Immunohistochemical Expression Pattern of FGFR1, FGFR2, RIP5, and HIP2 in Developing and Postnatal Kidneys of Dab1−/− (yotari) Mice
Abstract
1. Introduction
2. Results
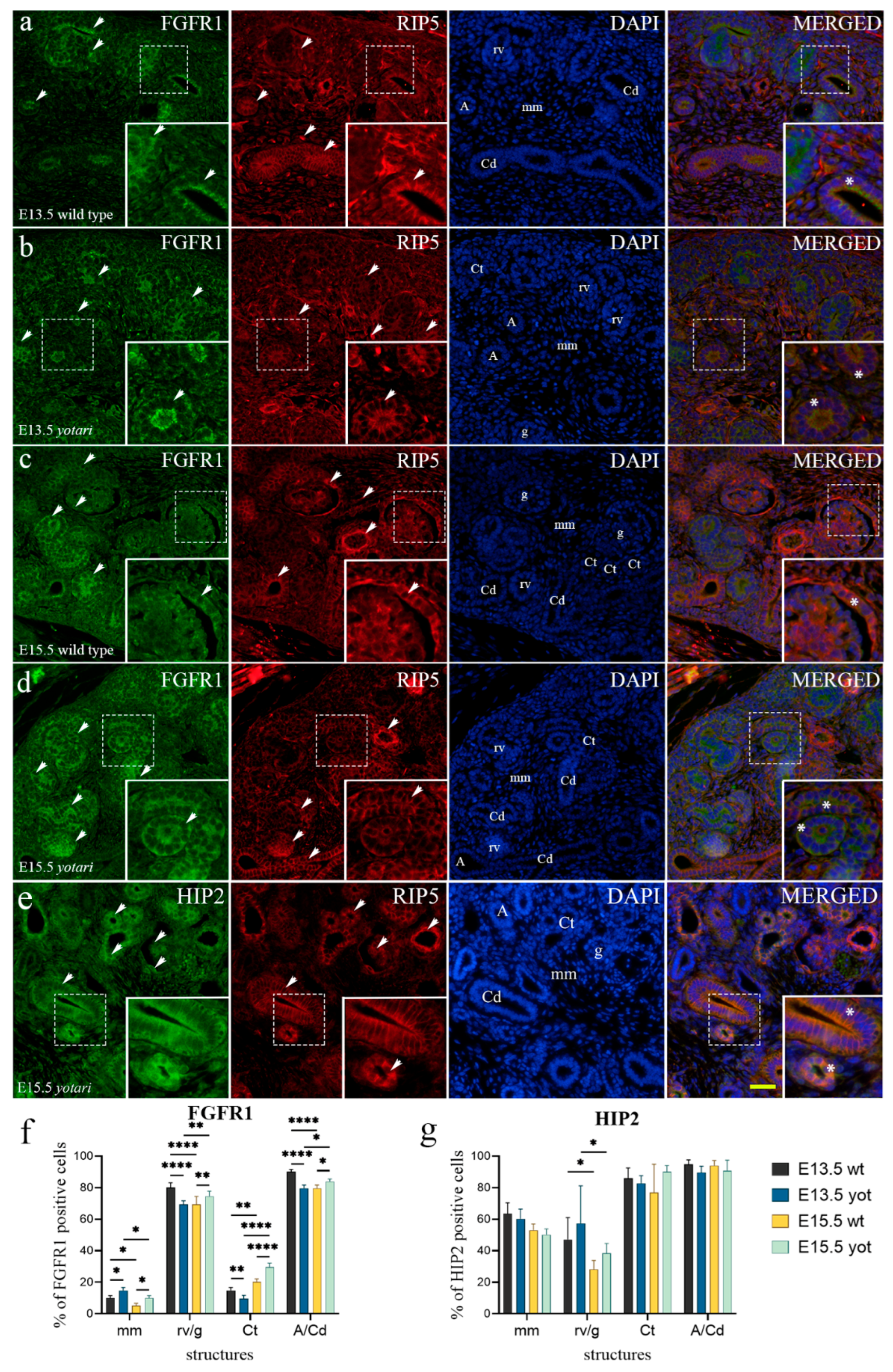
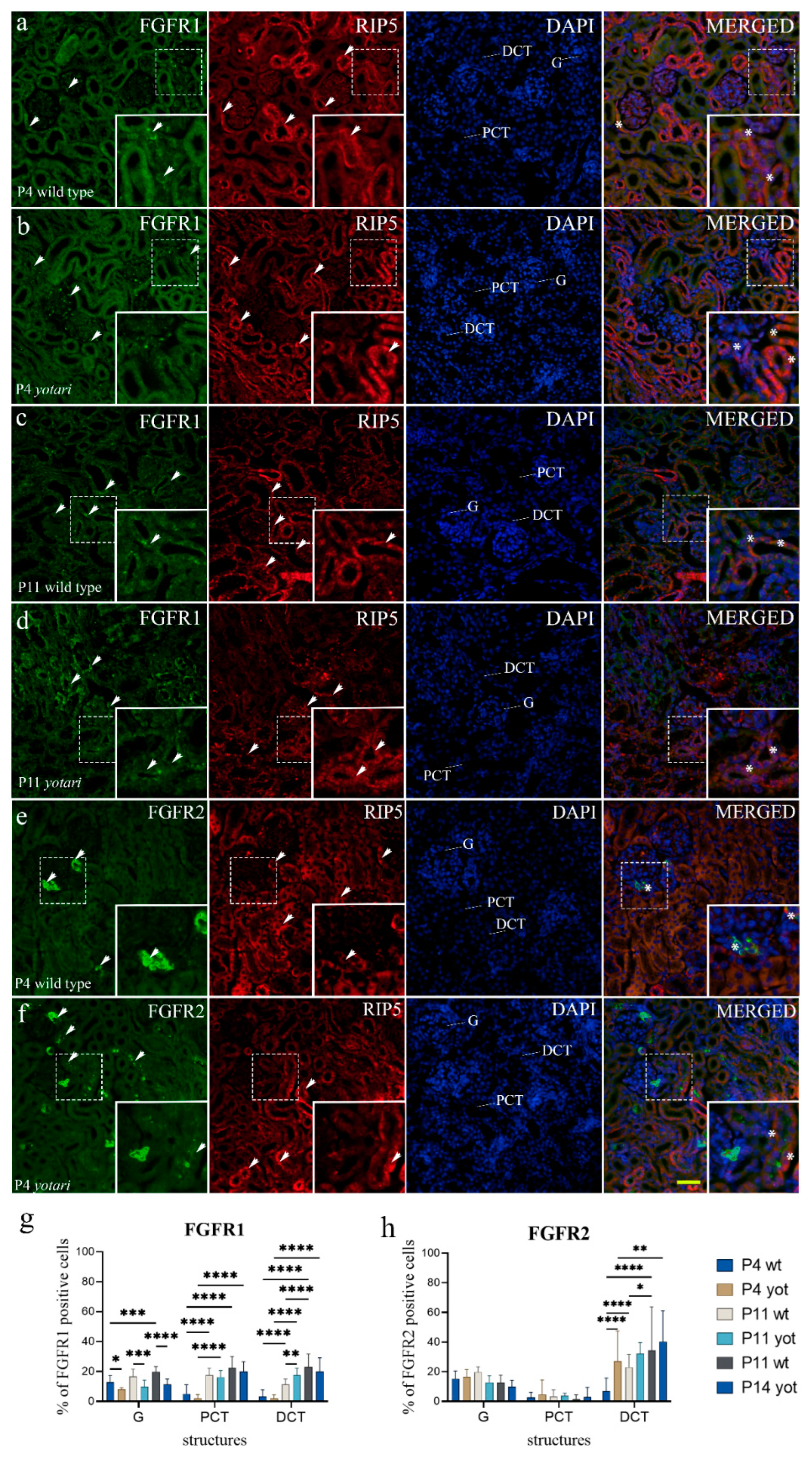
2.1. FGFR1 Expression
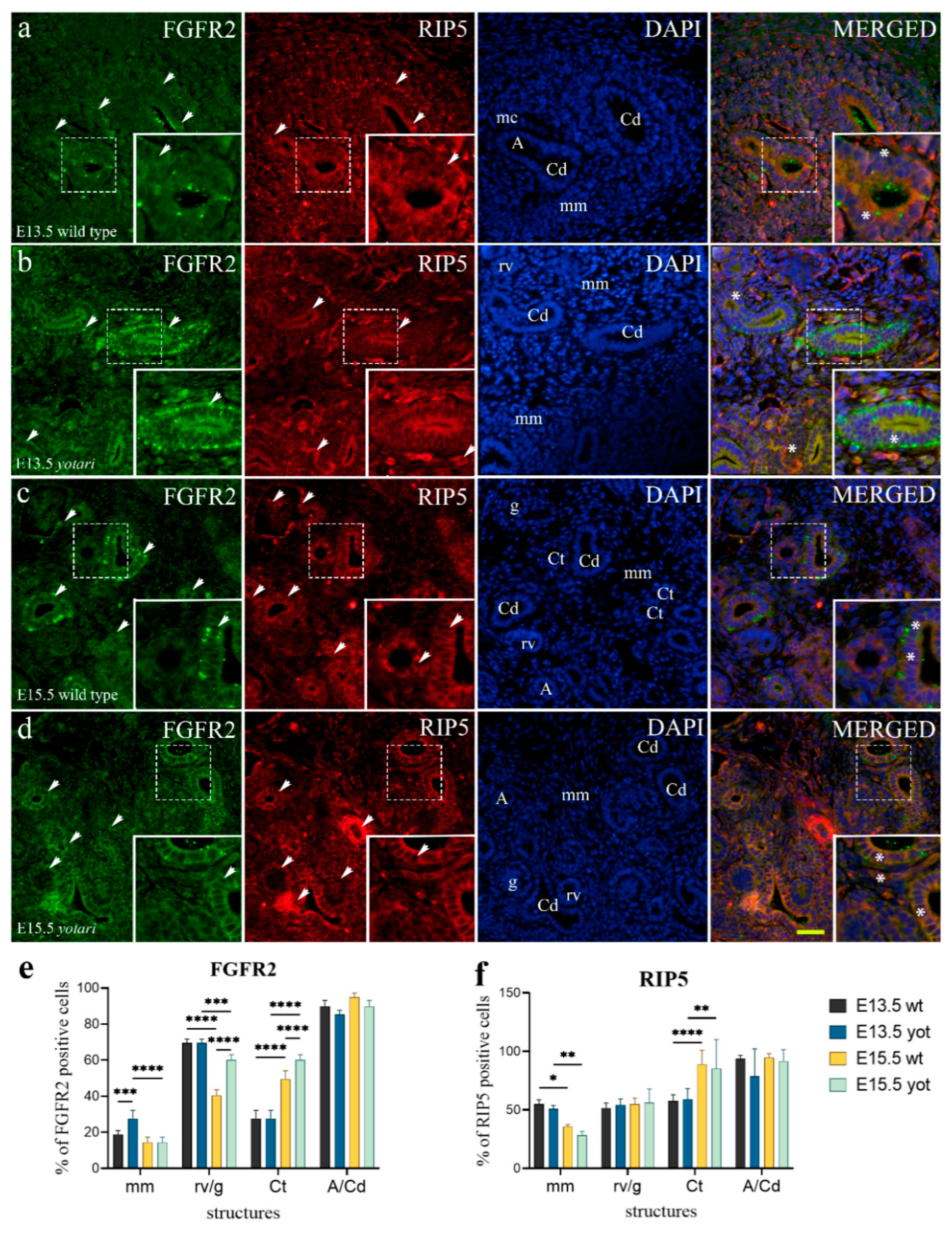
2.2. FGFR2 Expression
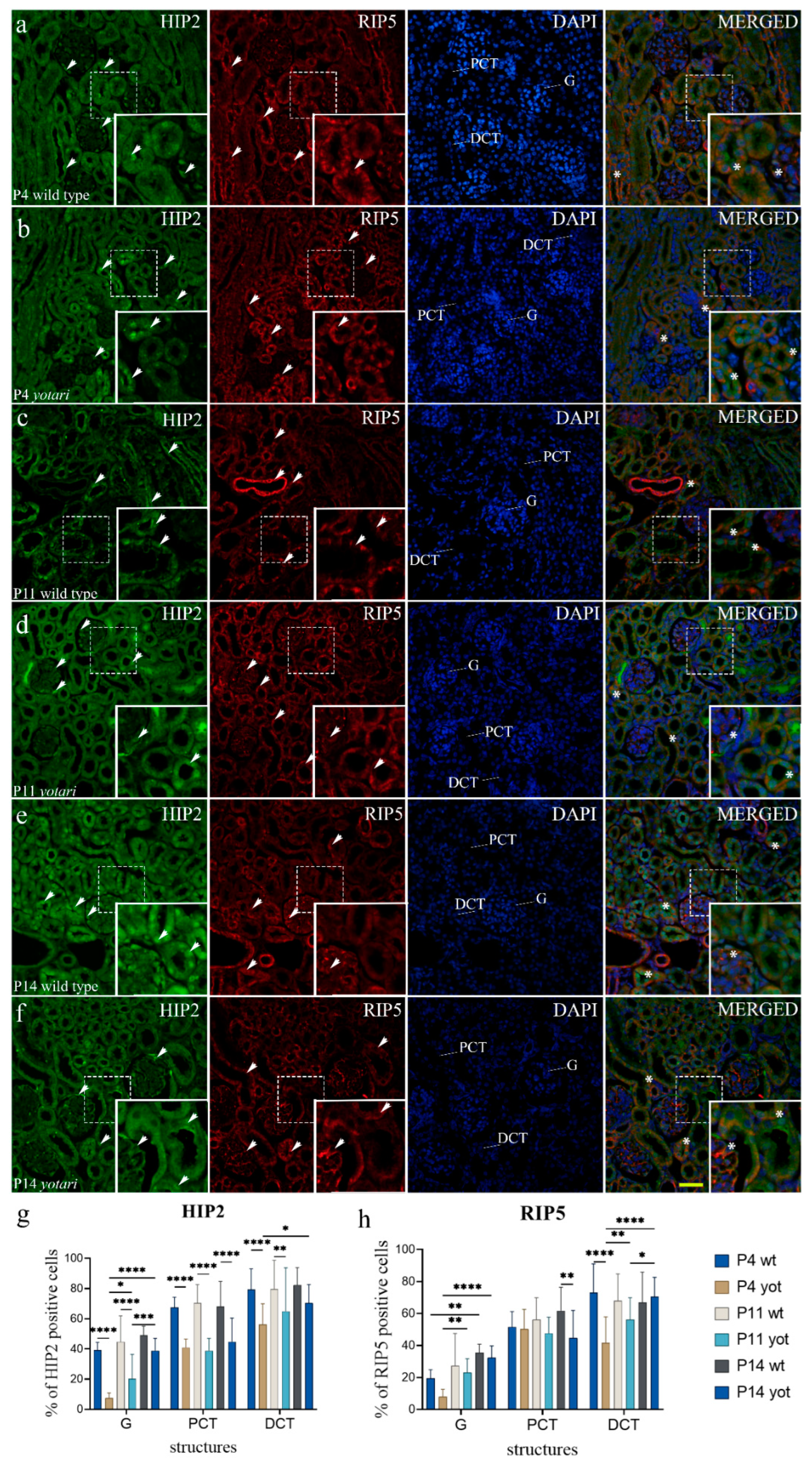
2.3. RIP5 Expression
2.4. HIP2 Expression
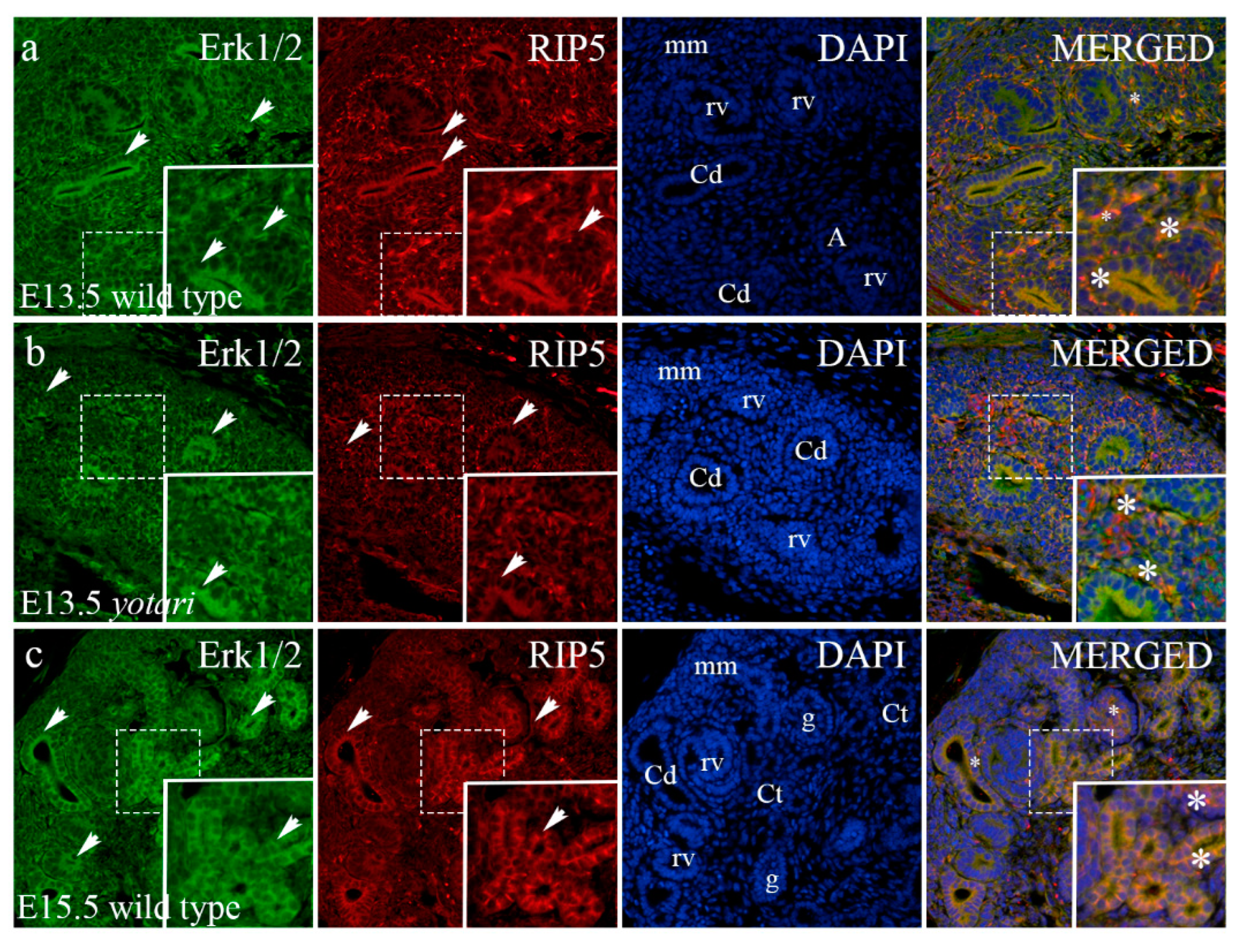
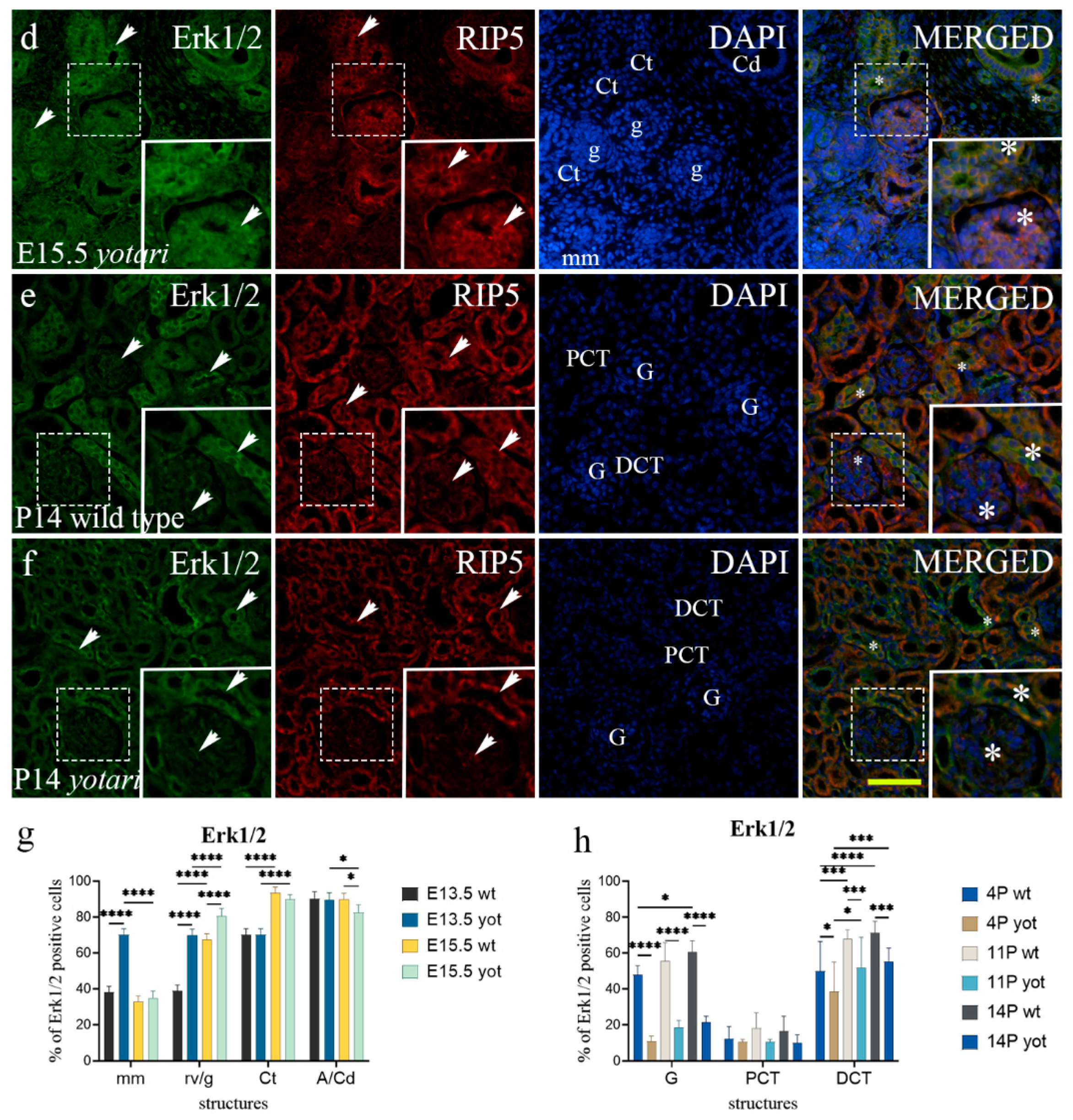
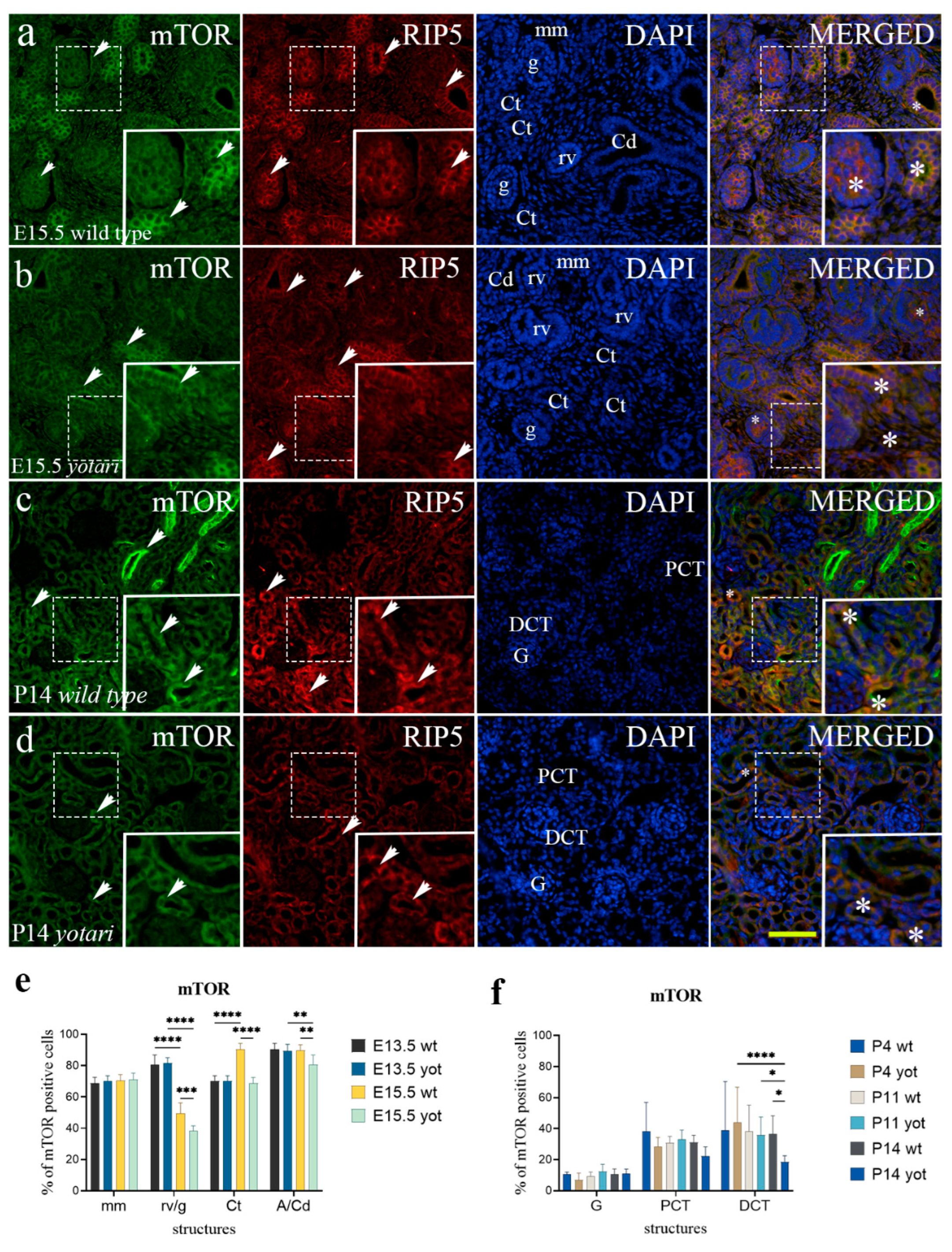
2.5. Erk1/2 and mTOR Expression
3. Discussion
4. Materials and Methods
4.1. Ethics
4.2. Generation of Dab1 Null Conventional Mutants and Sample Collection
4.3. Immunofluorescence
4.4. Data Acquisition and Analysis
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoneshima, H.; Nagata, E.; Matsumoto, M.; Yamada, M.; Nakajima, K.; Miyata, T.; Ogawa, M.; Mikoshiba, K. A novel neurological mutant mouse, yotari, which exhibits reeler-like phenotype but expresses CR-50 antigen/reelin. Neurosci. Res. 1997, 29, 217–223. [Google Scholar] [CrossRef]
- Racetin, A.; Filipovic, N.; Lozic, M.; Ogata, M.; Gudelj Ensor, L.; Kelam, N.; Kovacevic, P.; Watanabe, K.; Katsuyama, Y.; Saraga-Babic, M.; et al. A Homozygous Dab1(-/-) Is a Potential Novel Cause of Autosomal Recessive Congenital Anomalies of the Mice Kidney and Urinary Tract. Biomolecules 2021, 11, 609. [Google Scholar] [CrossRef] [PubMed]
- Lozic, M.; Filipovic, N.; Juric, M.; Kosovic, I.; Benzon, B.; Solic, I.; Kelam, N.; Racetin, A.; Watanabe, K.; Katsuyama, Y.; et al. Alteration of Cx37, Cx40, Cx43, Cx45, Panx1, and Renin Expression Patterns in Postnatal Kidneys of Dab1-/- (yotari) Mice. Int. J. Mol. Sci. 2021, 22, 1284. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Chen, X.; Zhu, K.; Zeng, P.; Ding, G. Dab1 Contributes to Angiotensin II-Induced Apoptosis via p38 Signaling Pathway in Podocytes. BioMed Res. Int. 2017, 2017, 2484303. [Google Scholar] [CrossRef]
- Racetin, A.; Juric, M.; Filipovic, N.; Solic, I.; Kosovic, I.; Glavina Durdov, M.; Kunac, N.; Zekic Tomas, S.; Saraga, M.; Soljic, V.; et al. Expression and localization of DAB1 and Reelin during normal human kidney development. Croat. Med. J. 2019, 60, 521–531. [Google Scholar] [CrossRef]
- Sheldon, M.; Rice, D.S.; D’Arcangelo, G.; Yoneshima, H.; Nakajima, K.; Mikoshiba, K.; Howell, B.W.; Cooper, J.A.; Goldowitz, D.; Curran, T. Scrambler and yotari disrupt the disabled gene and produce a reeler-like phenotype in mice. Nature 1997, 389, 730–733. [Google Scholar] [CrossRef]
- Howell, B.W.; Hawkes, R.; Soriano, P.; Cooper, J.A. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature 1997, 389, 733–737. [Google Scholar] [CrossRef]
- Loane, M.; Dolk, H.; Kelly, A.; Teljeur, C.; Greenlees, R.; Densem, J. Paper 4: EUROCAT statistical monitoring: Identification and investigation of ten year trends of congenital anomalies in Europe. Birth Defects Res. A Clin. Mol. Teratol. 2011, 91 (Suppl. S1), S31–S43. [Google Scholar] [CrossRef]
- Birth Defects Monitoring Program (BDMP)/Commission on Professional and Hospital Activities (CPHA) surveillance data, 1988–1991. Teratology 1993, 48, 658–675. [CrossRef]
- van der Ven, A.T.; Connaughton, D.M.; Ityel, H.; Mann, N.; Nakayama, M.; Chen, J.; Vivante, A.; Hwang, D.Y.; Schulz, J.; Braun, D.A.; et al. Whole-Exome Sequencing Identifies Causative Mutations in Families with Congenital Anomalies of the Kidney and Urinary Tract. J. Am. Soc. Nephrol. JASN 2018, 29, 2348–2361. [Google Scholar] [CrossRef]
- Sanna-Cherchi, S.; Sampogna, R.V.; Papeta, N.; Burgess, K.E.; Nees, S.N.; Perry, B.J.; Choi, M.; Bodria, M.; Liu, Y.; Weng, P.L.; et al. Mutations in DSTYK and dominant urinary tract malformations. N. Engl. J. Med. 2013, 369, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Becic, T.; Kero, D.; Vukojevic, K.; Mardesic, S.; Saraga-Babic, M. Growth factors FGF8 and FGF2 and their receptor FGFR1, transcriptional factors Msx-1 and MSX-2, and apoptotic factors p19 and RIP5 participate in the early human limb development. Acta Histochem. 2018, 120, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Racetin, A.; Raguz, F.; Durdov, M.G.; Kunac, N.; Saraga, M.; Sanna-Cherchi, S.; Soljic, V.; Martinovic, V.; Petricevic, J.; Kostic, S.; et al. Immunohistochemical expression pattern of RIP5, FGFR1, FGFR2 and HIP2 in the normal human kidney development. Acta Histochem. 2019, 121, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Touat, M.; Ileana, E.; Postel-Vinay, S.; Andre, F.; Soria, J.C. Targeting FGFR Signaling in Cancer. Clin. Cancer Res. 2015, 21, 2684–2694. [Google Scholar] [CrossRef] [PubMed]
- Bates, C.M. Role of fibroblast growth factor receptor signaling in kidney development. Pediatr. Nephrol. 2011, 26, 1373–1379. [Google Scholar] [CrossRef][Green Version]
- Ornitz, D.M.; Itoh, N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef]
- Turner, N.; Grose, R. Fibroblast growth factor signalling: From development to cancer. Nat. Rev. Cancer 2010, 10, 116–129. [Google Scholar] [CrossRef]
- Xie, Y.; Su, N.; Yang, J.; Tan, Q.; Huang, S.; Jin, M.; Ni, Z.; Zhang, B.; Zhang, D.; Luo, F.; et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target. Ther. 2020, 5, 181. [Google Scholar] [CrossRef]
- Brewer, J.R.; Mazot, P.; Soriano, P. Genetic insights into the mechanisms of Fgf signaling. Genes Dev. 2016, 30, 751–771. [Google Scholar] [CrossRef]
- Walker, K.A.; Sims-Lucas, S.; Bates, C.M. Fibroblast growth factor receptor signaling in kidney and lower urinary tract development. Pediatr. Nephrol. 2016, 31, 885–895. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Srivastava, S.P.; Hu, Q.; Gao, R.; Li, S.; Kitada, M.; Wu, G.; Koya, D.; Kanasaki, K. Endothelial FGFR1 (Fibroblast Growth Factor Receptor 1) Deficiency Contributes Differential Fibrogenic Effects in Kidney and Heart of Diabetic Mice. Hypertension 2020, 76, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.P.; Goodwin, J.E.; Tripathi, P.; Kanasaki, K.; Koya, D. Interactions among Long Non-Coding RNAs and microRNAs Influence Disease Phenotype in Diabetes and Diabetic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 6027. [Google Scholar] [CrossRef] [PubMed]
- Kanasaki, K. N-acetyl-seryl-aspartyl-lysyl-proline is a valuable endogenous antifibrotic peptide for kidney fibrosis in diabetes: An update and translational aspects. J. Diabetes Investig. 2020, 11, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Kanasaki, M.; Srivastava, S.P.; Nakamura, Y.; Ishigaki, Y.; Kitada, M.; Shi, S.; Kanasaki, K.; Koya, D. N-acetyl-seryl-aspartyl-lysyl-proline inhibits diabetes-associated kidney fibrosis and endothelial-mesenchymal transition. BioMed Res. Int. 2014, 2014, 696475. [Google Scholar] [CrossRef]
- Li, J.; Shi, S.; Srivastava, S.P.; Kitada, M.; Nagai, T.; Nitta, K.; Kohno, M.; Kanasaki, K.; Koya, D. FGFR1 is critical for the anti-endothelial mesenchymal transition effect of N-acetyl-seryl-aspartyl-lysyl-proline via induction of the MAP4K4 pathway. Cell Death Dis. 2017, 8, e2965. [Google Scholar] [CrossRef]
- Zhao, H.; Kegg, H.; Grady, S.; Truong, H.T.; Robinson, M.L.; Baum, M.; Bates, C.M. Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev. Biol. 2004, 276, 403–415. [Google Scholar] [CrossRef]
- Sims-Lucas, S.; Argyropoulos, C.; Kish, K.; McHugh, K.; Bertram, J.F.; Quigley, R.; Bates, C.M. Three-dimensional imaging reveals ureteric and mesenchymal defects in Fgfr2-mutant kidneys. J. Am. Soc. Nephrol. JASN 2009, 20, 2525–2533. [Google Scholar] [CrossRef]
- Sims-Lucas, S.; Cusack, B.; Baust, J.; Eswarakumar, V.P.; Masatoshi, H.; Takeuchi, A.; Bates, C.M. Fgfr1 and the IIIc isoform of Fgfr2 play critical roles in the metanephric mesenchyme mediating early inductive events in kidney development. Dev. Dyn. 2011, 240, 240–249. [Google Scholar] [CrossRef]
- Hains, D.; Sims-Lucas, S.; Kish, K.; Saha, M.; McHugh, K.; Bates, C.M. Role of fibroblast growth factor receptor 2 in kidney mesenchyme. Pediatr. Res. 2008, 64, 592–598. [Google Scholar] [CrossRef]
- Hains, D.S.; Sims-Lucas, S.; Carpenter, A.; Saha, M.; Murawski, I.; Kish, K.; Gupta, I.; McHugh, K.; Bates, C.M. High incidence of vesicoureteral reflux in mice with Fgfr2 deletion in kidney mesenchyma. J. Urol. 2010, 183, 2077–2084. [Google Scholar] [CrossRef][Green Version]
- Arman, E.; Haffner-Krausz, R.; Chen, Y.; Heath, J.K.; Lonai, P. Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proc. Natl. Acad. Sci. USA 1998, 95, 5082–5087. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Weinstein, M.; Li, C.; Naski, M.; Cohen, R.I.; Ornitz, D.M.; Leder, P.; Deng, C. Fibroblast growth factor receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8 and FGF10 is essential for limb induction. Development 1998, 125, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.P.; Harpal, K.; Henkemeyer, M.; Rossant, J. fgfr-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Genes Dev. 1994, 8, 3032–3044. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.X.; Wynshaw-Boris, A.; Shen, M.M.; Daugherty, C.; Ornitz, D.M.; Leder, P. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev. 1994, 8, 3045–3057. [Google Scholar] [CrossRef]
- Poladia, D.P.; Kish, K.; Kutay, B.; Bauer, J.; Baum, M.; Bates, C.M. Link between reduced nephron number and hypertension: Studies in a mutant mouse model. Pediatr. Res. 2006, 59, 489–493. [Google Scholar] [CrossRef]
- Celli, G.; LaRochelle, W.J.; Mackem, S.; Sharp, R.; Merlino, G. Soluble dominant-negative receptor uncovers essential roles for fibroblast growth factors in multi-organ induction and patterning. EMBO J. 1998, 17, 1642–1655. [Google Scholar] [CrossRef]
- Walker, K.A.; Ikeda, Y.; Zabbarova, I.; Schaefer, C.M.; Bushnell, D.; De Groat, W.C.; Kanai, A.; Bates, C.M. Fgfr2 is integral for bladder mesenchyme patterning and function. Am. J. Physiol. Ren. Physiol. 2015, 308, F888–F898. [Google Scholar] [CrossRef]
- Poladia, D.P.; Kish, K.; Kutay, B.; Hains, D.; Kegg, H.; Zhao, H.; Bates, C.M. Role of fibroblast growth factor receptors 1 and 2 in the metanephric mesenchyme. Dev. Biol. 2006, 291, 325–339. [Google Scholar] [CrossRef]
- Cohen, M.M., Jr.; Kreiborg, S. Visceral anomalies in the Apert syndrome. Am. J. Med. Genet. 1993, 45, 758–760. [Google Scholar] [CrossRef]
- Sergi, C.; Stein, H.; Heep, J.G.; Otto, H.F. A 19-week-old fetus with craniosynostosis, renal agenesis and gastroschisis: Case report and differential diagnosis. Pathol. Res. Pract. 1997, 193, 579–585. [Google Scholar] [CrossRef]
- Passos-Bueno, M.R.; Wilcox, W.R.; Jabs, E.W.; Sertie, A.L.; Alonso, L.G.; Kitoh, H. Clinical spectrum of fibroblast growth factor receptor mutations. Hum. Mutat. 1999, 14, 115–125. [Google Scholar] [CrossRef]
- Seyedzadeh, A.; Kompani, F.; Esmailie, E.; Samadzadeh, S.; Farshchi, B. High-grade vesicoureteral reflux in Pfeiffer syndrome. Urol. J. 2008, 5, 200–202. [Google Scholar] [PubMed]
- Pastar, V.; Lozic, M.; Kelam, N.; Filipovic, N.; Bernard, B.; Katsuyama, Y.; Vukojevic, K. Connexin Expression Is Altered in Liver Development of Yotari (dab1 -/-) Mice. Int. J. Mol. Sci. 2021, 22, 712. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Calvo-Jimenez, E.; Cossard, A.; Na, Y.; Cooper, J.A.; Jossin, Y. N-cadherin-regulated FGFR ubiquitination and degradation control mammalian neocortical projection neuron migration. Elife 2019, 8, e47673. [Google Scholar] [CrossRef] [PubMed]
- Hoe, H.S.; Harris, D.C.; Rebeck, G.W. Multiple pathways of apolipoprotein E signaling in primary neurons. J. Neurochem. 2005, 93, 145–155. [Google Scholar] [CrossRef]
- Lee, G.H.; Chhangawala, Z.; von Daake, S.; Savas, J.N.; Yates, J.R., 3rd; Comoletti, D.; D’Arcangelo, G. Reelin induces Erk1/2 signaling in cortical neurons through a non-canonical pathway. J. Biol. Chem. 2014, 289, 20307–20317. [Google Scholar] [CrossRef]
- Jossin, Y.; Goffinet, A.M. Reelin signals through phosphatidylinositol 3-kinase and Akt to control cortical development and through mTor to regulate dendritic growth. Mol. Cell Biol. 2007, 27, 7113–7124. [Google Scholar] [CrossRef]
- Zha, J.; Zhou, Q.; Xu, L.G.; Chen, D.; Li, L.; Zhai, Z.; Shu, H.B. RIP5 is a RIP-homologous inducer of cell death. Biochem. Biophys. Res. Commun. 2004, 319, 298–303. [Google Scholar] [CrossRef]
- Su, J.; Huang, P.; Qin, M.; Lu, Q.; Sang, X.; Cai, Y.; Wang, Y.; Liu, F.; Wu, R.; Wang, X.; et al. Reduction of HIP2 expression causes motor function impairment and increased vulnerability to dopaminergic degeneration in Parkinson’s disease models. Cell Death Dis. 2018, 9, 1020. [Google Scholar] [CrossRef]
- Wu, J.; Tian, B.; Yang, J.; Huo, H.; Song, Z.; Yu, J.; Gu, Y. Reduction of Hip2 suppresses gastric cancer cell proliferation, migration, invasion and tumorigenesis. Transl. Cancer Res. 2020, 9, 774–785. [Google Scholar] [CrossRef]
- Imai, H.; Shoji, H.; Ogata, M.; Kagawa, Y.; Owada, Y.; Miyakawa, T.; Sakimura, K.; Terashima, T.; Katsuyama, Y. Dorsal Forebrain-Specific Deficiency of Reelin-Dab1 Signal Causes Behavioral Abnormalities Related to Psychiatric Disorders. Cereb. Cortex 2017, 27, 3485–3501. [Google Scholar] [CrossRef] [PubMed]
- Cicchetti, D. Guidelines, Criteria, and Rules of Thumb for Evaluating Normed and Standardized Assessment Instrument in Psychology. Psychol. Assess. 1994, 6, 284–290. [Google Scholar] [CrossRef]
| Embryonic Day (E) | Animal | Structure | Antibody | |||
|---|---|---|---|---|---|---|
| FGFR1 | FGFR2 | RIP5 | HIP2 | |||
| E13.5 | wild type | mm | + | + | +++ | + |
| rv/g | ++ | ++ | + | + | ||
| Ct | + | + | + | ++ | ||
| A/Cd | ++ | +++ | ++ | ++ | ||
| yotari | mm | + | + | ++ | + | |
| rv/g | ++ | + | ++ | + | ||
| Ct | + | + | ++ | ++ | ||
| A/Cd | ++ | + | ++ | ++ | ||
| E15.5 | wild type | mm | + | + | + | + |
| rv/g | + | ++ | +++ | + | ||
| Ct | + | + | ++ | +++ | ||
| A/Cd | ++ | +++ | ++ | +++ | ||
| yotari | mm | + | −/+ | + | ++ | |
| rv/g | ++ | + | ++ | + | ||
| Ct | ++ | + | ++ | +++ | ||
| A/Cd | + | +++ | ++ | +++ | ||
| Postnatal Day (P) | Animal | Structure | Antibody | |||
|---|---|---|---|---|---|---|
| FGFR1 | FGFR2 | RIP5 | HIP2 | |||
| P4 | wild type | G | +/++ | +++ | +/++ | ++ |
| PCT | −/+ | + | +/++ | + | ||
| DCT | + | + | +++ | ++ | ||
| yotari | G | + | ++ | + | ++ | |
| PCT | + | + | +++ | + | ||
| DCT | + | ++ | + | + | ||
| P11 | wild type | G | ++ | +++ | ++ | + |
| PCT | ++ | + | ++ | ++ | ||
| DCT | ++ | +++ | ++ | ++ | ||
| yotari | G | ++ | ++ | ++ | ++/+++ | |
| PCT | ++ | + | ++ | ++ | ||
| DCT | ++ | +/++ | ++ | ++ | ||
| P14 | wild type | G | ++ | +++ | +++ | ++++ |
| PCT | ++/+++ | + | ++ | ++ | ||
| DCT | ++/+++ | +++ | +++ | ++ | ||
| yotari | G | + | +++ | +++ | ++ | |
| PCT | ++ | + | ++ | ++ | ||
| DCT | ++ | ++/+++ | +++ | ++ | ||
| Antibodies | Catalog Number | Host | Dilution | Source | |
|---|---|---|---|---|---|
| Primary | RIP5 (N-16) | sc-162109 | Goat | 1:50 | Santa Cruz Biotechnology, (Texas, TX, USA) |
| Flg (C-15) | sc-121 | Rabbit | 1:50 | Santa Cruz Biotechnology, (Texas, TX, USA) | |
| Bek (C-17) | sc-122 | Rabbit | 1:50 | Santa Cruz Biotechnology(Texas, TX, USA) | |
| HIP2 (D27C4) mAb | #8226 | Rabbit | 1:100 | Cell Signaling Technology (CST), (Danvers MA, USA) | |
| p44/42 MAPK (Erk1/2) (137F5) | CST-4695S | Rabbit | 1:250 | Cell Signaling Technology (CST), (Danvers MA, USA) | |
| mTOR | PA5-34663 | Rabbit | 1:100 | Thermo Fisher Scientific (Waltham, MA, USA) | |
| Human/Mouse/Rat Vimentin Antibody | AF2105 | Goat | 1:300 | R&DSystems (Minneapolis, MN, USA) | |
| Anti-nephrin Antibody (B-12) | sc-377246 | Mouse | 1:50 | Santa Cruz Biotechnology, (Texas, TX, USA) | |
| Secondary | Anti-Goat IgG, Alexa Fluor® 594 | 705-295-003 | Donkey | 1:400 | Jackson Immuno Research Laboratories, Inc., (Baltimore, PA, USA) |
| Anti-Rabbit IgG, Alexa Fluor® 488 | 711-545-152 | Donkey | 1:400 | Jackson Immuno Research Laboratories, Inc., (Baltimore, PA, USA) | |
| Anti-Goat IgG, Alexa Fluor® 488 | 705-545-003 | Donkey | 1:400 | Jackson Immuno Research Laboratories, Inc., (Baltimore, PA, USA) | |
| Anti-Mouse IgG, Alexa Fluor® 488 | 715-545-150 | Donkey | 1:400 | Jackson Immuno Research Laboratories, Inc., (Baltimore, PA, USA) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelam, N.; Racetin, A.; Katsuyama, Y.; Vukojević, K.; Kostić, S. Immunohistochemical Expression Pattern of FGFR1, FGFR2, RIP5, and HIP2 in Developing and Postnatal Kidneys of Dab1−/− (yotari) Mice. Int. J. Mol. Sci. 2022, 23, 2025. https://doi.org/10.3390/ijms23042025
Kelam N, Racetin A, Katsuyama Y, Vukojević K, Kostić S. Immunohistochemical Expression Pattern of FGFR1, FGFR2, RIP5, and HIP2 in Developing and Postnatal Kidneys of Dab1−/− (yotari) Mice. International Journal of Molecular Sciences. 2022; 23(4):2025. https://doi.org/10.3390/ijms23042025
Chicago/Turabian StyleKelam, Nela, Anita Racetin, Yu Katsuyama, Katarina Vukojević, and Sandra Kostić. 2022. "Immunohistochemical Expression Pattern of FGFR1, FGFR2, RIP5, and HIP2 in Developing and Postnatal Kidneys of Dab1−/− (yotari) Mice" International Journal of Molecular Sciences 23, no. 4: 2025. https://doi.org/10.3390/ijms23042025
APA StyleKelam, N., Racetin, A., Katsuyama, Y., Vukojević, K., & Kostić, S. (2022). Immunohistochemical Expression Pattern of FGFR1, FGFR2, RIP5, and HIP2 in Developing and Postnatal Kidneys of Dab1−/− (yotari) Mice. International Journal of Molecular Sciences, 23(4), 2025. https://doi.org/10.3390/ijms23042025








