Ginsenoside Rh4 Suppressed Metastasis of Lung Adenocarcinoma via Inhibiting JAK2/STAT3 Signaling
Abstract
1. Introduction
2. Results
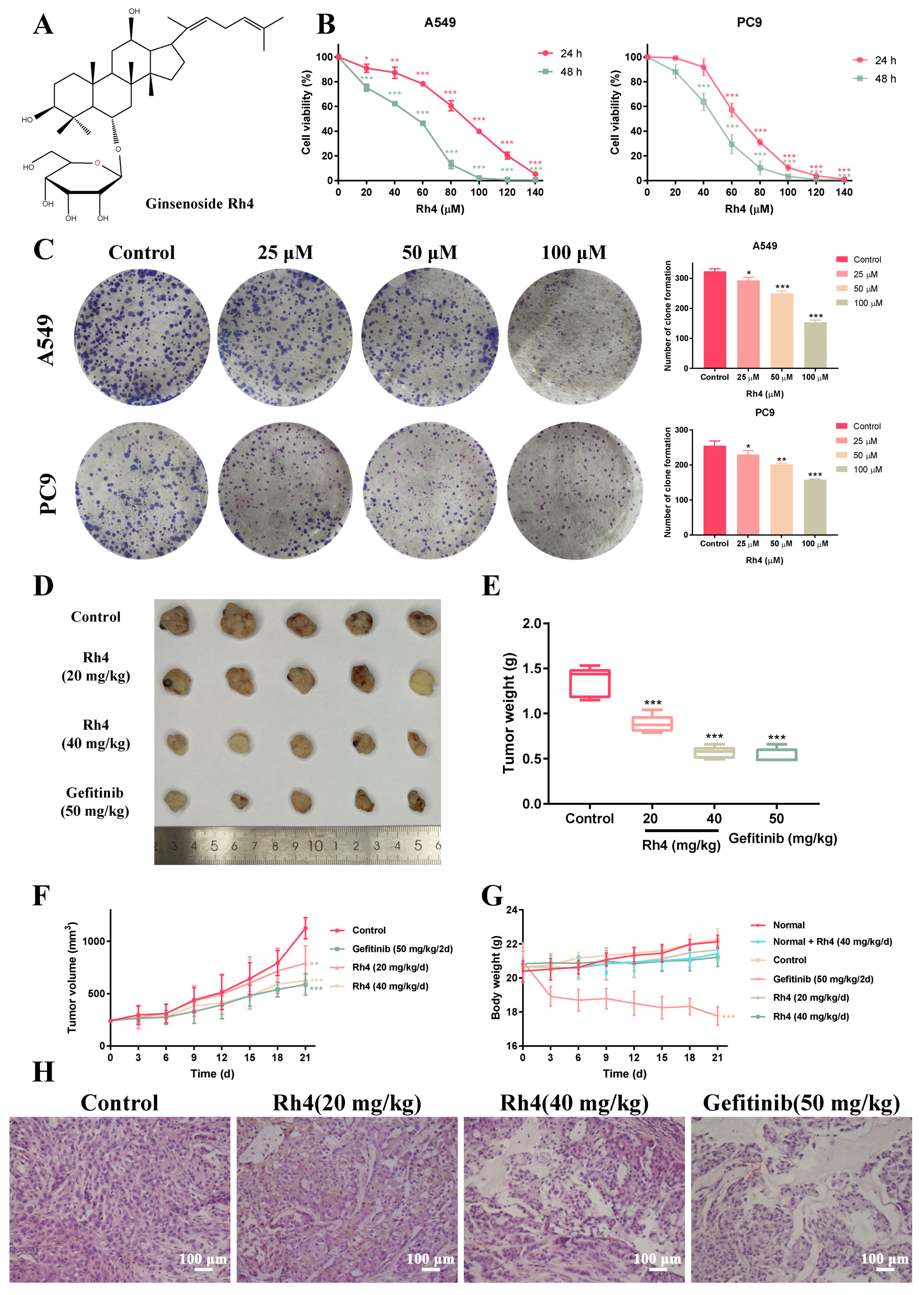
2.1. Rh4 Inhibited LAC Cell Growth In Vitro and In Vivo
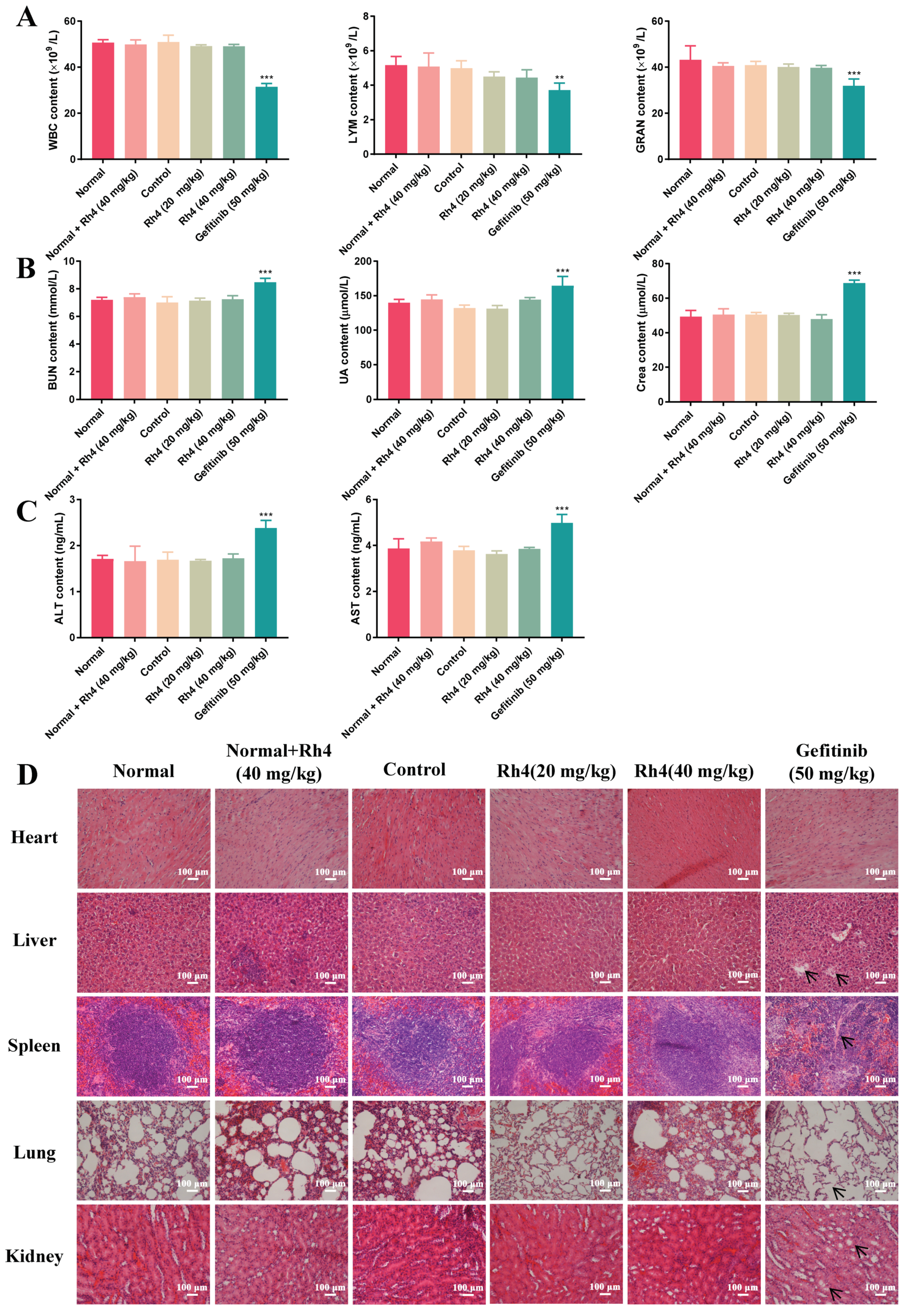
2.2. Low Toxicity Was Induced by Rh4 in Nude Mice
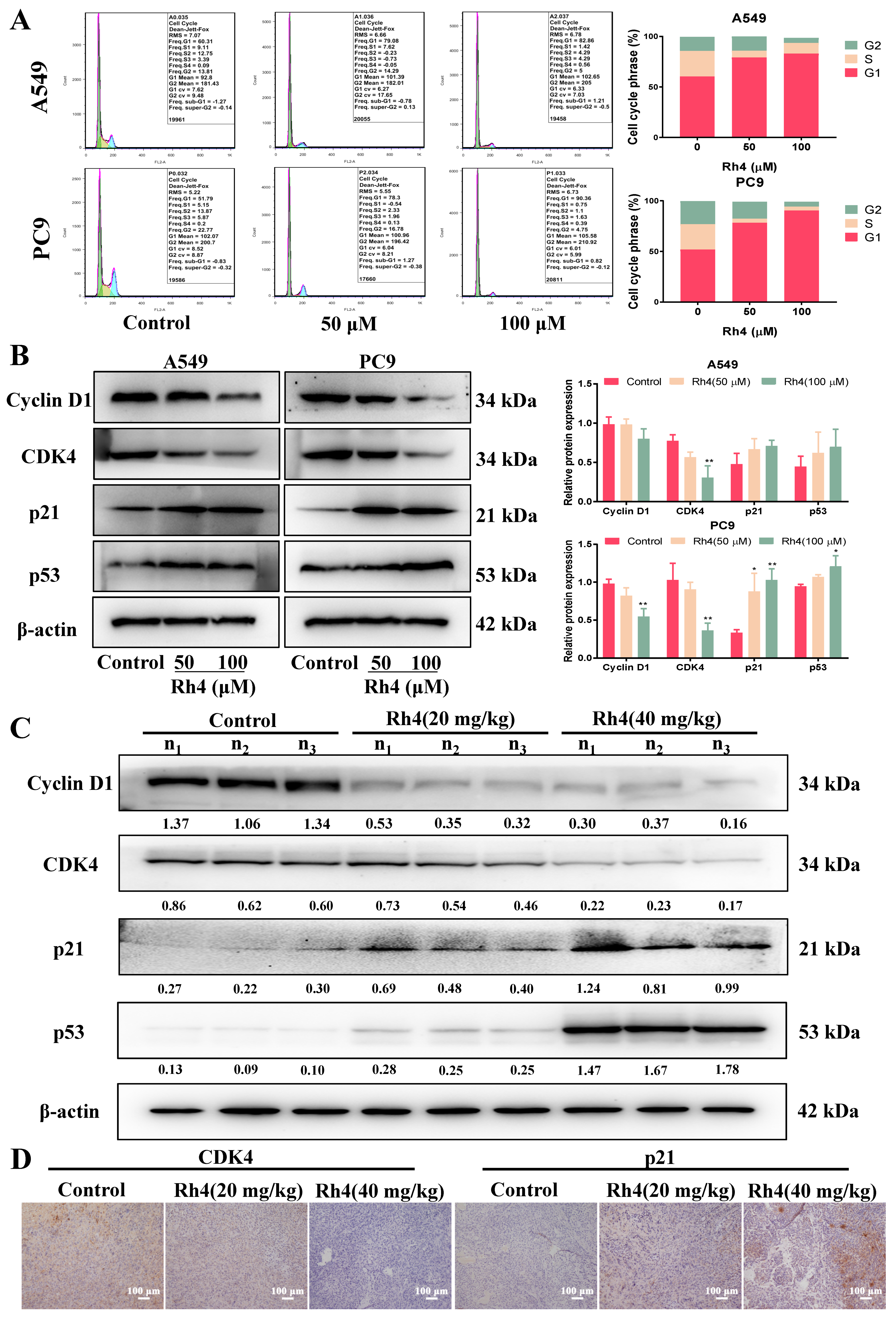
2.3. Rh4 Induced Cell Cycle Arrest in G1 Phase
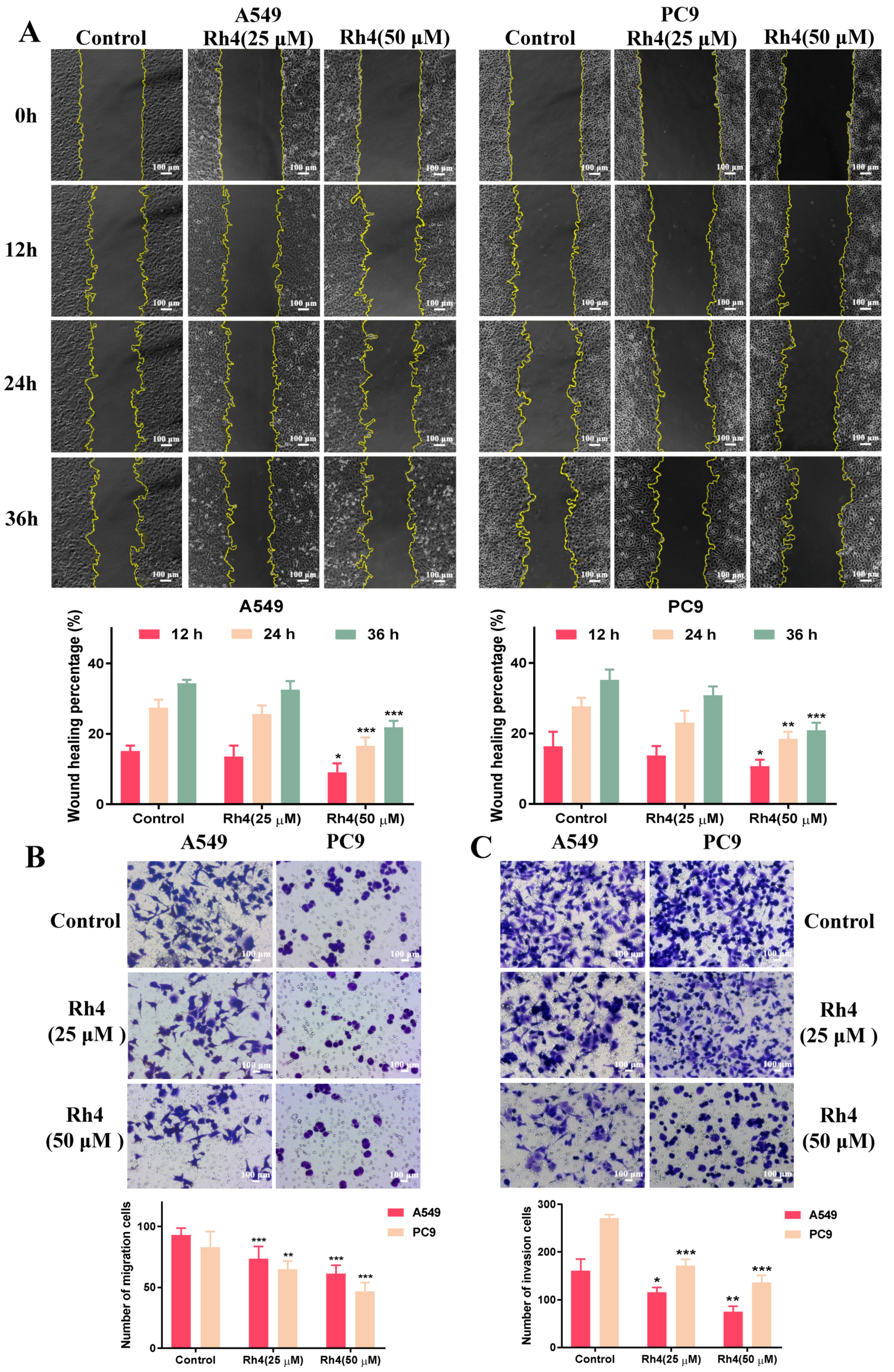
2.4. Rh4 Restrained Migration and Invasion of A549 and PC9
2.5. Rh4 Reversed EMT Induced by TGF-β1 In Vitro and In Vivo
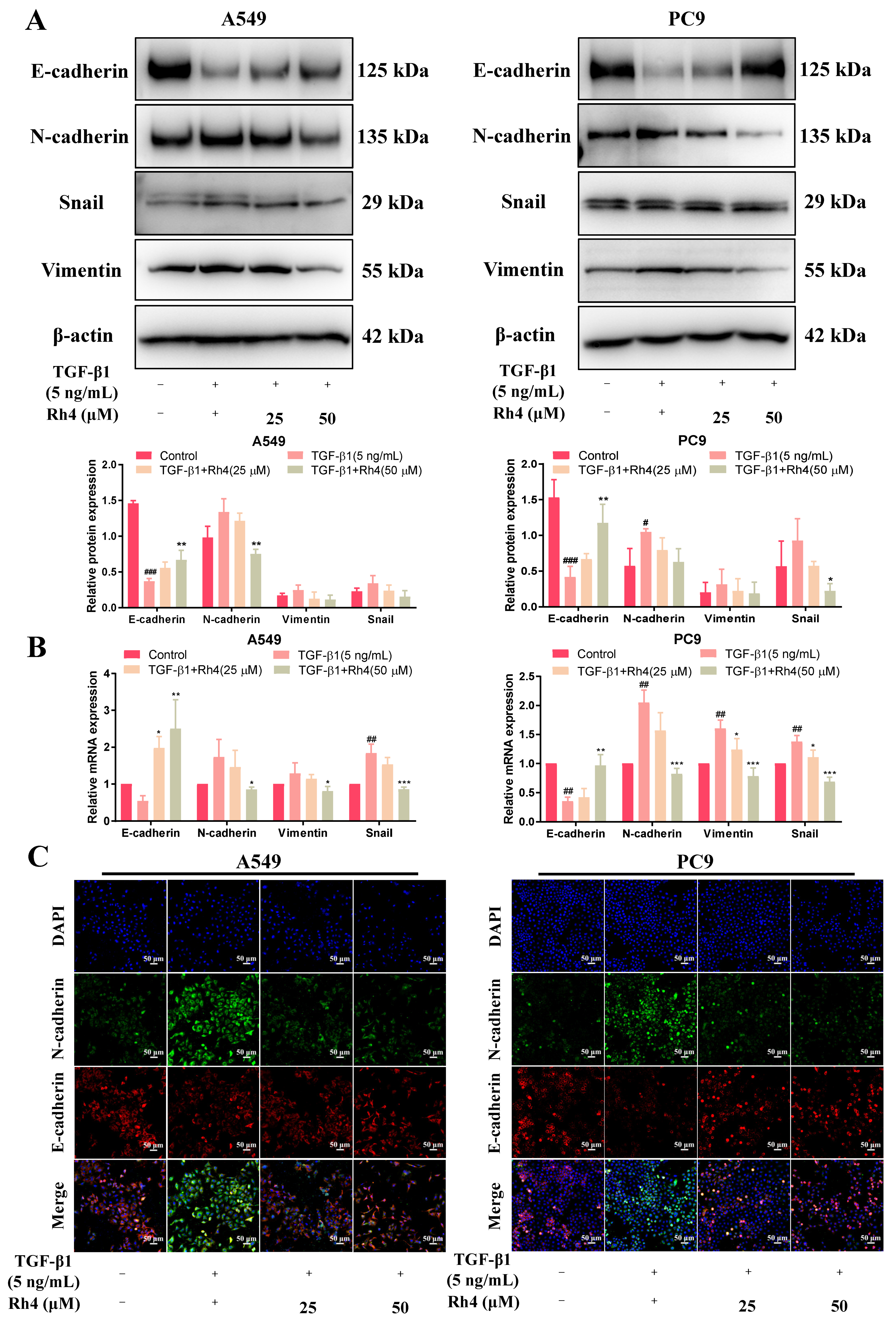
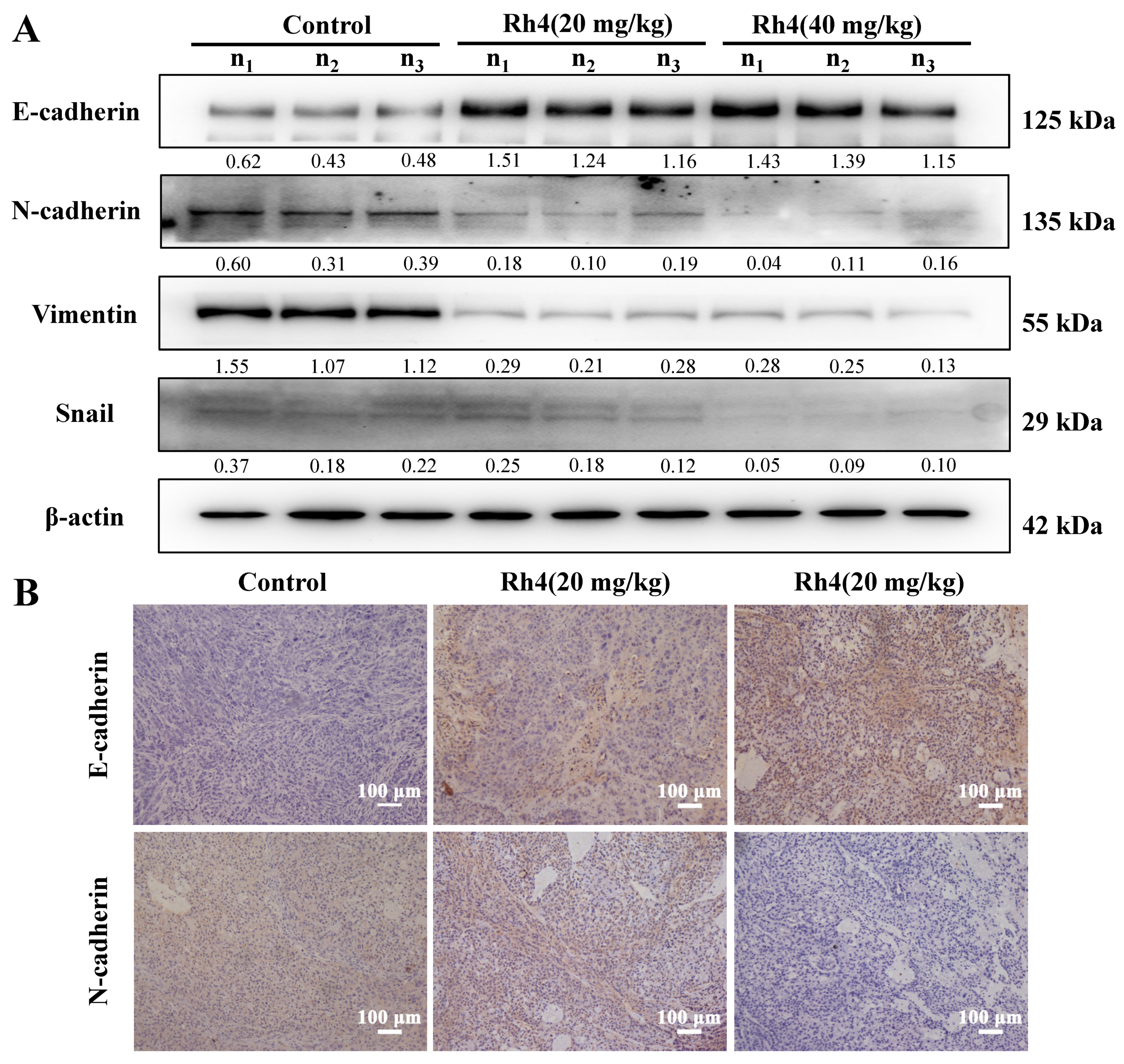
2.6. JAK2/STAT3 Was Involved in EMT Suppression by Rh4

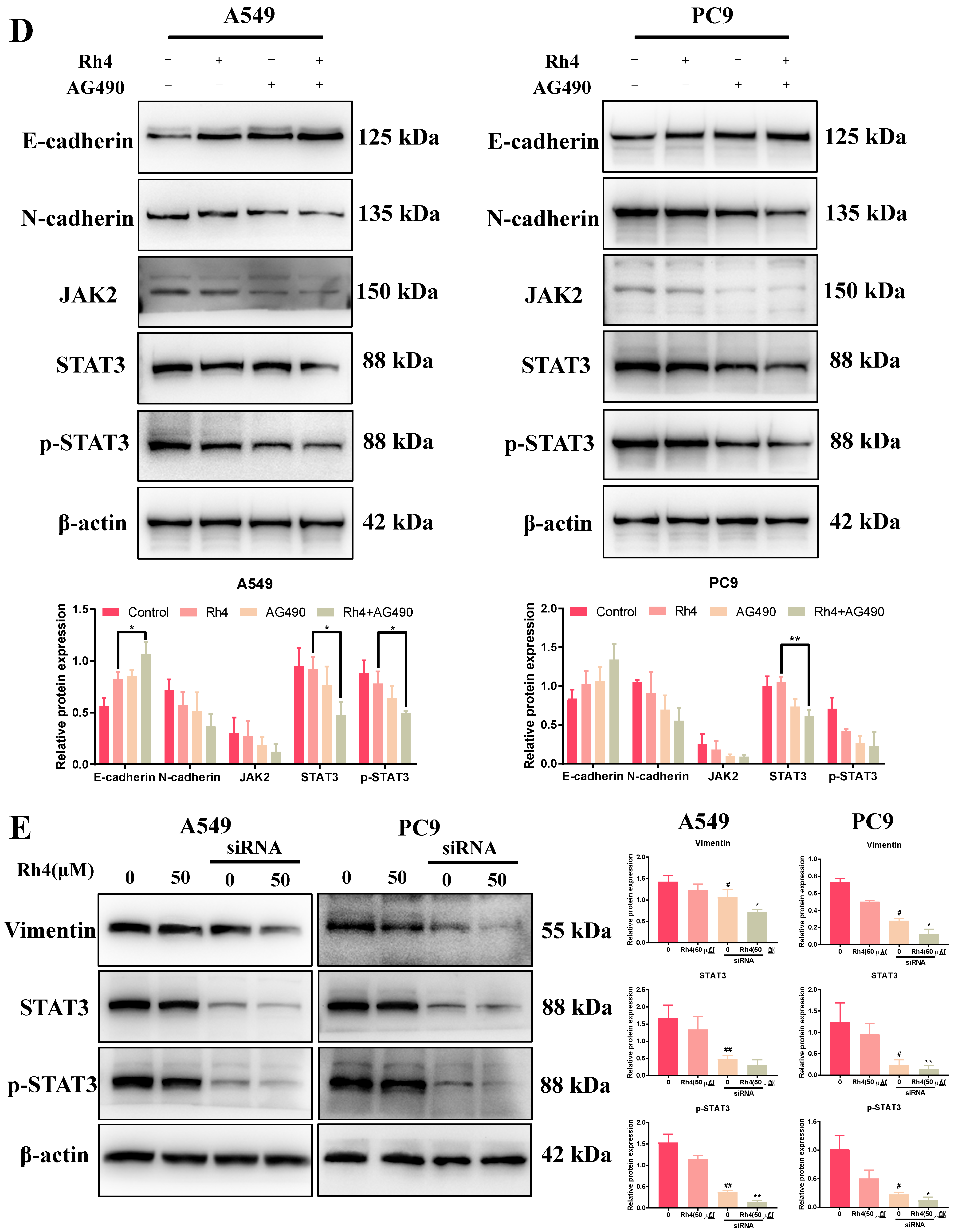
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Cell Culture
4.3. MTT Assay
4.4. Colony Formation Assay
4.5. Human LAC Xenograft Nude Mouse Model
4.6. Cell Cycle Assay by Flow Cytometry
4.7. Wound Healing Assay
4.8. Transwell Assay
4.9. Immunofluorescence Assay
4.10. Western Blot
4.11. Histopathology and Immunohistochemistry
4.12. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
4.13. Plasmids and siRNA Transfection
4.14. Hemogram Assay and Measurement of Biochemical Parameters
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, X.; Wang, Y.; Ren, F.; Sun, D.; Yan, Y.; Kong, X.; Bu, J.; Liu, M.; Xu, S. circRNA-002178 act as a ceRNA to promote PDL1/PD1 expression in lung adenocarcinoma. Cell Death Dis. 2020, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Piao, J.; Wang, Q.; Cui, X.; Meng, Z.; Jin, T.; Lin, Z. Paip1 predicts poor prognosis and promotes tumor progression through AKT/GSK-3β pathway in lung adenocarcinoma. Hum. Pathol. 2019, 86, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.P.; Massague, J. Cancer Metastasis: Building a Framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Sleeman, J.P. Complex networks orchestrate epithelial–mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006, 7, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Hlubek, F.; Spaderna, S.; Jung, A.; Kirchner, T.; Brabletz, T. β-Catenin activates a coordinated expression of the proinvasive factors laminin-5 γ2 chain and MT1-MMP in colorectal carcinomas. Int. J. Cancer 2004, 108, 321–326. [Google Scholar] [CrossRef]
- Bai, F.; Zhang, L.-H.; Liu, X.; Wang, C.; Zheng, C.; Sun, J.; Li, M.; Zhu, W.-G.; Pei, X.-H. GATA3 functions downstream of BRCA1 to suppress EMT in breast cancer. Theranostics 2021, 11, 8218–8233. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hodge, J.; Wang, J.; Wang, Y.; Wang, L.; Singh, U.P.; Li, Y.; Yao, Y.; Wang, D.; Ai, W.; et al. Emodin reduces Breast Cancer Lung Metastasis by suppressing Macrophage-induced Breast Cancer Cell Epithelial-mesenchymal transition and Cancer Stem Cell formation. Theranostics 2020, 10, 8365–8381. [Google Scholar] [CrossRef] [PubMed]
- Bonam, S.R.; Wu, Y.S.; Tunki, L.; Chellian, R.; Halmuthur, M.S.K.; Muller, S.; Pandy, V. What Has Come out from Phytomedicines and Herbal Edibles for the Treatment of Cancer? ChemMedChem 2018, 13, 1854–1872. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Z.; Anderson, S.; Du, W.; He, T.-C.; Yuan, C.-S. Red ginseng and cancer treatment. Chin. J. Nat. Med. 2016, 14, 7–16. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, H.W.; Yun, S.H.; Kim, S.J. Ginsenoside Rh2 upregulates long noncoding RNA STXBP5-AS1 to sponge microRNA-4425 in suppressing breast cancer cell proliferation. J. Ginseng Res. 2021, 45, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zuo, L.; Guo, D.; Chai, X.; Xu, J.; Cui, Z.; Wang, Z.; Hou, C. Ginsenoside Rg3 regulates DNA damage in non-small cell lung cancer cells by activating VRK1/P53BP1 pathway. Biomed. Pharmacother. 2019, 120, 109483. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Yang, X.; Zhao, L.; Hou, Z.; Zhang, R.; Zhang, F.; Ren, G. Relationship between antimicrobial activity and amphipathic structure of ginsenosides. Ind. Crops Prod. 2020, 143, 111929. [Google Scholar] [CrossRef]
- Tian, M.; Ma, P.; Zhang, Y.; Mi, Y.; Fan, D. Ginsenoside Rk3 alleviated DSS-induced ulcerative colitis by protecting colon barrier and inhibiting NLRP3 inflammasome pathway. Int. Immunopharmacol. 2020, 85, 106645. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Akter, R.; Rupa, E.J.; Awais, M.; Mathiyalagan, R.; Han, Y.; Kang, J.; Yang, D.C.; Kang, S.C. Role of Ginsenosides in Browning of White Adipose Tissue to Combat Obesity: A Narrative Review on Molecular Mechanism. Arch. Med. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, Y.; Yue, L.; Li, S.; Qi, X.; Zhao, H.; Yang, Y.; Zhang, C.; Yu, H. JNK pathway and relative transcriptional factor were involved in ginsenoside Rh2-mediated G1 growth arrest and apoptosis in human lung adenocarcinoma A549 cells. Genet. Mol. Res. 2016, 15, 15039003. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Du, X.; Xiong, M.; Cui, J.; Yang, Q.; Wang, W.; Chen, Y.; Zhang, T. Ginsenoside Rd attenuates breast cancer metastasis implicating derepressing microRNA-18a-regulated Smad2 expression. Sci. Rep. 2016, 6, srep33709. [Google Scholar] [CrossRef] [PubMed]
- Baek, N.-I.; Kim, N.; Lee, Y.; Park, J.; Lee, C.; Kim, S. Ginsenoside Rh4, A Genuine Dammarane Glycoside from Korean Red Ginseng. Planta Med. 1996, 62, 86–87. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Deng, J.; Fan, D.; Duan, Z.; Zhu, C.; Fu, R.; Wang, S. Ginsenoside Rh4 induces apoptosis and autophagic cell death through activation of the ROS/JNK/p53 pathway in colorectal cancer cells. Biochem. Pharmacol. 2018, 148, 64–74. [Google Scholar] [CrossRef]
- Deng, X.; Zhao, J.; Qu, L.; Duan, Z.; Fu, R.; Zhu, C.; Fan, D. Ginsenoside Rh4 suppresses aerobic glycolysis and the expression of PD-L1 via targeting AKT in esophageal cancer. Biochem. Pharmacol. 2020, 178, 114038. [Google Scholar] [CrossRef]
- Park, S.J.; Choi, Y.S.; Lee, S.; Lee, Y.J.; Hong, S.; Han, S.; Kim, B.-C. BIX02189 inhibits TGF-β1-induced lung cancer cell metastasis by directly targeting TGF-β type I receptor. Cancer Lett. 2016, 381, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, P.; Kim, T.; Kim, Y.; Song, B.G.; Park, Y.-T.; Choi, S.-J.; Yoon, C.H.; Lim, W.-C.; Ko, H.; et al. Ginsenosides Rk1 and Rg5 inhibit transforming growth factor-β1-induced epithelial-mesenchymal transition and suppress migration, invasion, anoikis resistance, and development of stem-like features in lung cancer. J. Ginseng Res. 2021, 45, 134–148. [Google Scholar] [CrossRef]
- Dai, G.; Sun, B.; Gong, T.; Pan, Z.; Meng, Q.; Ju, W. Ginsenoside Rb2 inhibits epithelial-mesenchymal transition of colorectal cancer cells by suppressing TGF-β/Smad signaling. Phytomedicine 2019, 56, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jin, J.; Xu, J.; Shao, Y.W.; Fan, Y. JAK2 variations and functions in lung adenocarcinoma. Tumor Biol. 2017, 39, 1010428317711140. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, T.; Wu, J.-C.; Luo, S.-Z.; Chen, R.; Lu, L.-G.; Xu, M.-Y. STAT3 aggravates TGF-β1-induced hepatic epithelial-to-mesenchymal transition and migration. Biomed. Pharmacother. 2018, 98, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Santillán-Benítez, J.G.; Mendieta-Zerón, H.; Gómez-Oliván, L.M.; Quiroz, A.O.; Torres-Juárez, J.J.; González-Bañales, J.M. JAK2, STAT3 and SOCS3 gene expression in women with and without breast cancer. Gene 2014, 547, 70–76. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, J.; Liu, G.; Huang, W.; Wei, X.; Wei, Z.; He, Y. STIP1 knockdown suppresses colorectal cancer cell proliferation, migration and invasion by inhibiting STAT3 pathway. Chem. Interact. 2021, 341, 109446. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Mohan, C.D.; Deivasigamani, A.; Jung, Y.Y.; Rangappa, S.; Basappa, S.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Garg, M.; et al. Brusatol suppresses STAT3-driven metastasis by downregulating epithelial-mesenchymal transition in hepatocellular carcinoma. J. Adv. Res. 2020, 26, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yuan, X.; Sun, T.; Fan, S.; Wang, J.; Zhou, Q.; Guo, W.; Ran, F.; Ge, Z.; Yang, H.; et al. Proteasome Inhibitor YSY01A Abrogates Constitutive STAT3 Signaling via Down-regulation of Gp130 and JAK2 in Human A549 Lung Cancer Cells. Front. Pharmacol. 2017, 8, 476. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, T.; Zhao, L.; Chen, W.; Hou, H.; Ye, Z.; Li, X. Ginsenoside 20(S)-Rg3 inhibits the Warburg effect through STAT3 pathways in ovarian cancer cells. Int. J. Oncol. 2014, 46, 775–781. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Chen, C.; Zhang, S.-Y.; Li, Y.; Jin, Y.-H. (20S) Ginsenoside Rh2 Inhibits STAT3/VEGF Signaling by Targeting Annexin A2. Int. J. Mol. Sci. 2021, 22, 9289. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Huynh, D.T.N.; Myung, C.-S.; Heo, K.-S. Ginsenoside Rh1 Prevents Migration and Invasion through Mitochondrial ROS-Mediated Inhibition of STAT3/NF-κB Signaling in MDA-MB-231 Cells. Int. J. Mol. Sci. 2021, 22, 10458. [Google Scholar] [CrossRef]
- Xiang, Y.; Liao, X.-H.; Yu, C.-X.; Yao, A.; Qin, H.; Li, J.-P.; Hu, P.; Li, H.; Guo, W.; Gu, C.-J.; et al. MiR-93-5p inhibits the EMT of breast cancer cells via targeting MKL-1 and STAT3. Exp. Cell Res. 2017, 357, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wu, P.; Zhao, W.; Wang, X.; Yang, J.; Huo, X.; Chen, J.; De, W.; Yang, F. Long noncoding RNA LINC00165-induced by STAT3 exerts oncogenic properties via interaction with Polycomb Repressive Complex 2 to promote EMT in gastric cancer. Biochem. Biophys. Res. Commun. 2018, 507, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Xu, X.; Wu, T.; Li, J.; Cao, G.; Li, Y.; Ji, Z. Knockdown of PYCR1 inhibits proliferation, drug resistance and EMT in colorectal cancer cells by regulating STAT3-Mediated p38 MAPK and NF-κB signalling pathway. Biochem. Biophys. Res. Commun. 2019, 520, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.P.; Tortelli, T.C.; Mancini, M.C.S.; Pavan, I.C.B.; Silva, L.G.S.; Severino, M.B.; Granato, D.C.; Pestana, N.F.; Ponte, L.G.S.; Peruca, G.F.; et al. STAT3 contributes to cisplatin resistance, modulating EMT markers, and the mTOR signaling in lung adenocarcinoma. Neoplasia 2021, 23, 1048–1058. [Google Scholar] [CrossRef]
- Liu, R.-Y.; Zeng, Y.; Lei, Z.; Wang, L.; Yang, H.; Liu, Z.; Zhao, J.; Zhang, H.-T. JAK/STAT3 signaling is required for TGF-β-induced epithelial-mesenchymal transition in lung cancer cells. Int. J. Oncol. 2014, 44, 1643–1651. [Google Scholar] [CrossRef]
- Bai, X.; Fu, R.; Duan, Z.; Liu, Y.; Zhu, C.; Fan, D. Ginsenoside Rh4 alleviates antibiotic-induced intestinal inflammation by regulating the TLR4-MyD88-MAPK pathway and gut microbiota composition. Food Funct. 2021, 12, 2874–2885. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, J.; Fan, D. G-Rh4 improves pancreatic β-cells dysfunction in vivo and in vitro by increased expression of Nrf2 and its target genes. Food Chem. Toxicol. 2021, 148, 111925. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Duan, Z.; Zhu, C.; Deng, J.; Fan, D. Anti-anemia effects of ginsenoside Rk3 and ginsenoside Rh4 on mice with ribavirin-induced anemia. Food Funct. 2018, 9, 2447–2455. [Google Scholar] [CrossRef]
- Duan, Z.; Wei, B.; Deng, J.; Mi, Y.; Dong, Y.; Zhu, C.; Fu, R.; Qu, L.; Fan, D. The anti-tumor effect of ginsenoside Rh4 in MCF-7 breast cancer cells in vitro and in vivo. Biochem. Biophys. Res. Commun. 2018, 499, 482–487. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Huysentruyt, L.C. On the Origin of Cancer Metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. The pathogenesis of cancer metastasis: The ’seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.-L.; Wang, B.-J.; Wang, L.; Shan, Y.-P.; Zou, H.; Song, R.-L.; Wang, T.; Gu, J.-H.; Yuan, Y.; Liu, X.-Z.; et al. ROS-Mediated Cell Cycle Arrest and Apoptosis Induced by Zearalenone in Mouse Sertoli Cells via ER Stress and the ATP/AMPK Pathway. Toxins 2018, 10, 24. [Google Scholar] [CrossRef]
- Shen, X.; Wu, Z.; Chen, S.; Chen, Y.; Xia, J.; Lv, Y.; Zhou, Y. Induction of G2/M phase arrest and apoptosis by ZGDHU-1 in A549 and RERF-LC-MA lung cancer cells. Oncol. Lett. 2016, 12, 989–994. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, M.; Yang, H. Negative growth regulators of the cell cycle machinery and cancer. Pathophysiology 2009, 16, 305–309. [Google Scholar] [CrossRef]
- Weinberg, R.A. Tumor Suppressor Genes. Science 1991, 254, 1138–1146. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, B.P. New insights of epithelial-mesenchymal transition in cancer metastasis. Acta Biochim. Biophys. Sin. 2008, 40, 643–650. [Google Scholar] [CrossRef]
- Aizawa, H.; Tagami, H. Delayed tissue necrosis due to mitomycin C. Acta Derm.-Venereol. 1987, 67, 364–366. [Google Scholar]
- Yang, J.; Mani, S.A.; Donaher, J.L.; Ramaswamy, S.; Itzykson, R.A.; Come, C.; Savagner, P.; Gitelman, I.; Richardson, A.; Weinberg, R.A. Twist, a Master Regulator of Morphogenesis, Plays an Essential Role in Tumor Metastasis. Cell 2004, 117, 927–939. [Google Scholar] [CrossRef]
- Miettinen, P.J.; Ebner, R.; Lopez, A.R.; Derynck, R. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: Involvement of type I receptors. J. Cell Biol. 1994, 127, 2021–2036. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Jarrett, J.A.; Chen, E.Y.; Eaton, D.H.; Bell, J.R.; Assoian, R.K.; Roberts, A.B.; Sporn, M.B.; Goeddel, D.V. Human transforming growth factor-β complementary DNA sequence and expression in normal and transformed cells. Nature 1985, 316, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Goeddel, D.V.; Ullrich, A.; Gutterman, J.U.; Williams, R.D.; Bringman, T.S.; Berger, W.H. Synthesis of messenger RNAs for transforming growth factors α and β and the epidermal growth factor receptor by human tumors. Cancer Res. 1987, 47, 707–712. [Google Scholar] [PubMed]
- Chiu, H.-C.; Chou, D.-L.; Huang, C.-T.; Lin, W.-H.; Lien, T.-W.; Yen, K.-J.; Hsu, J.T.-A. Suppression of Stat3 activity sensitizes gefitinib-resistant non-small cell lung cancer cells. Biochem. Pharmacol. 2011, 81, 1263–1270. [Google Scholar] [CrossRef]
- Wu, P.; Wu, D.; Zhao, L.; Huang, L.; Shen, G.; Chai, Y. Prognostic role of STAT3 in solid tumors: A systematic review and meta-analysis. Oncotarget 2016, 7, 19863–19883. [Google Scholar] [CrossRef]
- Chuang, C.-H.; Greenside, P.G.; Rogers, Z.N.; Brady, J.J.; Yang, D.; Ma, R.; Caswell, D.R.; Chiou, S.-H.; Winters, A.F.; Grüner, B.; et al. Molecular definition of a metastatic lung cancer state reveals a targetable CD109–Janus kinase–Stat axis. Nat. Med. 2017, 23, 291–300. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Ma, P.; Duan, Z.; Liu, Y.; Mi, Y.; Fan, D. Ginsenoside Rh4 Suppressed Metastasis of Lung Adenocarcinoma via Inhibiting JAK2/STAT3 Signaling. Int. J. Mol. Sci. 2022, 23, 2018. https://doi.org/10.3390/ijms23042018
Zhang Y, Ma P, Duan Z, Liu Y, Mi Y, Fan D. Ginsenoside Rh4 Suppressed Metastasis of Lung Adenocarcinoma via Inhibiting JAK2/STAT3 Signaling. International Journal of Molecular Sciences. 2022; 23(4):2018. https://doi.org/10.3390/ijms23042018
Chicago/Turabian StyleZhang, Yan, Pei Ma, Zhiguang Duan, Yannan Liu, Yu Mi, and Daidi Fan. 2022. "Ginsenoside Rh4 Suppressed Metastasis of Lung Adenocarcinoma via Inhibiting JAK2/STAT3 Signaling" International Journal of Molecular Sciences 23, no. 4: 2018. https://doi.org/10.3390/ijms23042018
APA StyleZhang, Y., Ma, P., Duan, Z., Liu, Y., Mi, Y., & Fan, D. (2022). Ginsenoside Rh4 Suppressed Metastasis of Lung Adenocarcinoma via Inhibiting JAK2/STAT3 Signaling. International Journal of Molecular Sciences, 23(4), 2018. https://doi.org/10.3390/ijms23042018





