The Effects of Stress and Diet on the “Brain–Gut” and “Gut–Brain” Pathways in Animal Models of Stress and Depression
Abstract
:1. Introduction
2. Animal Models of Stress and Depression: An Overview
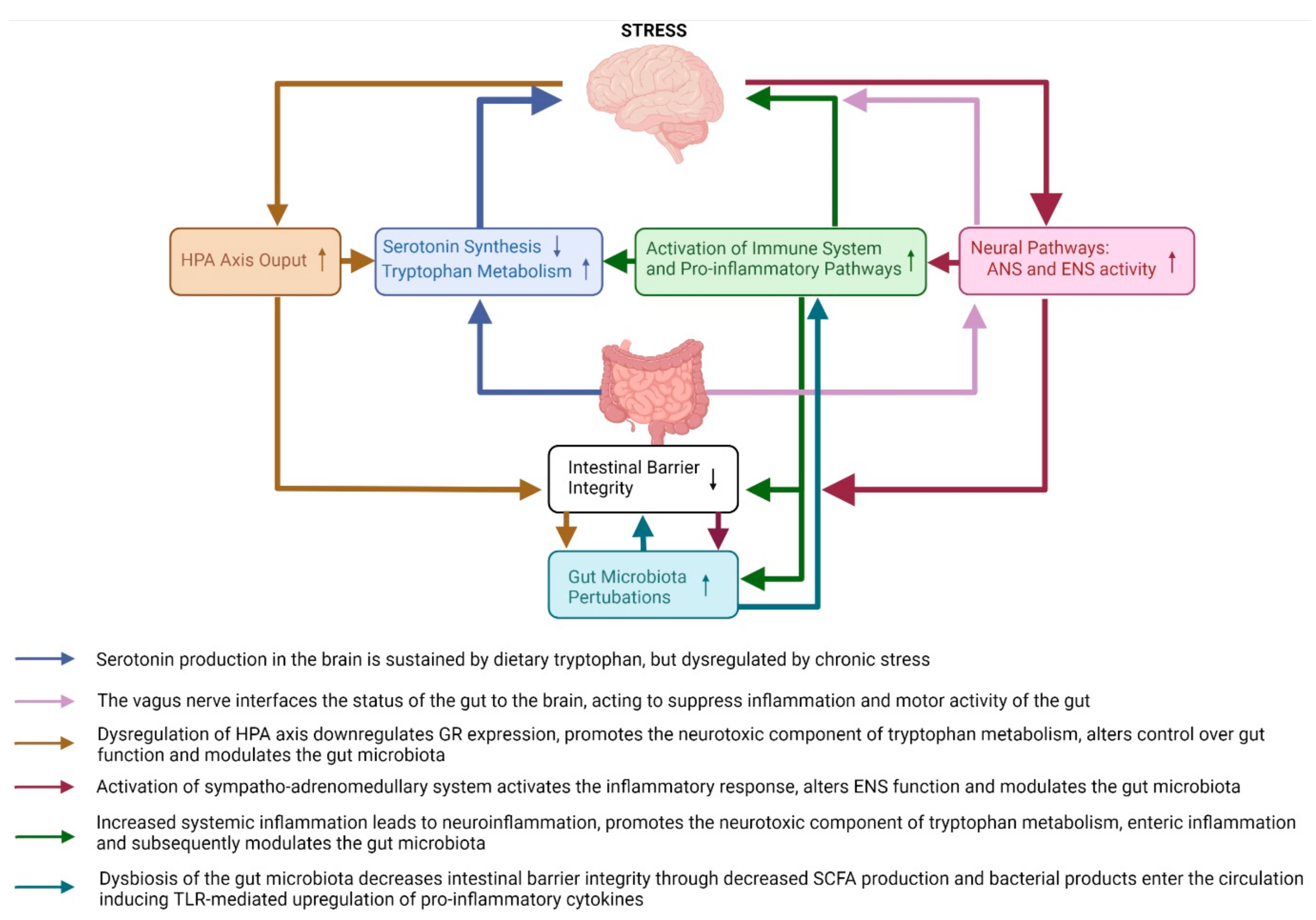
3. The Effects of Stress on the Bidirectional Pathways between the Brain and the Gut and Their Possible Contribution to Depressive Behaviours
3.1. Brain-to-Gut Pathways
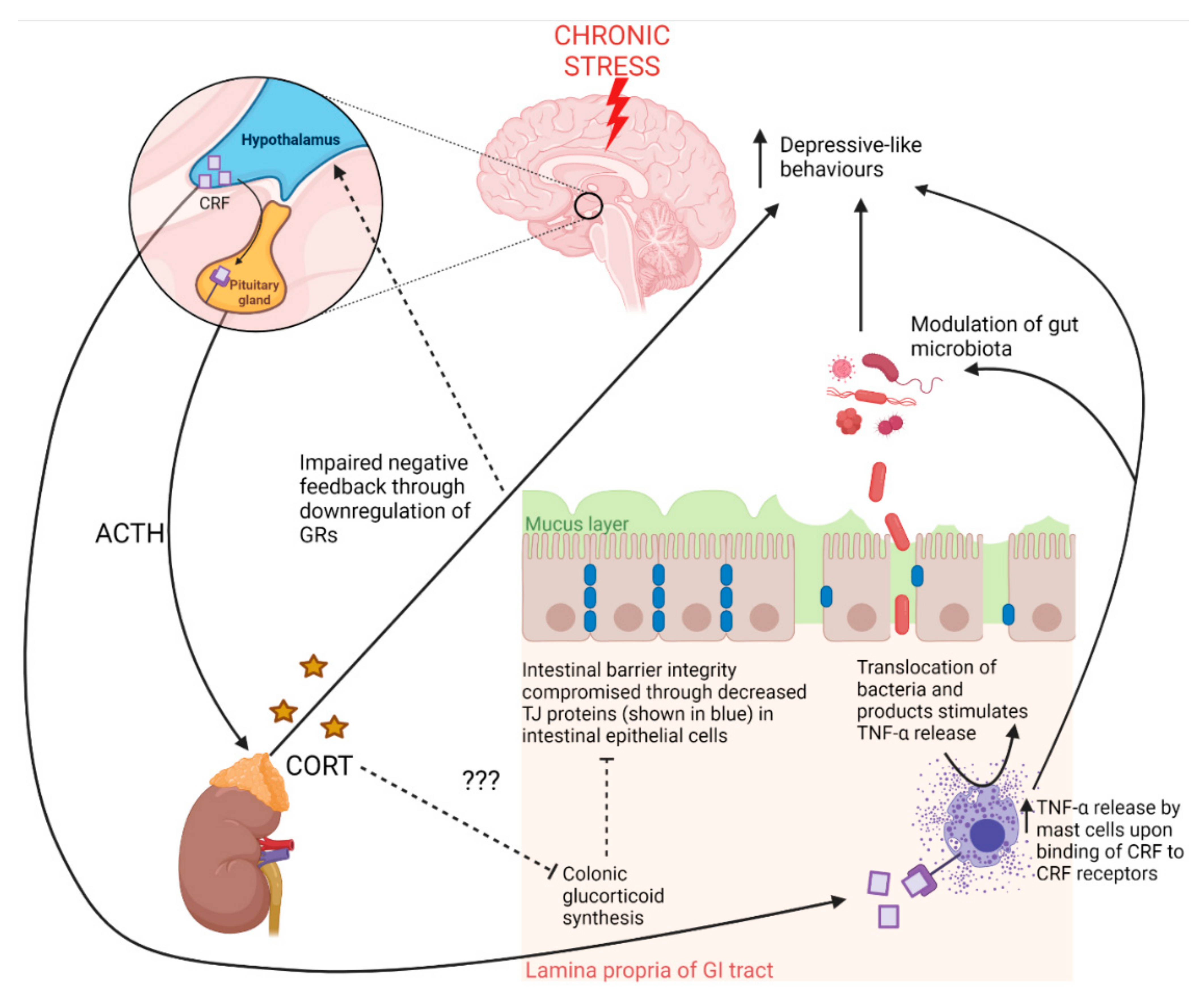
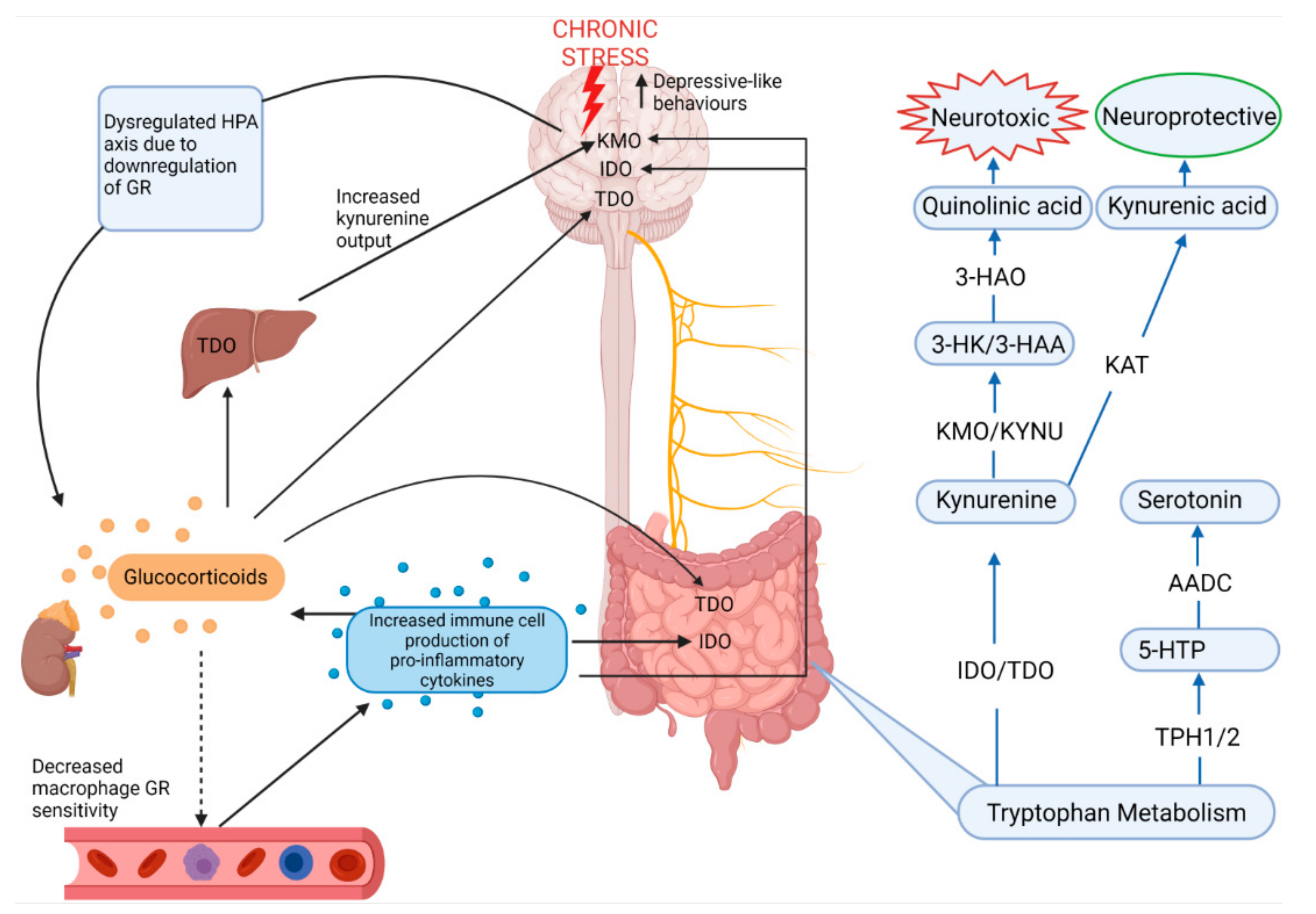
3.1.1. Neuroendocrine Pathways: HPA Axis
3.1.2. Neural Pathways
3.1.3. Serotonin and Tryptophan Pathways
3.2. Gut-to-Brain Pathways
3.2.1. Intestinal Barrier Integrity Pathways
3.2.2. Gut Microbiota Pathways
Gut Microbial Diversity
Gut Microbial Composition
4. The Effects of Diets on the “Gut–Brain” Pathways across Animal Models of Stress and Depression: Implications for Behaviour
4.1. Prebiotics and Probiotics Affect Neuroplasticty along the Gut–brain Axis
4.2. Diet, Stress and Intestinal Barrier Integrity
5. Conclusions and Future Directions
- Activation of HPA axis and ANS
- Inhibition of afferent vagal nerve fibres
- Increased intestinal, systemic inflammation and neuro-inflammation
- Dysregulation of tryptophan metabolism by upregulation of the kynurenine pathway in the brain, liver and gut
- Impairment of intestinal barrier integrity and reduction of mucus layer of the gut
- Gut microbial dysbiosis
- Prebiotics and probiotics show promise as dietary supplements which may alleviate depression pathophysiology
- Bifidobacteria and/or Lactobacilli supplementation restore neuroplasticity in key brain regions involved in depression
- Bifidobacteria and/or Lactobacilli supplementation restore intestinal barrier integrity and negative feedback in the HPA axis
- Westernised diets impair neuroplasticity in key brain regions involved in depression
- Westernised diets impair intestinal barrier integrity and the mucus layer of the gut
- Male bias exists in studies investigating the effects of stress on the multi-directional communication between the brain, the gut and gut microbiota
- The diversity and composition of the gut microbiota differ by sex, although further studies need to establish stress-induced alterations in females
- Probiotics reduce LPS-induced depressive-like behaviour in females and anxiety-like behaviour and stress activity associated with increased TLR4 expression in males
- Females may be more susceptible to the negative effects of high fat diets on the gut-brain axis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Czéh, B.; Fuchs, E.; Wiborg, O.; Simon, M. Animal models of major depression and their clinical implications. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.; Keshavan, M. The neurobiology of depression: An integrated view. Asian J. Psychiatry 2017, 27, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, B.H. Depression as a disease of modernity: Explanations for increasing prevalence. J. Affect. Disord. 2012, 140, 205–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milaneschi, Y.; Lamers, F.; Berk, M.; Penninx, B.W.J.B.P. Depression heterogeneity and its biological underpinnings: Toward immunometabolic depression. Biol. Psychiatry 2020, 88, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.G.; Goldenthal, A.R.; Uhlemann, A.-C.; Mann, J.J.; Miller, J.M.; Sublette, M.E. Systematic review of gut microbiota and major depression. Front. Psychiatry 2019, 10, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Timberlake, M.A.; Prall, K.; Dwivedi, Y. The recent progress in animal models of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 77, 99–109. [Google Scholar] [CrossRef]
- Ferrari, F.; Villa, R.F. The neurobiology of depression: An integrated overview from biological theories to clinical evidence. Mol. Neurobiol. 2017, 54, 4847–4865. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, G. Gut–brain axis and mood disorder. Front. Psychiatry 2018, 9, 223. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Hyland, N.P.; Dinan, T.G.; Cryan, J.F. Maternal separation as a model of brain–gut axis dysfunction. Psychopharmacology 2011, 214, 71–88. [Google Scholar] [CrossRef]
- Lyte, M.; Vulchanova, L.; Brown, D.R. Stress at the intestinal surface: Catecholamines and mucosa–bacteria interactions. Cell Tissue Res. 2011, 343, 23–32. [Google Scholar] [CrossRef]
- Bonaz, B.; Bazin, T.; Pellissier, S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef] [Green Version]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus nerve as modulator of the brain–gut axis in psychiatric and inflammatory disorders. Front. Psychiatry 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [PubMed] [Green Version]
- Taylor, A.M.; Holscher, H.D. A review of dietary and microbial connections to depression, anxiety, and stress. Nutr. Neurosci. 2020, 23, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Bear, T.L.; Dalziel, J.E.; Coad, J.; Roy, N.C.; Butts, C.A.; Gopal, P.K. The role of the gut microbiota in dietary interventions for depression and anxiety. Adv. Nutr. 2020, 11, 890–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opie, R.; Itsiopoulos, C.; Parletta, N.; Sanchez-Villegas, A.; Akbaraly, T.N.; Ruusunen, A.; Jacka, F. Dietary recommendations for the prevention of depression. Nutr. Neurosci. 2017, 20, 161–171. [Google Scholar] [CrossRef]
- Planchez, B.; Surget, A.; Belzung, C. Animal models of major depression: Drawbacks and challenges. J. Neural Transm. 2019, 126, 1383–1408. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.-Y.; Yin, C.-Y.; Zhu, L.-J.; Zhu, X.-H.; Xu, C.; Luo, C.-X.; Chen, H.; Zhu, D.-Y.; Zhou, Q.-G. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat. Protoc. 2018, 13, 1686–1698. [Google Scholar] [CrossRef]
- Sharma, S.; Rakoczy, S.; Brown-Borg, H. Assessment of spatial memory in mice. Life Sci. 2010, 87, 521–536. [Google Scholar] [CrossRef]
- Carvalho, C.; Herrmann, K.; Marques, T.A.; Knight, A. Time to abolish the forced swim test in rats for depression research? J. Appl. Anim. Ethics Res. 2021. [Google Scholar] [CrossRef]
- Oh, N.S.; Joung, J.Y.; Lee, J.Y.; Song, J.G.; Oh, S.; Kim, Y.; Kim, H.W.; Kim, S.H. Glycated milk protein fermented with Lactobacillus rhamnosus ameliorates the cognitive health of mice under mild-stress condition. Gut Microbes 2020, 11, 1643–1661. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.; Bagot, R.C. Defining valid chronic stress models for depression with female rodents. Biol. Psychiatry 2021, 90, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Amini-Khoei, H.; Haghani-Samani, E.; Beigi, M.; Soltani, A.; Mobini, G.R.; Balali-Dehkordi, S.; Haj-Mirzaian, A.; Rafieian-Kopaei, M.; Alizadeh, A.; Hojjati, M.R.; et al. On the role of corticosterone in behavioral disorders, microbiota composition alteration and neuroimmune response in adult male mice subjected to maternal separation stress. Int. Immunopharmacol. 2019, 66, 242–250. [Google Scholar] [CrossRef]
- Juruena, M.F. Early-life stress and hpa axis trigger recurrent adulthood depression. Epilepsy Behav. 2014, 38, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Pisu, M.G.; Garau, A.; Boero, G.; Biggio, F.; Pibiri, V.; Dore, R.; Locci, V.; Paci, E.; Porcu, P.; Serra, M. Sex differences in the outcome of juvenile social isolation on hpa axis function in rats. Neuroscience 2016, 320, 172–182. [Google Scholar] [CrossRef]
- Lundberg, S.; Abelson, K.S.P.; Nylander, I.; Roman, E. Few long-term consequences after prolonged maternal separation in female wistar rats. PLoS ONE 2017, 12, e0190042. [Google Scholar] [CrossRef] [Green Version]
- Galea, L.A.M.; McEwen, B.S.; Tanapat, P.; Deak, T.; Spencer, R.L.; Dhabhar, F.S. Sex differences in dendritic atrophy of ca3 pyramidal neurons in response to chronic restraint stress. Neuroscience 1997, 81, 689–697. [Google Scholar] [CrossRef]
- Deng, Y.; Zhou, M.; Wang, J.; Yao, J.; Yu, J.; Liu, W.; Wu, L.; Wang, J.; Gao, R. Involvement of the microbiota-gut-brain axis in chronic restraint stress: Disturbances of the kynurenine metabolic pathway in both the gut and brain. Gut Microbes 2021, 13, 1–16. [Google Scholar] [CrossRef]
- Gao, X.; Cao, Q.; Cheng, Y.; Zhao, D.; Wang, Z.; Yang, H.; Wu, Q.; You, L.; Wang, Y.; Lin, Y.; et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc. Natl. Acad. Sci. USA 2018, 115, E2960–E2969. [Google Scholar] [CrossRef] [Green Version]
- Kokras, N.; Dalla, C. Sex differences in animal models of psychiatric disorders. Br. J. Pharm. 2014, 171, 4595–4619. [Google Scholar] [CrossRef]
- Scholl, J.L.; Afzal, A.; Fox, L.C.; Watt, M.J.; Forster, G.L. Sex differences in anxiety-like behaviors in rats. Physiol. Behav. 2019, 211, 112670. [Google Scholar] [CrossRef] [PubMed]
- Vodička, M.; Ergang, P.; Hrnčíř, T.; Mikulecká, A.; Kvapilová, P.; Vagnerová, K.; Šestáková, B.; Fajstová, A.; Hermanová, P.; Hudcovic, T.; et al. Microbiota affects the expression of genes involved in hpa axis regulation and local metabolism of glucocorticoids in chronic psychosocial stress. Brain Behav. Immun. 2018, 73, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chang, J.; Long, N.; Beckwith, K.; Talhouarne, G.; Brooks, J.J.; Qu, M.H.; Ren, W.; Wood, J.D.; Cooper, S.; et al. Endogenous crf in rat large intestine mediates motor and secretory responses to stress. Neurogastroenterol. Motil. 2016, 28, 281–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, A.; Schmidt, C.; Brunner, T. Extra-adrenal glucocorticoid synthesis in the intestinal mucosa: Between immune homeostasis and immune escape. Front. Immunol. 2019, 10, 1438. [Google Scholar] [CrossRef]
- Seney, M.L.; Sibille, E. Sex differences in mood disorders: Perspectives from humans and rodent models. Biol. Sex Differ. 2014, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Mir, H.-D.; Milman, A.; Monnoye, M.; Douard, V.; Philippe, C.; Aubert, A.; Castanon, N.; Vancassel, S.; Guérineau, N.C.; Naudon, L.; et al. The gut microbiota metabolite indole increases emotional responses and adrenal medulla activity in chronically stressed male mice. Psychoneuroendocrinology 2020, 119, 104750. [Google Scholar] [CrossRef]
- Kostadinova, F.; Schwaderer, J.; Sebeo, V.; Brunner, T. Why does the gut synthesize glucocorticoids? Ann. Med. 2014, 46, 490–497. [Google Scholar] [CrossRef]
- Million, M.; Larauche, M. Stress, sex, and the enteric nervous system. Neurogastroenterol. Motil. 2016, 28, 1283–1289. [Google Scholar] [CrossRef] [Green Version]
- Hart, A.; Kamm, M.A. Mechanisms of initiation and perpetuation of gut inflammation by stress. Aliment. Pharmacol. Ther. 2002, 16, 2017–2028. [Google Scholar] [CrossRef]
- Bódi, N.; Szalai, Z.; Bagyánszki, M. Nitrergic enteric neurons in health and disease—Focus on animal models. Int. J. Mol. Sci. 2019, 20, 2003. [Google Scholar] [CrossRef] [Green Version]
- Marcondes Ávila, P.R.; Fiorot, M.; Michels, M.; Dominguini, D.; Abatti, M.; Vieira, A.; de Moura, A.B.; Behenck, J.P.; Borba, L.A.; Botelho, M.E.M.; et al. Effects of microbiota transplantation and the role of the vagus nerve in gut–brain axis in animals subjected to chronic mild stress. J. Affect. Disord. 2020, 277, 410–416. [Google Scholar] [CrossRef]
- Tian, P.; O’Riordan, K.J.; Lee, Y.-K.; Wang, G.; Zhao, J.; Zhang, H.; Cryan, J.F.; Chen, W. Towards a psychobiotic therapy for depression: Bifidobacterium breve ccfm1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol. Stress 2020, 12, 100216. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, J.; Gaurav, C.; Muhammad, U.; Chen, Y.; Li, X.; Chen, J.; Wang, Z. Cums and dexamethasone induce depression-like phenotypes in mice by differentially altering gut microbiota and triggering macroglia activation. Gen. Psychiatry 2021, 34, e100529. [Google Scholar] [CrossRef] [PubMed]
- Doney, E.; Cadoret, A.; Dion-Albert, L.; Lebel, M.; Menard, C. Inflammation-driven brain and gut barrier dysfunction in stress and mood disorders. Eur. J. Neurosci. 2021, 00, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Israeli, E.; Hershcovici, T.; Berenshtein, E.; Zannineli, G.; Wengrower, D.; Weiss, O.; Chevion, M.; Goldin, E. The effect of restraint stress on the normal colon and on intestinal inflammation in a model of experimental colitis. Dig. Dis. Sci. 2008, 53, 88–94. [Google Scholar] [CrossRef] [PubMed]
- González-Arancibia, C.; Urrutia-Piñones, J.; Illanes-González, J.; Martinez-Pinto, J.; Sotomayor-Zárate, R.; Julio-Pieper, M.; Bravo, J.A. Do your gut microbes affect your brain dopamine? Psychopharmacology 2019, 236, 1611–1622. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, D.; Tiano, S.; Esteban-Fernández, A.; Yuan, B.; Smith, C.; Brathwaite, J.; Jlayer, Z.; Wu, Q.; Simon, J.E. Prophylactic effect of flavanol rich preparation metabolites in promoting resilience to a mouse model of social stress. Transl. Psychiatry 2020, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Luo, C.; Zhang, W.; Chen, Y.; He, J.; Zhao, Q.; Zuo, R.; Wu, Y. Pro- and anti-inflammatory cytokines expression in rat’s brain and spleen exposed to chronic mild stress: Involvement in depression. Behav. Brain Res. 2011, 225, 135–141. [Google Scholar] [CrossRef]
- Keightley, P.C.; Koloski, N.A.; Talley, N.J. Pathways in gut-brain communication: Evidence for distinct gut-to-brain and brain-to-gut syndromes. Aust. N. Z. J. Psychiatry 2015, 49, 207–214. [Google Scholar] [CrossRef]
- Kraus, C.; Castrén, E.; Kasper, S.; Lanzenberger, R. Serotonin and neuroplasticity—Links between molecular, functional and structural pathophysiology in depression. Neurosci. Biobehav. Rev. 2017, 77, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wang, P.; Huang, L.; Li, P.; Zhang, D. Effects of regulating gut microbiota on the serotonin metabolism in the chronic unpredictable mild stress rat model. Neurogastroenterol. Motil. 2019, 31, e13677. [Google Scholar] [CrossRef] [Green Version]
- Lv, W.-J.; Wu, X.-L.; Chen, W.-Q.; Li, Y.-F.; Zhang, G.-F.; Chao, L.-M.; Zhou, J.-H.; Guo, A.; Liu, C.; Guo, S.-N. The gut microbiome modulates the changes in liver metabolism and in inflammatory processes in the brain of chronic unpredictable mild stress rats. Oxid. Med. Cell. Longev. 2019, 2019, 7902874. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Wang, F.; Hu, X.; Yang, C.; Xu, H.; Yao, Y.; Liu, J. Clostridium butyricum attenuates chronic unpredictable mild stress-induced depressive-like behavior in mice via the gut-brain axis. J. Agric. Food Chem. 2018, 66, 8415–8421. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Zou, R.; Song, L.; Zhang, X.; Jiang, B.; Wang, G.; Lee, Y.-K.; Zhao, J.; Zhang, H.; Chen, W. Ingestion of bifidobacterium longum subspecies infantis strain ccfm687 regulated emotional behavior and the central bdnf pathway in chronic stress-induced depressive mice through reshaping the gut microbiota. Food Funct. 2019, 10, 7588–7598. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Rohleder, N. Acute and chronic stress induced changes in sensitivity of peripheral inflammatory pathways to the signals of multiple stress systems—2011 curt richter award winner. Psychoneuroendocrinology 2012, 37, 307–316. [Google Scholar] [CrossRef]
- Caspani, G.; Kennedy, S.; Foster, J.A.; Swann, J. Gut microbial metabolites in depression: Understanding the biochemical mechanisms. Microb. Cell 2019, 6, 454–481. [Google Scholar] [CrossRef]
- Dehhaghi, M.; Kazemi Shariat Panahi, H.; Guillemin, G.J. Microorganisms, tryptophan metabolism, and kynurenine pathway: A complex interconnected loop influencing human health status. Int. J. Tryptophan Res. 2019, 12. [Google Scholar] [CrossRef] [Green Version]
- Li, C.-C.; Jiang, N.; Gan, L.; Zhao, M.-J.; Chang, Q.; Liu, X.-M.; Pan, R.-L. Peripheral and cerebral abnormalities of the tryptophan metabolism in the depression-like rats induced by chronic unpredicted mild stress. Neurochem. Int. 2020, 138, 104771. [Google Scholar] [CrossRef]
- Badawy, A.A.B. Kynurenine pathway of tryptophan metabolism: Regulatory and functional aspects. Int. J. Tryptophan Res. 2017, 10. [Google Scholar] [CrossRef] [Green Version]
- Silverman, M.N.; Sternberg, E.M. Glucocorticoid regulation of inflammation and its functional correlates: From hpa axis to glucocorticoid receptor dysfunction. Ann. N. Y. Acad. Sci. 2012, 1261, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Angoa-Pérez, M.; Zagorac, B.; Francescutti, D.M.; Theis, K.R.; Kuhn, D.M. Responses to chronic corticosterone on brain glucocorticoid receptors, adrenal gland, and gut microbiota in mice lacking neuronal serotonin. Brain Res. 2021, 1751, 147190. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Xue, F.; Yu, S.-F.; Li, X.-S.; Liu, L.; Jia, Y.-Y.; Yan, W.-J.; Tan, Q.-R.; Wang, H.-N.; Peng, Z.-W. Gut microbiota dysbiosis in depressed women: The association of symptom severity and microbiota function. J. Affect. Disord. 2021, 282, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Farzi, A.; Ke, X.; Yu, Y.; Chen, C.; Chen, S.; Yu, T.; Wang, H.; Li, Y. Oral administration of lactococcus lactis whh2078 alleviates depressive and anxiety symptoms in mice with induced chronic stress. Food Funct. 2022, 13, 957–969. [Google Scholar] [CrossRef]
- Liang, S.; Wang, T.; Hu, X.; Luo, J.; Li, W.; Wu, X.; Duan, Y.; Jin, F. Administration of lactobacillus helveticus ns8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 2015, 310, 561–577. [Google Scholar] [CrossRef]
- Wang, H.; He, S.; Xin, J.; Zhang, T.; Sun, N.; Li, L.; Ni, X.; Zeng, D.; Ma, H.; Bai, Y. Psychoactive effects of lactobacillus johnsonii against restraint stress-induced memory dysfunction in mice through modulating intestinal inflammation and permeability—A study based on the gut–brain axis hypothesis. Front. Pharmacol. 2021, 12, 1299. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.-L.; Wang, S.; Yen, J.-T.; Cheng, Y.-F.; Liao, C.-L.; Hsu, C.-C.; Wu, C.-C.; Tsai, Y.-C. Antidepressant-like activities of live and heat-killed Lactobacillus paracasei ps23 in chronic corticosterone-treated mice and possible mechanisms. Brain Res. 2019, 1711, 202–213. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, J.; Xiong, Y.; Zhang, X.; Lin, Y.; Liu, Z. Jasmine tea attenuates chronic unpredictable mild stress-induced depressive-like behavior in rats via the gut-brain axis. Nutrients 2021, 14, 99. [Google Scholar] [CrossRef]
- Xiao, Q.; Shu, R.; Wu, C.; Tong, Y.; Xiong, Z.; Zhou, J.; Yu, C.; Xie, X.; Fu, Z. Crocin-i alleviates the depression-like behaviors probably via modulating “microbiota-gut-brain” axis in mice exposed to chronic restraint stress. J. Affect. Disord. 2020, 276, 476–486. [Google Scholar] [CrossRef]
- Brun, P.; Gobbo, S.; Caputi, V.; Spagnol, L.; Schirato, G.; Pasqualin, M.; Levorato, E.; Palù, G.; Giron, M.C.; Castagliuolo, I. Toll like receptor-2 regulates production of glial-derived neurotrophic factors in murine intestinal smooth muscle cells. Mol. Cell. Neurosci. 2015, 68, 24–35. [Google Scholar] [CrossRef]
- Kunugi, H. Gut microbiota and pathophysiology of depressive disorder. Ann. Nutr. Metab. 2021, 77 (Suppl. 2), 11–20. [Google Scholar] [CrossRef] [PubMed]
- Segarra, M.; Aburto, M.R.; Acker-Palmer, A. Blood–brain barrier dynamics to maintain brain homeostasis. Trends Neurosci. 2021, 44, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.E.; Teixeira, A.L. Inflammation in psychiatric disorders: What comes first?: Inflammation in psychiatric disorders. Ann. N. Y. Acad. Sci. 2019, 1437, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Stenman, L.K.; Patterson, E.; Meunier, J.; Roman, F.J.; Lehtinen, M.J. Strain specific stress-modulating effects of candidate probiotics: A systematic screening in a mouse model of chronic restraint stress. Behav. Brain Res. 2020, 379, 112376. [Google Scholar] [CrossRef]
- Murray, E.; Sharma, R.; Smith, K.B.; Mar, K.D.; Barve, R.; Lukasik, M.; Pirwani, A.F.; Malette-Guyon, E.; Lamba, S.; Thomas, B.J.; et al. Probiotic consumption during puberty mitigates lps-induced immune responses and protects against stress-induced depression- and anxiety-like behaviors in adulthood in a sex-specific manner. Brain Behav. Immun. 2019, 81, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Nakamura, K.; Shimizu, Y.; Yokoi, Y.; Ohira, S.; Hagiwara, M.; Wang, Y.; Song, Y.; Aizawa, T.; Ayabe, T. Decrease of α-defensin impairs intestinal metabolite homeostasis via dysbiosis in mouse chronic social defeat stress model. Sci. Rep. 2021, 11, 9915. [Google Scholar] [CrossRef]
- Tian, T.; Xu, B.; Qin, Y.; Fan, L.; Chen, J.; Zheng, P.; Gong, X.; Wang, H.; Bai, M.; Pu, J.; et al. Clostridium butyricum miyairi 588 has preventive effects on chronic social defeat stress-induced depressive-like behaviour and modulates microglial activation in mice. Biochem. Biophys. Res. Commun. 2019, 516, 430–436. [Google Scholar] [CrossRef]
- Yamagishi, N.; Omata, Y.; Aoki-Yoshida, A.; Moriya, N.; Goto, T.; Toyoda, A.; Aoki, R.; Suzuki, C.; Takayama, Y. Comparison of gut tight junction gene expression in c57bl/6j and balb/c mice after chronic social defeat stress. Jpn. Agric. Res. Q. JARQ 2019, 53, 41–46. [Google Scholar] [CrossRef]
- Salzman, N.H.; Bevins, C.L. Dysbiosis—A consequence of paneth cell dysfunction. Semin. Immunol. 2013, 25, 334–341. [Google Scholar] [CrossRef]
- Fang, J.; Wang, H.; Zhou, Y.; Zhang, H.; Zhou, H.; Zhang, X. Slimy partners: The mucus barrier and gut microbiome in ulcerative colitis. Exp. Mol. Med. 2021, 53, 772–787. [Google Scholar] [CrossRef]
- Stahl, M.; Tremblay, S.; Montero, M.; Vogl, W.; Xia, L.; Jacobson, K.; Menendez, A.; Vallance, B.A. The muc2 mucin coats murine paneth cell granules and facilitates their content release and dispersion. Am. J. Physiol.-Gastrointest. Liver Physiol. 2018, 315, G195–G205. [Google Scholar] [CrossRef] [PubMed]
- Singhal, G.; Baune, B.T. Microglia: An interface between the loss of neuroplasticity and depression. Front. Cell. Neurosci. 2017, 11, 270. [Google Scholar] [CrossRef] [Green Version]
- Egerton, S.; Donoso, F.; Fitzgerald, P.; Gite, S.; Fouhy, F.; Whooley, J.; Dinan, T.G.; Cryan, J.F.; Culloty, S.C.; Ross, R.P.; et al. Investigating the potential of fish oil as a nutraceutical in an animal model of early life stress. Nutr. Neurosci. 2020, 1–23. [Google Scholar] [CrossRef]
- Methiwala, H.N.; Vaidya, B.; Addanki, V.K.; Bishnoi, M.; Sharma, S.S.; Kondepudi, K.K. Gut microbiota in mental health and depression: Role of pre/pro/synbiotics in their modulation. Food Funct. 2021, 12, 4284–4314. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Hreins, E.; Goldstone, A.P.; Brown, R.M.; Sumithran, P. The therapeutic potential of glp-1 analogues for stress-related eating and role of glp-1 in stress, emotion and mood: A review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 110, 110303. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Alvarez-Mon, M.A.; García-Montero, C.; Fraile-Martinez, O.; Guijarro, L.G.; Lahera, G.; Monserrat, J.; Valls, P.; Mora, F.; Rodríguez-Jiménez, R.; et al. Gut microbiota metabolites in major depressive disorder-deep insights into their pathophysiological role and potential translational applications. Metabolites 2022, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.M.-S.; Mohajeri, M.H. The role of gut bacterial metabolites in brain development, aging and disease. Nutrients 2021, 13, 732. [Google Scholar] [CrossRef]
- Kaiser, H.; Parker, E.; Hamrick, M.W. Kynurenine signaling through the aryl hydrocarbon receptor: Implications for aging and healthspan. Exp. Gerontol. 2020, 130, 110797. [Google Scholar] [CrossRef]
- Więdłocha, M.; Marcinowicz, P.; Janoska-Jaździk, M.; Szulc, A. Gut microbiota, kynurenine pathway and mental disorders—Review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 106, 110145. [Google Scholar] [CrossRef]
- Walter, J.; Armet, A.M.; Finlay, B.B.; Shanahan, F. Establishing or exaggerating causality for the gut microbiome: Lessons from human microbiota-associated rodents. Cell 2020, 180, 221–232. [Google Scholar] [CrossRef]
- Kelly, J.R.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-gut-microbiota axis: Challenges for translation in psychiatry. Ann. Epidemiol. 2016, 26, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Maltz, R.M.; Keirsey, J.; Kim, S.C.; Mackos, A.R.; Gharaibeh, R.Z.; Moore, C.C.; Xu, J.; Bakthavatchalu, V.; Somogyi, A.; Bailey, M.T. Prolonged restraint stressor exposure in outbred cd-1 mice impacts microbiota, colonic inflammation, and short chain fatty acids. PLoS ONE 2018, 13, e0196961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bridgewater, L.C.; Zhang, C.; Wu, Y.; Hu, W.; Zhang, Q.; Wang, J.; Li, S.; Zhao, L. Gender-based differences in host behavior and gut microbiota composition in response to high fat diet and stress in a mouse model. Sci. Rep. 2017, 7, 10776. [Google Scholar] [CrossRef] [Green Version]
- Kai, Z.; Yuko, F.; Lijia, C.; Youge, Q.; Yaoyu, P.; Siming, W.; Yukihiko, S.; Kenji, H. Abnormal composition of gut microbiota is associated with resilience versus susceptibility to inescapable electric stress. Transl. Psychiatry 2019, 9, 231. [Google Scholar]
- Li, H.; Xiang, Y.; Zhu, Z.; Wang, W.; Jiang, Z.; Zhao, M.; Cheng, S.; Pan, F.; Liu, D.; Ho, R.C.M.; et al. Rifaximin-mediated gut microbiota regulation modulates the function of microglia and protects against cums-induced depression-like behaviors in adolescent rat. J. Neuroinflamm. 2021, 18, 254. [Google Scholar] [CrossRef] [PubMed]
- Han, S.K.; Kim, J.K.; Park, H.S.; Shin, Y.J.; Kim, D.H. Chaihu-shugan-san (shihosogansan) alleviates restraint stress-generated anxiety and depression in mice by regulating nf-κb-mediated bdnf expression through the modulation of gut microbiota. Chin. Med. 2021, 16, 77. [Google Scholar] [CrossRef]
- Karen, C.; Shyu, D.J.H.; Rajan, K.E. Lactobacillus paracasei supplementation prevents early life stress-induced anxiety and depressive-like behavior in maternal separation model-possible involvement of microbiota-gut-brain axis in differential regulation of microrna124a/132 and glutamate receptors. Front. Neurosci. 2021, 15. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.-F.; Ma, C.-L.; Wei, H.; Li, B.-M.; Luo, J. Alleviation of anxiety/depressive-like behaviors and improvement of cognitive functions by Lactobacillus plantarum wlpl04 in chronically stressed mice. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 6613903. [Google Scholar] [CrossRef]
- Guo, Y.; Xie, J.-P.; Deng, K.; Li, X.; Yuan, Y.; Xuan, Q.; Xie, J.; He, X.-M.; Wang, Q.; Li, J.-J.; et al. Prophylactic effects of bifidobacterium adolescentis on anxiety and depression-like phenotypes after chronic stress: A role of the gut microbiota-inflammation axis. Front. Behav. Neurosci. 2019, 13, 126. [Google Scholar] [CrossRef]
- Szyszkowicz, J.K.; Wong, A.; Anisman, H.; Merali, Z.; Audet, M.-C. Implications of the gut microbiota in vulnerability to the social avoidance effects of chronic social defeat in male mice. Brain Behav. Immun. 2017, 66, 45–55. [Google Scholar] [CrossRef]
- Wu, E.; Song, J.; Pei, L.; Ling, Y. Comparison of the gut microbiota disturbance in rat models of irritable bowel syndrome induced by maternal separation and multiple early-life adversity. Front. Cell. Infect. Microbiol. 2021, 10, 581974. [Google Scholar]
- Barandouzi, Z.A.; Starkweather, A.R.; Henderson, W.A.; Gyamfi, A.; Cong, X.S. Altered composition of gut microbiota in depression: A systematic review. Front. Psychiatry 2020, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.; Yoo, J.Y.; Park, M.R.; Ryu, S.; Lee, W.J.; Choi, H.J.; Kang, M.K.; Kim, Y.; Oh, S. Ingestion of gouda cheese ameliorates the chronic unpredictable mild stress in mice. Food Sci. Anim. Resour. 2020, 40, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, J.; Huang, Y.; Tan, X.; Chai, T.; Wu, J.; Zhang, H.; Li, Y.; Hu, X.; Zheng, P.; Ji, P.; et al. Characterization of gut microbiome in mice model of depression with divergent response to escitalopram treatment. Transl. Psychiatry 2021, 11, 303. [Google Scholar] [CrossRef]
- Jaffe, D.H.; Rive, B.; Denee, T.R. The humanistic and economic burden of treatment-resistant depression in Europe: A cross-sectional study. BMC Psychiatry 2019, 19, 247. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Peng, Y.; Wang, J.; Ma, P.; Zhao, L.; Li, X. Total glycosides from stems of cistanche tubulosa alleviate depression-like behaviors: Bidirectional interaction of the phytochemicals and gut microbiota. Phytomedicine 2021, 83, 153471. [Google Scholar] [CrossRef]
- Gu, F.; Wu, Y.; Liu, Y.; Dou, M.; Jiang, Y.; Liang, H. Lactobacillus casei improves depression-like behavior in chronic unpredictable mild stress-induced rats by the bdnf-trkb signal pathway and the intestinal microbiota. Food Funct. 2020, 11, 6148–6157. [Google Scholar] [CrossRef]
- Song, X.; Wang, W.; Ding, S.; Liu, X.; Wang, Y.; Ma, H. Puerarin ameliorates depression-like behaviors of with chronic unpredictable mild stress mice by remodeling their gut microbiota. J. Affect. Disord. 2021, 290, 353–363. [Google Scholar] [CrossRef]
- Yu, M.; Jia, H.; Zhou, C.; Yang, Y.; Zhao, Y.; Yang, M.; Zou, Z. Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16s rrna gene sequencing and lc/ms-based metabolomics. J. Pharm. Biomed. Anal. 2017, 138, 231–239. [Google Scholar] [CrossRef]
- Zhang, W.; Qu, W.; Wang, H.; Yan, H. Antidepressants fluoxetine and amitriptyline induce alterations in intestinal microbiota and gut microbiome function in rats exposed to chronic unpredictable mild stress. Transl. Psychiatry 2021, 11, 131. [Google Scholar] [CrossRef]
- Hatton-Jones, K.M.; du Toit, E.F.; Cox, A.J. Effect of chronic restraint stress and western-diet feeding on colonic regulatory gene expression in mice. Neurogastroenterol. Motil. 2021, e14300. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.L.; Inserra, A.; Lewis, M.D.; Mastronardi, C.A.; Leong, L.; Choo, J.; Kentish, S.; Xie, P.; Morrison, M.; Wesselingh, S.L.; et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol. Psychiatry 2016, 21, 797–805. [Google Scholar] [CrossRef]
- Wu, M.; Tian, T.; Mao, Q.; Zou, T.; Zhou, C.-J.; Xie, J.; Chen, J.-J. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl. Psychiatry 2020, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Xiao, Q.; Xiong, Z.; Yu, C.; Zhou, J.; Fu, Z. Crocin-i ameliorates the disruption of lipid metabolism and dysbiosis of the gut microbiota induced by chronic corticosterone in mice. Food Funct. 2019, 10, 6779–6791. [Google Scholar] [CrossRef] [PubMed]
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.L.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Targeting the microbiota-gut-brain axis: Prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol. Psychiatry 2017, 82, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Jiang, P.; Lin, L.; Jiang, J.; Yu, B.; Rao, J.; Liu, H.; Wei, W.; Qiao, Y. Oral treatment with lactobacillus reuteri attenuates depressive-like behaviors and serotonin metabolism alterations induced by chronic social defeat stress. J. Psychiatr. Res. 2020, 122, 70–78. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, K.; Pu, Y.; Chang, L.; Wang, S.; Tan, Y.; Wang, X.; Zhang, J.; Ohnishi, T.; Yoshikawa, T.; et al. Betaine supplementation is associated with the resilience in mice after chronic social defeat stress: A role of brain–gut–microbiota axis. J. Affect. Disord. 2020, 272, 66–76. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.; Gasbarrini, A.; Mele, M. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wan, M.; Zhong, Y.; Gao, T.; Zhang, Y.; Yan, F.; Huang, D.; Wu, Y.; Weng, Z. Partially hydrolyzed guar gum modulates gut microbiota, regulates the levels of neurotransmitters, and prevents cums-induced depressive-like behavior in mice. Mol. Nutr. Food Res. 2021, 65, 2100146. [Google Scholar] [CrossRef]
- Lin, R.; Wang, Z.; Cao, J.; Gao, T.; Dong, Y.; Chen, Y. Role of melatonin in murine “restraint stress”-induced dysfunction of colonic microbiota. J. Microbiol. 2021, 59, 500–512. [Google Scholar] [CrossRef]
- Fukui, H.; Oshima, T.; Tanaka, Y.; Oikawa, Y.; Makizaki, Y.; Ohno, H.; Tomita, T.; Watari, J.; Miwa, H. Effect of probiotic bifidobacterium bifidum g9-1 on the relationship between gut microbiota profile and stress sensitivity in maternally separated rats. Sci. Rep. 2018, 8, 12384. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.C.; Vuong, H.E.; Luna, C.D.G.; Pronovost, G.N.; Aleksandrova, A.A.; Riley, N.G.; Vavilina, A.; McGinn, J.; Rendon, T.; Forrest, L.R.; et al. Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat. Microbiol. 2019, 4, 2064–2073. [Google Scholar] [CrossRef]
- Fidalgo, S.; Ivanov, D.K.; Wood, S.H. Serotonin: From top to bottom. Biogerontology 2012, 14, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Asano, Y.; Yoshihara, K.; Kimura-Todani, T.; Miyata, N.; Zhang, X.-T.; Takakura, S.; Aiba, Y.; Koga, Y.; Sudo, N. Regulation of gut luminal serotonin by commensal microbiota in mice. PLoS ONE 2017, 12, e0180745. [Google Scholar] [CrossRef]
- Yang, H.-L.; Li, M.-M.; Zhou, M.-F.; Xu, H.-S.; Huan, F.; Liu, N.; Gao, R.; Wang, J.; Zhang, N.; Jiang, L. Links between gut dysbiosis and neurotransmitter disturbance in chronic restraint stress-induced depressive behaviours: The role of inflammation. Inflammation 2021, 44, 2448–2462. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, S.A.; Kang, W.S.; Kim, J.W. Early-life stress modulates gut microbiota and peripheral and central inflammation in a sex-dependent manner. Int. J. Mol. Sci. 2021, 22, 1899. [Google Scholar] [CrossRef]
- Yang, C.; Fujita, Y.; Ren, Q.; Ma, M.; Dong, C.; Hashimoto, K. Bifidobacterium in the gut microbiota confer resilience to chronic social defeat stress in mice. Sci. Rep. 2017, 7, 45942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mika, A.; Day, H.E.W.; Martinez, A.; Rumian, N.L.; Greenwood, B.N.; Chichlowski, M.; Berg, B.M.; Fleshner, M. Early life diets with prebiotics and bioactive milk fractions attenuate the impact of stress on learned helplessness behaviours and alter gene expression within neural circuits important for stress resistance. Eur. J. Neurosci. 2017, 45, 342–357. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, N.; Park, J.H.; Nam, R.H.; Yoon, K.; Lee, D.H. Comparative analysis of ileal and cecal microbiota in aged rats. J. Cancer Prev. 2018, 23, 70. [Google Scholar] [CrossRef] [Green Version]
- Marteau, P.; Pochart, P.; Doré, J.; Béra-Maillet, C.; Bernalier, A.; Corthier, G. Comparative study of bacterial groups within the human cecal and fecal microbiota. Appl. Environ. Microbiol. 2001, 67, 4939–4942. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.; Verma, M.K.; Chauhan, N.S. A review of metabolic potential of human gut microbiome in human nutrition. Arch. Microbiol. 2018, 200, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, A.; Shimonishi, H.; Sato, M.; Usuda, K.; Ohsawa, N.; Nagaoka, K. Effects of non-purified and semi-purified commercial diets on behaviors, plasma corticosterone levels, and cecum microbiome in c57bl/6j mice. Neurosci. Lett. 2018, 670, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Kubota, Y.; Toyoda, A. Effects of diet quality on vulnerability to mild subchronic social defeat stress in mice. Nutr. Neurosci. 2016, 19, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Caracci, F.; Harary, J.; Simkovic, S.; Pasinetti, G.M. Grape-derived polyphenols ameliorate stress-induced depression by regulating synaptic plasticity. J. Agric. Food Chem. 2020, 68, 1808–1815. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Marx, W.; Dash, S.; Carney, R.; Teasdale, S.B.; Solmi, M.; Stubbs, B.; Schuch, F.B.; Carvalho, A.F.; Jacka, F.; et al. The effects of dietary improvement on symptoms of depression and anxiety: A meta-analysis of randomized controlled trials. Psychosom. Med. 2019, 81, 265–280. [Google Scholar] [CrossRef]
- Filosa, S.; Di Meo, F.; Crispi, S. Polyphenols-gut microbiota interplay and brain neuromodulation. Neural Regen. Res. 2018, 13, 2055–2059. [Google Scholar]
- Murakami, Y.; Saito, K. Species and cell types difference in tryptophan metabolism. Int. J. Tryptophan Res. 2013, 6, 47–54. [Google Scholar] [CrossRef]
- Moussaoui, N.; Larauche, M.; Biraud, M.; Molet, J.; Million, M.; Mayer, E.; Taché, Y. Limited nesting stress alters maternal behavior and in vivo intestinal permeability in male wistar pup rats. PLoS ONE 2016, 11, e0155037. [Google Scholar] [CrossRef] [Green Version]
- Lam, Y.Y.; Ha, C.W.Y.; Campbell, C.R.; Mitchell, A.J.; Dinudom, A.; Oscarsson, J.; Cook, D.I.; Hunt, N.H.; Caterson, I.D.; Holmes, A.J.; et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS ONE 2012, 7, e34233. [Google Scholar]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007, 50, 2374–2383. [Google Scholar] [CrossRef] [Green Version]
- Rincel, M.; Lépinay, A.L.; Delage, P.; Fioramonti, J.; Théodorou, V.S.; Layé, S.; Darnaudéry, M. Maternal high-fat diet prevents developmental programming by early-life stress. Transl. Psychiatry 2016, 6, e966. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, D.; Cecconello, A.L.; Partata, W.A.; de Fraga, L.S.; Ribeiro, M.F.M.; Guedes, R.P. The metabolic and neuroinflammatory changes induced by consuming a cafeteria diet are age-dependent. Nutr. Neurosci. 2019, 22, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Wait, J.; Burns, C.; Jones, T.; Harper, Z.; Allen, E.; Langley-Evans, S.C.; Voigt, J.-P. Early postnatal exposure to a cafeteria diet interferes with recency and spatial memory, but not open field habituation in adolescent rats. Dev. Psychobiol. 2021, 63, 572–581. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Rodrigues, M.E.; Bekhbat, M.; Houser, M.C.; Chang, J.; Walker, D.I.; Jones, D.P.; Oller do Nascimento, C.M.P.; Barnum, C.J.; Tansey, M.G. Chronic psychological stress and high-fat high-fructose diet disrupt metabolic and inflammatory gene networks in the brain, liver, and gut and promote behavioral deficits in mice. Brain Behav. Immun. 2017, 59, 158–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative effects of a high-fat diet on intestinal permeability: A review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef] [Green Version]
- del Bas, J.M.; Guirro, M.; Boqué, N.; Cereto, A.; Ras, R.; Crescenti, A.; Caimari, A.; Canela, N.; Arola, L. Alterations in gut microbiota associated with a cafeteria diet and the physiological consequences in the host. Int. J. Obes. 2018, 42, 746–754. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Martire, S.I.; Raipuria, M.; Mitchell, H.M.; Nielsen, S.; Westbrook, R.F.; Morris, M.J. Alternating or continuous exposure to cafeteria diet leads to similar shifts in gut microbiota compared to chow diet. Mol. Nutr. Food Res. 2017, 61, 1500815. [Google Scholar] [CrossRef] [Green Version]
- Ranyah Shaker, M.L.; Alfawaz, H.; Almnaizel, A.T.; Hassan, W.M.; Ramesa Shafi, B.; Nadine, M.S.M.; Bjørklund, G.; El-Ansary, A. High-fat diet-induced obesity and impairment of brain neurotransmitter pool. Transl. Neurosci. 2020, 11, 147–160. [Google Scholar]
- Saiyasit, N.; Chunchai, T.; Prus, D.; Suparan, K.; Pittayapong, P.; Apaijai, N.; Pratchayasakul, W.; Sripetchwandee, J.; Chattipakorn, M.D.P.D.N.; Chattipakorn, S.C. Gut dysbiosis develops before metabolic disturbance and cognitive decline in high-fat diet–induced obese condition. Nutrition 2020, 69, 110576. [Google Scholar] [CrossRef]


| Stress Model | Phylum | Sample Site | |||
|---|---|---|---|---|---|
| Firmicutes | Bacteroidetes | Actinobacteria | Proteobacteria | ||
| CUMS | Increased [21,42,51,52,54,64,68,106] | Increased [21,95,104,107,108,109] | Increased [34,42,52,54,108] | Increased [21,108] | Cecum [51,106] |
| Decreased [63,95,104,107,108,109] | Decreased [42,51,52,54,64,68] | Decreased [104] | Decreased [42] | Faecal pellets [21,42,52,54,63,64,68,95,103,104,107,108,109,110] | |
| No change [110] | No change [103,106,110] | - | No change [54] | ||
| CRS | - | Increased [69,98,99] | - | Increased [69,96,111] | Cecum [69,99,111] |
| Decreased [98,99] | - | Decreased [96,98] | - | Faecal pellets [28,96,98,112,113] | |
| No change [28,112,113] | No change [28,34,112,113] | No change [28,113] | No change [28,113] | ||
| MS | - | - | - | - | - |
| CORT | Increased [62,114] | Decreased [62,114] | Increased [114] | Cecum [114] | |
| CSDS | Decreased [76] | Increased [76] | Increased [76] | Increased [76,115] | Cecum [100,115] |
| - | - | Decreased [115] | Decreased [100,116] | Faecal pellets [76,117] | |
| No change [100,116,117] | No change [100,116] | - | - | Colonic content [116] | |
| LH | - | - | - | - | - |
| Stress Model | Genus | Sample Site | |||||
|---|---|---|---|---|---|---|---|
| Lactobacillus | Bacteroides | Clostridium | Bifidobacterium | Allobaculum | Turicibacter | ||
| CUMS | Increased [64,68] | Increased [21,68,104] | Increased [68] | Increased [42,108] | Increased [42,54] | - | Cecum [51] |
| Decreased [21,52,108,109] | Decreased [108,110] | Decreased [63] | Decreased [104] | - | Decreased [54] | Faecal pellets [21,42,52,54,63,64,68,103,104,108,109,110] | |
| - | - | No change [21] | - | - | No change [21] | ||
| CRS | Increased [125] | Increased [99,120] | - | - | - | - | Cecum [69,99] |
| Decreased [69,92,99] | Decreased [69] | Decreased [112] | Decreased [112] | Decreased [112,113] | Decreased [112] | Faecal pellets [112,113,120,125] | |
| Mid-colonic section [92] | |||||||
| MS | Increased [23] | Increased [101,121] | - | Increased [23] | - | - | Faecal pellets [23,83,97,101,121,126] |
| Decreased [97] | Decreased [97] | Decreased [101,121] | - | - | Decreased [101] | ||
| - | No change [83] | - | - | - | |||
| CORT | - | - | - | Decreased [67] | Decreased [114] | - | Cecum [114] |
| Faecal pellets [67] | |||||||
| CSDS | - | - | Increased [117] | - | - | - | Cecum [100,115,127] |
| Decreased [116] | - | - | Decreased [115] | Decreased [115,116] | Decreased [100] | Faecal pellets [117] | |
| - | No change [100,127] | No change [100] | No change [116] | - | - | Colonic content [116] | |
| LH | Increased [94] | - | Increased [94] | - | - | - | Faecal pellets [94,128] |
| No change [128] | - | ||||||
| Model, Duration and Species | Diet/Treatment | Gut Pathology | Behaviour | Possible Gut–brain Pathways | Authors |
|---|---|---|---|---|---|
| Chronic unpredictable mild stress (CUMS)—4 weeks Male Wistar rats | Standard diet + Orally gavaged fructo-oligosaccharides (FOS)/ galacto-oligosaccharides (GOS) and probiotics (Bifidobacterium longum and Lactobacillus rhamnosus) |
|
| Enteroendocrine alterations and perturbations in tryptophan metabolism | [51] |
| CUMS—4 weeks C57BL6 mice of unspecified sex | Standard diet + orally gavaged Bifidobacterium longum subspecies infantis strain CCFM687 in 10% skimmed milk solution at 109 CFU/mL daily for 6 weeks |
|
| Alterations to SCFA regulation of intestinal permeability affected systemic inflammation and HPA axis function, leading to changes in neuroplasticity in the frontal cortex | [54] |
| CUMS—4 weeks Male Sprague Dawley rats | Standard diet + orally gavaged Fluoxetine (1.82 mg/kg), green tea (64.8 mg/kg) or jasmine tea (21.6 mg/kg, 64.8 mg/kg and 194.4 mg/kg) |
|
| Alterations in peripheral (glucagon-like peptide 1) GLP-1 release in the gut with subsequent alterations in vagal-dependent central GLP-1 signalling in the brain | [68] |
| CUMS—4 weeks Male C57BL6 mice | Standard diet + orally gavaged saline, partially hydrolysed guar gum (PHGG) (600 mg/kg)), Fluoxetine (0.5 mg/kg) and PHGG (600 mg/kg) or Fluoxetine (1.0 mg/kg) |
|
| Alterations to SCFA regulation of intestinal permeability leading to changes in serotonergic and dopaminergic neurotransmission in the striatum and hippocampus | [63] |
| CUMS—5 weeks Male C57BL6 mice | Standard diet + orally gavaged CCFM105 Bifidobacterium breve (0.1 mL/10 g body weight) |
|
| Alterations to SCFA regulation of intestinal permeability and colonic enzymes involved in serotonin synthesis affected 5-hydroxytryptophan (5-HTP) levels. 5-HTP is capable of crossing the BBB, thus these alterations influenced neuroplasticity in the hippocampus. Alterations in HPA axis function may have also contributed the changes in brain and behaviour | [42] |
| CUMS—7 weeks Male C57BL6 mice | Standard diet or standard diet + glycated milk casein (Gc) or glycated milk casein fermented with Lactobacillus rhamnosus (FGc) |
|
| Alterations in colonic inflammation may modulate colitis pathology, affecting intestinal permeability and this may affect the transport of gut-derived molecules such as inflammatory mediators in circulation which can reach the brain. These alterations, which may also involve the HPA axis, may affect neuroplasticity in the brain | [21] |
| Chronic restraint stress (CRS)—1 week Male C57BL6 mice | Standard diet + orally gavaged saline or Lactobacillus johnsonii |
|
| Alterations in small bowel inflammation may modulate intestinal barrier integrity subsequently impacting hippocampal dopaminergic, serotonergic and GABAergic neurotransmission | [66] |
| CRS—2 weeks Male Swiss mice | Standard diet + orally gavaged Bifidobacterium longum BG0014, Bifidobacterium longum ssp. infantis Bl11471, Bifidobacterium animalis BL0005, Bifidobacterium animalis ssp. lactis 420, Lactobacillus paracasei Lpc-37, Lactobacillus salivarius Ls-33, Lactobacillus plantarum LP12418, Lactobacillus plantarum LP12151, Lactobacillus plantarum LP12407, Lactobacillus acidophilus LA11873, Lactobacillus rhamnosus LX11881 or Lactobacillus helveticus LH0138 (1 × 109 CFU/day for 5 weeks) |
|
| Alterations in behaviour modulated by changes in gut microbiota with impacts on GABAergic neurotransmission in the prefrontal cortex requiring further study | [74] |
| Chronic social defeat stress (CSDS)—10 days Male C57BL6 mice and male CD1 mice | Standard diet + Clostridium butyricum MIYAIRI 588 (>5 × 106/CFU) in drinking water for 4 weeks |
|
| Alterations in neuroinflammation and colonic inflammation modulated by alterations in the gut microbiota and intestinal barrier integrity | [77] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herselman, M.F.; Bailey, S.; Bobrovskaya, L. The Effects of Stress and Diet on the “Brain–Gut” and “Gut–Brain” Pathways in Animal Models of Stress and Depression. Int. J. Mol. Sci. 2022, 23, 2013. https://doi.org/10.3390/ijms23042013
Herselman MF, Bailey S, Bobrovskaya L. The Effects of Stress and Diet on the “Brain–Gut” and “Gut–Brain” Pathways in Animal Models of Stress and Depression. International Journal of Molecular Sciences. 2022; 23(4):2013. https://doi.org/10.3390/ijms23042013
Chicago/Turabian StyleHerselman, Mauritz F., Sheree Bailey, and Larisa Bobrovskaya. 2022. "The Effects of Stress and Diet on the “Brain–Gut” and “Gut–Brain” Pathways in Animal Models of Stress and Depression" International Journal of Molecular Sciences 23, no. 4: 2013. https://doi.org/10.3390/ijms23042013
APA StyleHerselman, M. F., Bailey, S., & Bobrovskaya, L. (2022). The Effects of Stress and Diet on the “Brain–Gut” and “Gut–Brain” Pathways in Animal Models of Stress and Depression. International Journal of Molecular Sciences, 23(4), 2013. https://doi.org/10.3390/ijms23042013







