Anthocyanins: Traditional Uses, Structural and Functional Variations, Approaches to Increase Yields and Products’ Quality, Hepatoprotection, Liver Longevity, and Commercial Products
Abstract
1. Perspective, Scope, and Methodology for Information Retrieval of Anthocyanins
2. Anthocyanins’ Aesthetics, and Plant Kingdom’s Distribution
3. Anthocyanins Roles in the Plants
4. Chemistry of the Anthocyanins, Structures, and the Structural Variants
5. Anthocyanins Extraction, Purification, and Structure Determinations
6. Anthocyanins Quality, and Contents’ Control through Spectro-Analytical and Chromatographic Techniques
7. Anthocyanins Biosynthesis, Modulation of the Enzymatic Synthesis, and Biotechnical Approach to Increase Anthocyanins Yields
8. Anthocyanins’ Synthesis and Semisynthetic Routes to Approach Newer Analogs
9. Herbal Medicines Traditional Uses, Toxicity, Liver Disorders, and Anthocyanins
10. Anthocyanins’ Metabolism in Liver
11. Anthocyanins-Based Broad-Spectrum Health Benefits
12. Anthocyanin’s Dietary-Intake and Deficiency, Nutrition, and Biological Importance
13. Anthocyanins and Liver Disorders
14. Anthocyanins’ Suggestive Roles through Hepatic Biomarkers Regulation, and Biomechanistics Outlook
| Plant’s Name | Used Extracts, and/or Pure Compounds | In Vivo/In Vitro Models and Bioactivity | Major Anthocyanins | Biomarkers, and Mode/Mechanism of Action | Refer |
|---|---|---|---|---|---|
| Morus alba, and species | Mulberry anthocyanins | CCl4 (carbon tetrachloride), in vivo model. Hepatoprotection | Cyanidin-3-O-β-glucoside | Decreased the ALT (alanine transaminase), AST (aspartate transaminase), hyaluronidase, hydroxyproline, and collagen type-III in the injured rats | [293] |
| Ipomoea batatas L. | Anthocyanins rich purple sweet potato extract | CCl4, in vivo model. Hepatoprotection | Peonidin-3-caffeoyl-feruloyl sophoroside-5-glucosid, peonidin 3-caffeoyl-p-hydroxy benzoyl sophoroside-5-glucoside, peonidin 3-dicaffeoyl sophoroside-5-glucoside | Reduced the AST and ALT enzymes and MDA (malondialdehyde) level; Increased the SOD (superoxide dismutase), and GSH (glutathione) levels compared to the injured CCl4 administered group of animals | [295] |
| Oryza sativa | Anthocyanins rich black rice bran extract | CCl4, in vivo model. Hepatoprotection | Cyanidin-3-O-β-glucoside, and peonidin-3-O-glucoside | Reduced aminotransferase activity in serum, enhanced SOD and glutathione peroxidase (GSH-Px) activities, thiobarbituric acid reactive substances (TBARS), and 8-hydroxy-20-deoxyguanosine levels significantly decreased as compared to the CCl4 intoxicated group. Liver histopathology confirmed pathological gains by ARBE administration | [186] |
| Ipomoea batatas | Anthocyanins rich fraction of purple sweet potato extract | In vivo, ethanol, acetaminophen, and, CCl4. Hepatoprotection, and treatment | 3-O-(6-O-trans-caffeyt-2-O-~-glucopyranosyl/3-glucopyranoside)-5-O-glucosides of cyanidin, and peonidin | Treatments of mice with anthocyanins fraction in dose dependent manner, and reduced the CYP2E1-dependent aniline hydroxylation, and CYP2E1 protein levels. Antioxidant effects on hepatic GSH level, and GSH S-transferase activity were up-regulated in FeCl2/ascorbate-induced lipid peroxidation in mouse liver homogenates, also showed superoxide radical scavenging activity. | [290,291] |
| Hibiscus sabdariffa L. | Anthocyanin-rich extract | In vivo, thioacetamide (TAA)-induced hepatotoxicity. Hepatoprotection | Cyanidine, delphinidin derivatives, cyanidin-3,5-O-di-glucoside, cyanidin-3-O-sophoroside-5-glucoside | Reduced the serum levels of ALA, AST, and hepatic malondialdehyde, decreased hepatic inflammatory markers, including TNF-α, interleukin-6, and INF-γ, decreased the immuno-positivity of NF kappa-B, and CYP2E1 in liver tissues | [185] |
| In vivo tert-BHP-induced cytotoxicity in rat | [176] | ||||
| CCl4 in vivo model. Hepatoprotection | [296] | ||||
| Aronia melanocarpa | Fruit juice | CCl4, N-nitroso diethyl amine, Paracetamol in vivo model. Hepatoprotection | Cyanidin-3-O-galactoside, cyanidin-3-O-arabinoside, cyanidin-3-O-xyloside and cyanidin-3-O-β-glucoside | Reduced necrotic changes in rat liver and inhibited increase of plasma AST and ALT activities, MDA formation induced by CCl4. Increased liver GSH contents. Decreased the activities of enzymatic markers of cytochrome P450, CYP1A1 and 1A2. | [297,298,299] |
| Justicia spicigera | Ethyl acetate fraction | CCl4 in vivo model. Hepatoprotection | Peonidin 3,5-O-di-glucoside, malvidin 3,5-O-di-glucoside, and petunidin 3,5-O-di-glucoside | Improvement in liver function indices and oxidative stress markers. Increased SOD and GSH, and decreased MDA. | [184] |
| Vaccinium sp. | Berry pomace extract | In vitro hepatic cell line HepG2 proliferation. Hepatic cells protection | Procyanidin dimers | Protects hepatic cells from oxidative damage. | [300] |
| Solanum tuberosum L. | Purple potato’s anthocyanins rich extract | In vivo, alcoholic liver disease mouse model. Hepatoprotection | Petunidin-3-coumaroyl-rutinoside-5-glucoside, peonidin-3-coumaroyl-rutinoside-5-glucoside, petunidin-3-O-glucoside, petunidin-3-rutinoside-5-glucoside, pelphinidin-3-coumaroyl-rutinoside-5-glucoside | Higher levels of SOD and reduced GSH enzymes, reduction in formation of malondialdehyde, protected against alcohol-induced detrimental levels, maneuvered the activity of cytochrome P450 2E1 (CYP2E1) | [301] |
| Ipomoea batatas | Anthocyanin fraction | Dimethyl nitrosamine-induced liver injury in rats. Hepatoprotection | Cyanidin-3-O-β-glucoside chloride, malvidin-3-O-glucoside, pelargonidin-3-O-glucoside chloride, and peonidine-3-O-glucoside chloride | Induced Nrf2 mediated antioxidant enzymes, and reduced the COX-2, and iNOS expressions, reduced inflammation through NF-KB inhibition | [302] |
| Hibiscus sabdariffa | Water extract, and anthocyanins | Paracetamol-induced hepatotoxicity in rats. Hepatoprotection | Anthocyanins | Increased GSH and SOD levels, decreased ALT and AST | [303] |
| Colocasia antiquorum | Ethanolic extract | Paracetamol, and CCl4 toxicated rats. Hepatoprotection | Cyanidin-3-O-β-glucoside, pelargonidin-3-O-glucoside and cyanidin-3-O-rhamnoside | Decreased ALT, and AST levels | [304] |
| Vaccinium myrtillus and Ribes nigrum | Anthocyanins-rich extracts | Acetaminophen-induced hepatotoxicity in rats. Hepatoprotection | Glycosides of cyanidin, peonidin, delphinidin, petunidin, and malvidin | Normalized activities of glutamate oxaloacetate and glutamate pyruvate transaminase, prevented APAP-induced plasmatic and tissue alterations in biomarkers of oxidative stress | [305] |
| Raphanus sativus L. (Red radish) | Anthocyanins fraction | CCl4 in vivo model. Hepatoprotection | Pelargonidin derivatives | Reversed the alteration of biochemical parameters to normal | [179] |
| Raphanus sativus L. var. niger | Fermented roots | In vivo model for the methionine, and choline-deficient, diet-induced non-alcoholic fatty liver in mice. Hepatoprotection | Pelargonidin derivatives | Decreased lipids in 3T3-L1 adipocytes by downregulating adipogenic transcription factors, sterol regulatory element-binding protein 1c, CCAAT/enhancer-binding protein α, peroxisome proliferator-activated receptor γ, and lipid accumulation-related genes adipocyte protein-2, as well as fatty acid synthase. Decreased ALT, AST, TG levels. Deceased expression of iNO synthase, suppression of the inactivation of macrophages, and Kupffer cells in liver. Inhibition of α-smooth muscle actin, transforming growth factor β-1, and collagen type-I α-1 chain leading to reduced liver fibrosis. | [306] |
| Raphanus sativus L. var. niger | Aqueous extract of roots | In vitro model in HepG2 cells. Hepatoprotection | Pelargonidin derivatives | Induced quinone reductase activity, and expression of multiple phase I, II detoxification enzymes in the HepG2 human hepatoma cell line | [307] |
| Malvaviscus arboreus Cav | Aerial parts extracts | CCl4 in vivo model. Hepatoprotection | Cyanidin-3-sambubioside | EtOAc (ethyl acetate), and CH2Cl2 (Dichloromethane) extracts significantly reduced the liver injury in rats as indicated by the reduced levels of ALT, AST, ALP, TB, and MDA, comparatively the EtOAc fraction enhanced total antioxidant capacity of liver at the maximum. | [308] |
| Cornus mas L. | Anthocyanins rich fraction | Lipid peroxidation, oxidative stress in the livers of cholesterol-fed rabbits | Delphinidin 3-O-galactoside, cyanidin-3-O-galactoside, Cyanidin-3-O-robinobioside, pelargonidin-3-O-galactoside, pelargonidin-3-O-robinobioside, cyanidin, and pelargonidin. | Decreased lipid peroxidation, decreased MDA levels, and reduced oxidative stress, an increase in liver GSH found. | [309] |
15. Anthocyanins Roles in Hepatocellular Longevity, Hepatic Carcinoma and Liver Cancer
16. Anthocyanins’ Structure Types, Hepatoprotection and the Evolving SAR
17. Commercialization and Anthocyanins-Based Products in the Market
18. Conclusions and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harborne, J.B.; Williams, C.A. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2001, 18, 310–333. [Google Scholar] [CrossRef] [PubMed]
- Varelis, P.; Melton, L.; Shahidi, F. Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 0128140453. [Google Scholar]
- Carle, R.; Schweiggert, R. Handbook on Natural Pigments in Food and Beverages: Industrial Applications for Improving Food Color; Woodhead Publishing: Sawston, UK, 2016; ISBN 0081003927. [Google Scholar]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- Fanning, K.; Edwards, D.; Netzel, M.; Stanley, R.; Netzel, G.; Russell, D.; Topp, B. Increasing anthocyanin content in Queen Garnet plum and correlations with in-field measures. Acta Hortic. 2013, 985, 97–104. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef] [PubMed]
- Siriwoharn, T.; Wrolstad, R.E.; Finn, C.E.; Pereira, C.B. Influence of cultivar, maturity, and sampling on blackberry (Rubus L. Hybrids) anthocyanins, polyphenolics, and antioxidant properties. J. Agric. Food Chem. 2004, 52, 8021–8030. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Sakakibara, H.; Iwata, R.; Ishii, T.; Sato, T.; Goda, T.; Shimoi, K.; Kumazawa, S. Anthocyanin composition and antioxidant activity of the crowberry (Empetrum nigrum) and other berries. J. Agric. Food Chem. 2008, 56, 4457–4462. [Google Scholar] [CrossRef]
- Wada, L.; Ou, B. Antioxidant activity and phenolic content of Oregon caneberries. J. Agric. Food Chem. 2002, 50, 3495–3500. [Google Scholar] [CrossRef]
- Hosseinian, F.S.; Beta, T. Saskatoon and wild blueberries have higher anthocyanin contents than other Manitoba berries. J. Agric. Food Chem. 2007, 55, 10832–10838. [Google Scholar] [CrossRef]
- Hiemori, M.; Koh, E.; Mitchell, A.E. Influence of cooking on anthocyanins in black rice (Oryza sativa L. japonica var. SBR). J. Agric. Food Chem. 2009, 57, 1908–1914. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Dao, L.T.; Full, G.H.; Wong, R.Y.; Harden, L.A.; Edwards, R.H.; Berrios, J.D.J. Characterization of black bean (Phaseolus vulgaris L.) anthocyanins. J. Agric. Food Chem. 1997, 45, 3395–3400. [Google Scholar] [CrossRef]
- Herrera-Sotero, M.Y.; Cruz-Hernández, C.D.; Trujillo-Carretero, C.; Rodríguez-Dorantes, M.; García-Galindo, H.S.; Chávez-Servia, J.L.; Oliart-Ros, R.M.; Guzmán-Gerónimo, R.I. Antioxidant and antiproliferative activity of blue corn and tortilla from native maize. Chem. Cent. J. 2017, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, S. The antioxidant power of purple corn: A research review. Altern. Complement. Ther. 2007, 13, 107–110. [Google Scholar] [CrossRef]
- Li, C.-Y.; Kim, H.-W.; Won, S.R.; Min, H.-K.; Park, K.-J.; Park, J.-Y.; Ahn, M.-S.; Rhee, H.-I. Corn husk as a potential source of anthocyanins. J. Agric. Food Chem. 2008, 56, 11413–11416. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Espada, A.C.; Wood, K.V.; Bordelon, B.; Watkins, B.A. Anthocyanin quantification and radical scavenging capacity of Concord, Norton, and Marechal Foch grapes and wines. J. Agric. Food Chem. 2004, 52, 6779–6786. [Google Scholar] [CrossRef] [PubMed]
- Ahmadiani, N.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Anthocyanins contents, profiles, and color characteristics of red cabbage extracts from different cultivars and maturity stages. J. Agric. Food Chem. 2014, 62, 7524–7531. [Google Scholar] [CrossRef]
- de Moura, C.; dos Reis, A.S.; da Silva, L.D.; de Lima, V.A.; Oldoni, T.L.C.; Pereira, C.; Carpes, S.T. Optimization of phenolic compounds extraction with antioxidant activity from açaí, blueberry and goji berry using response surface methodology. Emir. J. Food Agric. 2018, 30, 180–189. [Google Scholar]
- Ruiz, A.; Hermosín-Gutiérrez, I.; Vergara, C.; von Baer, D.; Zapata, M.; Hitschfeld, A.; Obando, L.; Mardones, C. Anthocyanin profiles in south Patagonian wild berries by HPLC-DAD-ESI-MS/MS. Food Res. Int. 2013, 51, 706–713. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Ruiz, A.; Mardones, C.; Vergara, C.; Hermosín-Gutiérrez, I.; von Baer, D.; Hinrichsen, P.; Rodriguez, R.; Arribillaga, D.; Dominguez, E. Analysis of hydroxycinnamic acids derivatives in calafate (Berberis microphylla G. Forst) berries by liquid chromatography with photodiode array and mass spectrometry detection. J. Chromatogr. A 2013, 1281, 38–45. [Google Scholar] [CrossRef]
- Scheuermann, E.; Seguel, I.; Montenegro, A.; Bustos, R.O.; Hormazábal, E.; Quiroz, A. Evolution of aroma compounds of murtilla fruits (Ugni molinae Turcz) during storage. J. Sci. Food Agric. 2008, 88, 485–492. [Google Scholar] [CrossRef]
- Pennington, J.A.T.; Fisher, R.A. Food component profiles for fruit and vegetable subgroups. J. Food Compos. Anal. 2010, 23, 411–418. [Google Scholar] [CrossRef]
- Lachman, J.; Orsák, M.; Pivec, V. Antioxidant contents and composition in some vegetables and their role in human nutrition. Hortic. Sci. 2000, 27, 65–78. [Google Scholar]
- Brito, A.; Areche, C.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanin characterization, total phenolic quantification and antioxidant features of some Chilean edible berry extracts. Molecules 2014, 19, 10936–10955. [Google Scholar] [CrossRef]
- Lees, D.-H.; Francis, F.J. Standardization of pigment analyses in cranberries. Hort Sci. 1972, 7, 83–84. [Google Scholar]
- De Rosso, V.V.; Hillebrand, S.; Montilla, E.C.; Bobbio, F.O.; Winterhalter, P.; Mercadante, A.Z. Determination of anthocyanins from acerola (Malpighia emarginata DC.) and açai (Euterpe oleracea Mart.) by HPLC–PDA–MS/MS. J. Food Compos. Anal. 2008, 21, 291–299. [Google Scholar] [CrossRef]
- Ella Missang, C.; Guyot, S.; Renard, C.M.G.C. Flavonols and anthocyanins of bush butter, Dacryodes edulis (G. Don) HJ Lam, fruit. Changes in their composition during ripening. J. Agric. Food Chem. 2003, 51, 7475–7480. [Google Scholar] [CrossRef] [PubMed]
- Veberic, R.; Slatnar, A.; Bizjak, J.; Stampar, F.; Mikulic-Petkovsek, M. Anthocyanin composition of different wild and cultivated berry species. LWT Food Sci. Technol. 2015, 60, 509–517. [Google Scholar] [CrossRef]
- Alappat, B.; Alappat, J. Anthocyanin Pigments: Beyond Aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef]
- Spinardi, A.; Cola, G.; Gardana, C.S.; Mignani, I. Variation of anthocyanin content and profile throughout fruit development and ripening of highbush blueberry cultivars grown at two different altitudes. Front. Plant Sci. 2019, 10, 1045. [Google Scholar] [CrossRef]
- Mannino, G.; Gentile, C.; Maffei, M.E. Chemical partitioning and DNA fingerprinting of some pistachio (Pistacia vera L.) varieties of different geographical origin. Phytochemistry 2019, 160, 40–47. [Google Scholar] [CrossRef]
- Mannino, G.; Gentile, C.; Ertani, A.; Serio, G.; Bertea, C.M. Anthocyanins: Biosynthesis, Distribution, Ecological Role, and Use of Biostimulants to Increase Their Content in Plant Foods—A Review. Agriculture 2021, 11, 212. [Google Scholar] [CrossRef]
- Gould, K.S.; Lister, C. Flavonoid functions in plants. In Flavonoids Chemistry, Biochemistry and Applications; CRC Press: Boca Raton, FL, USA, 2006; pp. 397–441. [Google Scholar]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Steyn, W.J.; Wand, S.J.E.; Holcroft, D.M.; Jacobs, G. Anthocyanins in vegetative tissues: A proposed unified function in photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef]
- Flamini, R.; Mattivi, F.; De Rosso, M.; Arapitsas, P.; Bavaresco, L. Advanced knowledge of three important classes of grape phenolics: Anthocyanins, stilbenes and flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Q.; Xia, L.; Hui, J.; Li, J.; Feng, Y.; Chen, Y. Preliminarily exploring of the association between sugars and anthocyanin accumulation in apricot fruit during ripening. Sci. Hortic. 2019, 248, 112–117. [Google Scholar] [CrossRef]
- Aza-Gonzalez, C.; Herrera-Isidrón, L.; Núñez-Palenius, H.G.; De La Vega, O.M.; Ochoa-Alejo, N. Anthocyanin accumulation and expression analysis of biosynthesis-related genes during chili pepper fruit development. Biol. Plant. 2013, 57, 49–55. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Durst, R.W.; Lee, J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci. Technol. 2005, 16, 423–428. [Google Scholar] [CrossRef]
- Yoshida, K.; Mori, M.; Kondo, T. Blue flower color development by anthocyanins: From chemical structure to cell physiology. Nat. Prod. Rep. 2009, 26, 884–915. [Google Scholar] [CrossRef] [PubMed]
- Horbowicz, M.; Kosson, R.; Grzesiuk, A.; Debski, H. Anthocyanins of fruits and vegetables-their occurrence, analysis and role in human nutrition. Veg. Crop. Res. Bull. 2008, 68, 5–22. [Google Scholar] [CrossRef]
- He, K.; Li, X.; Chen, X.; Ye, X.; Huang, J.; Jin, Y.; Li, P.; Deng, Y.; Jin, Q.; Shi, Q. Evaluation of antidiabetic potential of selected traditional Chinese medicines in STZ-induced diabetic mice. J. Ethnopharmacol. 2011, 137, 1135–1142. [Google Scholar] [CrossRef]
- Sims, C.A.; Morris, J.R. A comparison of the color components and color stability of red wine from Noble and Cabernet Sauvignon at various pH levels. Am. J. Enol. Vitic. 1985, 36, 181–184. [Google Scholar]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and modulating color by copigmentation: Insights from theory and experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef]
- Rein, M. Copigmentation Reactions and Color Stability of Berry Anthocyanins; University of Helsinki: Helsinki, Finland, 2005. [Google Scholar]
- Jiang, T.; Mao, Y.; Sui, L.; Yang, N.; Li, S.; Zhu, Z.; Wang, C.; Yin, S.; He, J.; He, Y. Degradation of anthocyanins and polymeric color formation during heat treatment of purple sweet potato extract at different pH. Food Chem. 2019, 274, 460–470. [Google Scholar] [CrossRef]
- Dincheva, I.; Badjakov, I. Assesment of the anthocyanin variation in bulgarian bilberry (Vaccinium myrtillus L.) and lingonberry (Vaccinium vitis-idaea L.). Int. J. Med. Pharm. Sci. 2016, 6, 39–50. [Google Scholar]
- Ambigaipalan, P.; de Camargo, A.C.; Shahidi, F. Identification of phenolic antioxidants and bioactives of pomegranate seeds following juice extraction using HPLC-DAD-ESI-MSn. Food Chem. 2017, 221, 1883–1894. [Google Scholar] [CrossRef]
- Nankar, A.N.; Dungan, B.; Paz, N.; Sudasinghe, N.; Schaub, T.; Holguin, F.O.; Pratt, R.C. Quantitative and qualitative evaluation of kernel anthocyanins from southwestern United States blue corn. J. Sci. Food Agric. 2016, 96, 4542–4552. [Google Scholar] [CrossRef]
- Sang, J.; Sang, J.; Ma, Q.; Hou, X.; Li, C. Extraction optimization and identification of anthocyanins from Nitraria tangutorun Bobr. seed meal and establishment of a green analytical method of anthocyanins. Food Chem. 2017, 218, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents–solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Jeong, K.M.; Zhao, J.; Jin, Y.; Heo, S.R.; Han, S.Y.; Lee, J. Highly efficient extraction of anthocyanins from grape skin using deep eutectic solvents as green and tunable media. Arch. Pharm. Res. 2015, 38, 2143–2152. [Google Scholar] [CrossRef]
- Bosiljkov, T.; Dujmić, F.; Bubalo, M.C.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Redovniković, I.R.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Da Silva, D.T.; Pauletto, R.; da Silva Cavalheiro, S.; Bochi, V.C.; Rodrigues, E.; Weber, J.; da Silva, C.d.B.; Morisso, F.D.P.; Barcia, M.T.; Emanuelli, T. Natural deep eutectic solvents as a biocompatible tool for the extraction of blueberry anthocyanins. J. Food Compos. Anal. 2020, 89, 103470. [Google Scholar] [CrossRef]
- Ongkowijoyo, P.; Luna-Vital, D.A.; de Mejia, E.G. Extraction techniques and analysis of anthocyanins from food sources by mass spectrometry: An update. Food Chem. 2018, 250, 113–126. [Google Scholar] [CrossRef]
- Jampani, C.; Naik, A.; Raghavarao, K. Purification of anthocyanins from jamun (Syzygium cumini L.) employing adsorption. Sep. Purif. Technol. 2014, 125, 170–178. [Google Scholar] [CrossRef]
- Heinonen, J.; Farahmandazad, H.; Vuorinen, A.; Kallio, H.; Yang, B.; Sainio, T. Extraction and purification of anthocyanins from purple-fleshed potato. Food Bioprod. Process. 2016, 99, 136–146. [Google Scholar] [CrossRef]
- Degenhardt, A.; Knapp, H.; Winterhalter, P. Separation and purification of anthocyanins by high-speed countercurrent chromatography and screening for antioxidant activity. J. Agric. Food Chem. 2000, 48, 338–343. [Google Scholar] [CrossRef]
- Friesen, J.B.; McAlpine, J.B.; Chen, S.-N.; Pauli, G.F. Countercurrent separation of natural products: An update. J. Nat. Prod. 2015, 78, 1765–1796. [Google Scholar] [CrossRef]
- Ying, L.; Jia-Ying, L.; Jing, L.; Mi-Lu, L.; Zhong-Hua, L. Preparative separation of anthocyanins from purple sweet potatoes by high-speed counter-current chromatography. Chin. J. Anal. Chem. 2011, 39, 851–856. [Google Scholar]
- Vatai, T.; Škerget, M.; Knez, Ž.; Kareth, S.; Wehowski, M.; Weidner, E. Extraction and formulation of anthocyanin-concentrates from grape residues. J. Supercrit. Fluids 2008, 45, 32–36. [Google Scholar] [CrossRef]
- Lao, F.; Giusti, M.M. Quantification of purple corn (Zea mays L.) anthocyanins using spectrophotometric and HPLC approaches: Method comparison and correlation. Food Anal. Methods 2016, 9, 1367–1380. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Fuleki, T.; Francis, F.J. Quantative methods for analysis. 2. Determination of total anthocyanin and degeadition index in cranberries. J. Food Sci. 1969, 33, 78–83. [Google Scholar] [CrossRef]
- Mazza, G.; Fukumoto, L.; Delaquis, P.; Girard, B.; Ewert, B. Anthocyanins, phenolics, and color of Cabernet franc, Merlot, and Pinot noir wines from British Columbia. J. Agric. Food Chem. 1999, 47, 4009–4017. [Google Scholar] [CrossRef]
- Sinela, A.; Rawat, N.; Mertz, C.; Achir, N.; Fulcrand, H.; Dornier, M. Anthocyanins degradation during storage of Hibiscus sabdariffa extract and evolution of its degradation products. Food Chem. 2017, 214, 234–241. [Google Scholar] [CrossRef]
- King, J.W.; Grabiel, R.D.; Wightman, J.D. Subcritical water extraction of anthocyanins from fruit berry substrates. In Proceedings of the 6th International Symposium on Supercritical Fluids, Versailles, France, 28–30 April 2003; Volume 1, pp. 28–30. [Google Scholar]
- Ju, Z.; Howard, L.R. Subcritical water and sulfured water extraction of anthocyanins and other phenolics from dried red grape skin. J. Food Sci. 2005, 70, S270–S276. [Google Scholar] [CrossRef]
- Schwarz, M.; Hillebrand, S.; Habben, S.; Degenhardt, A.; Winterhalter, P. Application of high-speed countercurrent chromatography to the large-scale isolation of anthocyanins. Biochem. Eng. J. 2003, 14, 179–189. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Chen, X.; Liu, Y.; Di, D. Preparative separation and purification of lycopene from tomato skins extracts by macroporous adsorption resins. Food Chem. 2010, 123, 1027–1034. [Google Scholar] [CrossRef]
- Petersson, E.V. Analysis of Acrylamide and Anthocyanins in Foods: Extraction Optimization for Challenging Analytes; Uppsala University: Uppsala, Sweden, 2009. [Google Scholar]
- Brauch, J.E.; Reuter, L.; Conrad, J.; Vogel, H.; Schweiggert, R.M.; Carle, R. Characterization of anthocyanins in novel Chilean maqui berry clones by HPLC–DAD–ESI/MSn and NMR-spectroscopy. J. Food Compos. Anal. 2017, 58, 16–22. [Google Scholar] [CrossRef]
- Stein-Chisholm, R.E.; Beaulieu, J.C.; Grimm, C.C.; Lloyd, S.W. LC–MS/MS and UPLC–UV evaluation of anthocyanins and anthocyanidins during rabbiteye blueberry juice processing. Beverages 2017, 3, 56. [Google Scholar] [CrossRef]
- Barnes, J.S.; Schug, K.A. Structural characterization of cyanidin-3,5-diglucoside and pelargonidin-3,5-diglucoside anthocyanins: Multi-dimensional fragmentation pathways using high performance liquid chromatography-electrospray ionization-ion trap-time of flight mass spectrometr. Int. J. Mass Spectrom. 2011, 308, 71–80. [Google Scholar] [CrossRef]
- Marković, J.M.D.; Baranac, J.M.; Brdarić, T.P. Electronic and infrared vibrational analysis of cyanidin–quercetin copigment complex. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 62, 673–680. [Google Scholar] [CrossRef]
- Mateus, N.; Silva, A.M.S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C.; de Freitas, V. Identification of anthocyanin-flavanol pigments in red wines by NMR and mass spectrometry. J. Agric. Food Chem. 2002, 50, 2110–2116. [Google Scholar] [CrossRef]
- Andersen, Ø.M.; Fossen, T. Characterization of anthocyanins by NMR. Curr. Protoc. Food Anal. Chem. 2003, 9, F1–F4. [Google Scholar] [CrossRef]
- Fraige, K.; Pereira-Filho, E.R.; Carrilho, E. Fingerprinting of anthocyanins from grapes produced in Brazil using HPLC–DAD–MS and exploratory analysis by principal component analysis. Food Chem. 2014, 145, 395–403. [Google Scholar] [CrossRef]
- Primetta, A.K.; Jaakola, L.; Ayaz, F.A.; Inceer, H.; Riihinen, K.R. Anthocyanin fingerprinting for authenticity studies of bilberry (Vaccinium myrtillus L.). Food Control 2013, 30, 662–667. [Google Scholar] [CrossRef]
- Mulabagal, V.; Calderón, A.I. Liquid chromatography/mass spectrometry based fingerprinting analysis and mass profiling of Euterpe oleracea (açaí) dietary supplement raw materials. Food Chem. 2012, 134, 1156–1164. [Google Scholar] [CrossRef]
- Mannino, G.; Di Stefano, V.; Lauria, A.; Pitonzo, R.; Gentile, C. Vaccinium macrocarpon (Cranberry)-based dietary supplements: Variation in mass uniformity, proanthocyanidin dosage and anthocyanin profile demonstrates quality control standard needed. Nutrients 2020, 12, 992. [Google Scholar] [CrossRef]
- Laitila, J.E.; Suvanto, J.; Salminen, J.-P. Liquid chromatography–tandem mass spectrometry reveals detailed chromatographic fingerprints of anthocyanins and anthocyanin adducts in red wine. Food Chem. 2019, 294, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Trikas, E.D.; Melidou, M.; Papi, R.M.; Zachariadis, G.A.; Kyriakidis, D.A. Extraction, separation and identification of anthocyanins from red wine by-product and their biological activities. J. Funct. Foods 2016, 25, 548–558. [Google Scholar] [CrossRef]
- Li, D.; Meng, X.; Li, B. Profiling of anthocyanins from blueberries produced in China using HPLC-DAD-MS and exploratory analysis by principal component analysis. J. Food Compos. Anal. 2016, 47, 1–7. [Google Scholar] [CrossRef]
- Poiana, M.-A. Enhancing oxidative stability of sunflower oil during convective and microwave heating using grape seed extract. Int. J. Mol. Sci. 2012, 13, 9240–9259. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Rana, J.; Li, Y. Separation, identification, quantification, and method validation of anthocyanins in botanical supplement raw materials by HPLC and HPLC− MS. J. Agric. Food Chem. 2001, 49, 3515–3521. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Barnes, J.S.; Nguyen, H.P.; Shen, S.; Schug, K.A. General method for extraction of blueberry anthocyanins and identification using high performance liquid chromatography–electrospray ionization-ion trap-time of flight-mass spectrometry. J. Chromatogr. A 2009, 1216, 4728–4735. [Google Scholar] [CrossRef]
- Mazza, G.; Cacace, J.E.; Kay, C.D. Methods of analysis for anthocyanins in plants and biological fluids. J. AOAC Int. 2004, 87, 129–145. [Google Scholar] [CrossRef]
- Heredia, F.J.; Francia-Aricha, E.M.; Rivas-Gonzalo, J.C.; Vicario, I.M.; Santos-Buelga, C. Chromatic characterization of anthocyanins from red grapes—I. pH effect. Food Chem. 1998, 63, 491–498. [Google Scholar] [CrossRef]
- Chandrasekhar, J.; Madhusudhan, M.C.; Raghavarao, K. Extraction of anthocyanins from red cabbage and purification using adsorption. Food Bioprod. Process. 2012, 90, 615–623. [Google Scholar] [CrossRef]
- Fuleki, T.; Francis, F.J. Quantitative methods for anthocyanins. 1. Extraction and determination of total anthocyanin in cranberries. J. Food Sci. 1968, 33, 72–77. [Google Scholar] [CrossRef]
- Zanatta, C.F.; Cuevas, E.; Bobbio, F.O.; Winterhalter, P.; Mercadante, A.Z. Determination of anthocyanins from camu-camu (Myrciaria dubia) by HPLC−PDA, HPLC−MS, and NMR. J. Agric. Food Chem. 2005, 53, 9531–9535. [Google Scholar] [CrossRef] [PubMed]
- Prasain, J.K.; Peng, N.; Dai, Y.; Moore, R.; Arabshahi, A.; Wilson, L.; Barnes, S.; Wyss, J.M.; Kim, H.; Watts, R.L. Liquid chromatography tandem mass spectrometry identification of proanthocyanidins in rat plasma after oral administration of grape seed extract. Phytomedicine 2009, 16, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Pompeu, D.R.; Silva, E.M.; Rogez, H. Optimisation of the solvent extraction of phenolic antioxidants from fruits of Euterpe oleracea using Response Surface Methodology. Bioresour. Technol. 2009, 100, 6076–6082. [Google Scholar] [CrossRef]
- Fan, G.; Han, Y.; Gu, Z.; Chen, D. Optimizing conditions for anthocyanins extraction from purple sweet potato using response surface methodology (RSM). LWT—Food Sci. Technol. 2008, 41, 155–160. [Google Scholar] [CrossRef]
- Silveira, S.T.; Daroit, D.J.; Sant’Anna, V.; Brandelli, A. Stability modeling of red pigments produced by Monascus purpureus in submerged cultivations with sugarcane bagasse. Food Bioprocess. Technol. 2013, 6, 1007–1014. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; pp. F1.2.1–F1.2.13. [Google Scholar]
- Pazmiño-Durán, E.A.; Giusti, M.M.; Wrolstad, R.E.; Glória, M.B.A. Anthocyanins from banana bracts (Musa X paradisiaca) as potential food colorants. Food Chem. 2001, 73, 327–332. [Google Scholar] [CrossRef]
- Eiro, M.J.; Heinonen, M. Anthocyanin color behavior and stability during storage: Effect of intermolecular copigmentation. J. Agric. Food Chem. 2002, 50, 7461–7466. [Google Scholar] [CrossRef]
- Jing, P.U.; Giusti, M.M. Effects of extraction conditions on improving the yield and quality of an anthocyanin-rich purple corn (Zea mays L.) color extract. J. Food Sci. 2007, 72, C363–C368. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, H.; Zhou, X.; Deng, Q.; Zhao, Y.; Zhao, C.; Gong, X. Optimization of the microwave-assisted extraction and antioxidant activities of anthocyanins from blackberry using a response surface methodology. RSC Adv. 2015, 5, 19686–19695. [Google Scholar] [CrossRef]
- Mollica, A.; Locatelli, M.; Macedonio, G.; Carradori, S.; Sobolev, A.P.; De Salvador, R.F.; Monti, S.M.; Buonanno, M.; Zengin, G.; Angeli, A. Microwave-assisted extraction, HPLC analysis, and inhibitory effects on carbonic anhydrase I, II, VA, and VII isoforms of 14 blueberry Italian cultivars. J. Enzym. Inhib. Med. Chem. 2016, 31, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ghareaghajlou, N.; Hallaj-Nezhadi, S.; Ghasempour, Z. Red cabbage anthocyanins: Stability, extraction, biological activities and applications in food systems. Food Chem. 2021, 365, 130482. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Yuesheng, D.; Ge, X.; Menglu, L.; Liya, D.; LiJia, A.; Zhilong, X. Extraction and purification of anthocyanins from the fruit residues of Vaccinium uliginosum Linn. J. Chromatogr. Sep. Tech. 2013, 4, 167. [Google Scholar] [CrossRef]
- Zannou, O.; Pashazadeh, H.; Ghellam, M.; Ibrahim, S.A.; Koca, I. Extraction of Anthocyanins from Borage (Echium amoenum) Flowers Using Choline Chloride and a Glycerol-Based, Deep Eutectic Solvent: Optimization, Antioxidant Activity, and In Vitro Bioavailability. Molecules 2022, 27, 134. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.-X.; Ning, K.-L.; Yu, D.-W.; Xu, Y.-S.; Wang, B.; Yang, F.; Gao, P.; Xia, W.-S. Effects of blanching on extraction and stability of anthocyanins from blueberry peel. J. Food Meas. Charact. 2020, 14, 2854–2861. [Google Scholar] [CrossRef]
- Ravanfar, R.; Tamadon, A.M.; Niakousari, M. Optimization of ultrasound assisted extraction of anthocyanins from red cabbage using Taguchi design method. J. Food Sci. Technol. 2015, 52, 8140–8147. [Google Scholar] [CrossRef] [PubMed]
- Nogales-Bueno, J.; Baca-Bocanegra, B.; Heredia, F.J.; Hernández-Hierro, J.M. Phenolic compounds extraction in enzymatic macerations of grape skins identified as low-level extractable total anthocyanin content. J. Food Sci. 2020, 85, 324–331. [Google Scholar] [CrossRef]
- Sharara, M.S. Copigmentation effect of some phenolic acids on stabilization of roselle (Hibiscus sabdariffa) anthocyanin extract. Am. J. Food Sci. Technol. 2017, 5, 45–52. [Google Scholar]
- Cheng, A.-X.; Han, X.-J.; Wu, Y.-F.; Lou, H.-X. The function and catalysis of 2-oxoglutarate-dependent oxygenases involved in plant flavonoid biosynthesis. Int. J. Mol. Sci. 2014, 15, 1080–1095. [Google Scholar] [CrossRef]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef]
- Passeri, V.; Koes, R.; Quattrocchio, F.M. New challenges for the design of high value plant products: Stabilization of anthocyanins in plant vacuoles. Front. Plant Sci. 2016, 7, 153. [Google Scholar] [CrossRef] [PubMed]
- Marinova, K.; Pourcel, L.; Weder, B.; Schwarz, M.; Barron, D.; Routaboul, J.-M.; Debeaujon, I.; Klein, M. The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+-antiporter active in proanthocyanidin-accumulating cells of the seed coat. Plant Cell 2007, 19, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Appelhagen, I.; Thiedig, K.; Nordholt, N.; Schmidt, N.; Huep, G.; Sagasser, M.; Weisshaar, B. Update on transparent testa mutants from Arabidopsis thaliana: Characterisation of new alleles from an isogenic collection. Planta 2014, 240, 955–970. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.S.; Tohge, T.; Fernie, A.R.; Grimm, B. The genomes uncoupled-dependent signalling pathway coordinates plastid biogenesis with the synthesis of anthocyanins. Philos. Trans. R. Soc. B 2020, 375, 20190403. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, M.; Chai, M.; He, Q.; Huang, X.; Zhao, L.; Qin, Y. Epigenetic regulation of anthocyanin biosynthesis by an antagonistic interaction between H2A. Z and H3K4me3. New Phytol. 2019, 221, 295–308. [Google Scholar] [CrossRef]
- Chaves-Silva, S.; Dos Santos, A.L.; Chalfun-Júnior, A.; Zhao, J.; Peres, L.E.P.; Benedito, V.A. Understanding the genetic regulation of anthocyanin biosynthesis in plants—Tools for breeding purple varieties of fruits and vegetables. Phytochemistry 2018, 153, 11–27. [Google Scholar] [CrossRef]
- Liu, J.; Osbourn, A.; Ma, P. MYB transcription factors as regulators of phenylpropanoid metabolism in plants. Mol. Plant 2015, 8, 689–708. [Google Scholar] [CrossRef]
- Shin, D.H.; Cho, M.; Choi, M.G.; Das, P.K.; Lee, S.-K.; Choi, S.-B.; Park, Y.-I. Identification of genes that may regulate the expression of the transcription factor production of anthocyanin pigment 1 (PAP1)/MYB75 involved in Arabidopsis anthocyanin biosynthesis. Plant Cell Rep. 2015, 34, 805–815. [Google Scholar] [CrossRef]
- Rowan, D.D.; Cao, M.; Lin-Wang, K.; Cooney, J.M.; Jensen, D.J.; Austin, P.T.; Hunt, M.B.; Norling, C.; Hellens, R.P.; Schaffer, R.J. Environmental regulation of leaf colour in red 35S: PAP1 Arabidopsis thaliana. New Phytol. 2009, 182, 102–115. [Google Scholar] [CrossRef]
- Bhargava, A.; Mansfield, S.D.; Hall, H.C.; Douglas, C.J.; Ellis, B.E. MYB75 functions in regulation of secondary cell wall formation in the Arabidopsis inflorescence stem. Plant Physiol. 2010, 154, 1428–1438. [Google Scholar] [CrossRef]
- Shi, M.-Z.; Xie, D.-Y. Biosynthesis and metabolic engineering of anthocyanins in Arabidopsis thaliana. Recent Pat. Biotechnol. 2014, 8, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Feyissa, D.N.; Løvdal, T.; Olsen, K.M.; Slimestad, R.; Lillo, C. The endogenous GL3, but not EGL3, gene is necessary for anthocyanin accumulation as induced by nitrogen depletion in Arabidopsis rosette stage leaves. Planta 2009, 230, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Olsen, K.M.; Slimestad, R.; Lea, U.S.; Brede, C.; Løvdal, T.; Ruoff, P.; Verheul, M.; Lillo, C. Temperature and nitrogen effects on regulators and products of the flavonoid pathway: Experimental and kinetic model studies. Plant. Cell Environ. 2009, 32, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Cominelli, E.; Gusmaroli, G.; Allegra, D.; Galbiati, M.; Wade, H.K.; Jenkins, G.I.; Tonelli, C. Expression analysis of anthocyanin regulatory genes in response to different light qualities in Arabidopsis thaliana. J. Plant Physiol. 2008, 165, 886–894. [Google Scholar] [CrossRef]
- Long, Y.; Schiefelbein, J. Novel TTG1 mutants modify root-hair pattern formation in Arabidopsis. Front. Plant Sci. 2020, 11, 383. [Google Scholar] [CrossRef]
- Walker, A.R.; Davison, P.A.; Bolognesi-Winfield, A.C.; James, C.M.; Srinivasan, N.; Blundell, T.L.; Esch, J.J.; Marks, M.D.; Gray, J.C. The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. Plant Cell 1999, 11, 1337–1349. [Google Scholar] [CrossRef]
- Fornalé, S.; Lopez, E.; Salazar-Henao, J.E.; Fernández-Nohales, P.; Rigau, J.; Caparros-Ruiz, D. AtMYB7, a new player in the regulation of UV-sunscreens in Arabidopsis thaliana. Plant Cell Physiol. 2014, 55, 507–516. [Google Scholar] [CrossRef]
- Zhu, H.-F.; Fitzsimmons, K.; Khandelwal, A.; Kranz, R.G. CPC, a single-repeat R3 MYB, is a negative regulator of anthocyanin biosynthesis in Arabidopsis. Mol. Plant 2009, 2, 790–802. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Song, Z.; Zhang, H. Repression of MYBL2 by both microRNA858a and HY5 leads to the activation of anthocyanin biosynthetic pathway in Arabidopsis. Mol. Plant 2016, 9, 1395–1405. [Google Scholar] [CrossRef]
- Yang, F.; Cai, J.; Yang, Y.; Liu, Z. Overexpression of microRNA828 reduces anthocyanin accumulation in Arabidopsis. Plant Cell Tissue Organ Cult. 2013, 115, 159–167. [Google Scholar] [CrossRef]
- Li, S.; Wang, W.; Gao, J.; Yin, K.; Wang, R.; Wang, C.; Petersen, M.; Mundy, J.; Qiu, J.-L. MYB75 phosphorylation by MPK4 is required for light-induced anthocyanin accumulation in Arabidopsis. Plant Cell 2016, 28, 2866–2883. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.; Schrader, A.; Kokkelink, L.; Falke, C.; Welter, B.; Iniesto, E.; Rubio, V.; Uhrig, J.F.; Hülskamp, M.; Hoecker, U. Light and the E3 ubiquitin ligase COP 1/SPA control the protein stability of the MYB transcription factors PAP 1 and PAP 2 involved in anthocyanin accumulation in Arabidopsis. Plant J. 2013, 74, 638–651. [Google Scholar] [CrossRef]
- González-Villagra, J.; Kurepin, L.V.; Reyes-Díaz, M.M. Evaluating the involvement and interaction of abscisic acid and miRNA156 in the induction of anthocyanin biosynthesis in drought-stressed plants. Planta 2017, 246, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Feyissa, B.A.; Arshad, M.; Gruber, M.Y.; Kohalmi, S.E.; Hannoufa, A. The interplay between miR156/SPL13 and DFR/WD40–1 regulate drought tolerance in alfalfa. BMC Plant Biol. 2019, 19, 434. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhao, X.; Chory, J. The Arabidopsis transcriptome responds specifically and dynamically to high light stress. Cell Rep. 2019, 29, 4186–4199. [Google Scholar] [CrossRef]
- Povero, G.; Gonzali, S.; Bassolino, L.; Mazzucato, A.; Perata, P. Transcriptional analysis in high-anthocyanin tomatoes reveals synergistic effect of Aft and atv genes. J. Plant Physiol. 2011, 168, 270–279. [Google Scholar] [CrossRef]
- Sun, C.; Deng, L.; Du, M.; Zhao, J.; Chen, Q.; Huang, T.; Jiang, H.; Li, C.-B.; Li, C. A transcriptional network promotes anthocyanin biosynthesis in tomato flesh. Mol. Plant 2020, 13, 42–58. [Google Scholar] [CrossRef]
- Butelli, E.; Titta, L.; Giorgio, M.; Mock, H.-P.; Matros, A.; Peterek, S.; Schijlen, E.G.W.M.; Hall, R.D.; Bovy, A.G.; Luo, J. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat. Biotechnol. 2008, 26, 1301–1308. [Google Scholar] [CrossRef]
- Bianco, A.; Cavarischia, C.; Guiso, M. Total synthesis of anthocyanidins via Heck reaction. Nat. Prod. Res. 2006, 20, 93–97. [Google Scholar] [CrossRef]
- Kraus, G.A.; Geraskin, I.M. Synthetic Anthocyanidins from Natural Benzopyrans. Nat. Prod. Commun. 2016, 11, 1649–1650. [Google Scholar] [CrossRef]
- Mas, T. A new and convenient one-step Synthesis of the natural 3-deoxyanthocyanidins apigeninidinand luteolinidin chlorides from 2, 4, 6-triacetoxybenzaldehyde. Synthesis 2003, 2003, 1878–1880. [Google Scholar] [CrossRef]
- Oyama, K.; Kimura, Y.; Iuchi, S.; Koga, N.; Yoshida, K.; Kondo, T. Conversion of flavonol glycoside to anthocyanin: An interpretation of the oxidation–reduction relationship of biosynthetic flavonoid-intermediates. RSC Adv. 2019, 9, 31435–31439. [Google Scholar] [CrossRef]
- Cruz, L.; Fernandes, I.; Evora, A.; De Freitas, V.; Mateus, N. Synthesis of the main red wine anthocyanin metabolite: Malvidin-3-O-β-glucuronide. Synlett 2017, 28, 593–596. [Google Scholar] [CrossRef]
- Zhang, Q.; Botting, N.P.; Kay, C. A gram scale synthesis of a multi-13C-labelled anthocyanin, [6,8,10,3′,5′-13C5] cyanidin-3-glucoside, for use in oral tracer studies in humans. Chem. Comm. 2011, 47, 10596–10598. [Google Scholar] [CrossRef]
- Bakstad, E. Method for the Synthesis of Anthocyanins. U.S. Patent US8513395B2, 20 August 2013. [Google Scholar]
- Ali, H.M.; Almagribi, W.; Al-Rashidi, M.N. Antiradical and reductant activities of anthocyanidins and anthocyanins, structure–activity relationship and synthesis. Food Chem. 2016, 194, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.; Mateus, N.; de Freitas, V. First chemical synthesis report of an anthocyanin metabolite with in vivo occurrence: Cyanidin-4′-O-methyl-3-glucoside. Tetrahedron Lett. 2013, 54, 2865–2869. [Google Scholar] [CrossRef]
- Barcena, H.S.; Chen, P.; Tuachi, A. Synthetic anthocyanidins and their antioxidant properties. SpringerPlus 2015, 4, 499. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, L.; Zhang, S.; Zhao, X.; Ni, H.; Song, X.; Wang, W.; Yao, L.; Zhao, X.; Fu, Y. Cyanidin-3-glucoside protects liver from oxidative damage through AMPK/Nrf2 mediated signaling pathway in vivo and in vitro. J. Funct. Foods 2020, 73, 104148. [Google Scholar] [CrossRef]
- Kondo, T.; Oyama, K.; Nakamura, S.; Yamakawa, D.; Tokuno, K.; Yoshida, K. Novel and efficient synthesis of cyanidin-3-O-β-d-glucoside from (+)-catechin via a flav-3-en-3-ol as a key intermediate. Org. Lett. 2006, 8, 3609–3612. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Pandey, R.P.; Darsandhari, S.; Parajuli, P.; Sohng, J.K. Combinatorial approach for improved cyanidin-3-O-glucoside production in Escherichia coli. Microb. Cell Fact. 2019, 18, 7. [Google Scholar] [CrossRef]
- Stickel, F.; Egerer, G.; Seitz, H.K. Hepatotoxicity of botanicals. Public Health Nutr. 2000, 3, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Stedman, C. Herbal hepatotoxicity. Semin. Liver Dis. 2002, 22, 195–206. [Google Scholar] [CrossRef]
- Navarro, V.J.; Khan, I.; Björnsson, E.; Seeff, L.B.; Serrano, J.; Hoofnagle, J.H. Liver injury from herbal and dietary supplements. Hepatology 2017, 65, 363–373. [Google Scholar] [CrossRef]
- Zheng, E.; Sandhu, N.; Navarro, V. Drug-induced liver injury secondary to herbal and dietary supplements. Clin. Liver Dis. 2020, 24, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Hartleb, M.; Biernat, L.; Kochel, A. Drug-induced liver damage—A three-year study of patients from one gastroenterological department. Med. Sci. Monit. 2002, 8, CR292–CR296. [Google Scholar] [PubMed]
- Voican, C.S.; Corruble, E.; Naveau, S.; Perlemuter, G. Antidepressant-induced liver injury: A review for clinicians. Am. J. Psychiatry 2014, 171, 404–415. [Google Scholar] [CrossRef]
- Park, S.H.; Ishino, R. Liver injury associated with antidepressants. Curr. Drug Saf. 2013, 8, 207–223. [Google Scholar] [CrossRef]
- Miele, L.; Liguori, A.; Marrone, G.; Biolato, M.; Araneo, C.; Vaccaro, F.G.; Gasbarrini, A.; Grieco, A. Fatty liver and drugs: The two sides of the same coin. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 86–94. [Google Scholar]
- Amacher, D.E.; Chalasani, N. Drug-induced hepatic steatosis. Semin. Liver Dis 2014, 34, 205–214. [Google Scholar]
- WebMD. Available online: https://www.webmd.com/search/search_results/default.aspx?query=liver diseases (accessed on 24 December 2021).
- Mataya, L.; Patel, N.; Azzam, R.K. Autoimmune liver diseases in children. Pediatr. Ann. 2018, 47, e452–e457. [Google Scholar] [CrossRef]
- Vilstrup, H.; Amodio, P.; Bajaj, J.; Cordoba, J.; Ferenci, P.; Mullen, K.D.; Weissenborn, K.; Wong, P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014, 60, 715–735. [Google Scholar] [CrossRef] [PubMed]
- Viveiros, K. The Role of Life Style Modifications in Comprehensive Non-Alcoholic Fatty Liver Disease Treatment. Clin. Liver Dis. 2021, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, R.K.; Chawla, Y.K. Herbal medicines for liver diseases. Dig. Dis. Sci. 2005, 50, 1807–1812. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R. Herbal Home Remedies; Lotus Press: Wisconsin, WA, USA, 2006; ISBN 8183820549. [Google Scholar]
- Konczak, I.; Zhang, W. Anthocyanins—More than nature’s colours. J. Biomed. Biotechnol. 2004, 2004, 239. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, D.; Jiménez-Ferrer, E.; Zamilpa, A.; Herrera-Arellano, A.; Tortoriello, J.; Alvarez, L. Inhibition of angiotensin convertin enzyme (ACE) activity by the anthocyanins delphinidin-and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. J. Ethnopharmacol. 2010, 127, 7–10. [Google Scholar] [CrossRef]
- Wang, C.-J.; Wang, J.-M.; Lin, W.-L.; Chu, C.-Y.; Chou, F.-P.; Tseng, T.-H. Protective effect of Hibiscus anthocyanins against tert-butyl hydroperoxide-induced hepatic toxicity in rats. Food Chem. Toxicol. 2000, 38, 411–416. [Google Scholar] [CrossRef]
- Mulabagal, V.; Wang, H.; Ngouajio, M.; Nair, M.G. Characterization and quantification of health beneficial anthocyanins in leaf chicory (Cichorium intybus) varieties. Eur. Food Res. Technol. 2009, 230, 47–53. [Google Scholar] [CrossRef]
- Chaves-López, C.; Yeimmy, P.-R.; Molina Hernandez, J.B.; Delgado Ospina, J.; Tovar, C.D.G.; Paparella, A. Anthocyanins in Folk Medicine: Local Traditions, Sources, Compounds and Related Aspects. In Anthocyanins; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2020; p. 141. [Google Scholar]
- Dash, R.N.; Habibuddin, M.; Baruah, D.B. Anthocyanins fraction of red radish (Raphanus sativus L.) protects hepatic damage induced by carbon tetrachloride in albino rats. J. Exp. Integr. Med. 2013, 3, 43–50. [Google Scholar] [CrossRef]
- Huo, Y. Mulberry Cultivation and Utilization in China; FAO Animal Production and Health Papers; FAO: Rome, Italy, 2000; pp. 11–44. [Google Scholar]
- Bagachi, A.; Semwal, A.; Bharadwaj, A. Traditional uses, phytochemistry and pharmacology of Morus alba Linn.: A review. J. Med. Plants Res. 2013, 7, 461–469. [Google Scholar]
- Dinda, B.; Kyriakopoulos, A.M.; Dinda, S.; Zoumpourlis, V.; Thomaidis, N.S.; Velegraki, A.; Markopoulos, C.; Dinda, M. Cornus mas L. (cornelian cherry), an important European and Asian traditional food and medicine: Ethnomedicine, phytochemistry and pharmacology for its commercial utilization in drug industry. J. Ethnopharmacol. 2016, 193, 670–690. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Raji, H.O.; Muritala, H.F.; Ojewuyi, O.B.; Yakubu, M.T. Anthocyanin extract of Lannea microcarpa fruits stall oxidative rout associated with aflatoxin B1 hepatocarcinogenesis. Food Biosci. 2013, 4, 58–67. [Google Scholar] [CrossRef]
- Awad, N.E.; Abdelkawy, M.A.; Hamed, M.A.; Souleman, A.M.A.; Abdelrahman, E.H.; Ramadan, N.S. Antioxidant and hepatoprotective effects of Justicia spicigera ethyl acetate fraction and characterization of its anthocyanin content. Int. J. Pharm. Pharm. Sci. 2015, 7, 91–96. [Google Scholar]
- Ezzat, S.M.; Salama, M.M.; Seif el-Din, S.H.; Saleh, S.; El-Lakkany, N.M.; Hammam, O.A.; Salem, M.B.; Botros, S.S. Metabolic profile and hepatoprotective activity of the anthocyanin-rich extract of Hibiscus sabdariffa calyces. Pharm. Biol. 2016, 54, 3172–3181. [Google Scholar] [CrossRef]
- Hou, F.; Zhang, R.; Zhang, M.; Su, D.; Wei, Z.; Deng, Y.; Zhang, Y.; Chi, J.; Tang, X. Hepatoprotective and antioxidant activity of anthocyanins in black rice bran on carbon tetrachloride-induced liver injury in mice. J. Funct. Foods 2013, 5, 1705–1713. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Choi, J.H.; Choi, J.M.; Chung, Y.C.; Jeong, H.G. Protective mechanisms of anthocyanins from purple sweet potato against tert-butyl hydroperoxide-induced hepatotoxicity. Food Chem. Toxicol. 2011, 49, 2081–2089. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.J.; Kroon, P.A.; Hollands, W.J.; Walls, R.; Jenkins, G.; Kay, C.D.; Cassidy, A. Cardiovascular disease risk biomarkers and liver and kidney function are not altered in postmenopausal women after ingesting an elderberry extract rich in anthocyanins for 12 weeks. J. Nutr. 2009, 139, 2266–2271. [Google Scholar] [CrossRef]
- Kalt, W.; Blumberg, J.B.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.E.; Graf, B.A.; O’Leary, J.M.; Milbury, P.E. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J. Agric. Food Chem. 2008, 56, 705–712. [Google Scholar] [CrossRef]
- Tsuda, T.; Horio, F.; Osawa, T. The role of anthocyanins as an antioxidant under oxidative stress in rats. Biofactors 2000, 13, 133–139. [Google Scholar] [CrossRef]
- Belwal, T.; Nabavi, S.F.; Nabavi, S.M.; Habtemariam, S. Dietary anthocyanins and insulin resistance: When food becomes a medicine. Nutrients 2017, 9, 1111. [Google Scholar] [CrossRef]
- Ávila, M.; Hidalgo, M.; Sánchez-Moreno, C.; Pelaez, C.; Requena, T.; de Pascual-Teresa, S. Bioconversion of anthocyanin glycosides by Bifidobacteria and Lactobacillus. Food Res. Int. 2009, 42, 1453–1461. [Google Scholar] [CrossRef]
- Keppler, K.; Humpf, H.-U. Metabolism of anthocyanins and their phenolic degradation products by the intestinal microflora. Bioorg. Med. Chem. 2005, 13, 5195–5205. [Google Scholar] [CrossRef]
- Aura, A.-M.; Martin-Lopez, P.; O’Leary, K.A.; Williamson, G.; Oksman-Caldentey, K.-M.; Poutanen, K.; Santos-Buelga, C. In vitro metabolism of anthocyanins by human gut microflora. Eur. J. Nutr. 2005, 44, 133–142. [Google Scholar] [CrossRef]
- Kay, C.D.; Mazza, G.; Holub, B.J.; Wang, J. Anthocyanin metabolites in human urine and serum. Br. J. Nutr. 2004, 91, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Les, F.; Cásedas, G.; Gómez, C.; Moliner, C.; Valero, M.S.; López, V. The role of anthocyanins as antidiabetic agents: From molecular mechanisms to in vivo and human studies. J. Physiol. Biochem. 2021, 77, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, P.; Scazzocchio, B.; Varì, R.; Santangelo, C.; D’Archivio, M.; Silecchia, G.; Iacovelli, A.; Giovannini, C.; Masella, R. Effect of protocatechuic acid on insulin responsiveness and inflammation in visceral adipose tissue from obese individuals: Possible role for PTP1B. Int. J. Obes. 2018, 42, 2012–2021. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.A.; Suddek, G.M.; Rahim, M.A.; Abdelrahman, R.S. The protective effect of protocatechuic acid on hepatotoxicity induced by cisplatin in mice. Life Sci. 2021, 277, 119485. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-L.; Hsieh, Y.-J.; Chou, F.-P.; Wang, C.-J.; Cheng, M.-T.; Tseng, T.-H. Hibiscus protocatechuic acid inhibits lipopolysaccharide-induced rat hepatic damage. Arch. Toxicol. 2003, 77, 42–47. [Google Scholar]
- Owumi, S.E.; Ajijola, I.J.; Agbeti, O.M. Hepatorenal protective effects of protocatechuic acid in rats administered with anticancer drug methotrexate. Hum. Exp. Toxicol. 2019, 38, 1254–1265. [Google Scholar] [CrossRef]
- Fu, R.; Zhou, J.; Wang, R.; Sun, R.; Feng, D.; Wang, Z.; Zhao, Y.; Lv, L.; Tian, X.; Yao, J. Protocatechuic acid-mediated miR-219a-5p activation inhibits the p66shc oxidant pathway to alleviate alcoholic liver injury. Oxid. Med. Cell. Longev. 2019, 2019, 3527809. [Google Scholar] [CrossRef]
- Andersen, Ø.M.; Jordheim, M. Basic anthocyanin chemistry and dietary sources. In Anthocyanins in Health and Disease; Wallace, T.C., Giusti, M.M., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 13–90. [Google Scholar]
- Muth, E.R.; Laurent, J.M.; Jasper, P. The effect of bilberry nutritional supplementation on night visual acuity and contrast sensitivity. Altern. Med. Rev. 2000, 5, 164–173. [Google Scholar]
- Wallace, T.C.; Giusti, M.M. (Eds.) Anthocyanins in cardiovascular disease prevention. In Anthocyanins in Health and Disease; CRC Press: Boca Raton, FL, USA, 2014; pp. 165–197. [Google Scholar]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef] [PubMed]
- Delporte, C.; Backhouse, N.; Inostroza, V.; Aguirre, M.C.; Peredo, N.; Silva, X.; Negrete, R.; Miranda, H.F. Analgesic activity of Ugni molinae (murtilla) in mice models of acute pain. J. Ethnopharmacol. 2007, 112, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.H.; Kim, J.M.; Choi, C.Y.; Kim, C.Y.; Park, K.H. Ginkgo biloba extract and bilberry anthocyanins improve visual function in patients with normal tension glaucoma. J. Med. Food 2012, 15, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Lila, M.A. Anthocyanins and human health: An in vitro investigative approach. J. Biomed. Biotechnol. 2004, 2004, 306. [Google Scholar] [CrossRef]
- Tsuda, T. Dietary anthocyanin-rich plants: Biochemical basis and recent progress in health benefits studies. Mol. Nutr. Food Res. 2012, 56, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, A.; Dzierżanowski, T. Targeting inflammation by anthocyanins as the novel therapeutic potential for chronic diseases: An update. Molecules 2021, 26, 4380. [Google Scholar] [CrossRef]
- Titta, L.; Trinei, M.; Stendardo, M.; Berniakovich, I.; Petroni, K.; Tonelli, C.; Riso, P.; Porrini, M.; Minucci, S.; Pelicci, P.G. Blood orange juice inhibits fat accumulation in mice. Int. J. Obes. 2010, 34, 578–588. [Google Scholar] [CrossRef]
- Cappellini, F.; Marinelli, A.; Toccaceli, M.; Tonelli, C.; Petroni, K. Anthocyanins: From Mechanisms of Regulation in Plants to Health Benefits in Foods. Front. Plant Sci. 2021, 12, 748049. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Alawadh, S.H.; Bouazzaoui, A.; Alhowail, A.H.; Mohammed, H.A. Anthocyanins rich pomegranate cream as a topical formulation with anti-aging activity. J. Dermatol. Treat. 2021, 32, 983–990. [Google Scholar] [CrossRef]
- Wallace, T.C.; Giusti, M.M. (Eds.) Role of Anthocyanins in Skin Aging and UV Induced Skin Damage. In Anthocyanins in Health and Disease; CRC Press: Boca Raton, FL, USA, 2013; Chapter 11; ISBN 1439894760. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Fedirko, V.; Trichopoulou, A.; González, C.A.; Bamia, C.; Trepo, E.; Nöthlings, U.; Duarte-Salles, T.; Serafini, M.; Bredsdorff, L. Dietary flavonoid, lignan and antioxidant capacity and risk of hepatocellular carcinoma in the European prospective investigation into cancer and nutrition study. Int. J. Cancer 2013, 133, 2429–2443. [Google Scholar] [CrossRef] [PubMed]
- Escribano-Bailón, M.T.; Alcalde-Eon, C.; Muñoz, O.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanins in berries of maqui [Aristotelia chilensis (Mol.) Stuntz]. Phytochem. Anal. 2006, 17, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Mariangel, E.; Díaz, M.R.; Alvarez, W.L.; Bensch, E.; Schalchli, H.; Ibarra, P. The antioxidant properties of calafate (Berberis microphylla) fruits from four different locations in southern Chile. Cienc. Investig. Agrar. 2013, 40, 161–170. [Google Scholar] [CrossRef]
- Ruiz, A.; Hermosin-Gutierrez, I.; Mardones, C.; Vergara, C.; Herlitz, E.; Vega, M.; Dorau, C.; Winterhalter, P.; von Baer, D. Polyphenols and antioxidant activity of calafate (Berberis microphylla) fruits and other native berries from Southern Chile. J. Agric. Food Chem. 2010, 58, 6081–6089. [Google Scholar] [CrossRef]
- Shi, N.; Chen, X.; Chen, T. Anthocyanins in Colorectal Cancer Prevention Review. Antioxidants 2021, 10, 1600. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy on Diet, Physical Activity and Health; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of anthocyanins (E 163) as a food additive. EFSA J. 2013, 11, 3145. [Google Scholar]
- Kuhnau, J. The flavonoids. A class of semi-essential food components: Their role in human nutrition. World Rev. Nutr. Diet. 1976, 24, 117–191. [Google Scholar]
- Sebastian, R.S.; Wilkinson Enns, C.; Goldman, J.D.; Martin, C.L.; Steinfeldt, L.C.; Murayi, T.; Moshfegh, A.J. A new database facilitates characterization of flavonoid intake, sources, and positive associations with diet quality among US adults. J. Nutr. 2015, 145, 1239–1248. [Google Scholar] [CrossRef]
- Wang, S.; Lay, S.; Yu, H.; Shen, S. Dietary guidelines for Chinese residents (2016): Comments and comparisons. J. Zhejiang Univ. B 2016, 17, 649–656. [Google Scholar] [CrossRef]
- Wallace, T.C.; Blumberg, J.B.; Johnson, E.J.; Shao, A. Dietary bioactives: Establishing a scientific framework for recommended intakes. Adv. Nutr. 2015, 6, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Rimac Brnčić, S.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; de Pascual-Teresa, S. A review of factors affecting anthocyanin bioavailability: Possible implications for the inter-individual variability. Foods 2020, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Mülleder, U.; Murkovic, M.; Pfannhauser, W. Urinary excretion of cyanidin glycosides. J. Biochem. Biophys. Methods 2002, 53, 61–66. [Google Scholar] [CrossRef]
- Bub, A.; Watzl, B.; Heeb, D.; Rechkemmer, G.; Briviba, K. Malvidin-3-glucoside bioavailability in humans after ingestion of red wine, dealcoholized red wine and red grape juice. Eur. J. Nutr. 2001, 40, 113–120. [Google Scholar] [CrossRef]
- Cao, G.; Prior, R.L. Anthocyanins are detected in human plasma after oral administration of an elderberry extract. Clin. Chem. 1999, 45, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Fang, J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Bioavailability of anthocyanins and derivatives. J. Funct. Foods 2014, 7, 54–66. [Google Scholar] [CrossRef]
- Kay, C.D.; Pereira-Caro, G.; Ludwig, I.A.; Clifford, M.N.; Crozier, A. Anthocyanins and flavanones are more bioavailable than previously perceived: A review of recent evidence. Annu. Rev. Food Sci. Technol. 2017, 8, 155–180. [Google Scholar] [CrossRef]
- Yousuf, B.; Gul, K.; Wani, A.A.; Singh, P. Health benefits of anthocyanins and their encapsulation for potential use in food systems: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2223–2230. [Google Scholar] [CrossRef]
- Mahdavi, S.A.; Jafari, S.M.; Assadpoor, E.; Dehnad, D. Microencapsulation optimization of natural anthocyanins with maltodextrin, gum Arabic and gelatin. Int. J. Biol. Macromol. 2016, 85, 379–385. [Google Scholar] [CrossRef]
- Chen, B.-H.; Stephen Inbaraj, B. Nanoemulsion and nanoliposome based strategies for improving anthocyanin stability and bioavailability. Nutrients 2019, 11, 1052. [Google Scholar] [CrossRef] [PubMed]
- Novotny, J.A.; Clevidence, B.A.; Kurilich, A.C. Anthocyanin kinetics are dependent on anthocyanin structure. Br. J. Nutr. 2012, 107, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-O-glucoside: A 13C-tracer study. Am. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Dupeyrón, D.; Kawakami, M.; Rieumont, J.; Carlos Carvalho, J. Formulation and characterization of anthocyanins-loaded nanoparticles. Curr. Drug Deliv. 2017, 14, 54–64. [Google Scholar] [CrossRef]
- Miyazawa, T.; Nakagawa, K.; Kudo, M.; Muraishi, K.; Someya, K. Direct intestinal absorption of red fruit anthocyanins, cyanidin-3-glucoside and cyanidin-3, 5-diglucoside, into rats and humans. J. Agric. Food Chem. 1999, 47, 1083–1091. [Google Scholar] [CrossRef]
- Hou, D.-X. Potential mechanisms of cancer chemoprevention by anthocyanins. Curr. Mol. Med. 2003, 3, 149–159. [Google Scholar] [CrossRef]
- Meiers, S.; Kemény, M.; Weyand, U.; Gastpar, R.; von Angerer, E.; Marko, D. The anthocyanidins cyanidin and delphinidin are potent inhibitors of the epidermal growth-factor receptor. J. Agric. Food Chem. 2001, 49, 958–962. [Google Scholar] [CrossRef]
- Fang, J. Classification of fruits based on anthocyanin types and relevance to their health effects. Nutrition 2015, 31, 1301–1306. [Google Scholar] [CrossRef]
- Batista, Â.G.; Mendonça, M.C.P.; Soares, E.S.; da Silva-Maia, J.K.; Dionísio, A.P.; Sartori, C.R.; da Cruz-Höfling, M.A.; Júnior, M.R.M. Syzygium malaccense fruit supplementation protects mice brain against high-fat diet impairment and improves cognitive functions. J. Funct. Foods 2020, 65, 103745. [Google Scholar] [CrossRef]
- Bensalem, J.; Dudonné, S.; Gaudout, D.; Servant, L.; Calon, F.; Desjardins, Y.; Layé, S.; Lafenetre, P.; Pallet, V. Polyphenol-rich extract from grape and blueberry attenuates cognitive decline and improves neuronal function in aged mice. J. Nutr. Sci. 2018, 7, e19. [Google Scholar] [CrossRef]
- Whyte, A.R.; Schafer, G.; Williams, C.M. Cognitive effects following acute wild blueberry supplementation in 7-to 10-year-old children. Eur. J. Nutr. 2016, 55, 2151–2162. [Google Scholar] [CrossRef] [PubMed]
- Whyte, A.R.; Williams, C.M. Effects of a single dose of a flavonoid-rich blueberry drink on memory in 8 to 10 y old children. Nutrition 2015, 31, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Igwe, E.O.; Charlton, K.E.; Roodenrys, S.; Kent, K.; Fanning, K.; Netzel, M.E. Anthocyanin-rich plum juice reduces ambulatory blood pressure but not acute cognitive function in younger and older adults: A pilot crossover dose-timing study. Nutr. Res. 2017, 47, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Lamport, D.J.; Lawton, C.L.; Merat, N.; Jamson, H.; Myrissa, K.; Hofman, D.; Chadwick, H.K.; Quadt, F.; Wightman, J.D.; Dye, L. Concord grape juice, cognitive function, and driving performance: A 12-wk, placebo-controlled, randomized crossover trial in mothers of preteen children. Am. J. Clin. Nutr. 2016, 103, 775–783. [Google Scholar] [CrossRef]
- Kent, K.; Charlton, K.; Roodenrys, S.; Batterham, M.; Potter, J.; Traynor, V.; Gilbert, H.; Morgan, O.; Richards, R. Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia. Eur. J. Nutr. 2017, 56, 333–341. [Google Scholar] [CrossRef]
- McNamara, R.K.; Kalt, W.; Shidler, M.D.; McDonald, J.; Summer, S.S.; Stein, A.L.; Stover, A.N.; Krikorian, R. Cognitive response to fish oil, blueberry, and combined supplementation in older adults with subjective cognitive impairment. Neurobiol. Aging 2018, 64, 147–156. [Google Scholar] [CrossRef]
- Boespflug, E.L.; Eliassen, J.C.; Dudley, J.A.; Shidler, M.D.; Kalt, W.; Summer, S.S.; Stein, A.L.; Stover, A.N.; Krikorian, R. Enhanced neural activation with blueberry supplementation in mild cognitive impairment. Nutr. Neurosci. 2018, 21, 297–305. [Google Scholar] [CrossRef]
- Neshat, S.Y.; Quiroz, V.M.; Wang, Y.; Tamayo, S.; Doloff, J.C. Liver Disease: Induction, Progression, Immunological Mechanisms, and Therapeutic Interventions. Int. J. Mol. Sci. 2021, 22, 6777. [Google Scholar] [CrossRef]
- Fausto, N. Liver regeneration. J. Hepatol. 2000, 32, 19–31. [Google Scholar] [CrossRef]
- Sánchez-Valle, V.; Chavez-Tapia, N.C.; Uribe, M.; Méndez-Sánchez, N. Role of oxidative stress and molecular changes in liver fibrosis: A review. Curr. Med. Chem. 2012, 19, 4850–4860. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Unnikrishnan, A.; Deepa, S.S.; Liu, Y.; Li, Y.; Ikeno, Y.; Sosnowska, D.; Van Remmen, H.; Richardson, A. A new role for oxidative stress in aging: The accelerated aging phenotype in Sod1−/− mice is correlated to increased cellular senescence. Redox Biol. 2017, 11, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hong, M.; Tan, H.-Y.; Wang, N.; Feng, Y. Insights into the role and interdependence of oxidative stress and inflammation in liver diseases. Oxid. Med. Cell. Longev. 2016, 2016, 4234061. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Lauridsen, C. From oxidative stress to inflammation: Redox balance and immune system. Poult. Sci. 2019, 98, 4240–4246. [Google Scholar] [CrossRef]
- Castellani, R.; Hirai, K.; Aliev, G.; Drew, K.L.; Nunomura, A.; Takeda, A.; Cash, A.D.; Obrenovich, M.E.; Perry, G.; Smith, M.A. Role of mitochondrial dysfunction in Alzheimer’s disease. J. Neurosci. Res. 2002, 70, 357–360. [Google Scholar] [CrossRef]
- Yusuf, M.; Khan, M.; Robaian, M.A.; Khan, R.A. Biomechanistic insights into the roles of oxidative stress in generating complex neurological disorders. Biol. Chem. 2018, 399, 305–319. [Google Scholar] [CrossRef]
- Cichoż-Lach, H. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082. [Google Scholar] [CrossRef]
- Wu, X.; Kang, J.; Xie, C.; Burris, R.; Ferguson, M.E.; Badger, T.M.; Nagarajan, S. Dietary blueberries attenuate atherosclerosis in apolipoprotein E-deficient mice by upregulating antioxidant enzyme expression. J. Nutr. 2010, 140, 1628–1632. [Google Scholar] [CrossRef]
- Feagins, L.A.; Flores, A.; Arriens, C.; Park, C.; Crook, T.; Reimold, A.; Brown, G. Nonalcoholic fatty liver disease: A potential consequence of tumor necrosis factor-inhibitor therapy. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1154. [Google Scholar] [CrossRef]
- Coffin, C.S.; Fraser, H.F.; Panaccione, R.; Ghosh, S. Liver diseases associated with anti-tumor necrosis factor-alpha (TNF-α) use for inflammatory bowel disease. Inflamm. Bowel Dis. 2011, 17, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, B.-E.; Wink, M. Medicinal Plants of the World; CABI: Wallingford, UK, 2018; ISBN 1786393255. [Google Scholar]
- Aboelsoud, N.H. Herbal medicine in ancient Egypt. J. Med. Plants Res. 2010, 4, 82–86. [Google Scholar]
- Jain, S.K. Ethnobotany and research in medicinal plants in India. Ethnobot. Search New Drugs 1994, 185, 153–168. [Google Scholar]
- Mohammed, S.A.A.; Eldeeb, H.M.; Mohammed, H.A.; Al-Omar, M.S.; Almahmoud, S.A.; El-Readi, M.Z.; Ragab, E.A.; Sulaiman, G.M.; Aly, M.S.A.; Khan, R.A. Roles of Suaeda vermiculata Aqueous-Ethanolic Extract, Its Subsequent Fractions, and the Isolated Compounds in Hepatoprotection against Paracetamol-Induced Toxicity as Compared to Silymarin. Oxid. Med. Cell. Longev. 2021, 2021, 6174897. [Google Scholar] [CrossRef] [PubMed]
- Mu, T.; Sun, H.; Zhang, M.; Wang, C. Chapter 6—Sweet Potato Anthocyanins. In Sweet Potato Processing Technology; Mu, T., Sun, H., Zhang, M., Wang, C., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 279–355. ISBN 978-0-12-812871-8. [Google Scholar]
- Danielewski, M.; Matuszewska, A.; Nowak, B.; Kucharska, A.Z.; Sozański, T. The effects of natural iridoids and anthocyanins on selected parameters of liver and cardiovascular system functions. Oxid. Med. Cell. Longev. 2020, 2020, 2735790. [Google Scholar] [CrossRef]
- Jia, Y.; Kim, J.-Y.; Jun, H.; Kim, S.-J.; Lee, J.-H.; Hoang, M.H.; Kim, H.S.; Chang, H.I.; Hwang, K.-Y.; Um, S.-J. Cyanidin is an agonistic ligand for peroxisome proliferator-activated receptor-alpha reducing hepatic lipid. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2013, 1831, 698–708. [Google Scholar] [CrossRef]
- Chang, J.-J.; Hsu, M.-J.; Huang, H.-P.; Chung, D.-J.; Chang, Y.-C.; Wang, C.-J. Mulberry anthocyanins inhibit oleic acid induced lipid accumulation by reduction of lipogenesis and promotion of hepatic lipid clearance. J. Agric. Food Chem. 2013, 61, 6069–6076. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Choi, J.H.; Han, E.H.; Kim, H.G.; Wee, J.-H.; Jung, K.O.; Jung, K.H.; Kwon, K.; Jeong, T.C.; Chung, Y.C. Purple sweet potato anthocyanins attenuate hepatic lipid accumulation through activating adenosine monophosphate–activated protein kinase in human HepG2 cells and obese mice. Nutr. Res. 2011, 31, 896–906. [Google Scholar] [CrossRef]
- Zhu, W.; Jia, Q.; Wang, Y.; Zhang, Y.; Xia, M. The anthocyanin cyanidin-3-O-β-glucoside, a flavonoid, increases hepatic glutathione synthesis and protects hepatocytes against reactive oxygen species during hyperglycemia: Involvement of a cAMP–PKA-dependent signaling pathway. Free Radic. Biol. Med. 2012, 52, 314–327. [Google Scholar] [CrossRef]
- Pei, L.; Wan, T.; Wang, S.; Ye, M.; Qiu, Y.; Jiang, R.; Pang, N.; Huang, Y.; Zhou, Y.; Jiang, X. Cyanidin-3-O-β-glucoside regulates the activation and the secretion of adipokines from brown adipose tissue and alleviates diet induced fatty liver. Biomed. Pharmacother. 2018, 105, 625–632. [Google Scholar] [CrossRef]
- Sangsefidi, Z.S.; Mozaffari-Khosravi, H.; Sarkhosh-Khorasani, S.; Hosseinzadeh, M. The effect of anthocyanins supplementation on liver enzymes: A systematic review and meta-analysis of randomized clinical trials. Food Sci. Nutr. 2021, 9, 3954–3970. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, A.; Zhao, L.; Wang, Y.; Pan, F.; Hao, S.; Zhang, H.; Iftikhar, A.; Usman, M. Dietary anthocyanins as potential natural modulators for the prevention and treatment of non-alcoholic fatty liver disease: A comprehensive review. Food Res. Int. 2021, 142, 110180. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Luo, Z.; Guo, Z.; Wang, X.; Ding, M.; Wang, W.; Shen, X.; Zhao, Y. Multiple Roles of Black Raspberry Anthocyanins Protecting against Alcoholic Liver Disease. Molecules 2021, 26, 2313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.-W.; Chen, F.-X.; Di Li, W.-H.L.; Guo, H.-H. A CONSORT-compliant, randomized, double-blind, placebo-controlled pilot trial of purified anthocyanin in patients with nonalcoholic fatty liver disease. Medicine 2015, 94, e758. [Google Scholar] [CrossRef]
- Sun, H.; Mu, T.; Liu, X.; Zhang, M.; Chen, J. Purple sweet potato (Ipomoea batatas L.) anthocyanins: Preventive effect on acute and subacute alcoholic liver damage and dealcoholic effect. J. Agric. Food Chem. 2014, 62, 2364–2373. [Google Scholar] [CrossRef]
- Castera, L. Steatosis, insulin resistance and fibrosis progression in chronic hepatitis C. Minerva Gastroenterol. Dietol. 2006, 52, 125–134. [Google Scholar]
- Powell, E.E.; Jonsson, J.R.; Clouston, A.D. Steatosis: Co-factor in other liver diseases. Hepatology 2005, 42, 5–13. [Google Scholar] [CrossRef]
- Powell, E.E.; Jonsson, J.R.; Clouston, A.D. Metabolic factors and non-alcoholic fatty liver disease as co-factors in other liver diseases. Dig. Dis. 2010, 28, 186–191. [Google Scholar] [CrossRef]
- Clouston, A.D.; Jonsson, J.R.; Powell, E.E. Steatosis as a cofactor in other liver diseases: Hepatitis C virus, alcohol, hemochromatosis, and others. Clin. Liver Dis. 2007, 11, 173–189. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Ren, G.; Yang, R.; Chen, J.; Xiang, X.; Qin, H.; Chen, J. Inhibitory effect of delphinidin on oxidative stress induced by H2O2 in HepG2 cells. Oxid. Med. Cell. Longev. 2020, 2020, 4694760. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Y.; Tao, X.; Zhang, M.; Sun, A. Protective effect of blueberry anthocyanins in a CCL4-induced liver cell model. LWT—Food Sci. Technol. 2015, 60, 1105–1112. [Google Scholar] [CrossRef]
- Choi, J.H.; Choi, C.Y.; Lee, K.J.; Hwang, Y.P.; Chung, Y.C.; Jeong, H.G. Hepatoprotective effects of an anthocyanin fraction from purple-fleshed sweet potato against acetaminophen-induced liver damage in mice. J. Med. Food 2009, 12, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, J.; Wang, Z.; Gao, H.; Su, L.; Xie, J.; Chen, X.; Liang, H.; Wang, C.; Han, Y. Oral hepatoprotective ability evaluation of purple sweet potato anthocyanins on acute and chronic chemical liver injuries. Cell Biochem. Biophys. 2014, 69, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Hinson, J.A.; Reid, A.B.; McCullough, S.S.; James, L.P. Acetaminophen-induced hepatotoxicity: Role of metabolic activation, reactive oxygen/nitrogen species, and mitochondrial permeability transition. Drug Metab. Rev. 2004, 36, 805–822. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Jia, S.; Yuan, K. Protective effect and mechanism of action of mulberry marc anthocyanins on carbon tetrachloride-induced liver fibrosis in rats. J. Funct. Foods 2016, 24, 595–601. [Google Scholar] [CrossRef]
- Haseeb, A.; Chen, D.; Haqqi, T.M. Delphinidin inhibits IL-1β-induced activation of NF-κB by modulating the phosphorylation of IRAK-1Ser376 in human articular chondrocytes. Rheumatology 2013, 52, 998–1008. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Zhou, Q.; Luo, C.-L.; Deng, A.-P.; Zhang, Z.-C.; Zhang, J.-L. Characterization and hepatoprotective activity of anthocyanins from purple sweet potato (Ipomoea batatas L. cultivar Eshu No. 8). J. Food Drug Anal. 2017, 25, 607–618. [Google Scholar] [CrossRef]
- Onyesom, I.; Mordi, J.; Opajobi, A.O.; Esume, C.O. Hepatoprotective Potentials of Hibiscus rosasinensis Petal anthocyanin Extracts against Carbon tetrachloride-Induced Acute Liver Damage in Wistar Rats. Sudan J. Med. Sci. 2008, 3, 33–36. [Google Scholar] [CrossRef]
- Valcheva-Kuzmanova, S.; Borisova, P.; Galunska, B.; Krasnaliev, I.; Belcheva, A. Hepatoprotective effect of the natural fruit juice from Aronia melanocarpa on carbon tetrachloride-induced acute liver damage in rats. Exp. Toxicol. Pathol. 2004, 56, 195–201. [Google Scholar] [CrossRef]
- Valcheva-Kuzmanova, S. Comparative Study of the Protective Effect of Fruit Juice and Quercetin in a Model of Paracetamol-Induced Hepatotoxicity in Rats. J. Biomed. Clin. Res. 2015, 8, 118–123. [Google Scholar] [CrossRef][Green Version]
- Krajka-Kuźniak, V.; Szaefer, H.; Ignatowicz, E.; Adamska, T.; Oszmiański, J.; Baer-Dubowska, W. Effect of Chokeberry (Aronia melanocarpa) Juice on the Metabolic Activation and Detoxication of Carcinogenic N-Nitrosodiethylamine in Rat Liver. J. Agric. Food Chem. 2009, 57, 5071–5077. [Google Scholar] [CrossRef] [PubMed]
- Muceniece, R.; Klavins, L.; Kviesis, J.; Jekabsons, K.; Rembergs, R.; Saleniece, K.; Dzirkale, Z.; Saulite, L.; Riekstina, U.; Klavins, M. Antioxidative, hypoglycaemic and hepatoprotective properties of five Vaccinium spp. berry pomace extracts. J. Berry Res. 2019, 9, 267–282. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, C.; Wang, J.; Xie, W.; Wang, M.; Li, X.; Zhang, X. Purple potato (Solanum tuberosum L.) anthocyanins attenuate alcohol-induced hepatic injury by enhancing antioxidant defense. J. Nat. Med. 2016, 70, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Choi, J.H.; Yun, H.J.; Han, E.H.; Kim, H.G.; Kim, J.Y.; Park, B.H.; Khanal, T.; Choi, J.M.; Chung, Y.C. Anthocyanins from purple sweet potato attenuate dimethylnitrosamine-induced liver injury in rats by inducing Nrf2-mediated antioxidant enzymes and reducing COX-2 and iNOS expression. Food Chem. Toxicol. 2011, 49, 93–99. [Google Scholar] [CrossRef]
- Ali, B.H.; Mousa, H.M.; El-Mougy, S. The effect of a water extract and anthocyanins of Hibiscus sabdariffa L. on paracetamol-induced hepatoxicity in rats. Phyther. Res. 2003, 17, 56–59. [Google Scholar] [CrossRef]
- Tuse, T.A.; Harle, U.N.; Bore, V.V. Hepatoprotective activity of Colocasia antiquorum against experimentally induced liver injury in rats. Malyasian J. Pharm. Sci. 2009, 7, 99–112. [Google Scholar]
- Cristani, M.; Speciale, A.; Mancari, F.; Arcoraci, T.; Ferrari, D.; Fratantonio, D.; Saija, A.; Cimino, F.; Trombetta, D. Protective activity of an anthocyanin-rich extract from bilberries and blackcurrants on acute acetaminophen-induced hepatotoxicity in rats. Nat. Prod. Res. 2016, 30, 2845–2849. [Google Scholar] [CrossRef]
- Ahn, M.; Kim, J.; Choi, Y.; Ekanayake, P.; Chun, J.; Yang, D.; Kim, G.-O.; Shin, T. Fermented black radish (Raphanus sativus L. var. niger) attenuates methionine and choline deficient diet-induced nonalcoholic fatty liver disease in mice. Food Sci. Nutr. 2019, 7, 3327–3337. [Google Scholar] [CrossRef]
- Hanlon, P.R.; Robbins, M.G.; Hammon, L.D.; Barnes, D.M. Aqueous extract from the vegetative portion of Spanish black radish (Raphanus sativus L. var. niger) induces detoxification enzyme expression in HepG2 cells. J. Funct. Foods 2009, 1, 356–365. [Google Scholar] [CrossRef]
- Abdelhafez, O.H.; Fawzy, M.A.; Fahim, J.R.; Desoukey, S.Y.; Krischke, M.; Mueller, M.J.; Abdelmohsen, U.R. Hepatoprotective potential of Malvaviscus arboreus against carbon tetrachloride-induced liver injury in rats. PLoS ONE 2018, 13, e0202362. [Google Scholar] [CrossRef]
- Sozański, T.; Kucharska, A.Z.; Dzimira, S.; Magdalan, J.; Szumny, D.; Matuszewska, A.; Nowak, B.; Piórecki, N.; Szeląg, A.; Trocha, M. Loganic acid and anthocyanins from cornelian cherry (Cornus mas L.) fruits modulate diet-induced atherosclerosis and redox status in rabbits. Adv. Clin. Exp. Med. 2018, 27, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, G.; Zhang, X.; Xu, D.; Gao, J.; Fan, J. Anthocyanins delay ageing-related degenerative changes in the liver. Plant Foods Hum. Nutr. 2017, 72, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Gong, C.; Song, H.; Cui, Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Longo, L.; Platini, F.; Scardino, A.; Alabiso, O.; Vasapollo, G.; Tessitore, L. Autophagy inhibition enhances anthocyanin-induced apoptosis in hepatocellular carcinoma. Mol. Cancer Ther. 2008, 7, 2476–2485. [Google Scholar] [CrossRef]
- Fisher, C.D.; Lickteig, A.J.; Augustine, L.M.; Ranger-Moore, J.; Jackson, J.P.; Ferguson, S.S.; Cherrington, N.J. Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab. Dispos. 2009, 37, 2087–2094. [Google Scholar] [CrossRef]
- Ogu, C.C.; Maxa, J.L. Drug interactions due to cytochrome P450. Baylor Univ. Med. Cent. Proc. 2000, 13, 421–423. [Google Scholar] [CrossRef]
- Albertolle, M.E.; Phan, T.T.N.; Pozzi, A.; Guengerich, F.P. Sulfenylation of human liver and kidney microsomal cytochromes P450 and other drug-metabolizing enzymes as a response to redox alteration. Mol. Cell. Proteom. 2018, 17, 889–900. [Google Scholar] [CrossRef]
- Hanioka, N.; Jinno, H.; Nishimura, T.; Ando, M. Changes in cytochrome P450 enzymes by 1, 1-dichloroethylene in rat liver and kidney. Arch. Toxicol. 1997, 72, 9–16. [Google Scholar] [CrossRef]
- Albertolle, M.E.; Kim, D.; Nagy, L.D.; Yun, C.-H.; Pozzi, A.; Savas, Ü.; Johnson, E.F.; Guengerich, F.P. Heme–thiolate sulfenylation of human cytochrome P450 4A11 functions as a redox switch for catalytic inhibition. J. Biol. Chem. 2017, 292, 11230–11242. [Google Scholar] [CrossRef]
- Tomankova, V.; Anzenbacher, P.; Anzenbacherova, E. Effects of obesity on liver cytochromes P450 in various animal models. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc 2017, 161, 144–151. [Google Scholar] [CrossRef]
- Elfaki, I.; Mir, R.; Almutairi, F.M.; Duhier, F.M.A. Cytochrome P450: Polymorphisms and roles in cancer, diabetes and atherosclerosis. Asian Pac. J. Cancer Prev. 2018, 19, 2057. [Google Scholar] [PubMed]
- Zhang, J.; Giampieri, F.; Afrin, S.; Battino, M.; Zheng, X.; Reboredo-Rodriguez, P. Structure-stability relationship of anthocyanins under cell culture condition. Int. J. Food Sci. Nutr. 2019, 70, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.L.; Chen, Z.J.; Bai, X.S.; Ding, C.; Long, T.J.; Wei, F.G.; Miao, K.R. Structure–activity relationships of anthocyanidin glycosylation. Mol. Divers. 2014, 18, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Cyboran-Mikołajczyk, S.; Jurkiewicz, P.; Hof, M.; Kleszczyńska, H. The impact of O-glycosylation on cyanidin interaction with POPC membranes: Structure-activity relationship. Molecules 2018, 23, 2771. [Google Scholar] [CrossRef]
- Cyboran-Mikołajczyk, S.; Solarska-Ściuk, K.; Mieszała, K.; Glatzel-Plucińska, N.; Matczak, K.; Kleszczyńska, H. The Impact of O-Glycosylation on Cyanidin Interaction with RBCs and HMEC-1 Cells—Structure–Activity Relationships. Int. J. Mol. Sci. 2019, 20, 1928. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. Int. Sch. Res. Not. 2014, 2014, 952943. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Kumar, C.S. Syringic acid (SA)—A review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef]
- Gheena, S.; Ezhilarasan, D. Syringic acid triggers reactive oxygen species–mediated cytotoxicity in HepG2 cells. Hum. Exp. Toxicol. 2019, 38, 694–702. [Google Scholar] [CrossRef]
- Market Data Forecast. Available online: https://www.marketdataforecast.com/market-reports/anthocyanins-market (accessed on 24 December 2021).
- Melini, V.; Panfili, G.; Fratianni, A.; Acquistucci, R. Bioactive compounds in rice on Italian market: Pigmented varieties as a source of carotenoids, total phenolic compounds and anthocyanins, before and after cooking. Food Chem. 2019, 277, 119–127. [Google Scholar] [CrossRef]
- Clark, K.S. 7 Best Anthocyanin Supplements—Welder Genius. Available online: https://bizbrainers.com/ (accessed on 24 December 2021).
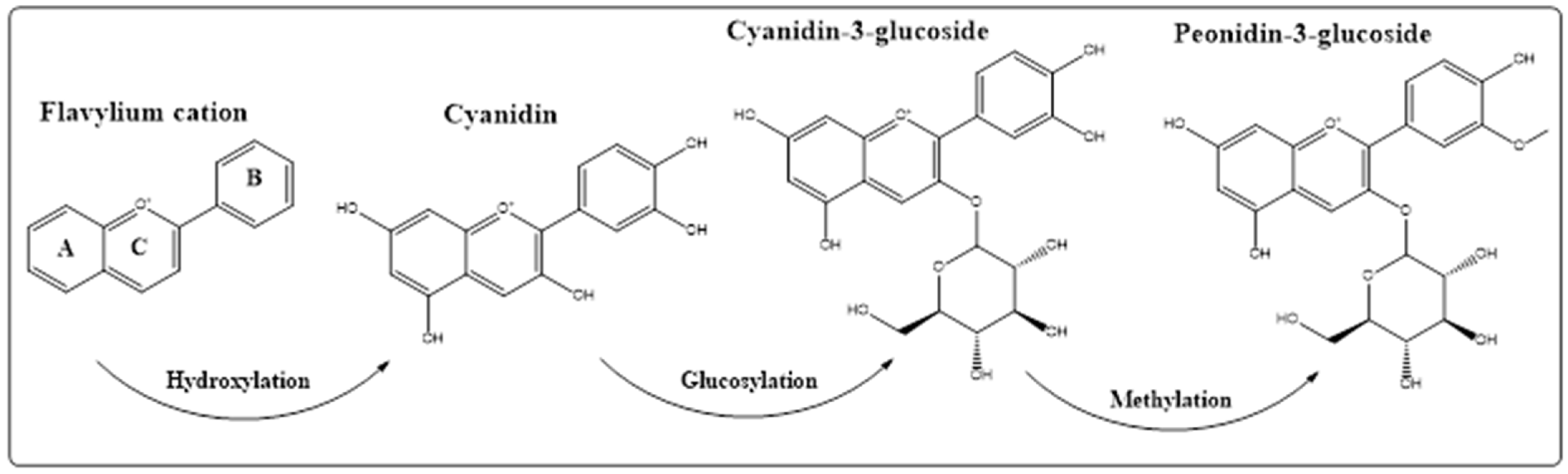
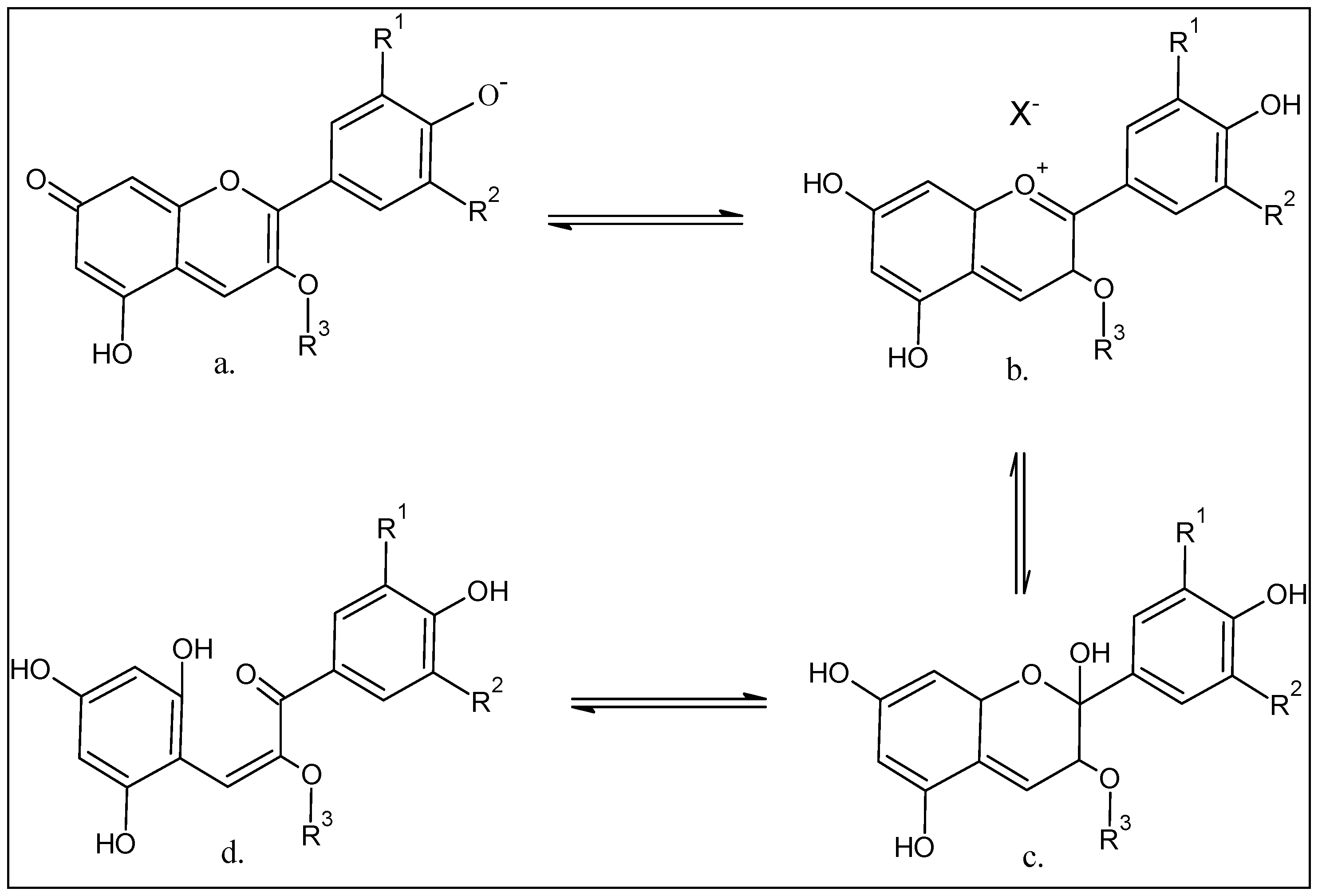
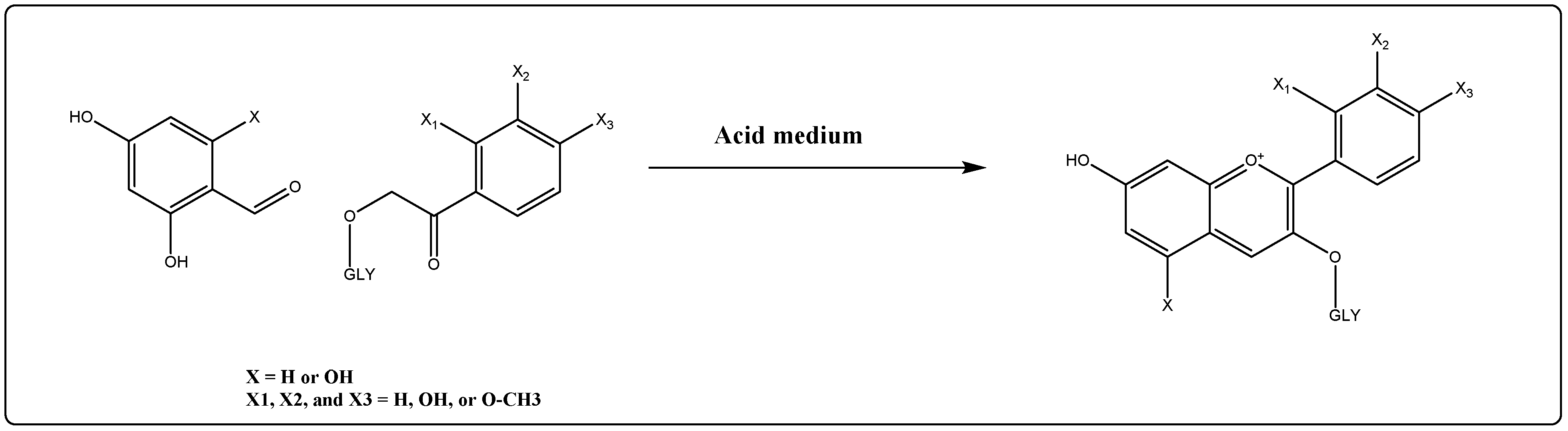
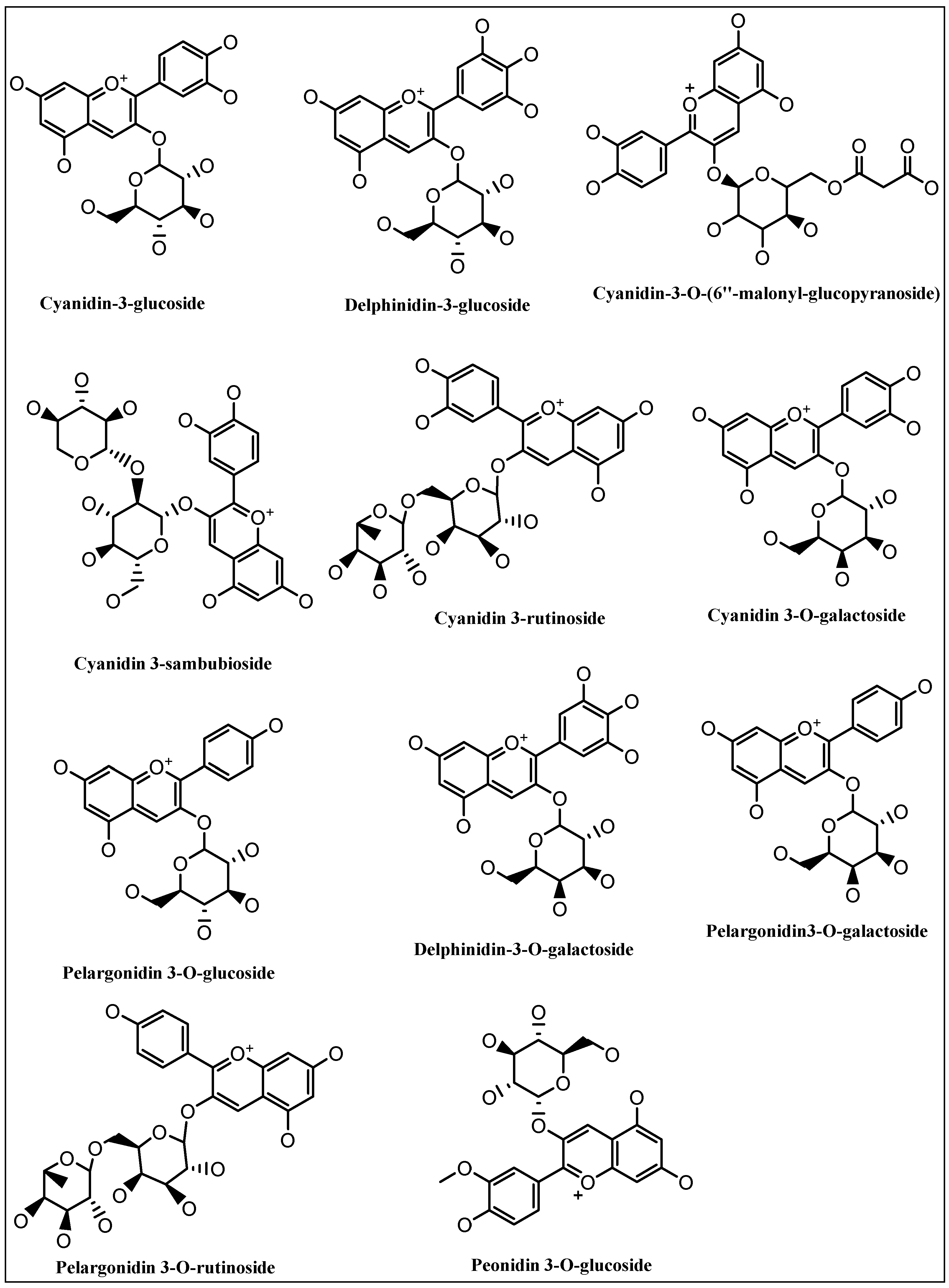
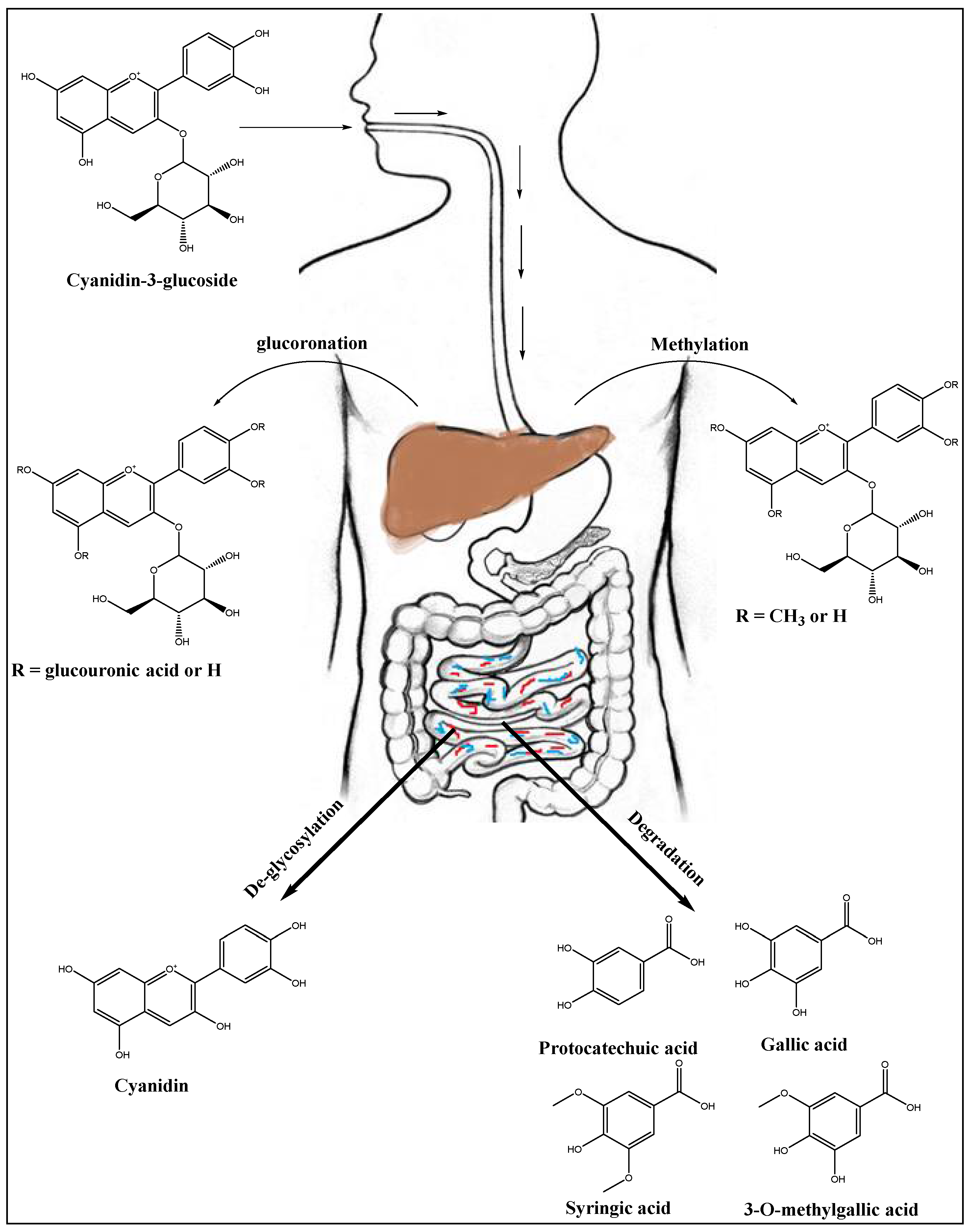

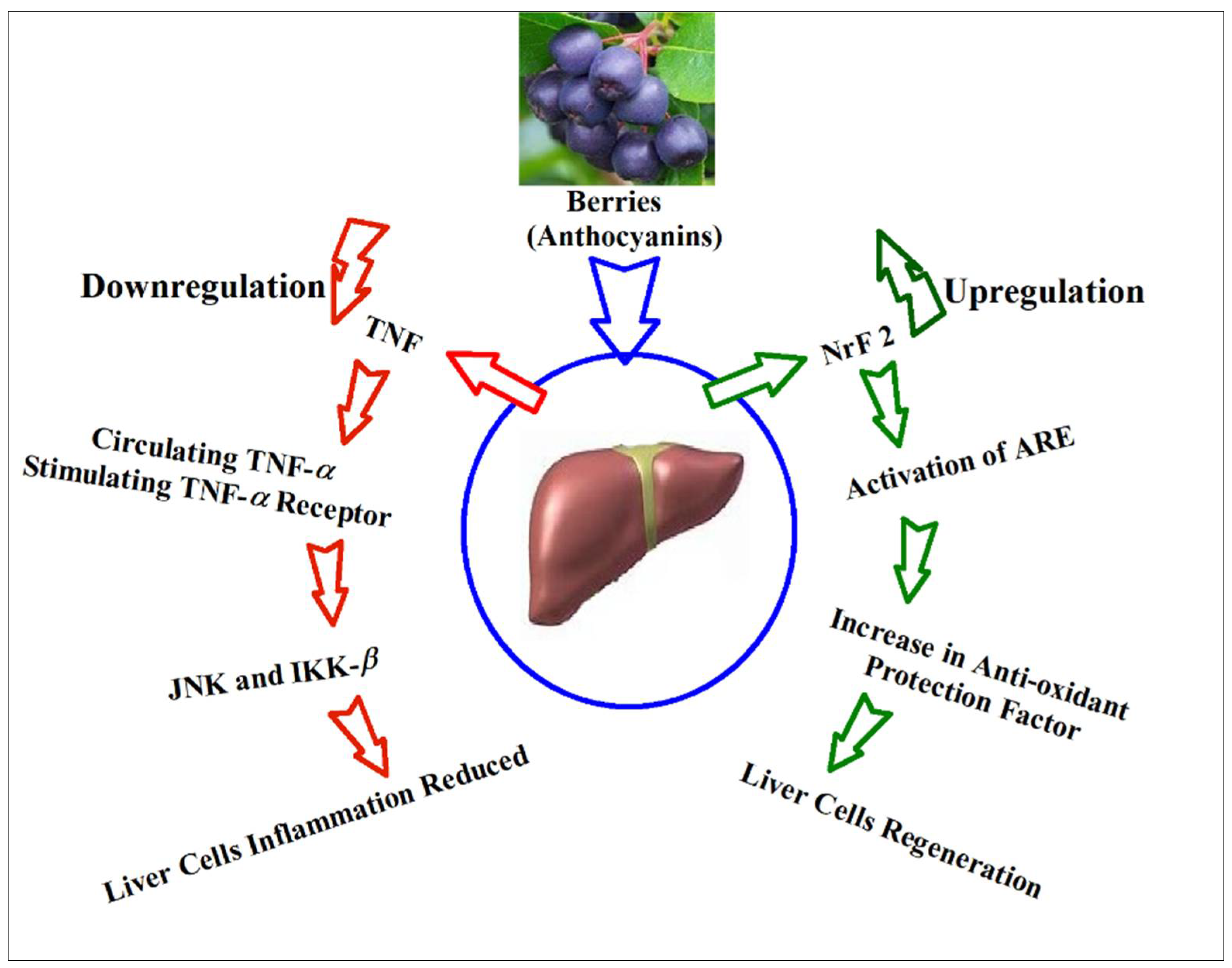
| Plant’s Name | Folklore Medicinal Uses, Other than Liver Disorders | Plant Parts Used | Major Identified Anthocyanins | Refer |
|---|---|---|---|---|
| Hibiscus sabdariffa | Hypertension, pyrexia | Calyx, Epicalyx | Cyanidin-3-O-β-glucoside, and delphinidin-3-glucoside | [175,176] |
| Cichorium intybus | Inflammation | Leaves | Cyanidin-3-O-(6″-malonyl-β-glucopyranoside) | [177] |
| Garcinia indica | Male digestion, flatulence, and constipation. | Fruits | Cyanidin-3-O-β-glucoside, and cyanidin-3-O-sambubioside | [178] |
| Raphanus sativus | Roots | Pelargonidin derivatives | [179] | |
| Morus alba (Mulberry), & other species | Cardiovascular diseases, nephritis, thirsty, constipation | Fruits | Cyanidin-3-O-rutinoside, cyanidin-3-O-glucoside | [180,181] |
| Cornus mas (cornelian cherry) | Diabetes, diarrhea, fevers, rheumatic complains, skin diseases and urinary tract infections | Fruits | Cyanidin-3-O-galactoside, pelargonidin-3-O-galactoside, delphinidin-3-O-galactoside, cyanidin-3-O-rutinoside, pelargonidin-3-O-glucoside, pelargonidin-3-O-rutinoside, pegonidin-3-O-glucoside | [182] |
| Lannea microcarpa | Scurvy, rickets and cough. | Fruits | Cyanidin-3-O-(2-O-β-D-xylopyranosyl)-β-D-galactopyranoside, and cyanidin-3-O-β-D-galactopyranoside. | [183] |
| Anthocyanins | Experimental Protocol | Mode of Action | Refer |
|---|---|---|---|
| Cyanidin-3-O-β-glucoside | In vivo CCl4-induced liver damage in mice and in vitro H2O2-induced oxidative stress in HepG2 cells apoptosis | Enhance the antioxidant enzymes activities and upregulating Nrf2-antioxidant pathway. | [156] |
| Delphinidin | In vitro H2O2-induced oxidative stress in HepG2 Cells | Enhance the expression of Nrf2 and promoted Nrf2 nuclear translocation. Increase expression of antioxidant protein HO-1 (Nrf2-related phase II enzyme heme oxygenase-1). Alleviate the reduction of Nrf2 protein levels and the accumulation of intracellular ROS levels in Nrf2 knockdown HepG2 cells. | [288] |
| Mixture of cyanidin-3-O-β-glucoside, delphinidin-3-O-rutinoside, and malvidin-3-O-galactoside | In vivo CCl4-induced human embryonic-liver (L-02) cells toxicity | Reduce the percentage of hypo-diploid cells and decrease in caspase-3 protein expression | [289] |
| Cyanidin-3-O-β-glucoside, and peonidin-3-O-glucoside | In vitro human embryo non-malignant liver tissue cell line (L-02). Hepatoprotection | Exhibited higher cell viability, decreased aminotransferase activity and enhanced cellular antioxidant status. Furthermore, Cy-3-G showed much stronger hepatoprotective activity than Pn-3-G at the same concentration. | [186] |
| No. | Supplier/Company | Product’s Name | Plants/Declared Anthocyanins | Company’s Address |
|---|---|---|---|---|
| 1. | Solgar | Bilberry Berry Extract | Vaccinium myrtillus | https://www.solgar.com/ |
| 2. | Sports Research | Blueberry Concentrate | Vaccinium cyanococcus | https://sportsresearch.com/ |
| 3. | Life Extension | Tart Cherry Extract | Prunus cerasus | https://www.lifeextension.com/ |
| 4. | Navitas Organics | Organic-Maqui Powder, Tart Berry | Anthocyanins contents | https://navitasorganics.com/ |
| 5. | Nature’s Plus | Black Cherry | 0.8 % Anthocyanins | https://naturesplus.com/ |
| 6. | NOW Foods | Super Antioxidants | Bilberry Extract | https://www.nowfoods.com/ |
| 7. | Natural Factors | BlueRich Super Strength | Blueberry concentrate | https://naturalfactors.com/en-ca/ |
| 8. | Nature’s Truth | Bilberry | Bilberry concentrate | https://naturestruth.com/ |
| 9. | Vision Alive | Bilberries, blueberries, from black currant, Maqui berry, saffron, and astaxanthin | https://visionalive.net/products.html | |
| 10. | New Nordic | Blueberry Eyebright | Conc. blue berry extract | https://newnordic.com/ |
| 11. | Vitabiotics | Bilberry, Lutein | 500 mg Bilberry | http://www.vitabiotics.com/ |
| 12. | Sambucol | Syrup Black-Elderberry | Elderberry extract 1.9/10 mL | https://sambucol.co.uk/ |
| 13. | Fairvital | Aronia Capsules | 20% anthocyanins | https://www.fairvital.com/en/index |
| 14. | TruNature | Blueberry | Blueberry extract 1000 mg | http://trunature.com/ |
| 15. | TruNature | One per day Cranberry | Cranberry 650 mg | http://trunature.com/ |
| 16. | Life Extension | Optimized Cran-Max | Cranberry whole fruit conc. | https://www.lifeextension.com/ |
| 17. | Life Extension | Garcinia HCA | G. cambogia fruits extract | https://www.lifeextension.com/ |
| 18. | Georgia’s Natural | Organic Carrot Juice | Purple carrot juice 99% | https://georgiasnatural.com/ge/intro |
| 19. | Santarome | Bio Black Radish | Black radish juice | https://en.santarome.fr/ |
| 20. | PipingRock | Hibiscus Flower | H sabdariffa 11 mg in extract | https://sa.pipingrock.com/brand/piping-rock |
| 21. | Swanson | Hibiscus Flower | Hibiscus sabdariffa | https://swansoneurope.com/ |
| 22. | Bluebonnet | Skinny Garcinia | Garcinia fruit rind extract | https://bluebonnetnutrition.com/ |
| 23. | Ceregumil | Vid-Master Plant | Vaccinium corymbosum | https://sa.carethy.net/ceregumil |
| 24. | Source Naturals | Pomegranate Extract | Punica granatum extract | https://www.sourcenaturals.com/ |
| 25. | AllMax | Nutrition Liver D-Tox | Artichoke extract | https://allmaxnutrition.com/ |
| 26. | PureClinica | Anti-oxidant Bilberry | Bilberry extract | https://pureclinica.com/ |
| 27. | SYM Nutrition | Bilberry | 25% Anthocyanins | https://symnutrition.com/ |
| 28. | Vision Smart Center | Blackcurrant Anthocyanins | 210 mg Anthocyanins, cyanidin, and delphinidin based | https://visionsmartcenter.com// |
| 29. | Designs for health | Grape Seeds and Grape Skin | Anthocyanins and grapes’ polyphenol anti-oxidant blend | https://designsforhealth.com/ |
| 30. | Berry Supplements | Anti-oxidant Nutritional Supplement | Aronia berry powder | https://bulksupplements.com/ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, H.A.; Khan, R.A. Anthocyanins: Traditional Uses, Structural and Functional Variations, Approaches to Increase Yields and Products’ Quality, Hepatoprotection, Liver Longevity, and Commercial Products. Int. J. Mol. Sci. 2022, 23, 2149. https://doi.org/10.3390/ijms23042149
Mohammed HA, Khan RA. Anthocyanins: Traditional Uses, Structural and Functional Variations, Approaches to Increase Yields and Products’ Quality, Hepatoprotection, Liver Longevity, and Commercial Products. International Journal of Molecular Sciences. 2022; 23(4):2149. https://doi.org/10.3390/ijms23042149
Chicago/Turabian StyleMohammed, Hamdoon A., and Riaz A. Khan. 2022. "Anthocyanins: Traditional Uses, Structural and Functional Variations, Approaches to Increase Yields and Products’ Quality, Hepatoprotection, Liver Longevity, and Commercial Products" International Journal of Molecular Sciences 23, no. 4: 2149. https://doi.org/10.3390/ijms23042149
APA StyleMohammed, H. A., & Khan, R. A. (2022). Anthocyanins: Traditional Uses, Structural and Functional Variations, Approaches to Increase Yields and Products’ Quality, Hepatoprotection, Liver Longevity, and Commercial Products. International Journal of Molecular Sciences, 23(4), 2149. https://doi.org/10.3390/ijms23042149







