of rodents. Aging 2011, 3, 1110–1119. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.-W.; Xu, X.-C.; Liu, T.; Yuan, S. Mitochondrion-Permeable Antioxidants to Treat ROS-Burst-Mediated Acute Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 6859523. [Google Scholar] [CrossRef] [Green Version]
- Feniouk, B.A.; Skulachev, V. Cellular and Molecular Mechanisms of Action of Mitochondria-Targeted Antioxidants. Curr. Aging Sci. 2017, 10, 41–48. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).