Cannabidiol Downregulates Myocardial de Novo Ceramide Synthesis Pathway in a Rat Model of High-Fat Diet-Induced Obesity
Abstract
:1. Introduction
2. Results
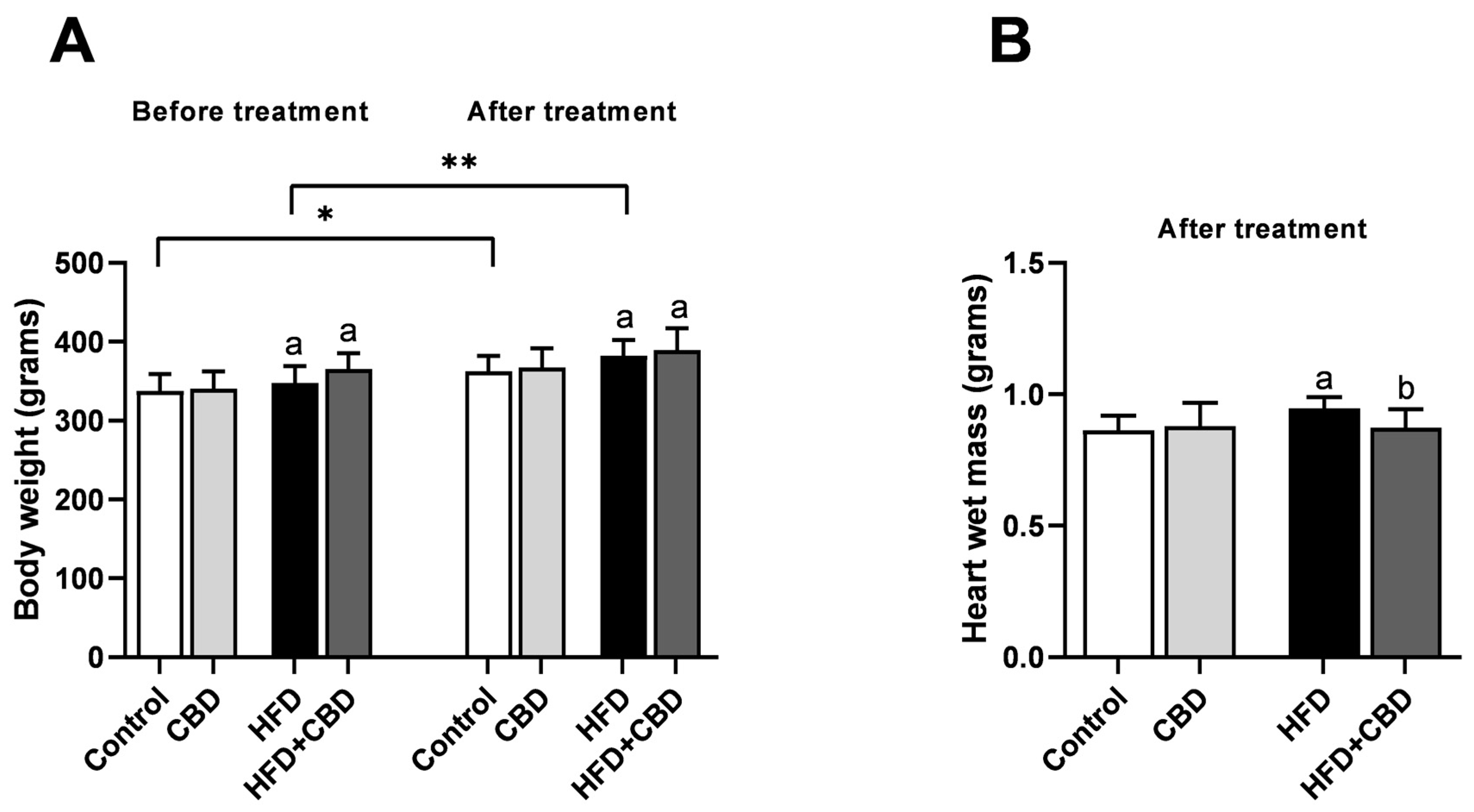
2.1. General Characteristic of the Experimental Model
2.2. Effects of 2-Week CBD Administration on the Plasma Sphinganine, Sphinganine-1-Phosphate, Ceramide, Sphingosine, and Sphingosine-1-Phosphate Contents in Both the Standard Chow and High-Fat Diet-Fed Rats
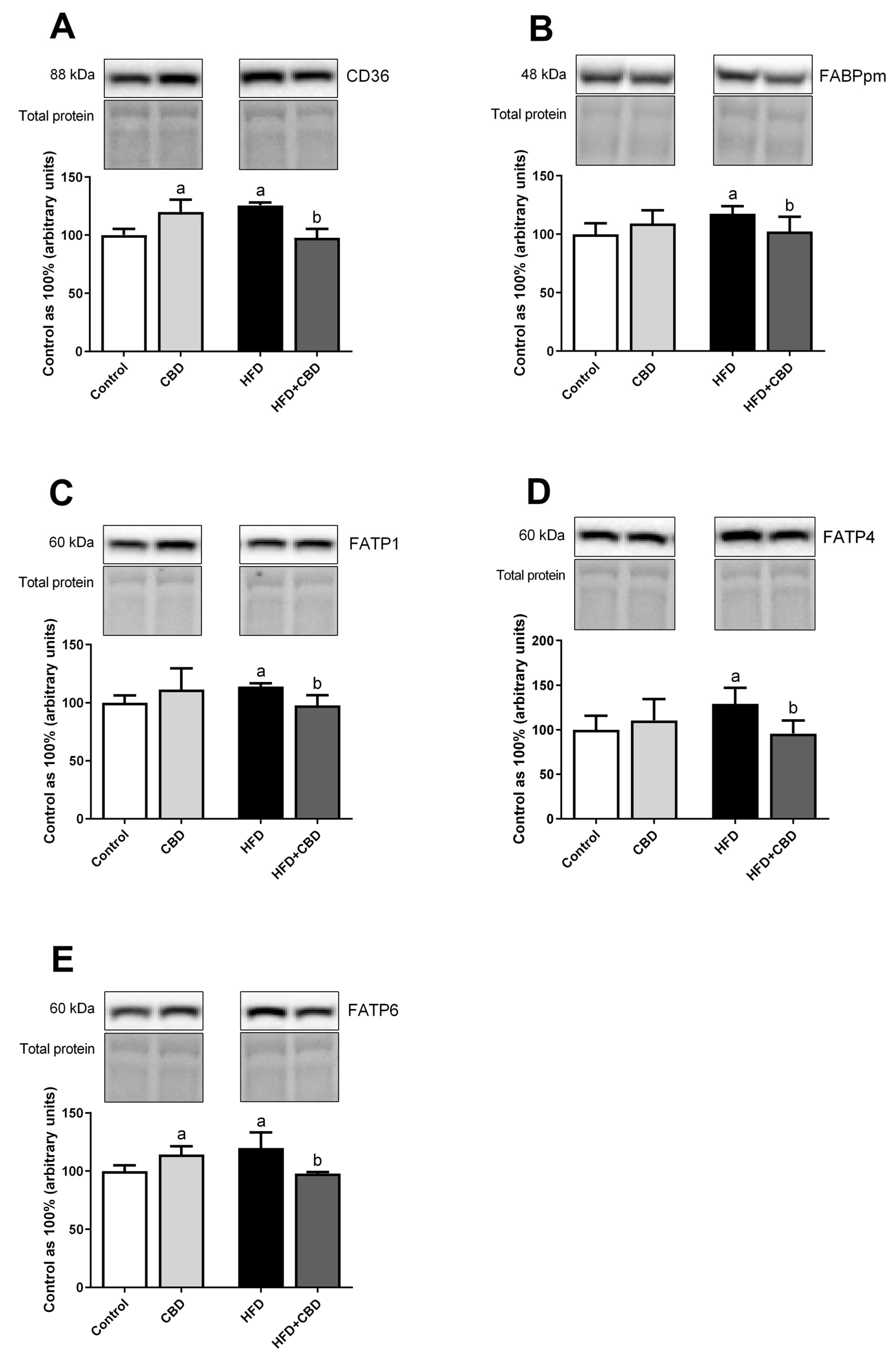
2.3. Effects of 2-Week CBD Administration on the Total Myocardial Expression of Fatty Acid Transporters in Both the Standard Chow and High-Fat Diet-Fed Rats
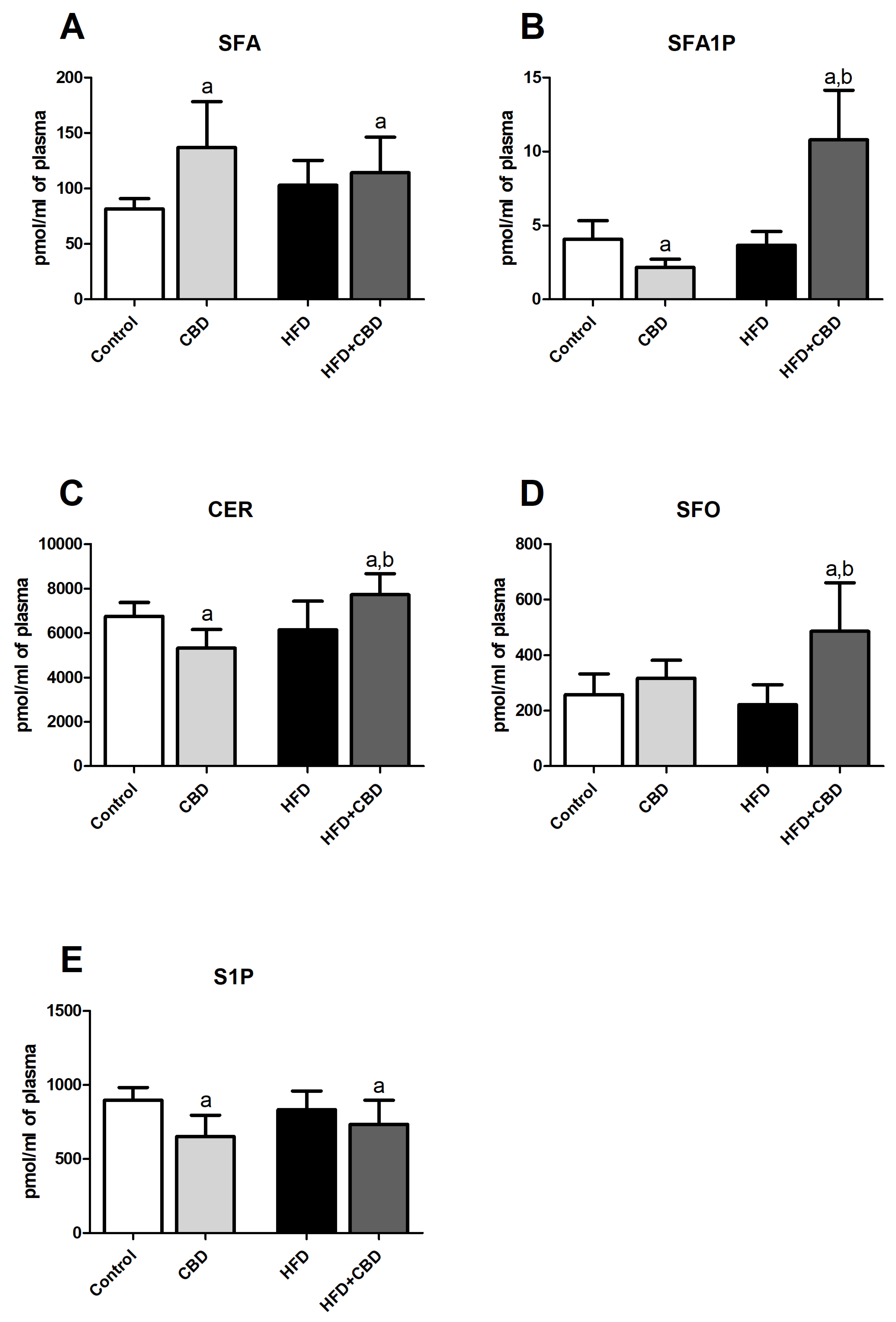
2.4. Effects of 2-Week CBD Administration on the Myocardial Sphinganine, Sphinganine-1-Phosphate, Ceramide, Sphingosine, Sphingosine-1-Phosphate, and Sphingomyelin Contents in Both the Standard Chow and High-Fat Diet-Fed Rats
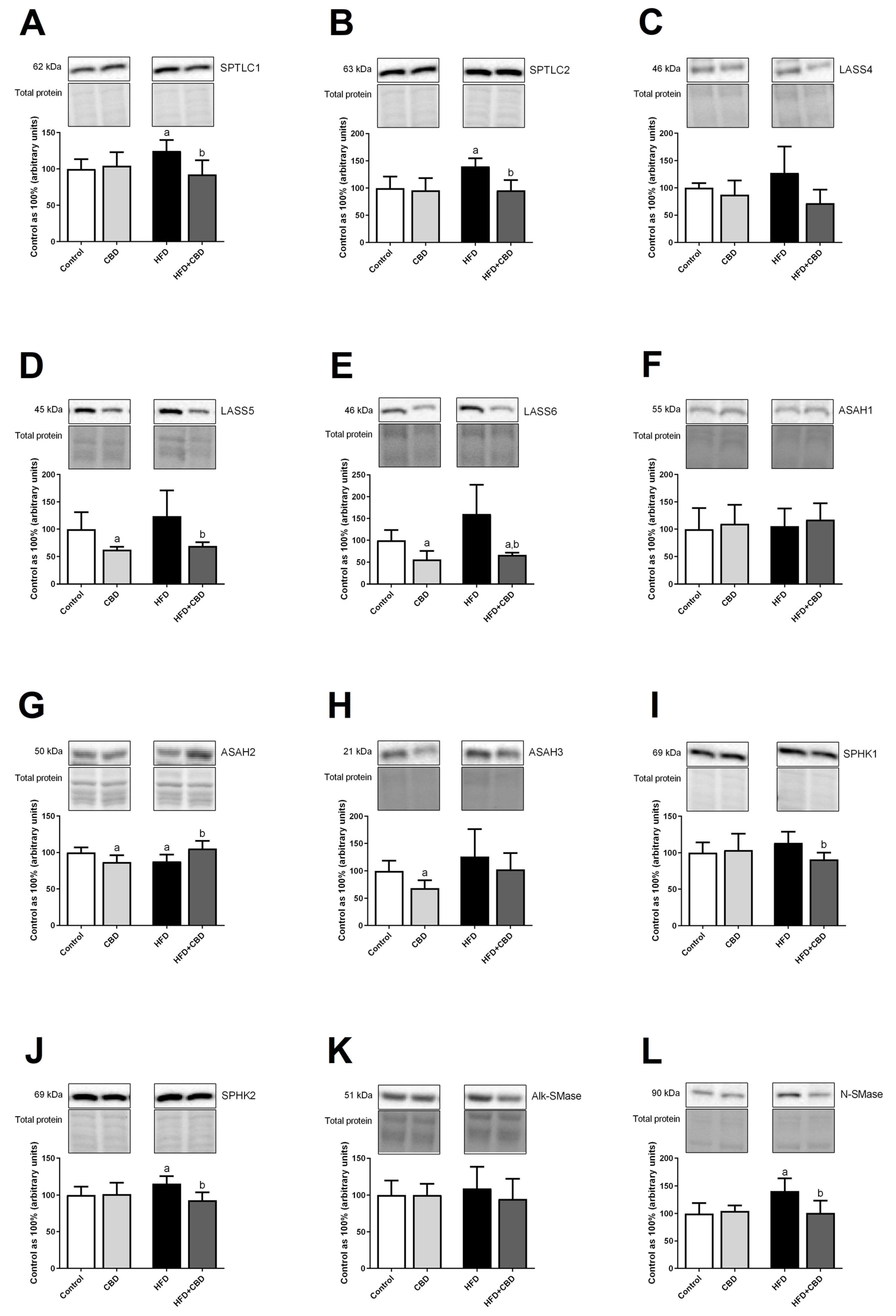
2.5. Effects of 2-Week CBD Administration on the Total Myocardial Expression of Enzymes Involved in the Sphingolipid Metabolism in Both the Standard Chow and High-Fat Diet-Fed Rats
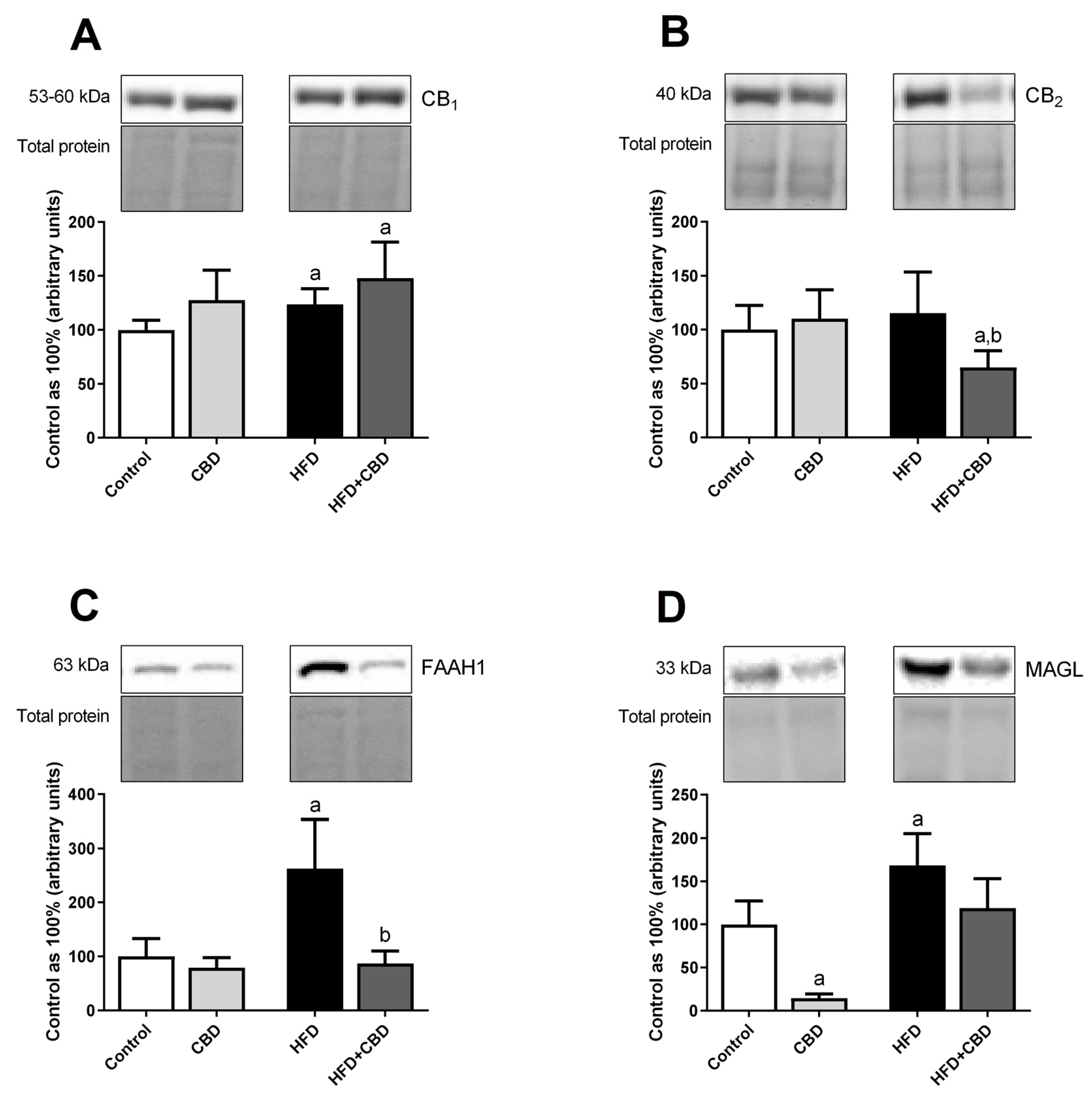
2.6. Effects of 2-Week CBD Administration on the Total Myocardial Expression of the Endocannabinoid System Components in Both the Standard Chow and High-Fat Diet-Fed Rats
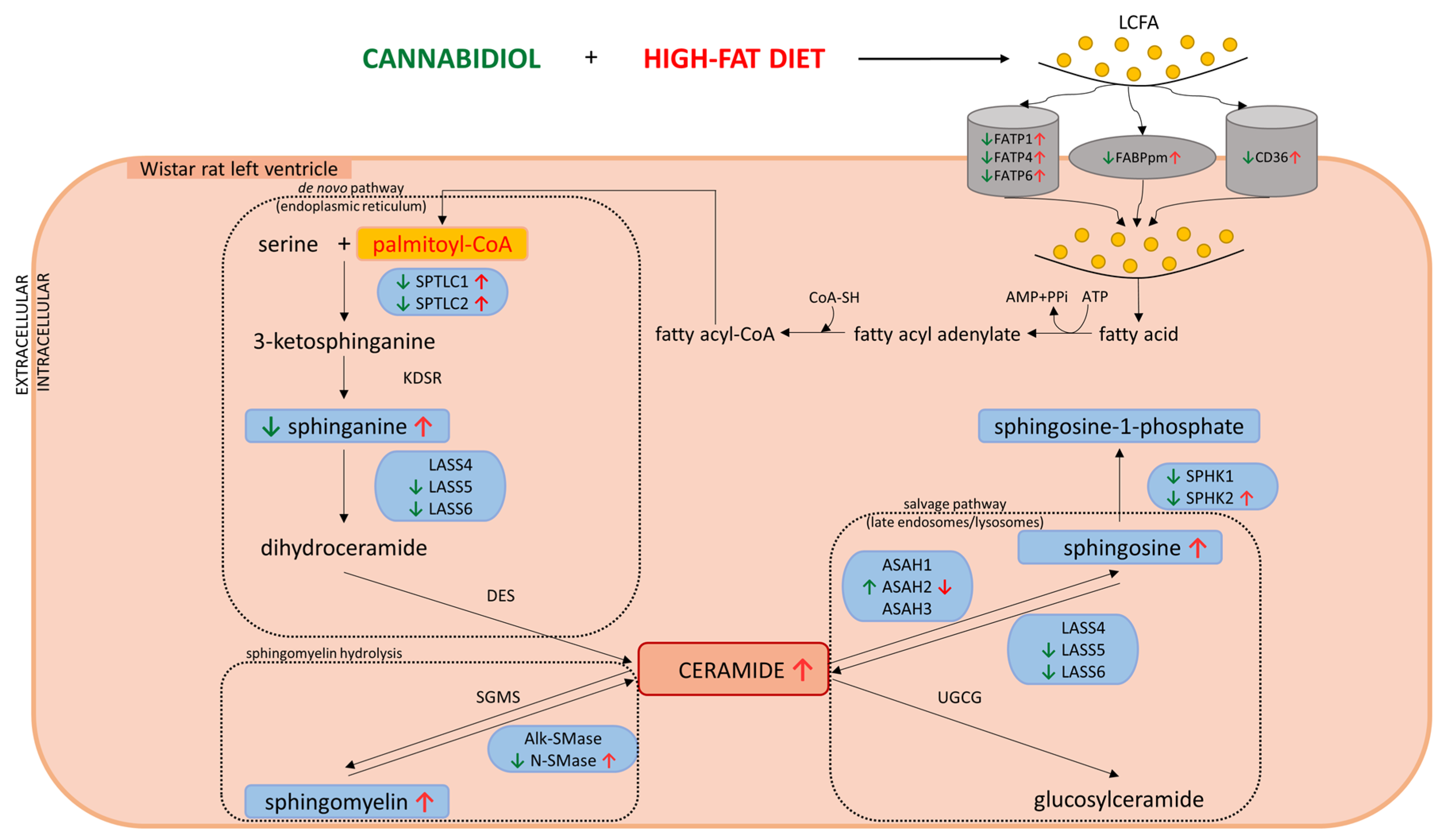
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Protocol
4.2. Plasma and Heart Tissue Lipid Analysis
4.3. Western Blotting
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karwi, Q.G.; Uddin, G.M.; Ho, K.L.; Lopaschuk, G.D. Loss of Metabolic Flexibility in the Failing Heart. Front. Cardiovasc. Med. 2018, 5, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabowski, A.; Górski, J.; Glatz, J.F.; Luiken, J.J.; Bonen, A. Protein-mediated Fatty Acid Uptake in the Heart. Curr. Cardiol. Rev. 2008, 4, 12–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.; Jaswal, J.S.; Stanley, W.C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef] [PubMed]
- Briggs, M.A.; Petersen, K.S.; Kris-Etherton, P.M. Saturated Fatty Acids and Cardiovascular Disease: Replacements for Saturated Fat to Reduce Cardiovascular Risk. Healthcare 2017, 5, 29. [Google Scholar] [CrossRef] [Green Version]
- Bandet, C.L.; Tan-Chen, S.; Bourron, O.; Le Stunff, H.; Hajduch, E. Sphingolipid Metabolism: New Insight into Ceramide-Induced Lipotoxicity in Muscle Cells. Int. J. Mol. Sci. 2019, 20, 479. [Google Scholar] [CrossRef] [Green Version]
- Park, T.S.; Goldberg, I.J. Sphingolipids, lipotoxic cardiomyopathy, and cardiac failure. Heart Fail. Clin. 2012, 8, 633–641. [Google Scholar] [CrossRef] [Green Version]
- Spijkers, L.J.; van den Akker, R.F.; Janssen, B.J.; Debets, J.J.; De Mey, J.G.; Stroes, E.S.; van den Born, B.J.; Wijesinghe, D.S.; Chalfant, C.E.; MacAleese, L.; et al. Hypertension is associated with marked alterations in sphingolipid biology: A potential role for ceramide. PLoS ONE 2011, 6, e21817. [Google Scholar] [CrossRef] [Green Version]
- Pacher, P.; Steffens, S. The emerging role of the endocannabinoid system in cardiovascular disease. Semin. Immunopathol. 2009, 31, 63–77. [Google Scholar] [CrossRef] [Green Version]
- Kunos, G.; Osei-Hyiaman, D.; Liu, J.; Godlewski, G.; Bátkai, S. Endocannabinoids and the control of energy homeostasis. J. Biol. Chem. 2008, 283, 33021–33025. [Google Scholar] [CrossRef] [Green Version]
- Di Marzo, V.; Silvestri, C. Lifestyle and Metabolic Syndrome: Contribution of the Endocannabinoidome. Nutrients 2019, 11, 1956. [Google Scholar] [CrossRef] [Green Version]
- Chye, Y.; Christensen, E.; Solowij, N.; Yücel, M. The Endocannabinoid System and Cannabidiol’s Promise for the Treatment of Substance Use Disorder. Front. Psychiatry 2019, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Piscitelli, F.; Mechoulam, R. Cannabinoids and endocannabinoids in metabolic disorders with focus on diabetes. Handb. Exp. Pharmacol. 2011, 203, 75–104. [Google Scholar] [CrossRef]
- Eid, B.G. Cannabinoids for Treating Cardiovascular Disorders: Putting Together a Complex Puzzle. J. Microsc. Ultrastruct. 2018, 6, 171–176. [Google Scholar] [CrossRef]
- Durst, R.; Danenberg, H.; Gallily, R.; Mechoulam, R.; Meir, K.; Grad, E.; Beeri, R.; Pugatsch, T.; Tarsish, E.; Lotan, C. Cannabidiol, a nonpsychoactive Cannabis constituent, protects against myocardial ischemic reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3602–H3607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Patel, V.; Saito, K.; Matsumoto, S.; Kashiwaya, Y.; Horváth, B.; Mukhopadhyay, B.; Becker, L.; et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 2115–2125. [Google Scholar] [CrossRef] [Green Version]
- Kitatani, K.; Idkowiak-Baldys, J.; Hannun, Y.A. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal 2008, 20, 1010–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watt, M.J.; Barnett, A.C.; Bruce, C.R.; Schenk, S.; Horowitz, J.F.; Hoy, A.J. Regulation of plasma ceramide levels with fatty acid oversupply: Evidence that the liver detects and secretes de novo synthesised ceramide. Diabetologia 2012, 55, 2741–2746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuzmenko, D.I.; Klimentyeva, T.K. Role of Ceramide in Apoptosis and Development of Insulin Resistance. Biochemistry 2016, 81, 913–927. [Google Scholar] [CrossRef]
- Aburasayn, H.; Al Batran, R.; Ussher, J.R. Targeting ceramide metabolism in obesity. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E423–E435. [Google Scholar] [CrossRef]
- Parra, V.; Eisner, V.; Chiong, M.; Criollo, A.; Moraga, F.; Garcia, A.; Härtel, S.; Jaimovich, E.; Zorzano, A.; Hidalgo, C.; et al. Changes in mitochondrial dynamics during ceramide-induced cardiomyocyte early apoptosis. Cardiovasc. Res. 2008, 77, 387–397. [Google Scholar] [CrossRef] [Green Version]
- Law, B.A.; Liao, X.; Moore, K.S.; Southard, A.; Roddy, P.; Ji, R.; Szulc, Z.; Bielawska, A.; Schulze, P.C.; Cowart, L.A. Lipotoxic very-long-chain ceramides cause mitochondrial dysfunction, oxidative stress, and cell death in cardiomyocytes. FASEB J. 2018, 32, 1403–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bielawska, A.E.; Shapiro, J.P.; Jiang, L.; Melkonyan, H.S.; Piot, C.; Wolfe, C.L.; Tomei, L.D.; Hannun, Y.A.; Umansky, S.R. Ceramide is involved in triggering of cardiomyocyte apoptosis induced by ischemia and reperfusion. Am. J. Pathol. 1997, 151, 1257–1263. [Google Scholar] [PubMed]
- Vestri, A.; Pierucci, F.; Frati, A.; Monaco, L.; Meacci, E. Sphingosine 1-Phosphate Receptors: Do They Have a Therapeutic Potential in Cardiac Fibrosis? Front. Pharmacol. 2017, 8, 296. [Google Scholar] [CrossRef] [Green Version]
- Cantalupo, A.; Gargiulo, A.; Dautaj, E.; Liu, C.; Zhang, Y.; Hla, T.; Di Lorenzo, A. S1PR1 (Sphingosine-1-Phosphate Receptor 1) Signaling Regulates Blood Flow and Pressure. Hypertension 2017, 70, 426–434. [Google Scholar] [CrossRef]
- Jin, Z.Q.; Zhou, H.Z.; Zhu, P.; Honbo, N.; Mochly-Rosen, D.; Messing, R.O.; Goetzl, E.J.; Karliner, J.S.; Gray, M.O. Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKC epsilon knockout mouse hearts. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1970–H1977. [Google Scholar] [CrossRef] [Green Version]
- Baranowska-Kuczko, M.; Kozłowska, H.; Kloza, M.; Kusaczuk, M.; Harasim-Symbor, E.; Biernacki, M.; Kasacka, I.; Malinowska, B. Vasoprotective Endothelial Effects of Chronic Cannabidiol Treatment and Its Influence on the Endocannabinoid System in Rats with Primary and Secondary Hypertension. Pharmaceuticals 2021, 14, 1120. [Google Scholar] [CrossRef]
- Remiszewski, P.; Jarocka-Karpowicz, I.; Biernacki, M.; Jastrząb, A.; Schlicker, E.; Toczek, M.; Harasim-Symbor, E.; Pędzińska-Betiuk, A.; Malinowska, B. Chronic Cannabidiol Administration Fails to Diminish Blood Pressure in Rats with Primary and Secondary Hypertension Despite Its Effects on Cardiac and Plasma Endocannabinoid System, Oxidative Stress and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 1295. [Google Scholar] [CrossRef] [Green Version]
- Burstein, S.; Hunter, S.A.; Renzulli, L. Stimulation of sphingomyelin hydrolysis by cannabidiol in fibroblasts from a Niemann-Pick patient. Biochem. Biophys. Res. Commun. 1984, 121, 168–173. [Google Scholar] [CrossRef]
- Pavoine, C.; Pecker, F. Sphingomyelinases: Their regulation and roles in cardiovascular pathophysiology. Cardiovasc. Res. 2009, 82, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Klevstig, M.; Ståhlman, M.; Lundqvist, A.; Scharin Täng, M.; Fogelstrand, P.; Adiels, M.; Andersson, L.; Kolesnick, R.; Jeppsson, A.; Borén, J.; et al. Targeting acid sphingomyelinase reduces cardiac ceramide accumulation in the post-ischemic heart. J. Mol. Cell Cardiol. 2016, 93, 69–72. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, O.M.; Discher, D.J.; Bishopric, N.H.; Webster, K.A. Rapid activation of neutral sphingomyelinase by hypoxia-reoxygenation of cardiac myocytes. Circ. Res. 2000, 86, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Luiken, J.J.; Schaap, F.G.; van Nieuwenhoven, F.A.; van der Vusse, G.J.; Bonen, A.; Glatz, J.F. Cellular fatty acid transport in heart and skeletal muscle as facilitated by proteins. Lipids 1999, 34, S169–S175. [Google Scholar] [CrossRef]
- Glatz, J.F.; Luiken, J.J.; Bonen, A. Membrane fatty acid transporters as regulators of lipid metabolism: Implications for metabolic disease. Physiol. Rev. 2010, 90, 367–417. [Google Scholar] [CrossRef] [Green Version]
- Luiken, J.J.; Arumugam, Y.; Bell, R.C.; Calles-Escandon, J.; Tandon, N.N.; Glatz, J.F.; Bonen, A. Changes in fatty acid transport and transporters are related to the severity of insulin deficiency. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E612–E621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lago-Fernandez, A.; Zarzo-Arias, S.; Jagerovic, N.; Morales, P. Relevance of Peroxisome Proliferator Activated Receptors in Multitarget Paradigm Associated with the Endocannabinoid System. Int. J. Mol. Sci. 2021, 22, 1001. [Google Scholar] [CrossRef]
- Kalinowska, A.; Górski, J.; Harasim, E.; Harasiuk, D.; Bonen, A.; Chabowski, A. Differential effects of chronic, in vivo, PPAR’s stimulation on the myocardial subcellular redistribution of FAT/CD36 and FABPpm. FEBS Lett. 2009, 583, 2527–2534. [Google Scholar] [CrossRef] [Green Version]
- Silvestri, C.; Paris, D.; Martella, A.; Melck, D.; Guadagnino, I.; Cawthorne, M.; Motta, A.; Di Marzo, V. Two non-psychoactive cannabinoids reduce intracellular lipid levels and inhibit hepatosteatosis. J. Hepatol. 2015, 62, 1382–1390. [Google Scholar] [CrossRef]
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as novel anti-inflammatory drugs. Future Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef] [Green Version]
- Devinsky, O.; Cilio, M.R.; Cross, H.; Fernandez-Ruiz, J.; French, J.; Hill, C.; Katz, R.; Di Marzo, V.; Jutras-Aswad, D.; Notcutt, W.G.; et al. Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014, 55, 791–802. [Google Scholar] [CrossRef] [Green Version]
- Watson, J.E.; Kim, J.S.; Das, A. Emerging class of omega-3 fatty acid endocannabinoids & their derivatives. Prostaglandins Other Lipid Mediat. 2019, 143, 106337. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, L.; Harvey-White, J.; Osei-Hyiaman, D.; Razdan, R.; Gong, Q.; Chan, A.C.; Zhou, Z.; Huang, B.X.; Kim, H.Y.; et al. A biosynthetic pathway for anandamide. Proc. Natl. Acad. Sci. USA 2006, 103, 13345–13350. [Google Scholar] [CrossRef] [Green Version]
- Saghatelian, A.; Trauger, S.A.; Want, E.J.; Hawkins, E.G.; Siuzdak, G.; Cravatt, B.F. Assignment of endogenous substrates to enzymes by global metabolite profiling. Biochemistry 2004, 43, 14332–14339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, J.; Gaetani, S.; Oveisi, F.; Lo Verme, J.; Serrano, A.; Rodríguez De Fonseca, F.; Rosengarth, A.; Luecke, H.; Di Giacomo, B.; Tarzia, G.; et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature 2003, 425, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Puhl, S.L. Cannabinoid-sensitive receptors in cardiac physiology and ischaemia. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118462. [Google Scholar] [CrossRef] [PubMed]
- Hiley, C.R. Endocannabinoids and the heart. J. Cardiovasc. Pharmacol. 2009, 53, 267–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bátkai, S.; Rajesh, M.; Mukhopadhyay, P.; Haskó, G.; Liaudet, L.; Cravatt, B.F.; Csiszár, A.; Ungvári, Z.; Pacher, P. Decreased age-related cardiac dysfunction, myocardial nitrative stress, inflammatory gene expression, and apoptosis in mice lacking fatty acid amide hydrolase. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H909–H918. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, M.; Zabielski, P.; Blachnio, A.; Gorski, J. Effect of exercise duration on ceramide metabolism in the rat heart. Acta Physiol. 2008, 192, 519–529. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Konstantynowicz-Nowicka, K.; Harasim, E.; Baranowski, M.; Chabowski, A. New evidence for the role of ceramide in the development of hepatic insulin resistance. PLoS ONE 2015, 10, e0116858. [Google Scholar] [CrossRef] [Green Version]
- Gilda, J.E.; Gomes, A.V. Stain-Free total protein staining is a superior loading control to β-actin for Western blots. Anal. Biochem. 2013, 440, 186–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charytoniuk, T.; Sztolsztener, K.; Bielawiec, P.; Chabowski, A.; Konstantynowicz-Nowicka, K.; Harasim-Symbor, E. Cannabidiol Downregulates Myocardial de Novo Ceramide Synthesis Pathway in a Rat Model of High-Fat Diet-Induced Obesity. Int. J. Mol. Sci. 2022, 23, 2232. https://doi.org/10.3390/ijms23042232
Charytoniuk T, Sztolsztener K, Bielawiec P, Chabowski A, Konstantynowicz-Nowicka K, Harasim-Symbor E. Cannabidiol Downregulates Myocardial de Novo Ceramide Synthesis Pathway in a Rat Model of High-Fat Diet-Induced Obesity. International Journal of Molecular Sciences. 2022; 23(4):2232. https://doi.org/10.3390/ijms23042232
Chicago/Turabian StyleCharytoniuk, Tomasz, Klaudia Sztolsztener, Patrycja Bielawiec, Adrian Chabowski, Karolina Konstantynowicz-Nowicka, and Ewa Harasim-Symbor. 2022. "Cannabidiol Downregulates Myocardial de Novo Ceramide Synthesis Pathway in a Rat Model of High-Fat Diet-Induced Obesity" International Journal of Molecular Sciences 23, no. 4: 2232. https://doi.org/10.3390/ijms23042232
APA StyleCharytoniuk, T., Sztolsztener, K., Bielawiec, P., Chabowski, A., Konstantynowicz-Nowicka, K., & Harasim-Symbor, E. (2022). Cannabidiol Downregulates Myocardial de Novo Ceramide Synthesis Pathway in a Rat Model of High-Fat Diet-Induced Obesity. International Journal of Molecular Sciences, 23(4), 2232. https://doi.org/10.3390/ijms23042232





