In Vivo Metabolic Responses to Different Formulations of Amino Acid Mixtures for the Treatment of Phenylketonuria (PKU)
Abstract
:1. Introduction
2. Results
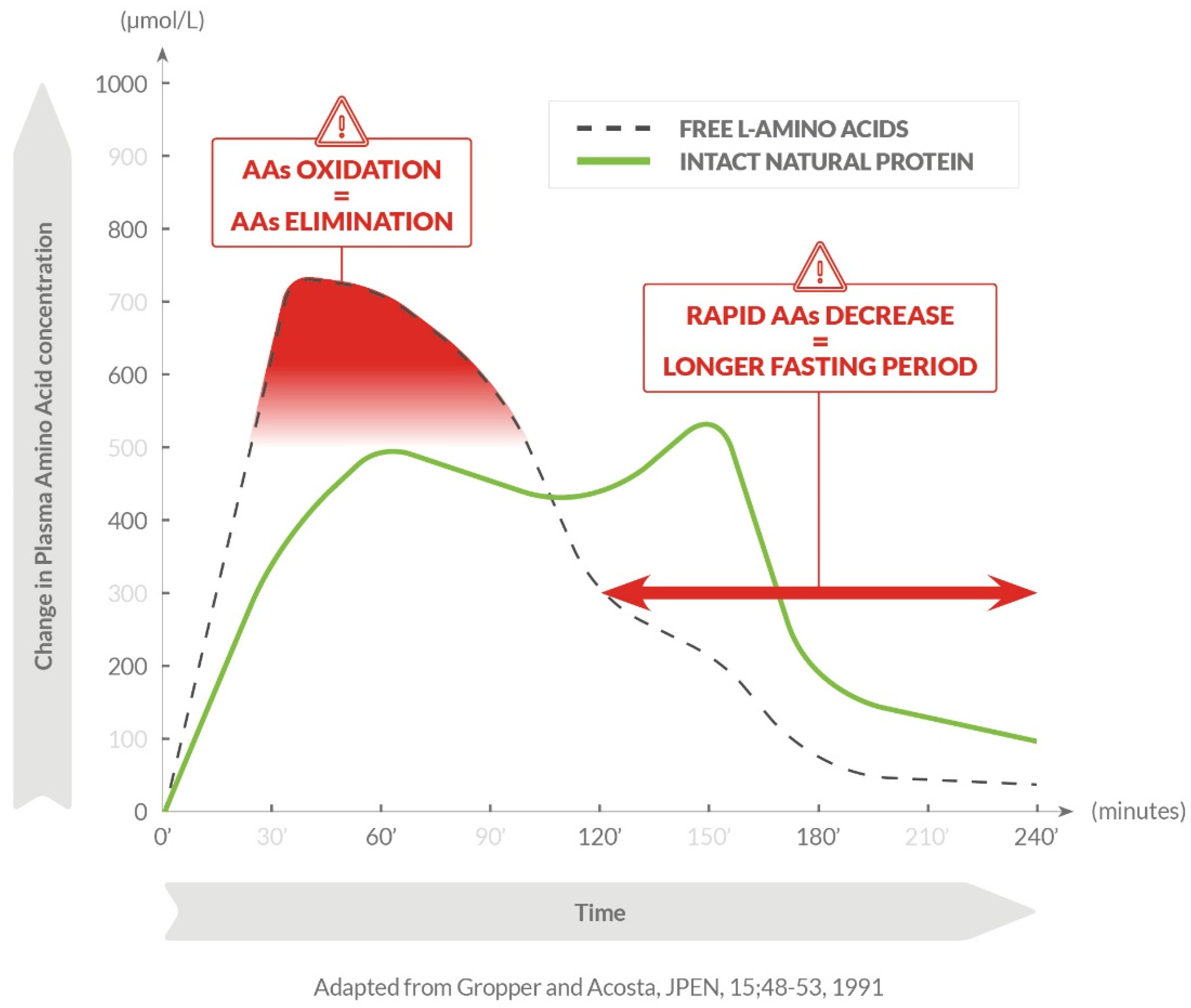
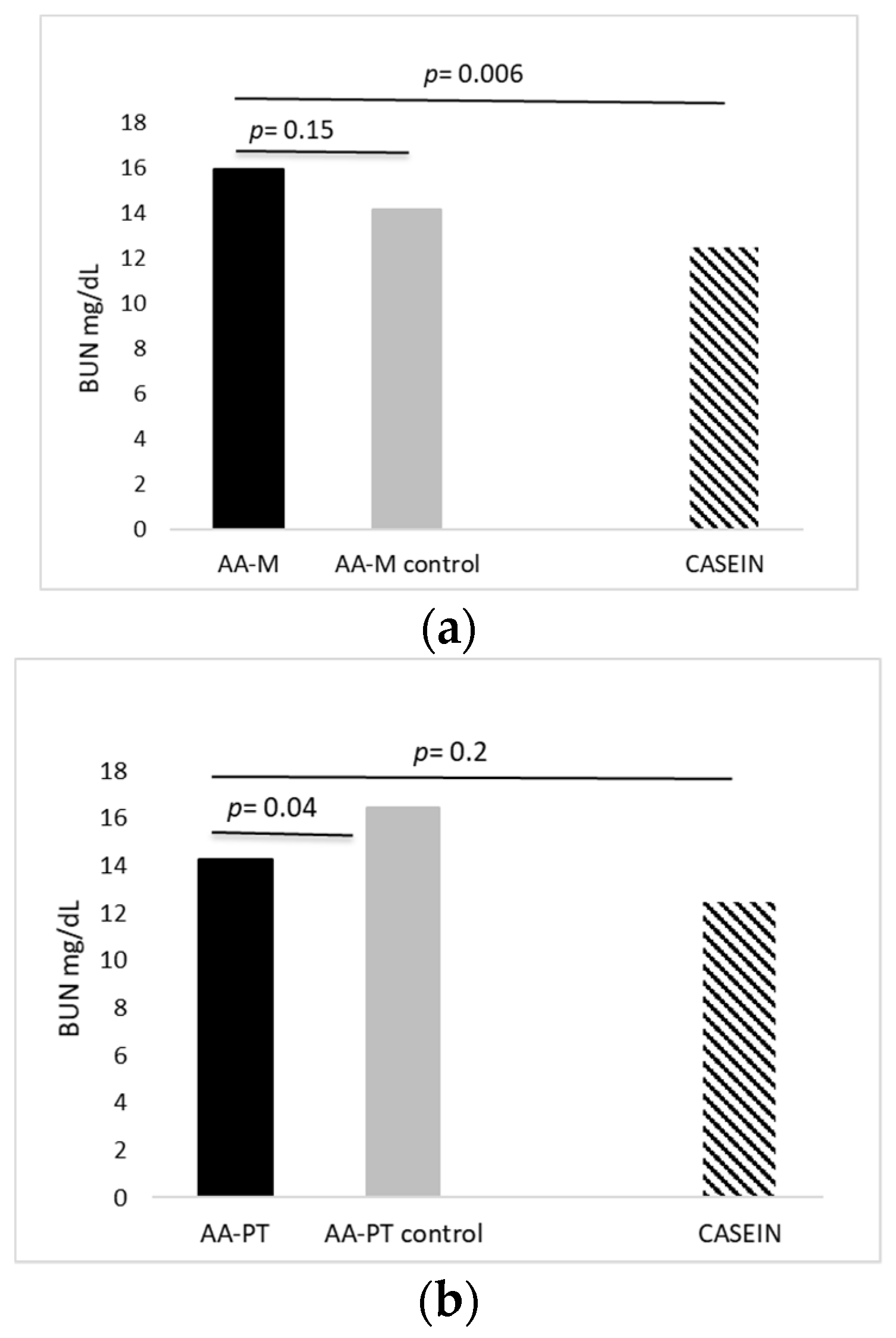
2.1. Acute Effects of AA Formulations on AA Oxidation (BUN)
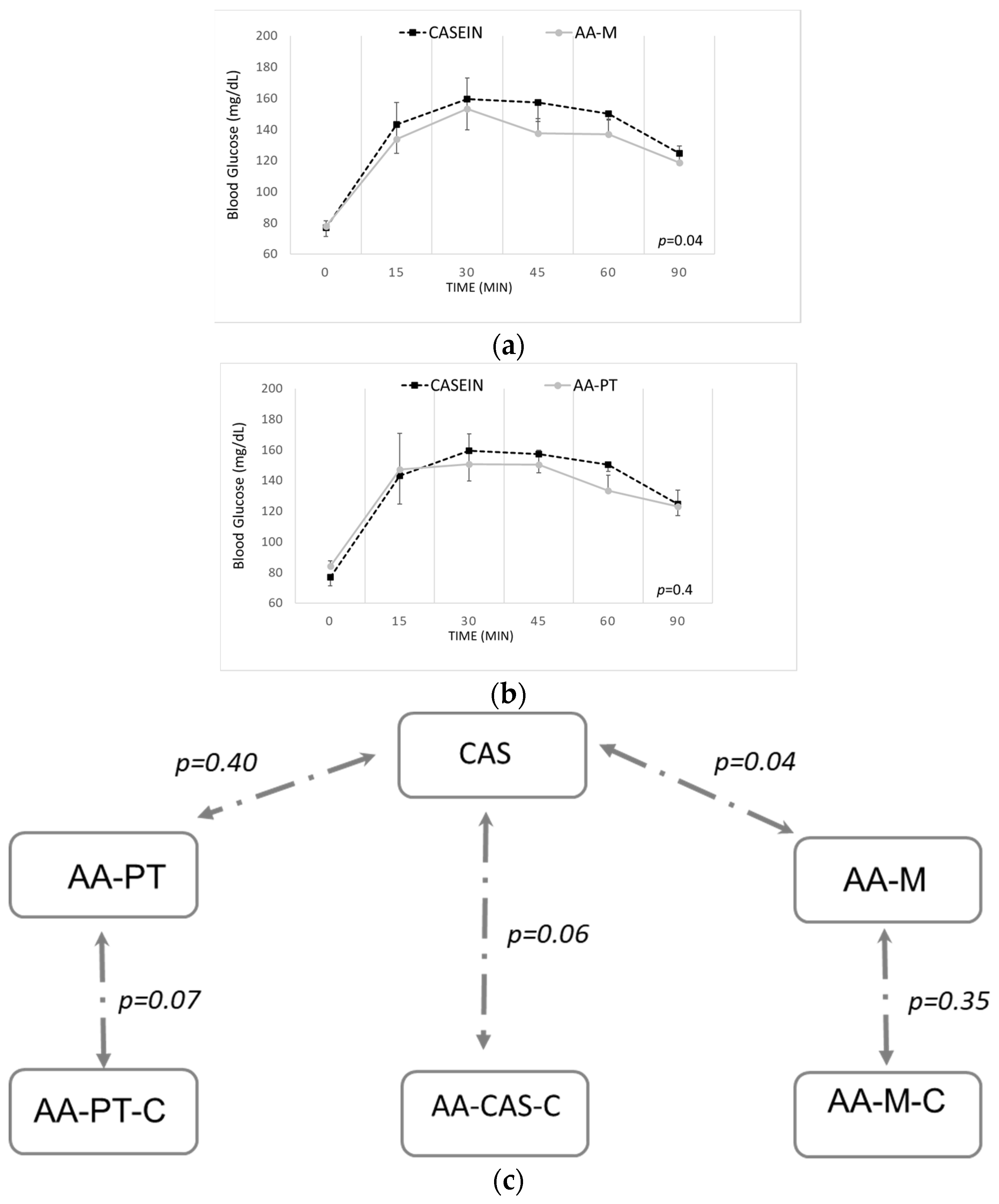
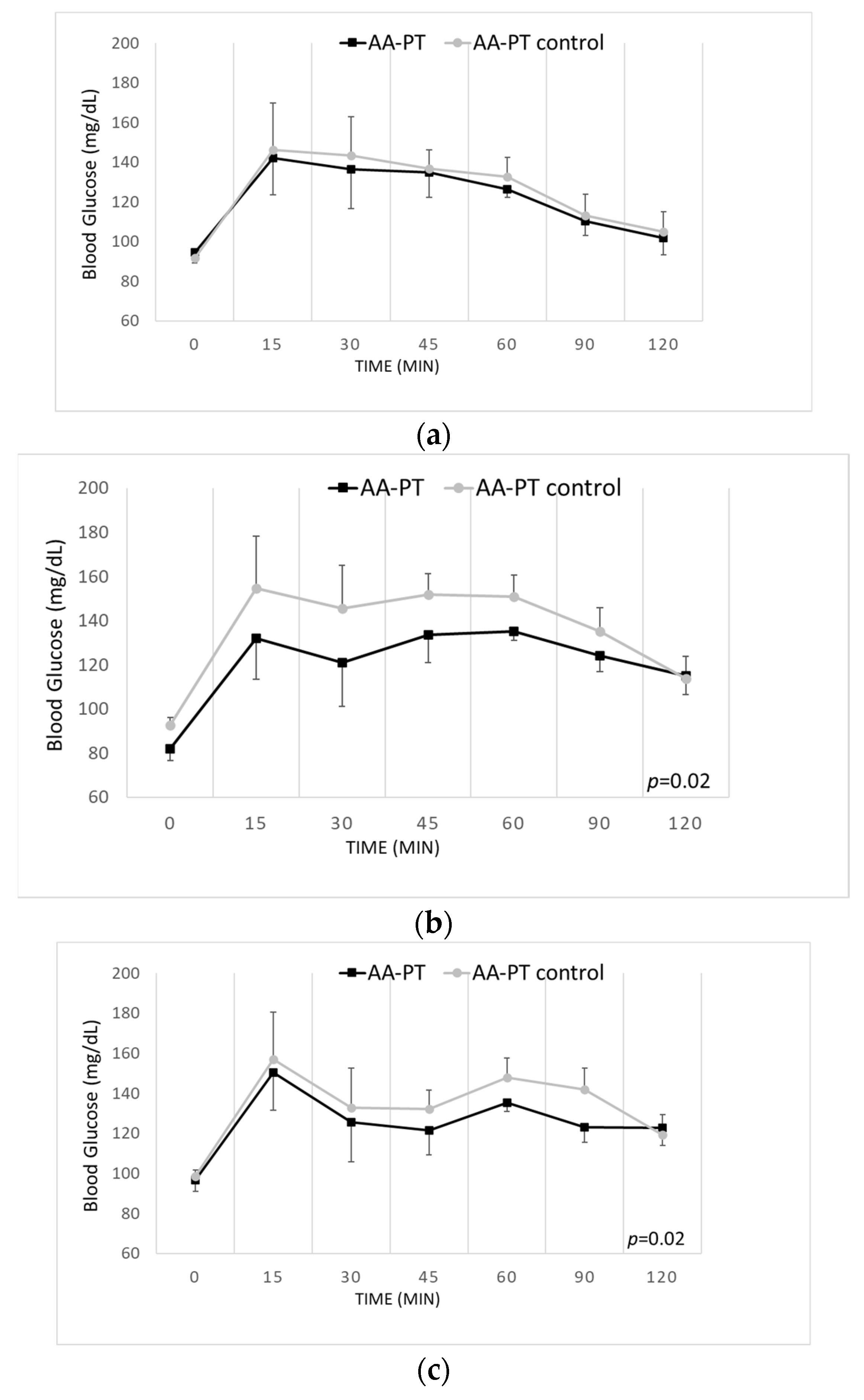
2.2. Acute Effects on Blood Glucose and Other Biomarkers by AA Formulations and Casein
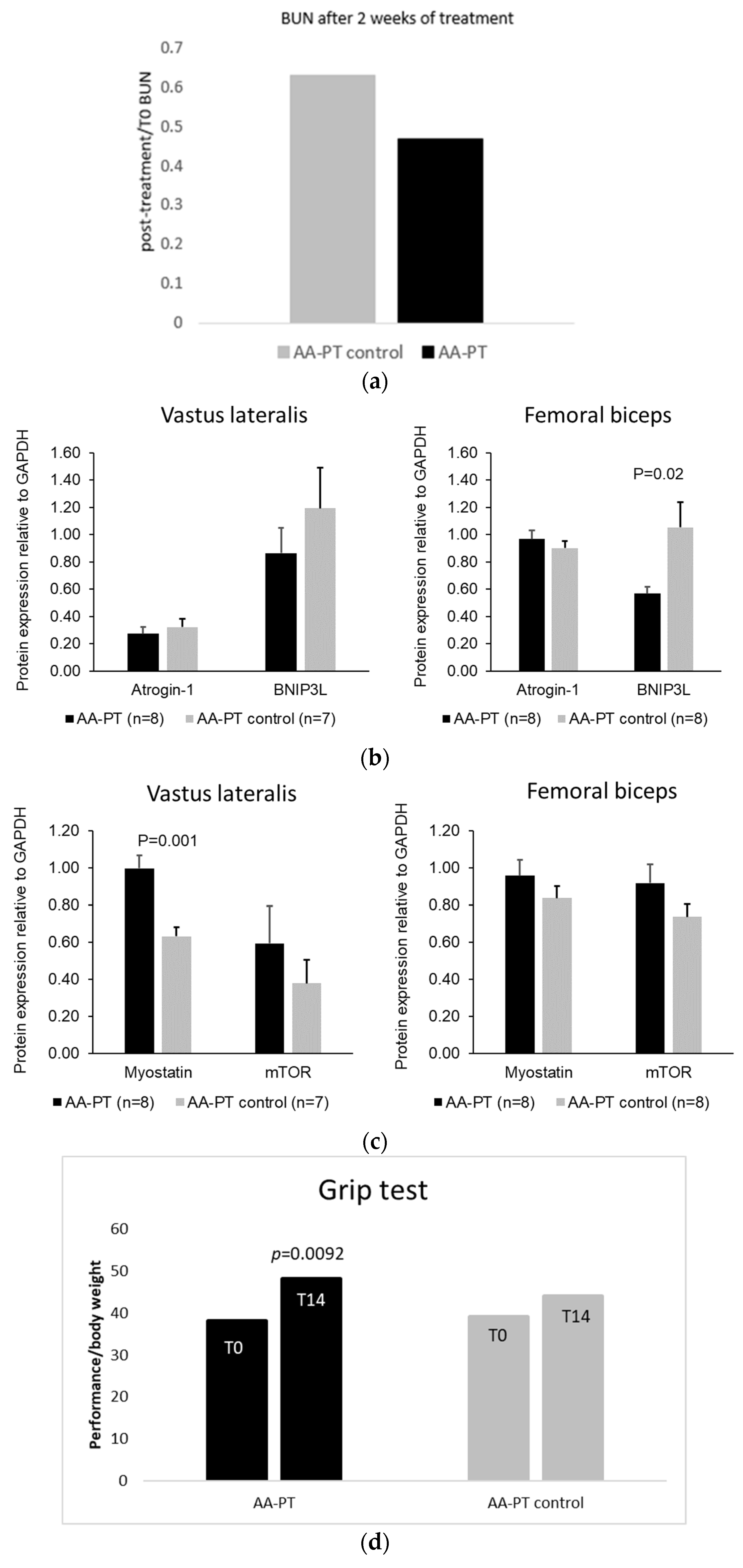
2.3. Long Term Effect of AA Formulations on Muscle
2.4. Long-Term Effects of the Different AA Formulations on Glucose Tolerance
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Procedures
4.2. Acute Experiment
Acute Metabolic Marker Analysis
4.3. Long-Term Experiment
4.3.1. Grip Test
4.3.2. Semi-Quantitative Western Blot of Muscle Protein Expression
4.3.3. Glucose Tolerance Test
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Williams, R.A.; Mamotte, C.D.S.; Burnett, J.R. Phenylketonuria: An Inborn Error of Phenylalanine Metabolism. Clin. Biochem. Rev. 2008, 29, 31–41. [Google Scholar]
- van Spronsen, F.J.; Blau, N.; Harding, C.; Burlina, A.; Longo, N.; Bosch, A.M. Phenylketonuria. Nat. Rev. Dis. Prim. 2021, 7, 36. [Google Scholar] [CrossRef]
- Van Wegberg, A.M.J.; Macdonald, A.; Ahring, K.; BéLanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef] [Green Version]
- Rocha, J.C.; Macdonald, A. Treatment options and dietary supplements for patients with phenylketonuria. Expert Opin. Orphan Drugs 2018, 6, 667–681. [Google Scholar] [CrossRef]
- Daly, A.; Evans, S.; Pinto, A.; Ashmore, C.; MacDonald, A. Protein Substitutes in PKU; Their Historical Evolution. Nutrients 2021, 13, 484. [Google Scholar] [CrossRef]
- MacDonald, A.; Singh, R.H.; Rocha, J.C.; Van Spronsen, F.J. Optimising amino acid absorption: Essential to improve nitrogen balance and metabolic control in phenylketonuria. Nutr. Res. Rev. 2018, 32, 70–78. [Google Scholar] [CrossRef]
- Brown, C.S.; Lichter-Konecki, U. Phenylketonuria (PKU): A problem solved? Mol. Genet. Metab. Rep. 2016, 6, 8–12. [Google Scholar] [CrossRef]
- Ilgaz, F.; Pinto, A.; Gökmen-Özel, H.; Rocha, J.C.; Van Dam, E.; Ahring, K.; Bélanger-Quintana, A.; Dokoupil, K.; Karabulut, E.; Macdonald, A. Long-Term Growth in Phenylketonuria: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2070. [Google Scholar] [CrossRef] [Green Version]
- Giarratana, N.; Gallina, G.; Panzeri, V.; Frangi, A.; Canobbio, A.; Reiner, G. A New Phe-Free Protein Substitute Engineered to Allow a Physiological Absorption of Free Amino Acids for Phenylketonuria. J. Inborn Errors Metab. Screen. 2018, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Pena, M.J.; Costa, R.; Rodrigues, I.; Martins, S.; Guimarães, J.T.; Faria, A.; Calhau, C.; Rocha, J.C.; Borges, N. Unveiling the Metabolic Effects of Glycomacropeptide. Int. J. Mol. Sci. 2021, 22, 9731. [Google Scholar] [CrossRef]
- Dangin, M.; Boirie, Y.; Garcia-Rodenas, C.; Gachon, P.; Fauquant, J.; Callier, P.; Ballèvre, O.; Beaufrère, B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am. J. Physiol. Metab. 2001, 280, E340–E348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacroix, M.; Bos, C.; Léonil, J.; Airinei, G.; Luengo, C.; Daré, S.; Benamouzig, R.; Fouillet, H.; Fauquant, J.; Tomé, D.; et al. Compared with casein or total milk protein, digestion of milk soluble proteins is too rapid to sustain the anabolic postprandial amino acid requirement. Am. J. Clin. Nutr. 2006, 84, 1070–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordano, M.; Castellino, P.; A DeFronzo, R. Differential responsiveness of protein synthesis and degradation to amino acid availability in humans. Diabetes 1996, 45, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Whang, K.Y.; Easter, R.A. Blood Urea Nitrogen as an Index of Feed Efficiency and Lean Growth Potential in Growing-Finishing Swine. Asian-Australas. J. Anim. Sci. 2000, 13, 811–816. [Google Scholar] [CrossRef]
- Mönch, E.; Herrmann, M.E.; Brösicke, H.; Schöffer, A.; Keller, M. Utilisation of amino acid mixtures in adolescents with phenylketonuria. Eur. J. Pediatr. 1996, 155 (Supp. 1), S115–S120. [Google Scholar] [CrossRef]
- Singh, R.H.; Cunningham, A.C.; Mofidi, S.; Douglas, T.D.; Frazier, D.M.; Hook, D.G.; Jeffers, L.; McCune, H.; Moseley, K.D.; Ogata, B.; et al. Updated, web-based nutrition management guideline for PKU: An evidence and consensus based approach. Mol. Genet. Metab. 2016, 118, 72–83. [Google Scholar] [CrossRef]
- Scheinin, M.; Barassi, A.; Junnila, J.; Lovró, Z.; Reiner, G.; Sarkkinen, E.; Macdonald, A. Amino Acid Plasma Profiles from a Prolonged-Release Protein Substitute for Phenylketonuria: A Randomized, Single-Dose, Four-Way Crossover Trial in Healthy Volunteers. Nutriens 2020, 12, 1653. [Google Scholar] [CrossRef]
- Scheinin, M.; Junnila, J.; Reiner, G.; MacDonald, A.; Muntau, A.C. Nitrogen Balance after the Administration of a Prolonged-Release Protein Substitute for Phenylketonuria as a Single Dose in Healthy Volunteers. Nutriens 2021, 13, 3189. [Google Scholar] [CrossRef]
- Veeneman, J.M.; Kingma, H.A.; Stellaard, F.; De Jong, P.E.; Reijngoud, D.-J.; Huisman, R.M. Comparison of amino acid oxidation and urea metabolism in haemodialysis patients during fasting and meal intake. Nephrol. Dial. Transplant. 2004, 19, 1533–1541. [Google Scholar] [CrossRef] [Green Version]
- Taillandier, D.; Polge, C. Skeletal muscle atrogenes: From rodent models to human pathologies. Biochimie 2019, 166, 251–269. [Google Scholar] [CrossRef]
- Casey, L. Caring for children with phenylketonuria. Can. Fam. Physician 2013, 59, 837–840. [Google Scholar] [PubMed]
- Van Spronsen, F.J.; Van Rijn, M.; Van Dijk, T.; Smit, G.P.; Reijngoud, D.J.; Berger, R.; Heymans, H.S. Plasma phenylalanine and tyrosine responses to different nutritional conditions (fasting/postprandial) in patients with phenylketonuria: Effect of sample timing. Pediatrics 1993, 92, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Solon-Biet, S.M.; Cogger, V.C.; Pulpitel, T.; Wahl, D.; Clark, X.; Bagley, E.E.; Gregoriou, G.C.; Senior, A.M.; Wang, Q.-P.; Brandon, A.E.; et al. Branched-chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat. Metab. 2019, 1, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Pena, M.J.; Guerreiro, S.G.; Rocha, J.C.; Morais, T.; Pereira, S.S.; Monteiro, M.; Borges, N. Acute effect of an amino acid mixture in the rat glycemic profile. J. Cell. Biochem. 2019, 120, 13056–13065. [Google Scholar] [CrossRef]
- Couce, M.L.; Sánchez-Pintos, P.; Vitoria, I.; De Castro, M.-J.; Aldámiz-Echevarría, L.; Correcher, P.; Fernández-Marmiesse, A.; Roca, I.; Hermida, A.; Martínez-Olmos, M.; et al. Carbohydrate status in patients with phenylketonuria. Orphanet J. Rare Dis. 2018, 13, 103. [Google Scholar] [CrossRef] [Green Version]
- van Rijn, M.; Hoeksma, M.; Sauer, P.; Szczerbak, B.; Gross, M.; Reijngoud, D.-J.; van Spronsen, F. Protein metabolism in adult patients with phenylketonuria. Nutrition 2007, 23, 445–453. [Google Scholar] [CrossRef]
- Thompson, G.N.; Pacy, P.J.; E Watts, R.W.; Halliday, D. Protein Metabolism in Phenylketonuria and Lesch-Nyhan Syndrome. Pediatr. Res. 1990, 28, 240–246. [Google Scholar] [CrossRef] [Green Version]
- Wolfensohn, S.; Lloyd, M. Handbook of Laboratory Animal Management and Welfare, 2nd ed.; Wiley: Hoboken, NJ, USA, 1998. [Google Scholar]
- Wang, Z.; Yang, Y.; Xiang, X.; Zhu, Y.; Men, J.; He, M. Estimation of the normal range of blood glucose in rats. Wei Sheng Yan Jiu J. Hyg. Res. 2010, 39, 133–137, 142. [Google Scholar]
- Montilla-García, Á.; Tejada, M.Á.; Perazzoli, G.; Entrena, J.M.; Portillo-Salido, E.; Fernández-Segura, E.; Cañizares, F.J.; Cobos, E.J. Grip strength in mice with joint inflammation: A rheumatology function test sensitive to pain and analgesia. Neuropharmacology 2017, 125, 231–242. [Google Scholar] [CrossRef]
- Gumucio, J.P.; Mendias, C.L. Atrogin-1, MuRF-1, and sarcopenia. Endocrinology 2013, 43, 12–21. [Google Scholar] [CrossRef]
- Zhang, J.; A Ney, P. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 2009, 16, 939–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elkina, Y.; Von Haehling, S.; Anker, S.D.; Springer, J. The role of myostatin in muscle wasting: An overview. J. Cachexia Sarcopenia Muscle 2011, 2, 143–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, M.-S. mTOR as a Key Regulator in Maintaining Skeletal Muscle Mass. Front. Physiol. 2017, 8, 788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bass, J.; Wilkinson, D.J.; Rankin, D.; Phillips, B.; Szewczyk, N.; Smith, K.; Atherton, P.J. An overview of technical considerations for Western blotting applications to physiological research. Scand. J. Med. Sci. Sports 2017, 27, 4–25. [Google Scholar] [CrossRef]
- MacDonald, E.M.; Andres-Mateos, E.; Mejias-Estevez, R.; Simmers, J.L.; Park, J.-S.; Ying, S.; Hoke, A.; Lee, S.-J.; Cohn, R.D. Denervation atrophy is independent from Akt and mTOR activation and is not rescued by myostatin inhibition. Dis. Model. Mech. 2014, 7, 471–481. [Google Scholar] [CrossRef] [Green Version]
- Tezze, C.; Romanello, V.; Desbats, M.A.; Fadini, G.P.; Albiero, M.; Favaro, G.; Ciciliot, S.; Soriano, M.E.; Morbidoni, V.; Cerqua, C.; et al. Age-Associated Loss of OPA1 in Muscle Impacts Muscle Mass, Metabolic Homeostasis, Systemic Inflammation, and Epithelial Senescence. Cell Metab. 2017, 25, 1374–1389. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giarratana, N.; Giardino, L.; Bighinati, A.; Reiner, G.; Rocha, J.C. In Vivo Metabolic Responses to Different Formulations of Amino Acid Mixtures for the Treatment of Phenylketonuria (PKU). Int. J. Mol. Sci. 2022, 23, 2227. https://doi.org/10.3390/ijms23042227
Giarratana N, Giardino L, Bighinati A, Reiner G, Rocha JC. In Vivo Metabolic Responses to Different Formulations of Amino Acid Mixtures for the Treatment of Phenylketonuria (PKU). International Journal of Molecular Sciences. 2022; 23(4):2227. https://doi.org/10.3390/ijms23042227
Chicago/Turabian StyleGiarratana, Nadia, Luciana Giardino, Andrea Bighinati, Giorgio Reiner, and Júlio César Rocha. 2022. "In Vivo Metabolic Responses to Different Formulations of Amino Acid Mixtures for the Treatment of Phenylketonuria (PKU)" International Journal of Molecular Sciences 23, no. 4: 2227. https://doi.org/10.3390/ijms23042227
APA StyleGiarratana, N., Giardino, L., Bighinati, A., Reiner, G., & Rocha, J. C. (2022). In Vivo Metabolic Responses to Different Formulations of Amino Acid Mixtures for the Treatment of Phenylketonuria (PKU). International Journal of Molecular Sciences, 23(4), 2227. https://doi.org/10.3390/ijms23042227






