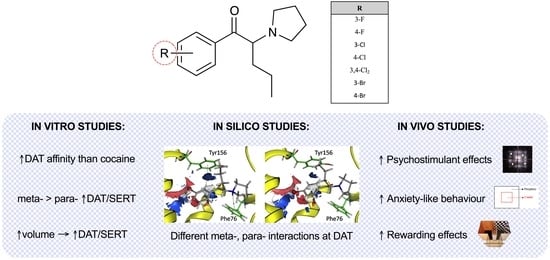
Neuropsychopharmacology of Emerging Drugs of Abuse: meta- and para-Halogen-Ring-Substituted α-PVP (“flakka”) Derivatives
Abstract
:1. Introduction
2. Results
2.1. Effect of α-PVP Derivatives on Monoamine Uptake Inhibition in Transfected HEK293 Cells
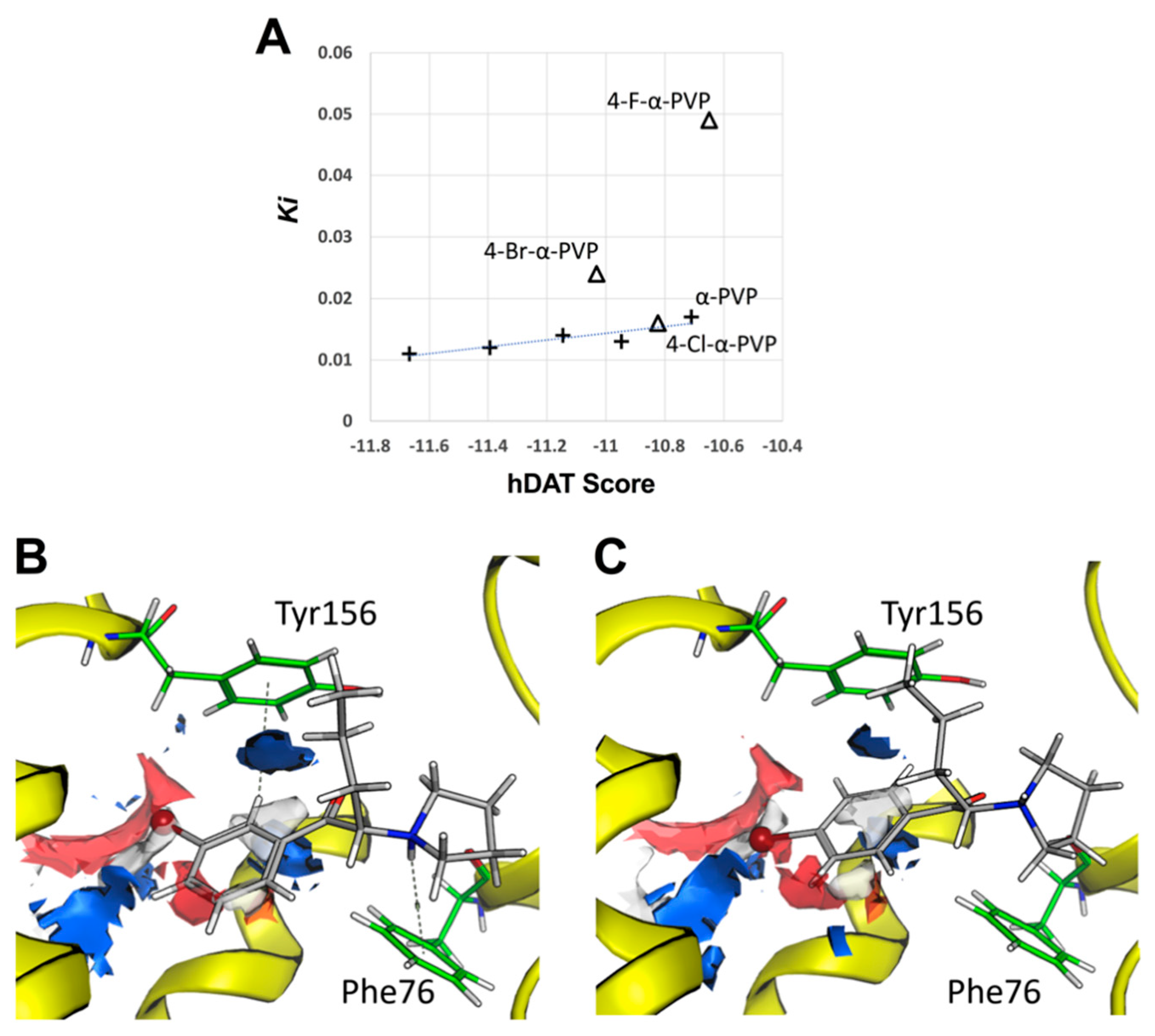
2.2. Effect of α-PVP Derivatives on Transporter Binding Affinities
2.3. Molecular Docking of α-PVP Derivatives at hDAT
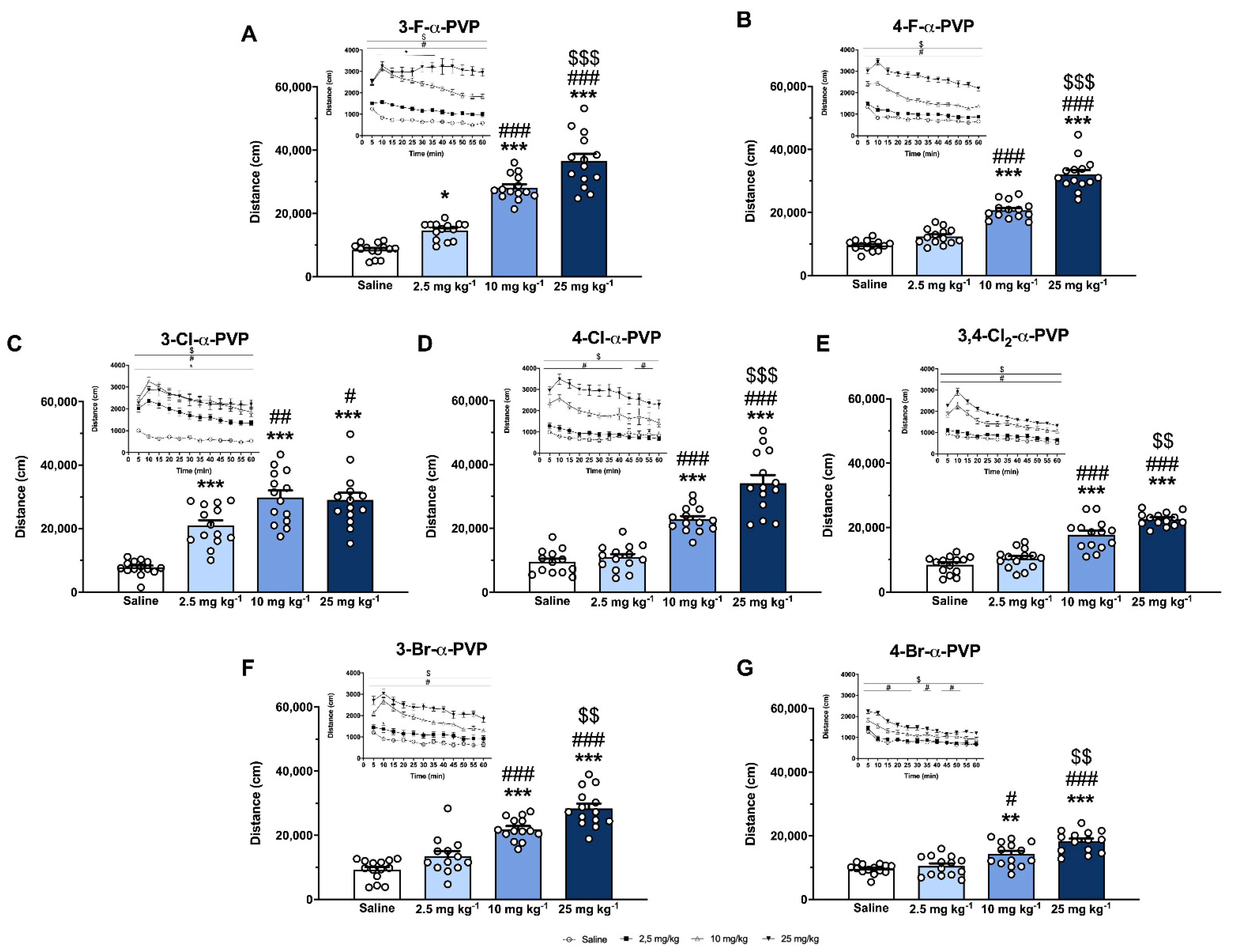
2.4. Effect of α-PVP Derivatives on HLA
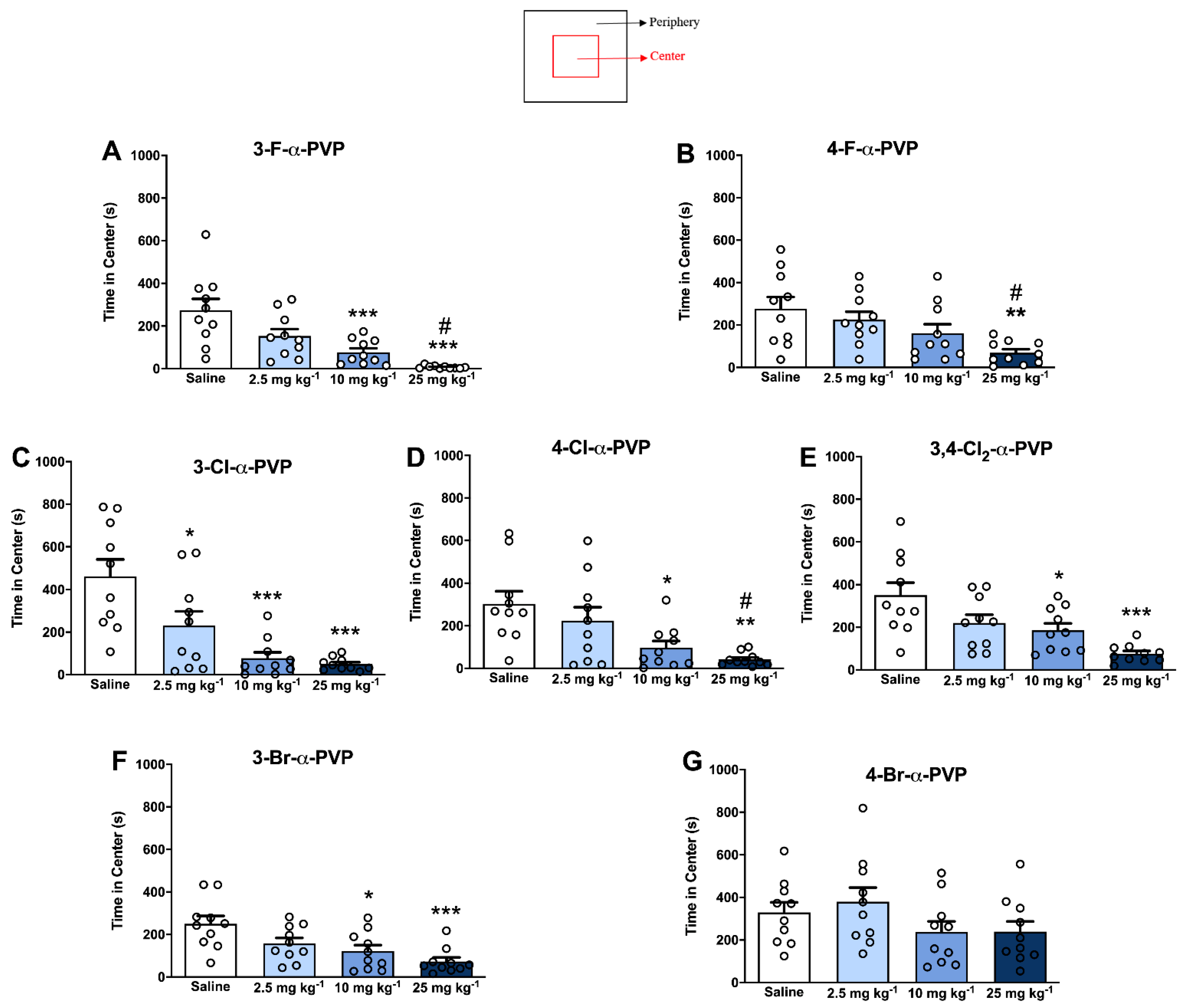
2.5. Effect of α-PVP Derivatives on Anxiety-like Behavior
2.6. Effect of α-PVP Derivatives on CPP
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Drugs and Materials
4.3. Uptake Inhibition and Transporter Binding Assays in HEK293 Cells
4.3.1. Cell Culture and Membrane Preparation
4.3.2. Uptake Inhibition Assays
4.3.3. Transporter Binding Assays
4.4. Molecular Docking of α-PVP Derivatives at hDAT
4.5. Horizontal Locomotor Activity (HLA)
4.6. Open Field Test: Center vs. Periphery
4.7. Conditioned Place Preference (CPP)
4.8. Data and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). European Drug Report: Trends and Developments; Publications Office of the European Union: Luxembourg, 2021. [CrossRef]
- United Nations Office on Drugs and Crime (UNODC). World Drug Report 2021. Global Overview: Drug Demand Drug Supply; Sales No. E.21.XI.8; United Nations Publication: Vienna, Austria, 2021; ISBN 9789211483611. [Google Scholar]
- Majchrzak, M.; Celiński, R.; Kuś, P.; Kowalska, T.; Sajewicz, M. The newest cathinone derivatives as designer drugs: An analytical and toxicological review. Forensic Toxicol. 2018, 36, 33–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunt, T.M.; Poortman, A.; Niesink, R.J.M.; van den Brink, W. Instability of the ecstasy market and a new kid on the block: Mephedrone. J. Psychopharmacol. 2011, 25, 1543–1547. [Google Scholar] [CrossRef] [PubMed]
- Drug Enforcement Administration (DEA). Establishment of drug codes for 26 substances. Final Rule. Fed. Regist. 2013, 78, 664–666. [Google Scholar]
- Odoardi, S.; Romolo, F.S.; Strano-Rossi, S. A snapshot on NPS in Italy: Distribution of drugs in seized materials analysed in an Italian forensic laboratory in the period 2013–2015. Forensic Sci. Int. 2016, 265, 116–120. [Google Scholar] [CrossRef]
- Grifell, M.; Ventura, M.; Carbón, X.; Quintana, P.; Galindo, L.; Palma, Á.; Fornis, I.; Gil, C.; Farre, M.; Torrens, M. Patterns of use and toxicity of new para-halogenated substituted cathinones: 4-CMC (clephedrone), 4-CEC (4-chloroethcatinone) and 4-BMC (brephedrone). Hum. Psychopharmacol. Clin. Exp. 2017, 32, e2621. [Google Scholar] [CrossRef] [Green Version]
- Machado, Y.; Coelho Neto, J.; Barbosa, P.E.N.; Lordeiro, R.A.; Alves, R.B. Brephedrone: A new psychoactive substance seized in Brazil. Forensic Sci. Int. 2017, 275, 302–307. [Google Scholar] [CrossRef]
- Luethi, D.; Liechti, M.E. Designer drugs: Mechanism of action and adverse effects. Arch. Toxicol. 2020, 94, 1085–1133. [Google Scholar] [CrossRef] [Green Version]
- La Maida, N.; Di Trana, A.; Giorgetti, R.; Tagliabracci, A.; Busardò, F.P.; Huestis, M.A. A Review of Synthetic Cathinone-Related Fatalities From 2017 to 2020. Ther. Drug Monit. 2021, 43, 52–68. [Google Scholar] [CrossRef]
- Katselou, M.; Papoutsis, I.; Nikolaou, P.; Spiliopoulou, C.; Athanaselis, S. a-PVP (“flakka”’): A new synthetic cathinone invades the drug arena. Forensic Toxicol. 2016, 34, 41–50. [Google Scholar] [CrossRef]
- Patocka, J.; Zhao, B.; Wu, W.; Klimova, B.; Valis, M.; Nepovimova, E.; Kuca, K. Flakka: New Dangerous Synthetic Cathinone on the Drug Scene. Int. J. Mol. Sci. 2020, 21, 8185. [Google Scholar] [CrossRef]
- Gannon, B.M.; Baumann, M.H.; Walther, D.; Jimenez-Morigosa, C.; Sulima, A.; Rice, K.C.; Collins, G.T. The abuse-related effects of pyrrolidine-containing cathinones are related to their potency and selectivity to inhibit the dopamine transporter. Neuropsychopharmacology 2018, 43, 2399–2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duart-Castells, L.; Nadal-Gratacós, N.; Muralter, M.; Puster, B.; Berzosa, X.; Estrada-Tejedor, R.; Niello, M.; Bhat, S.; Pubill, D.; Camarasa, J.; et al. Role of amino terminal substitutions in the pharmacological, rewarding and psychostimulant profiles of novel synthetic cathinones. Neuropharmacology 2021, 186, 108475. [Google Scholar] [CrossRef]
- Eshleman, A.J.; Wolfrum, K.M.; Reed, J.F.; Kim, S.O.; Swanson, T.; Johnson, R.A.; Janowsky, A. Structure-Activity Relationships of Substituted Cathinones, with Transporter Binding, Uptake, and Release. J. Pharmacol. Exp. Ther. 2017, 360, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Cameron, K.; Kolanos, R.; Vekariya, R.; Verkariya, R.; De Felice, L.; Glennon, R.A. Mephedrone and methylenedioxypyrovalerone (MDPV), major constituents of “bath salts,” produce opposite effects at the human dopamine transporter. Psychopharmacology 2013, 227, 493–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatch, M.B.; Dolan, S.B.; Forster, M.J. Comparative Behavioral Pharmacology of Three Pyrrolidine-Containing Synthetic Cathinone Derivatives. J. Pharmacol. Exp. Ther. 2015, 354, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Huskinson, S.L.; Naylor, J.E.; Townsend, E.A.; Rowlett, J.K.; Blough, B.E.; Freeman, K.B. Self-administration and behavioral economics of second-generation synthetic cathinones in male rats. Psychopharmacology 2017, 234, 589–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, H.; Saka, K.; Nakajima, M.; Maeda, H.; Kuroda, R.; Igarashi, A.; Tsujimura-Ito, T.; Nara, A.; Komori, M.; Yoshida, K.-I. Sudden death after sustained restraint following self-administration of the designer drug α-pyrrolidinovalerophenone. Int. J. Cardiol. 2014, 172, 263–265. [Google Scholar] [CrossRef]
- Potocka-Banaś, B.; Janus, T.; Majdanik, S.; Banaś, T.; Dembińska, T.; Borowiak, K. Fatal Intoxication with α-PVP, a Synthetic Cathinone Derivative. J. Forensic Sci. 2017, 62, 553–556. [Google Scholar] [CrossRef]
- EMCDDA-Europol Joint Report on a New Psychoactive Substance: 1-Phenyl-2-(1-pyrrolidinyl)-1-Pentanone (α-PVP); Joint Reports; Publications Office of the European Union: Luxembourg, 2015. [CrossRef]
- Drug Enforcement Administration (DEA). Temporary Placement of 10 Synthetic Cathinones into Schedule I. Available online: https://www.deadiversion.usdoj.gov/fed_regs/rules/2014/fr0128.htm (accessed on 10 September 2021).
- Uchiyama, N.; Matsuda, S.; Kawamura, M.; Shimokawa, Y.; Kikura-Hanajiri, R.; Aritake, K.; Urade, Y.; Goda, Y. Characterization of four new designer drugs, 5-chloro-NNEI, NNEI indazole analog, α-PHPP and α-POP, with 11 newly distributed designer drugs in illegal products. Forensic Sci. Int. 2014, 243, 1–13. [Google Scholar] [CrossRef]
- Beck, O.; Bäckberg, M.; Signell, P.; Helander, A. Intoxications in the STRIDA project involving a panorama of psychostimulant pyrovalerone derivatives, MDPV copycats. Clin. Toxicol. 2018, 56, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Richeval, C.; Dumestre-Toulet, V.; Wiart, J.-F.; Vanhoye, X.; Humbert, L.; Nachon-Phanithavong, M.; Allorge, D.; Gaulier, J.-M. New psychoactive substances in oral fluid of drivers around a music festival in south-west France in 2017. Forensic Sci. Int. 2019, 297, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, A.; Adachi, N.; Shojo, H. Detection of 4-FMC, 4-MeO-α-PVP, 4-F-α-PVP, and PV8 in blood in a forensic case using liquid chromatography-electrospray ionization linear ion trap mass spectrometry. Forensic Sci. Int. 2021, 325, 110888. [Google Scholar] [CrossRef] [PubMed]
- Kuś, P.; Kusz, J.; Książek, M.; Pieprzyca, E.; Rojkiewicz, M. Spectroscopic characterization and crystal structures of two cathinone derivatives: 1-(4-chlorophenyl)-2-(1-pyrrolidinyl)-pentan-1-one (4-chloro-α-PVP) sulfate and 1-(4-methylphenyl)-2-(dimethylamino)-propan-1-one (4-MDMC) hydrochloride salts, seized on illicit drug market. Forensic Toxicol. 2018, 36, 178–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Błażewicz, A.; Bednarek, E.; Sitkowski, J.; Popławska, M.; Stypułkowska, K.; Bocian, W.; Kozerski, L. Identification and structural characterization of four novel synthetic cathinones: α-methylaminohexanophenone (hexedrone, HEX), 4-bromoethcathinone (4-BEC), 4-chloro-α-pyrrolidinopropiophenone (4-Cl-PPP), and 4-bromo-α-pyrrolidinopentiophenone (4-Br-PVP) after their seizures. Forensic Toxicol. 2017, 35, 317–332. [Google Scholar] [CrossRef]
- Pieprzyca, E.; Skowronek, R.; Czekaj, P. Toxicological Analysis of Cases of Mixed Poisonings with Synthetic Cathinones and other Drugs of Abuse. Online ahead of print. J. Anal. Toxicol. 2021, bkab119. [Google Scholar] [CrossRef]
- Drug Enforcement Administration (DEA). Temporary Placement of N-Ethylhexedrone, [alpha]-PHP, 4-MEAP, MPHP, PV8, and 4-Chloro-[alpha]-PVP in Schedule I. Available online: https://www.deadiversion.usdoj.gov/fed_regs/rules/2019/fr0501.htm (accessed on 13 September 2021).
- Meltzer, P.C.; Butler, D.; Deschamps, J.R.; Madras, B.K. 1- (4-Methylphenyl) -2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) Analogues: A Promising Class of Monoamine Uptake Inhibitors. J. Med. Chem. 2006, 49, 1420–1432. [Google Scholar] [CrossRef] [Green Version]
- Eshleman, A.J.; Nagarajan, S.; Wolfrum, K.M.; Reed, J.F.; Swanson, T.L.; Nilsen, A.; Janowsky, A. Structure-activity relationships of bath salt components: Substituted cathinones and benzofurans at biogenic amine transporters. Psychopharmacology 2019, 236, 939–952. [Google Scholar] [CrossRef]
- Wojcieszak, J.; Kuczyńska, K.; Zawilska, J.B. Four Synthetic Cathinones: 3-Chloromethcathinone, 4-Chloromethcathinone, 4-Fluoro-α-Pyrrolidinopentiophenone, and 4-Methoxy-α-Pyrrolidinopentiophenone Produce Changes in the Spontaneous Locomotor Activity and Motor Performance in Mice with Varied Profiles. Neurotox Res. 2020, 38, 536–551. [Google Scholar] [CrossRef]
- Titov, O.I.; Shulga, D.A.; Palyulin, V.A.; Zefirov, N.S. Perspectives of Halogen Bonding Description in Scoring Functions and QSAR/QSPR: Substituent Effects in Aromatic Core. Mol. Inform. 2015, 34, 404–416. [Google Scholar] [CrossRef]
- Eshleman, A.J.; Wolfrum, K.M.; Hatfield, M.G.; Johnson, R.A.; Murphy, K.V.; Janowsky, A. Substituted methcathinones differ in transporter and receptor interactions. Biochem. Pharmacol. 2013, 85, 1803–1815. [Google Scholar] [CrossRef] [Green Version]
- Negus, S.S.; Banks, M.L. Decoding the Structure of Abuse Potential for New Psychoactive Substances: Structure-Activity Relationships for Abuse-Related Effects of 4-Substituted Methcathinone Analogs. Curr. Top. Behav. Neurosci. 2017, 32, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Walther, D.; Shalabi, A.R.; Baumann, M.H.; Glennon, R.A. Systematic structure-activity studies on selected 2-, 3-, and 4-monosubstituted synthetic methcathinone analogs as monoamine transporter releasing agents. ACS Chem. Neurosci. 2019, 10, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Bonano, J.S.; Banks, M.L.; Kolanos, R.; Sakloth, F.; Barnier, M.L.; Glennon, R.A.; Cozzi, N.V.; Partilla, J.S.; Baumann, M.H.; Negus, S.S. Quantitative structure-activity relationship analysis of the pharmacology of para-substituted methcathinone analogues. Br. J. Pharmacol. 2015, 172, 2433–2444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luethi, D.; Walter, M.; Zhou, X.; Rudin, D.; Krähenbühl, S.; Liechti, M.E. Para-Halogenation Affects Monoamine Transporter Inhibition Properties and Hepatocellular Toxicity of Amphetamines and Methcathinones. Front. Pharmacol. 2019, 10, 438. [Google Scholar] [CrossRef]
- Rickli, A.; Hoener, M.C.; Liechti, M.E. Monoamine transporter and receptor interaction profiles of novel psychoactive substances: Para-halogenated amphetamines and pyrovalerone cathinones. Eur. Neuropsychopharmacol. 2015, 25, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. 2015, 96, e52434. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Lin, Z.; Tao, X.; Huang, Z.; Zhang, Y.; Zheng, S.; Wang, H.; Rao, Y. Effects of N-ethylpentylone on locomotor activity and anxiety-like behavior in rats. Behav. Pharmacol. 2019, 30, 500–505. [Google Scholar] [CrossRef]
- Štefková, K.; Židková, M.; Horsley, R.R.; Pinterová, N.; Šíchová, K.; Uttl, L.; Balíková, M.; Danda, H.; Kuchař, M.; Páleníček, T. Pharmacokinetic, Ambulatory, and Hyperthermic Effects of 3,4-Methylenedioxy-N-Methylcathinone (Methylone) in Rats. Front. Psychiatry 2017, 8, 232. [Google Scholar] [CrossRef] [Green Version]
- Pezze, M.A.; Feldon, J. Mesolimbic dopaminergic pathways in fear conditioning. Prog. Neurobiol. 2004, 74, 301–320. [Google Scholar] [CrossRef]
- Vicario, C.M.; Rafal, R.D.; Martino, D.; Avenanti, A. Core, social and moral disgust are bounded: A review on behavioral and neural bases of repugnance in clinical disorders. Neurosci. Biobehav. Rev. 2017, 80, 185–200. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Guo, W. Emotional Roles of Mono-Aminergic Neurotransmitters in Major Depressive Disorder and Anxiety Disorders. Front. Psychol. 2018, 9, 2201. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.M.; Gorman, A.L.; Dunn, A.J.; Goeders, N.E. Anxiogenic effects of acute and chronic cocaine administration: Neurochemical and behavioral studies. Pharmacol. Biochem. Behav. 1992, 41, 643–650. [Google Scholar] [CrossRef]
- Biała, G.; Kruk, M. Amphetamine-induced anxiety-related behavior in animal models. Pharmacol. Rep. 2007, 59, 636–644. [Google Scholar] [PubMed]
- Ortman, H.A.; Newby, M.L.; Acevedo, J.; Siegel, J.A. The acute effects of multiple doses of methamphetamine on locomotor activity and anxiety-like behavior in adolescent and adult mice. Behav. Brain Res. 2021, 405, 113186. [Google Scholar] [CrossRef] [PubMed]
- Riley, A.L.; Nelson, K.H.; To, P.; López-Arnau, R.; Xu, P.; Wang, D.; Wang, Y.; Shen, H.-W.; Kuhn, D.M.; Angoa-Perez, M.; et al. Abuse potential and toxicity of the synthetic cathinones (i.e., “Bath salts”). Neurosci. Biobehav. Rev. 2020, 110, 150–173. [Google Scholar] [CrossRef]
- Bardo, M.T.; Bevins, R.A. Conditioned place preference: What does it add to our preclinical understanding of drug reward? Psychopharmacology 2000, 153, 31–43. [Google Scholar] [CrossRef] [Green Version]
- Tzschentke, T.M. Measuring reward with the conditioned place preference (CPP) paradigm: Update of the last decade. Addict. Biol. 2007, 12, 227–462. [Google Scholar] [CrossRef]
- López-Arnau, R.; Luján, M.A.; Duart-Castells, L.; Pubill, D.; Camarasa, J.; Valverde, O.; Escubedo, E. Exposure of adolescent mice to 3,4-methylenedioxypyrovalerone increases the psychostimulant, rewarding and reinforcing effects of cocaine in adulthood. Br. J. Pharmacol. 2017, 174, 1161–1173. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Sonders, M.S.; Ukairo, O.T.; Scott, H.; Kloetzel, M.K.; Surratt, C.K. Dissociation of high-affinity cocaine analog binding and dopamine uptake inhibition at the dopamine transporter. Mol. Pharmacol. 2003, 64, 430–439. [Google Scholar] [CrossRef]
- Ukairo, O.T.; Bondi, C.D.; Newman, A.H.; Kulkarni, S.S.; Kozikowski, A.P.; Pan, S.; Surratt, C.K. Recognition of benztropine by the dopamine transporter (DAT) differs from that of the classical dopamine uptake inhibitors cocaine, methylphenidate, and mazindol as a function of a DAT transmembrane 1 aspartic acid residue. J. Pharmacol. Exp. Ther. 2005, 314, 575–583. [Google Scholar] [CrossRef] [Green Version]
- Newman, A.H.; Kulkarni, S. Probes for the dopamine transporter: New leads toward a cocaine-abuse therapeutic—A focus on analogues of benztropine and rimcazole. Med. Res. Rev. 2002, 22, 429–464. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.I.; Kopajtic, T.A.; Koffarnus, M.; Newman, A.H.; Katz, J.L. Identification of a dopamine transporter ligand that blocks the stimulant effects of cocaine. J. Neurosci. 2005, 25, 1889–1893. [Google Scholar] [CrossRef]
- Niello, M.; Sideromenos, S.; Gradisch, R.; O’Shea, R.; Schwazer, J.; Sandtner, W.; Maier, J.; Jäntsch, K.; Lupica, C.; Hoffman, A.; et al. Psychomotor stimulant effects of α-pyrrolidinovalerophenone (αPVP) enantiomers correlate with drug binding kinetics at the dopamine transporter. Res. Sq. 2021, 1–25. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Exp. Physiol. 2020, 105, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.P.; Luf, A.; Nagy, C.; Holy, M.; Schmid, R.; Freissmuth, M.; Sitte, H.H. Application of a Combined Approach to Identify New Psychoactive Street Drugs and Decipher Their Mechanisms at Monoamine Transporters. Curr. Top. Behav. Neurosci. 2016, 32, 333–350. [Google Scholar] [CrossRef]
- Nadal-Gratacós, N.; Alberto-Silva, A.S.; Rodríguez-Soler, M.; Urquizu, E.; Espinosa-Velasco, M.; Jäntsch, K.; Holy, M.; Batllori, X.; Berzosa, X.; Pubill, D.; et al. Structure–Activity Relationship of Novel Second-Generation Synthetic Cathinones: Mechanism of Action, Locomotion, Reward, and Immediate-Early Genes. Front. Pharmacol. 2021, 12, 2766. [Google Scholar] [CrossRef] [PubMed]
- Sucic, S.; Dallinger, S.; Zdrazil, B.; Weissensteiner, R.; Jørgensen, T.N.; Holy, M.; Kudlacek, O.; Seidel, S.; Cha, J.H.; Gether, U.; et al. The N terminus of monoamine transporters is a lever required for the action of amphetamines. J. Biol. Chem. 2010, 285, 10924–10938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Penmatsa, A.; Gouaux, E. Neurotransmitter and psychostimulant recognition by the dopamine transporter. Nature 2015, 521, 322–327. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.-C.; Prusoff, W.H. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar] [CrossRef]
- Romanek, K.; Stenzel, J.; Schmoll, S.; Schrettl, V.; Geith, S.; Eyer, F.; Rabe, C. Synthetic cathinones in Southern Germany—characteristics of users, substance-patterns, co-ingestions, and complications. Clin. Toxicol. 2017, 55, 573–578. [Google Scholar] [CrossRef]
- Weinstein, A.M.; Rosca, P.; Fattore, L.; London, E.D. Synthetic Cathinone and Cannabinoid Designer Drugs Pose a Major Risk for Public Health. Front. Psychiatry 2017, 8, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauritzen, G.; Nordfjærn, T. Changes in opiate and stimulant use through 10 years: The role of contextual factors, mental health disorders and psychosocial factors in a prospective SUD treatment cohort study. PLoS ONE 2018, 13, e0190381. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Transfected HEK293 Cells | ||||||
|---|---|---|---|---|---|---|
| Monoamine Uptake Inhibition | Transporter Binding Affinities | |||||
| Compound | [3H]MPP+ Uptake at hDAT | [3H]5-HT Uptake at hSERT | hDAT/hSERT Inhibition Ratio | [3H]WIN35,428 Binding at hDAT | [3H]Imipramine Binding at hSERT | hDAT/hSERT Affinity Ratio |
| α-PVP | 0.129 (±0.002) | >100 | 3418 | 0.017 (±0.001) | >100 | 8514 |
| 3-F-α-PVP | 0.132 (±0.016) | >100 | 1819 | 0.014 (±0.001) | 63.53 (±7.62) | 4540 |
| 4-F-α-PVP | 0.154 (±0.019) | >100 | 1366 | 0.049 (±0.004) | 57.88 (±4.27) | 1173 |
| 3-Cl-α-PVP | 0.349 (±0.033) | 33.33 (±2.46) | 95 | 0.013 (± 0.001) | 16.43 (±0.50) | 1234 |
| 4-Cl-α-PVP | 0.391 (±0.025) | 15.17 (±0.24) | 38 | 0.016 (± 0.001) | 7.90 (±0.18) | 478 |
| 3,4-Cl-α-PVP | 0.266 (±0.012) | 12.74 (±0.28) | 47 | 0.011 (± 0.001) | 4.99 (±0.27) | 462 |
| 3-Br-α-PVP | 0.117 (±0.016) | 39.96 (±6.38) | 341 | 0.012 (±0.002) | 5.60 (±0.31) | 470 |
| 4-Br-α-PVP | 0.156 (±0.012) | 23.11 (±2.27) | 148 | 0.024 (±0.001) | 1.25 (±0.09) | 52 |
| Cocaine | 0.231 (±0.013) | 1.85 (±0.28) | 7.98 | 0.283 (±0.04) | 0.54 (±0.03) | 1.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadal-Gratacós, N.; Lleixà, E.; Gibert-Serramià, M.; Estrada-Tejedor, R.; Berzosa, X.; Batllori, X.; Pubill, D.; Camarasa, J.; Escubedo, E.; López-Arnau, R. Neuropsychopharmacology of Emerging Drugs of Abuse: meta- and para-Halogen-Ring-Substituted α-PVP (“flakka”) Derivatives. Int. J. Mol. Sci. 2022, 23, 2226. https://doi.org/10.3390/ijms23042226
Nadal-Gratacós N, Lleixà E, Gibert-Serramià M, Estrada-Tejedor R, Berzosa X, Batllori X, Pubill D, Camarasa J, Escubedo E, López-Arnau R. Neuropsychopharmacology of Emerging Drugs of Abuse: meta- and para-Halogen-Ring-Substituted α-PVP (“flakka”) Derivatives. International Journal of Molecular Sciences. 2022; 23(4):2226. https://doi.org/10.3390/ijms23042226
Chicago/Turabian StyleNadal-Gratacós, Núria, Esther Lleixà, Mónica Gibert-Serramià, Roger Estrada-Tejedor, Xavier Berzosa, Xavier Batllori, David Pubill, Jordi Camarasa, Elena Escubedo, and Raúl López-Arnau. 2022. "Neuropsychopharmacology of Emerging Drugs of Abuse: meta- and para-Halogen-Ring-Substituted α-PVP (“flakka”) Derivatives" International Journal of Molecular Sciences 23, no. 4: 2226. https://doi.org/10.3390/ijms23042226
APA StyleNadal-Gratacós, N., Lleixà, E., Gibert-Serramià, M., Estrada-Tejedor, R., Berzosa, X., Batllori, X., Pubill, D., Camarasa, J., Escubedo, E., & López-Arnau, R. (2022). Neuropsychopharmacology of Emerging Drugs of Abuse: meta- and para-Halogen-Ring-Substituted α-PVP (“flakka”) Derivatives. International Journal of Molecular Sciences, 23(4), 2226. https://doi.org/10.3390/ijms23042226