The Impact of Early Saliva Interaction on Dental Implants and Biomaterials for Oral Regeneration: An Overview
Abstract
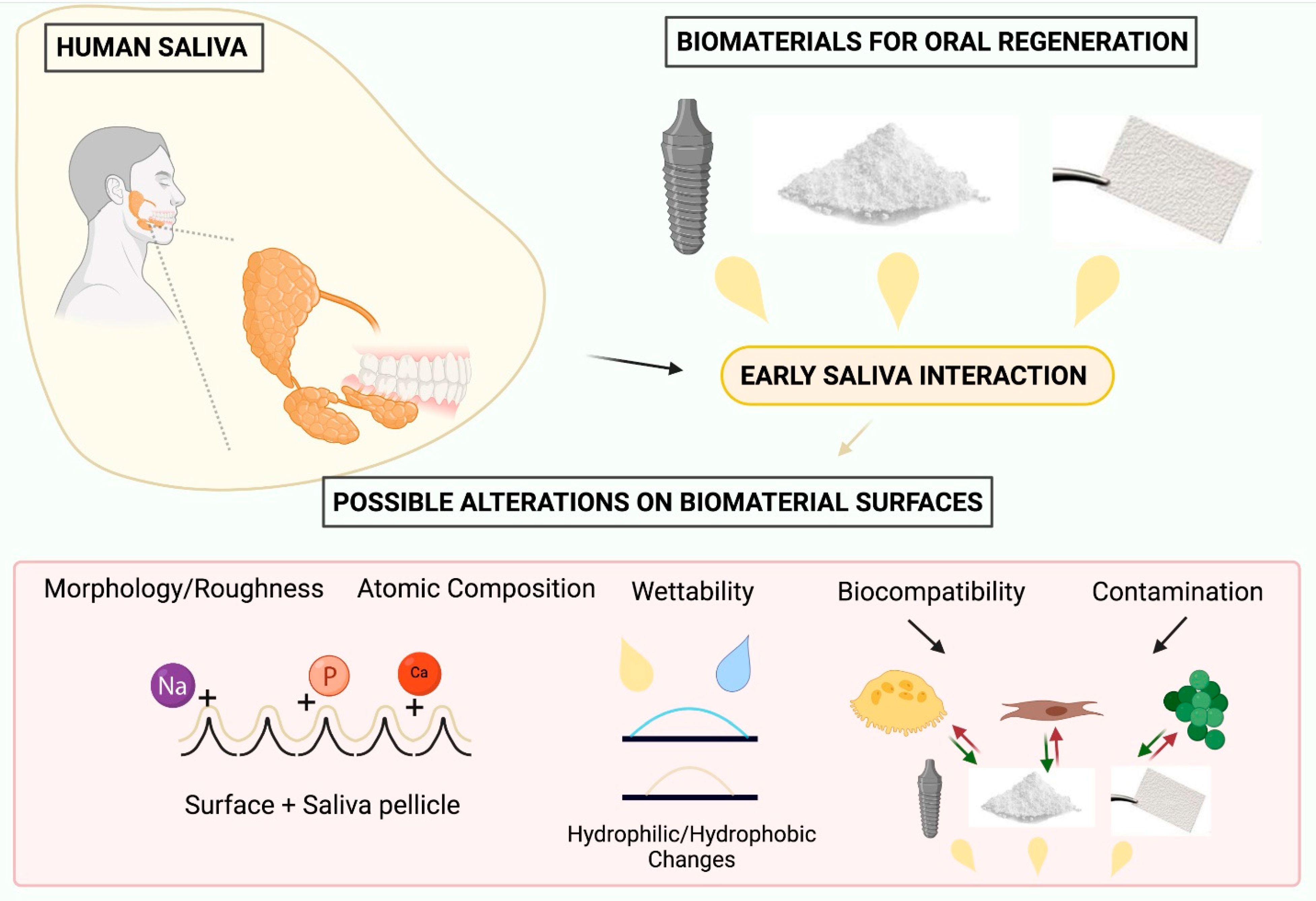
:1. Introduction
2. Search Strategy
3. Saliva Composition
4. Effects of Saliva Interaction on the Physical–Chemical Properties of Biomaterials for Oral Regeneration
4.1. Biomaterial Morphology/Roughness
4.2. Biomaterial Surface Composition
4.3. Biomaterial Wettability
5. Effects of Saliva Interaction on Biocompatibility Properties of Biomaterials
5.1. Biocompatibility
5.1.1. Dental Implants
5.1.2. Membranes and Bone Substitutes
5.2. The Role of Saliva and Bacterial Contamination
5.2.1. Dental Implants
5.2.2. Membranes and Bone Substitutes
6. Limitations of Studies Applying Saliva-Contaminated Biomaterials
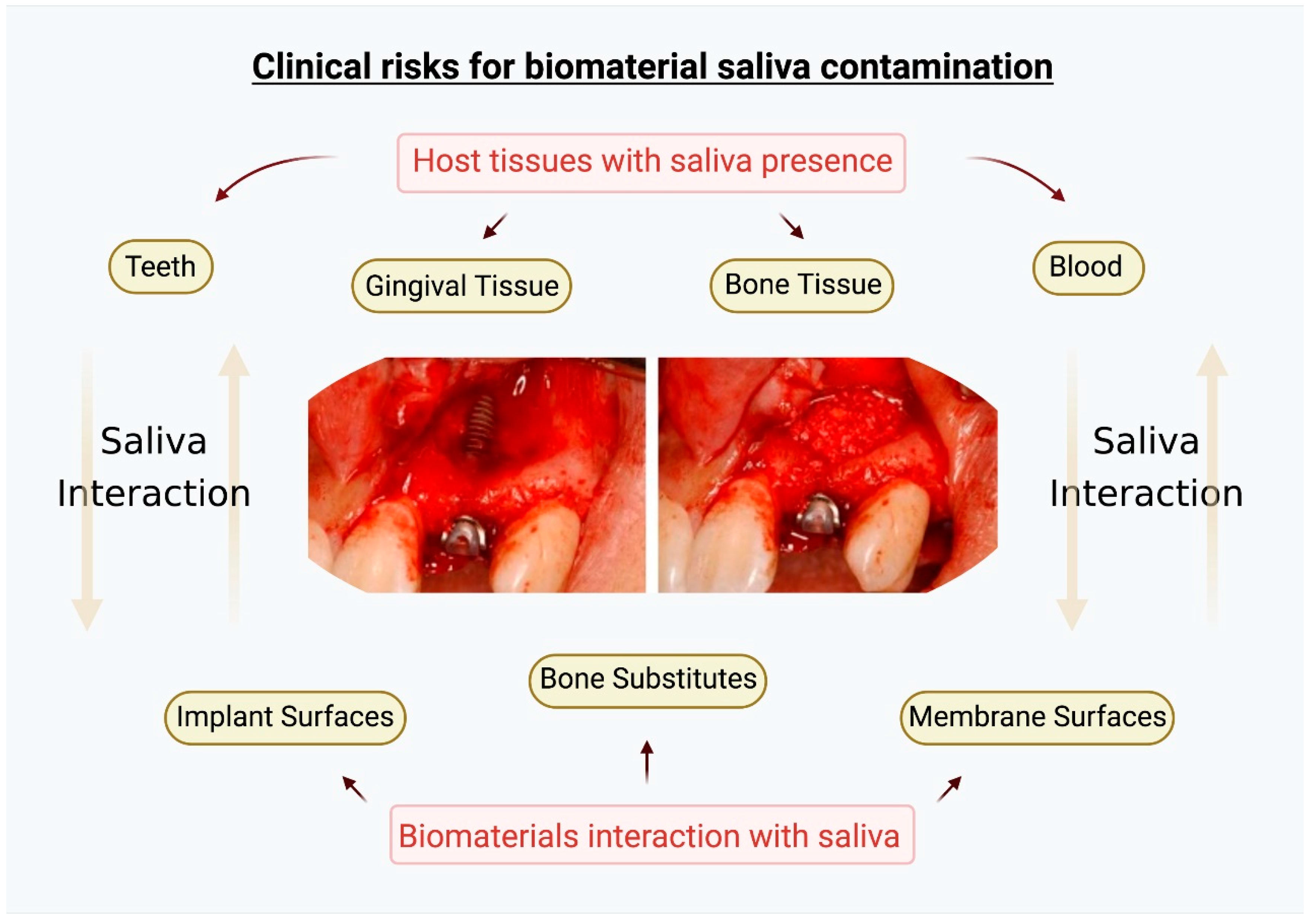
7. Clinical Significance
8. Conclusions
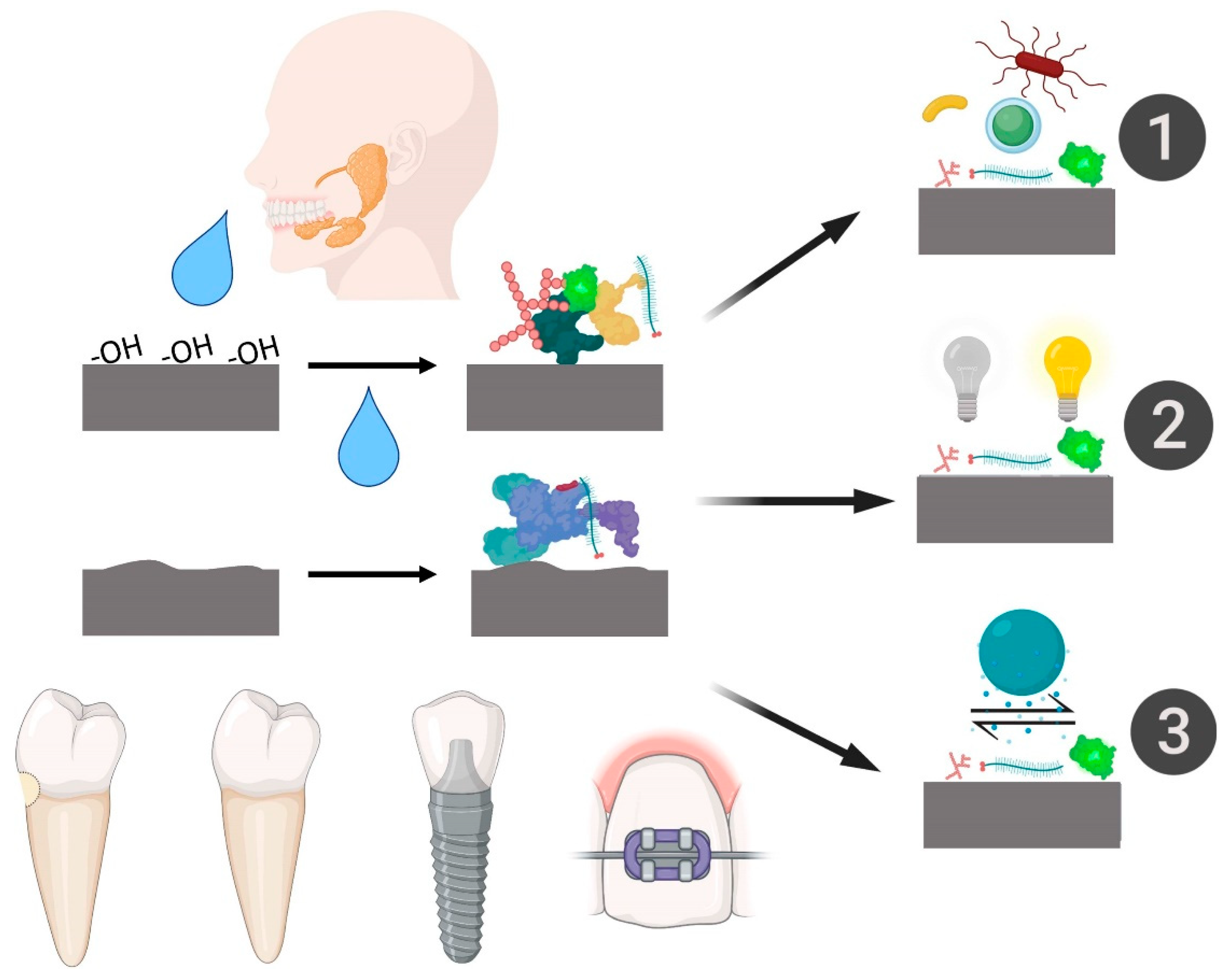
- Salivary pellicle formation over biomaterials is an extremely quick and natural process that occurs within the first minute of interaction with saliva. The pellicle thickness depends on the exposure time to saliva and on the physical–chemical properties of the substrate.
- Accordingly to the physical–chemical studies explored, hydrophilic and hydrophobic characteristics are clearly altered by the interaction with saliva, causing substantial changes in biomaterials with surfaces designed for rapid healing. Moreover, rougher biomaterial surfaces showed high salivary protein adsorption.
- Accordingly to the basic biological studies analyzed, biomaterial biocompatibility with different types of cells is significantly impaired after saliva interaction compared to biomaterials noncontaminated with saliva. In addition, salivary pellicle formation promoted specific conditions for bacterial adhesion and proliferation.
- Clinically, there are no studies demonstrating that early saliva interaction is a factor for direct biomaterial rejections or infections. However, the saliva interaction can alter early biological responses at the surgical site that should be prevented. Efforts to control saliva invasion in surgical sites involving biomaterials for oral regeneration must be maximized to maintain all the basic physical–chemical–biological properties of the biomaterials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fischer, N.G.; Aparicio, C. The salivary pellicle on dental biomaterials. Colloids Surf. B Biointerfaces 2021, 200, 111570. [Google Scholar] [CrossRef] [PubMed]
- Mandel, I.D. The functions of saliva. J. Dent. Res. 1987, 66, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Laguna, L.; Fiszman, S.; Tarrega, A. Saliva matters: Reviewing the role of saliva in the rheology and tribology of liquid and semisolid foods. Relation to in-mouth perception. Food Hydrocoll. 2021, 116, 106660. [Google Scholar] [CrossRef]
- Carpenter, G.H. The secretion, components, and properties of saliva. Annu. Rev. Food Sci. Technol. 2013, 4, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Hannig, M. Ultrastructural investigation of pellicle morphogenesis at two different intraoral sites during a 24-h period. Clin. Oral Investig. 1999, 3, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, D.; Yakubov, G.E.; Stokes, J.R.; Williamson, A.M.; Fuller, G.G. Interaction of human whole saliva and astringent dietary compounds investigated by interfacial shear rheology. Food Hydrocoll. 2008, 22, 1068–1078. [Google Scholar] [CrossRef]
- Kunrath, M.F.; Muradás, T.C.; Penha, N.; Campos, M.M. Innovative surfaces and alloys for dental implants: What about biointerface-safety concerns? Dent. Mater. 2021, 37, 1447–1462. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46, 92–102. [Google Scholar] [CrossRef]
- Omar, O.; Elgali, I.; Dahlin, C.; Thomsen, P. Barrier membranes: More than the barrier effect? J. Clin. Periodontol. 2019, 46, 103–123. [Google Scholar] [CrossRef] [Green Version]
- Kunrath, M.; dos Santos, R.; de Oliveira, S.; Hubler, R.; Sesterheim, P.; Teixeira, E. Osteoblastic cell behavior and early bacterial adhesion on macro-, micro-, and nanostructured titanium surfaces for biomedical implant applications. Int. J. Oral Maxillofac. Implants 2020, 35, 773–781. [Google Scholar] [CrossRef]
- Li, J.J.; Dunstan, C.R.; Entezari, A.; Li, Q.; Steck, R.; Saifzadeh, S.; Sadeghpour, A.; Field, J.R.; Akey, A.; Vielreicher, M.; et al. A novel bone substitute with high bioactivity, strength, and porosity for repairing large and load-bearing bone defects. Adv. Healthc. Mater. 2019, 8, 1801298. [Google Scholar] [CrossRef] [PubMed]
- De Santana, R.B.; de Mattos, C.M.L.; Francischone, C.E.; Van Dyke, T. Superficial topography and porosity of an absorbable barrier membrane impacts soft tissue response in guided bone regeneration. J. Periodontol. 2010, 81, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Hicklin, S.P.; Schneebeli, E.; Chappuis, V.; Janner, S.F.M.; Buser, D.; Brägger, U. Early loading of titanium dental implants with an intra-operatively conditioned hydrophilic implant surface after 21 days of healing. Clin. Oral Implants Res. 2016, 27, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Kunrath, M.F.; Hubler, R.; Silva, R.M.; Barros, M.; Teixeira, E.R.; Correia, A. Influence of saliva interaction on surface properties manufactured for rapid osseointegration in dental implants. Biofouling 2021, 37, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Zuanazzi, D.; Xiao, Y.; Siqueira, W.L. Evaluating protein binding specificity of titanium surfaces through mass spectrometry–based proteomics. Clin. Oral Investig. 2021, 25, 2281–2296. [Google Scholar] [CrossRef] [PubMed]
- Mystkowska, J.; Niemirowicz-Laskowska, K.; Łysik, D.; Tokajuk, G.; Dąbrowski, J.R.; Bucki, R. The role of oral cavity biofilm on metallic biomaterial surface destruction–corrosion and friction aspects. Int. J. Mol. Sci. 2018, 19, 743. [Google Scholar] [CrossRef] [Green Version]
- Dawes, C.; Pedersen, A.M.L.; Villa, A.; Ekström, J.; Proctor, G.B.; Vissink, A.; Aframian, D.; McGowan, R.; Aliko, A.; Narayana, N.; et al. The functions of human saliva: A review sponsored by the World Workshop on Oral Medicine VI. Arch. Oral Biol. 2015, 60, 863–874. [Google Scholar] [CrossRef]
- Li, J.; Helmerhorst, E.J.; Leone, C.W.; Troxler, R.F.; Yaskell, T.; Haffajee, A.D.; Socransky, S.S.; Oppenheim, F.G. Identification of early microbial colonizers in human dental biofilm. J. Appl. Microbiol. 2004, 97, 1311–1318. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Wiedmann-Al-Ahmad, M.; Carvalho, C.; Lang, M.; Follo, M.; Braun, G.; Wittmer, A.; Mülhaupt, R.; Hellwig, E. Bacterial and Candida albicansadhesion on rapid prototyping-produced 3D-scaffolds manufactured as bone replacement materials. J. Biomed. Mater. Res. A 2008, 87A, 933–943. [Google Scholar] [CrossRef]
- Álvarez, S.; Leiva-Sabadini, C.; Schuh, C.M.A.P.; Aguayo, S. Bacterial adhesion to collagens: Implications for biofilm formation and disease progression in the oral cavity. Crit. Rev. Microbiol. 2021, 48, 83–95. [Google Scholar] [CrossRef]
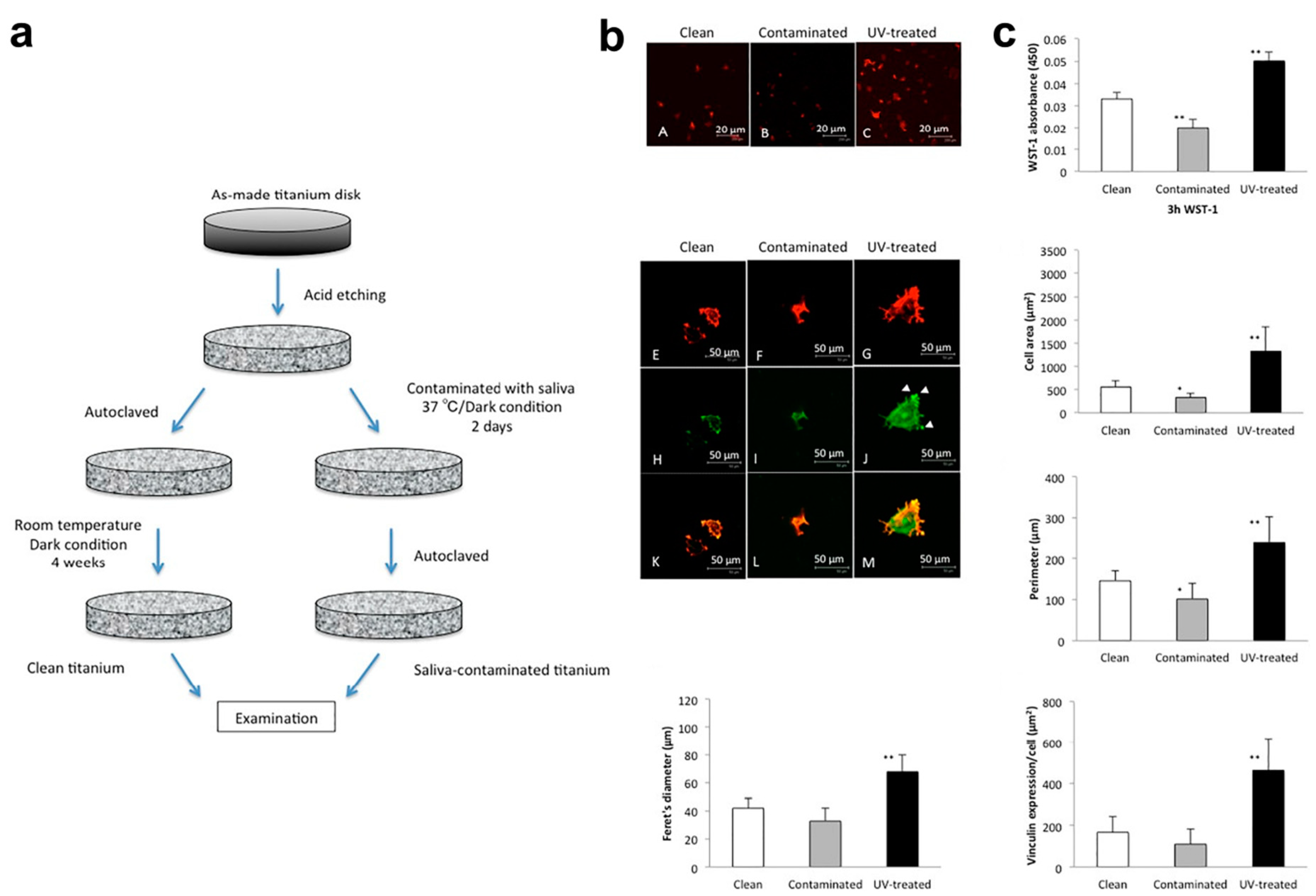
- Hirota, M.; Ikeda, T.; Sugita, Y.; Ishijima, M.; Hirota, S.; Ogawa, T. Impaired osteoblastic behavior and function on saliva-contaminated titanium and its restoration by UV treatment. Mater. Sci. Eng. C 2019, 100, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Kunrath, M.F.; Gupta, S.; Lorusso, F.; Scarano, A.; Noumbissi, S. Oral tissue interactions and cellular response zirconia implant-prosthetic components: A critical review. Materials 2021, 14, 2825. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Scarano, E.; Fiorita, A.; Picciotti, P.M.; Passali, G.C.; Calò, L.; Cabras, T.; Inzitari, R.; Fanali, C.; Messana, I.; Castagnola, M.; et al. Proteomics of saliva: Personal experience. Acta Otorhinolaryngol. Ital. 2010, 30, 125–130. [Google Scholar]
- Amani, H.; Arzaghi, H.; Bayandori, M.; Dezfuli, A.S.; Pazoki-Toroudi, H.; Shafiee, A.; Moradi, L. Controlling cell behavior through the design of biomaterial surfaces: A focus on surface modification techniques. Adv. Mater. Interfaces 2019, 6, 1900572. [Google Scholar] [CrossRef] [Green Version]
- Canullo, L.; Genova, T.; Rakic, M.; Sculean, A.; Miron, R.; Muzzi, M.; Carossa, S.; Mussano, F. Effects of argon plasma treatment on the osteoconductivity of bone grafting materials. Clin. Oral Investig. 2019, 24, 2611–2623. [Google Scholar] [CrossRef]
- Kunrath, M.F.; Diz, F.M.; Magini, R.; Galárraga-Vinueza, M.E. Nanointeraction: The profound influence of nanostructured and nano-drug delivery biomedical implant surfaces on cell behavior. Adv. Colloid Interface Sci. 2020, 284, 102265. [Google Scholar] [CrossRef]
- Marenzi, G.; Impero, F.; Scherillo, F.; Sammartino, J.C.; Squillace, A.; Spagnuolo, G. Effect of different surface treatments on titanium dental implant micro-morphology. Materials 2019, 12, 733. [Google Scholar] [CrossRef] [Green Version]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.M.G.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implants Res. 2009, 20, 172–184. [Google Scholar] [CrossRef]
- Anselme, K.; Bigerelle, M. Topography effects of pure titanium substrates on human osteoblast long-term adhesion. Acta Biomater. 2005, 1, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Watari, F.; Zhu, Y.; Uo, M.; Akasaka, T.; Wang, W.; Xu, G.; Cui, F. The degradation of the three layered nano-carbonated hydroxyapatite/collagen/PLGA composite membrane in vitro. Den. Mater. 2007, 23, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, Y.W.; Soares, R.V.; Assis, M.A.L.; Zenóbio, E.G.; Girundi, F.M.D.S. Titanium surface roughing treatments contribute to higher interaction with salivary proteins MG2 and lactoferrin. J. Contemp. Dent. Pract. 2015, 16, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernández, M.; Hannig, M.; García-Pérez, V.I.; Olivares-Navarrete, R.; Fecher-Trost, C.; Almaguer-Flores, A. Roughness and wettability of titanium implant surfaces modify the salivary pellicle composition. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1017–1028. [Google Scholar] [CrossRef]
- Souza, J.G.S.; Bertolini, M.; Costa, R.C.; Lima, C.V.; Barão, V.A.R. Proteomic profile of the saliva and plasma protein layer adsorbed on Ti–Zr alloy: The effect of sandblasted and acid-etched surface treatment. Biofouling 2020, 36, 428–441. [Google Scholar] [CrossRef]
- Mareci, D.; Ungureanu, G.; Aelenei, D.M.; Rosca, J.C.M. Electrochemical characteristics of titanium based biomaterials in artificial saliva. Mater. Corros. 2007, 58, 848–856. [Google Scholar] [CrossRef]
- Barão, V.A.R.; Mathew, M.T.; Assunção, W.G.; Yuan, J.C.C.; Wimmer, M.A.; Sukotjo, C. Stability of cp-Ti and Ti-6Al-4V alloy for dental implants as a function of saliva pH—An electrochemical study. Clin. Oral Implants Res. 2011, 23, 1055–1062. [Google Scholar] [CrossRef]
- Abey, S.; Mathew, M.T.; Lee, D.J.; Knoernschild, K.L.; Wimmer, M.A.; Sukotjo, C. Electrochemical behavior of titanium in artificial saliva: Influence of pH. J. Oral Implantol. 2014, 40, 3–10. [Google Scholar] [CrossRef]
- Sobczak-Kupiec, A.; Olender, E.; Malina, D.; Tyliszczak, B. Effect of calcination parameters on behavior of bone hydroxyapatite in artificial saliva and its biosafety. Mater. Chem. Phys. 2018, 206, 158–165. [Google Scholar] [CrossRef]
- De Aza, P. Reactivity of a wollastonite–tricalcium phosphate Bioeutectic® ceramic in human parotid saliva. Biomaterials 2000, 21, 1735–1741. [Google Scholar] [CrossRef]
- Gibbins, H.L.; Yakubov, G.E.; Proctor, G.B.; Wilson, S.; Carpenter, G.H. What interactions drive the salivary mucosal pellicle formation? Colloids Surf. B Biointerfaces 2014, 120, 184–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassilakos, N.; Arnebrant, T.; Glantz, P.O. Adsorption of whole saliva onto hydrophilic and hydrophobic solid surfaces: Influence of concentration, ionic strength and pH. Eur. J. Oral Sci. 1992, 100, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, I.E.; Lindh, L. The composition of enamel salivary films is different from the ones formed on dental materials. Biofouling 2009, 25, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Xu, F.; Lee, S. Human saliva and model saliva at bulk to adsorbed phases—Similarities and differences. Adv. Colloid Interface Sci. 2019, 273, 102034. [Google Scholar] [CrossRef] [PubMed]
- Schweikl, H.; Hiller, K.A.; Carl, U.; Schweiger, R.; Eidt, A.; Ruhl, S.; Müller, R.; Schmalz, G. Salivary protein adsorption and Streptococccus gordonii adhesion to dental material surfaces. Dent. Mater. 2013, 29, 1080–1089. [Google Scholar] [CrossRef]
- Müller, R.; Gröger, G.; Hiller, K.A.; Schmalz, G.; Ruhl, S. Fluorescence-based bacterial overlay method for simultaneous in situ quantification of surface-attached bacteria. Appl. Environ. Microbiol. 2007, 73, 2653–2660. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Peng, X.; Zhou, X.; Li, M.; Ren, B.; Cheng, L. Influence of bio-aging on corrosion behavior of different implant materials. Clin. Implant Dent. Relat. Res. 2019, 21, 1225–1234. [Google Scholar] [CrossRef]
- Kunrath, M.F.; Vargas, A.L.M.; Sesterheim, P.; Teixeira, E.R.; Hubler, R. Extension of hydrophilicity stability by reactive plasma treatment and wet storage on TiO(2) nanotube surfaces for biomedical implant applications. J. R. Soc. Interface 2020, 17, 20200650. [Google Scholar] [CrossRef]
- Ikumi, R.; Miyahara, T.; Akino, N.; Tachikawa, N.; Kasugai, S. Guided bone regeneration using a hydrophilic membrane made of unsintered hydroxyapatite and poly(L-lactic acid) in a rat bone-defect model. Dent. Mater. J. 2018, 37, 912–918. [Google Scholar] [CrossRef] [Green Version]
- Zigterman, B.G.R.; Van den Borre, C.; Braem, A.; Mommaerts, M.Y. Titanium surface modifications and their soft-tissue interface on nonkeratinized soft tissues—A systematic review (Review). Biointerphases 2019, 14, 040802. [Google Scholar] [CrossRef]
- Checchi, V.; Generali, L.; Generali, P. Isolation through rubber dam to prevent COVID-19 exposure during flapless trans-crestal sinus lift procedures. J. Oral Implantol. 2021, 47, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Shams, N.; Ghasemi, M.; Sadatmansouri, S.; Bonakdar, S. Morphology and differentiation of MG63 osteoblast cells on saliva contaminated implant surfaces. J. Dent. 2015, 12, 424–429. [Google Scholar]
- Zöller, G.O.; Zentner, A. Initial attachment of human gingival fibroblast-like cells in vitro to titanium surfaces oretreated with saliva and serum. Clin. Oral Implants Res. 1996, 7, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Ma, D.; Bolscher, J.G.M.; Nazmi, K.; Veerman, E.C.I.; Bikker, F.J.; Sun, P.; Lin, H.; Wu, G. Human salivary histatin-1 promotes osteogenic cell spreading on both bio-inert substrates and titanium SLA surfaces. Front. Bioeng. Biotechnol. 2020, 8, 584410. [Google Scholar] [CrossRef]
- Caballé-Serrano, J.; Cvikl, B.; Bosshardt, D.D.; Buser, D.; Lussi, A.; Gruber, R. Saliva suppresses osteoclastogenesis in murine bone marrow cultures. J. Dent. Res. 2015, 94, 192–200. [Google Scholar] [CrossRef]
- Jinno, Y.; Johansson, K.; Stocchero, M.; Toia, M.; Galli, S.; Stavropoulos, A.; Becktor, J.P. Impact of salivary contamination during placement of implants with simultaneous bony augmentation in iliac bone in sheep. Br. J. Oral Maxillofac. Surg. 2019, 57, 1131–1136. [Google Scholar] [CrossRef]
- Proksch, S.; Steinberg, T.; Keller, C.; Wolkewitz, M.; Wiedmann-Al-Ahmad, M.; Finkenzeller, G.; Hannig, C.; Hellwig, E.; Al-Ahmad, A. Human saliva exposure modulates bone cell performance in vitro. Clin. Oral Investig. 2011, 16, 69–77. [Google Scholar] [CrossRef]
- Heaney, T.G. Inhibition of attachment of human gingival fibroblast-like cells in vitro by saliva and salivary-sulfated glycoprotein in the presence of serum. J. Periodontol. 1990, 61, 504–509. [Google Scholar] [CrossRef]
- Pourgonabadi, S.; Müller, H.D.; Mendes, J.R.; Gruber, R. Saliva initiates the formation of pro-inflammatory macrophages in vitro. Arch. Oral Biol. 2017, 73, 295–301. [Google Scholar] [CrossRef]
- Sun, P.; Shi, A.; Shen, C.; Liu, Y.; Wu, G.; Feng, J. Human salivary histatin-1 (Hst1) promotes bone morphogenetic protein 2 (BMP2)-induced osteogenesis and angiogenesis. FEBS Open Bio 2020, 10, 1503–1515. [Google Scholar] [CrossRef]
- Mi, B.; Chen, L.; Xiong, Y.; Yan, C.; Xue, H.; Panayi, A.C.; Liu, J.; Hu, L.; Hu, Y.; Cao, F.; et al. Saliva exosomes-derived UBE2O mRNA promotes angiogenesis in cutaneous wounds by targeting SMAD6. J. Nanobiotechnol. 2020, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Geurts, J.; Arts, J.J.C.; Walenkamp, G.H.I.M. Bone graft substitutes in active or suspected infection. Contra-indicated or not? Injury 2011, 42, S82–S86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, M.H.; Van Der Hoeven, J.S. The growth of oral bacteria on saliva. J. Dent. Res. 1987, 66, 498–505. [Google Scholar] [CrossRef]
- Cavalcanti, Y.W.; Wilson, M.; Lewis, M.; Williams, D.; Senna, P.M.; Del-Bel-Cury, A.A.; Silva, W.J.D. Salivary pellicles equalise surfaces’ charges and modulate the virulence of Candida albicans biofilm. Arch. Oral Biol. 2016, 66, 129–140. [Google Scholar] [CrossRef] [Green Version]
- Bürgers, R.; Hahnel, S.; Reichert, T.E.; Rosentritt, M.; Behr, M.; Gerlach, T.; Handel, G.; Gosau, M. Adhesion of Candida albicans to various dental implant surfaces and the influence of salivary pellicle proteins. Acta Biomater. 2010, 6, 2307–2313. [Google Scholar] [CrossRef]
- Dorkhan, M.; Svensäter, G.; Davies, J.R. Salivary pellicles on titanium and their effect on metabolic activity in Streptococcus oralis. BMC Oral Health 2013, 13, 32. [Google Scholar] [CrossRef] [Green Version]
- Kolenbrander, P.E.; Palmer, R.J.; Periasamy, S.; Jakubovics, N.S. Oral multispecies biofilm development and the key role of cell–cell distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef]
- Dorkhan, M.; Chávez de Paz, L.E.; Skepö, M.; Svensäter, G.; Davies, J.R. Effects of saliva or serum coating on adherence of Streptococcus oralis strains to titanium. Microbiology 2012, 158, 390–397. [Google Scholar] [CrossRef] [Green Version]
- Mabboux, F.; Ponsonnet, L.; Morrier, J.J.; Jaffrezic, N.; Barsotti, O. Surface free energy and bacterial retention to saliva-coated dental implant materials—An in vitro study. Colloids Surf. B Biointerfaces 2004, 39, 199–205. [Google Scholar] [CrossRef]
- Hauser-Gerspach, I.; Kulik, E.M.; Weiger, R.; Decker, E.M.; Ohle, C.V.; Meyer, J. Adhesion of Streptococcus sanguinis to dental implant and restorative materials in vitro. Dent. Mater. J. 2007, 26, 361–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunrath, M.F.; Leal, B.F.; Hubler, R.; de Oliveira, S.D.; Teixeira, E.R. Antibacterial potential associated with drug-delivery built TiO2 nanotubes in biomedical implants. AMB Express 2019, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Kunrath, M.F.; Monteiro, M.S.G.; Gupta, S.; Hubler, R.; de Oliveira, S.D. Influence of titanium and zirconia modified surfaces for rapid healing on adhesion and biofilm formation of Staphylococcus epidermidis. Arch. Oral Biol. 2020, 117, 104824. [Google Scholar] [CrossRef] [PubMed]
- Sinjari, B.; D’Addazio, G.; De Tullio, I.; Traini, T.; Caputi, S. Peri-implant bone resorption during healing abutment placement: The effect of a 0.20% Chrolhexidine gel vs. placebo—A randomized double blind controlled human study. BioMed Res. Int. 2018, 2018, 5326340. [Google Scholar] [CrossRef] [Green Version]
- D’Ercole, S.; D’Addazio, G.; Di Ludovico, S.; Traini, T.; Di Giulio, M.; Sinjari, B. Porphyromonas Gingivalis load is balanced by 0.20% Chrolhexidine gel. A randomized, double-blind, controlled, microbiological and immunohistochemical human study. J. Clin. Med. 2020, 9, 284. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Shang, L.; Brandt, B.W.; Buijs, M.J.; Roffel, S.; van Loveren, C.; Crielaard, W.; Gibbs, S.; Deng, D.M. Saliva-derived microcosm biofilms grown on different oral surfaces in vitro. NPJ Biofilms Microbiomes 2021, 7, 74. [Google Scholar] [CrossRef]
- Mukai, Y.; Torii, M.; Urushibara, Y.; Kawai, T.; Takahashi, Y.; Maeda, N.; Ohkubo, C.; Ohshima, T. Analysis of plaque microbiota and salivary proteins adhering to dental materials. J. Oral Biosci. 2020, 62, 182–188. [Google Scholar] [CrossRef]
- Carlén, A.; Rüdiger, S.G.; Loggner, I.; Olsson, J. Bacteria-binding plasma proteins in pellicles formed on hydroxyapatitein vitroand on teethin vivo. Oral Microbiol. Immunol. 2003, 18, 203–207. [Google Scholar] [CrossRef]
- Lee, B.S.; Chen, Y.J.; Wei, T.C.; Ma, T.L.; Chang, C.C. Comparison of antibacterial adhesion when salivary pellicle is coated on both poly (2-hydroxyethyl-methacrylate)-and polyethylene-glycol-methacrylate-grafted poly (methyl methacrylate). Int. J. Mol. Sci. 2018, 19, 2764. [Google Scholar] [CrossRef] [Green Version]
- Größner-Schreiber, B.; Griepentrog, M.; Haustein, I.; Müller, W.D.; Briedigkeit, H.; Göbel, U.B.; Lange, K.P. Plaque formation on surface modified dental implants. Clin. Oral Implants Res. 2001, 12, 543–551. [Google Scholar] [CrossRef]
- Lima, E.M.C.X.; Koo, H.; Smith, A.M.V.; Rosalen, P.L.; Cury, A.A.D.B. Adsorption of salivary and serum proteins, and bacterial adherence on titanium and zirconia ceramic surfaces. Clin. Oral Implants Res. 2008, 19, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Turri, A.; Čirgić, E.; Shah, F.A.; Hoffman, M.; Omar, O.; Dahlin, C.; Trobos, M. Early plaque formation on PTFE membranes with expanded or dense surface structures applied in the oral cavity of human volunteers. Clin. Exp. Dent. Res. 2021, 7, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Baumann, T.; Kozik, J.; Lussi, A.; Carvalho, T.S. Erosion protection conferred by whole human saliva, dialysed saliva, and artificial saliva. Sci. Rep. 2016, 6, 34760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, N.; Marques, J.; Esteves, E.; Fernandes, M.; Mendes, V.M.; Afonso, Â.; Dias, S.; Pereira, J.P.; Manadas, B.; Correia, M.J.; et al. Protein quality assessment on saliva samples for biobanking purposes. Biopreservation Biobanking 2016, 14, 289–297. [Google Scholar] [CrossRef]
- Avila, M.; Ojcius, D.M.; Yilmaz, O. The oral microbiota: Living with a permanent guest. DNA Cell Biol. 2009, 28, 405–411. [Google Scholar] [CrossRef]

| Protein | Main Function | Percentages |
|---|---|---|
| Mucin | Protection, lubrication, bolus, inhibition of demineralization | ~20% |
| Amylase | Digestion | ~25% |
| bPRP (basic proline-rich proteins | Lubrication and remineralization | ~20% |
| “S” Cystatins | Protection | ~8% |
| aPRP (acidic proline-rich proteins) | Lubrication and remineralization | ~12% |
| gPRP (glycosylated proline-rich proteins) | Lubrication and remineralization | ~5% |
| Immunoglobulins | Protection | ~5% |
| Reference | Study Model | Cells or Animals Employed | Findings | Biomaterial Applied |
|---|---|---|---|---|
| Zhou et al. [47] | In vitro | HGFs cell seemed over the surfaces. | Decreased adhesion and proliferation of HGF cells after bioaging in saliva. | Dental implant surfaces. |
| Shams et al. [52] | In vitro | MG63 human osteoblasts. | Saliva contamination altered morphology and proliferation of osteoblasts. | Dental implant surfaces. |
| Kunrath et al. [14] | In vitro | Osteoblast cell line MC3T3-E1. | Saliva interaction reduced the viability of osteoblast cell line. | Dental implant surfaces. |
| Hirota et al. [21] | In vitro | Bone marrow cells from rats. | Saliva contamination impaired osteoblastic behavior. | Dental implant surfaces. |
| Zöller and Zentner [53] | In vitro | Human gingival fibroblasts-like cells. | Saliva contaminated surfaces had less fibroblast adhesion and proliferation. | Dental implant surfaces. |
| Sun et al. [54] | In vitro | Osteoblast cell line MC3T3-E1. | Histatin-1 was added to titanium surfaces promoting spreading of osteogenic cells. | Dental implant surfaces. |
| Jinno et al. [56] | In vivo | Sheep. | Contaminated saliva from a human with periodontitis was interacted (15s) with the implants before insertion. Osseointegration was prejudiced regarding BIC measurements by saliva contamination. | Dental implants. |
| Sun et al. [60] | In vivo | Sprague–Dawley rats. | The study proposed the addition of histatin-1 (saliva protein) to absorbable collagen sponge. The results showed high bone volume when the functionalized membrane was applied. | Membranes. |
| Proksch et al. [57] | In vitro | Murine MC3T3 osteoblasts. | Saliva interaction hampers the osteoblast behavior. Decreased level of proliferation, alkaline phosphatase and differentiation were verified in groups with saliva. | No biomaterial applied. Cells were exposed directly to culture mediums with or without saliva. |
| Heaney [58] | In vitro | Human gingival fibroblasts. | Saliva interaction decreased the cell adherence to the substrate. | No biomaterial applied. Cells were exposed directly to plastic wells with or without saliva. |
| Pourgonabadi et al. [59] | In vitro | Bone marrow cultures and RAW 264.7 mouse macrophages. | Saliva activated polarization into proinflammatory M1 macrophages. | No biomaterial applied. Cells were exposed directly to culture mediums with or without saliva. |
| Mi et al. [61] | In vitro and in vivo | Human umbilical vein endothelial cells. | The study proposed the application of saliva-derived exosomes in created skin wound in mouse. The results enhanced wound healing through promotion of angiogenesis. | Wound healing. |
| Reference | Study Model | Bacterial Information | Results | Biomaterial Applied |
|---|---|---|---|---|
| Gröbner-Schereiber et al. [80] | In vitro | Streptococcus mutans; Streptococcus sanguis | Saliva had no significant influence on the adherence of the specific strains. | Dental implant surfaces. |
| Mabboux et al. [70] | In vitro | S. sanguinis; S. Constellatus | Results showed that the physical–chemical properties of bacterial cells were influential on the bacterial adherence to surfaces with saliva contact. | Dental implant surfaces. |
| Hauser-Gerspach et al. [71] | In vitro | S. sanguinis | The bacterial vitality depends on the physical–chemical properties of the substrate. | Dental implant surface |
| Bürgers et al. [66] | In vitro | Candida albicans | Mucin protein serves as a receptor for C. albicans adherence and albumin may act as a blocker for this specific adhesion. | Dental implant surfaces. |
| Zhou et al. [47] | In vitro | S. sanguinis | Bacterial adhesion was promoted by bioaging in saliva. | Dental implant surface. |
| Dorkhan et al. [67] | In vitro | S. oralis | Saliva pellicle enhanced the bacterial metabolic activity. | Dental implant surfaces. |
| Dorkhan et al. [69] | In vitro | S. oralis | Saliva pellicle associated with rougher surfaces promoted high bacterial adherence. | Dental implant surfaces. |
| Cavalcanti et al. [65] | In vitro | C. albicans | Saliva contamination induced high virulence for C. albicans. | Dental implant surfaces. |
| Lima et al. [81] | In vitro | S. mutans; Actinomyces naeslundii | Saliva exposure did not create significant attachment of bacteria compared to noncontaminated surfaces with saliva. | Dental implant surfaces. |
| Li et al. [76] | In vitro | Natural saliva (wide number of microorganisms) | The substrate is significant to the proliferation of microorganisms. Biotic substrates promote rich environment for bacterial growth. | Different materials for oral regeneration (natural tissues, titanium and hydroxyapatite). |
| Mukai et al. [77] | Clinical | Human saliva (Wide number of microorganisms) | The study showed nonsignificance between the specificity of bacteria attached to each material. However, all materials demonstrated bacterial adhesion after contamination with saliva. | Different biomaterials for oral regeneration. |
| Carlen et al. [78] | In vitro | P. gingivalis; F. nucleation; A. naeslundii; A. viscosuos | The study suggested that the salivary pellicle could mediate the adhesion of bacteria present in gingivitis and periodontitis. | Hydroxyapatite beads. |
| Lee et al. [79] | In vitro | E. coli and S. mutans | Saliva pellicle did not promote bacterial proliferation. The material showed antibacterial properties even when saliva-coated. | Materials for oral rehabilitation (PMMA). |
| Turri et al. [82] | Clinical study | Biofilm oral flora; Investigation focused on Staphylococcus spp. | The membrane exposure to the oral cavity promoted a higher presence of bacteria compared to teeth surfaces exposed under the same conditions. | Membranes for guided oral regeneration (e-PTFE and d-PTFE). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunrath, M.F.; Dahlin, C. The Impact of Early Saliva Interaction on Dental Implants and Biomaterials for Oral Regeneration: An Overview. Int. J. Mol. Sci. 2022, 23, 2024. https://doi.org/10.3390/ijms23042024
Kunrath MF, Dahlin C. The Impact of Early Saliva Interaction on Dental Implants and Biomaterials for Oral Regeneration: An Overview. International Journal of Molecular Sciences. 2022; 23(4):2024. https://doi.org/10.3390/ijms23042024
Chicago/Turabian StyleKunrath, Marcel Ferreira, and Christer Dahlin. 2022. "The Impact of Early Saliva Interaction on Dental Implants and Biomaterials for Oral Regeneration: An Overview" International Journal of Molecular Sciences 23, no. 4: 2024. https://doi.org/10.3390/ijms23042024
APA StyleKunrath, M. F., & Dahlin, C. (2022). The Impact of Early Saliva Interaction on Dental Implants and Biomaterials for Oral Regeneration: An Overview. International Journal of Molecular Sciences, 23(4), 2024. https://doi.org/10.3390/ijms23042024







